Abstract
Maladaptive avoidance behaviors are seen in many stress-related psychiatric illnesses. Patients with these illnesses favor passive, avoidant coping strategies rather than adaptive, active coping strategies. Preclinically, coping strategy can be measured in rats using the shock-probe defensive burying test, wherein rats receive a shock from an electrified probe inserted into a test cage that mimics their home cage environment, and behavioral output (immobility or burying) is recorded for 15 min following the shock. Immobility in response to the perceived threat of the shock-probe, associated with elevated stress hormone levels, is regarded as a passive, maladaptive coping strategy. In opposition, burying the probe is associated with lower stress hormone levels and is considered an active, adaptive coping style. In rats, chronic stress induces a shift from active to passive coping in this test (i.e., proportionally less burying and more immobility), modeling the avoidant symptoms presented across many stress-related psychiatric illnesses. The stress-induced shifts in coping style and overall behavioral reactivity to the shock-probe provide a unique and well-validated measure of not only an anxiety-like behavioral response but also coping strategy selection in rat models of psychiatric illness.
Keywords: Active coping, Passive coping, Stress, Behavior, Anxiety, Coping strategy, Avoidance, Animal models
Background
In addition to the “fight, flight, or freeze” response, rats were reported to engage in a specific defensive behavior in response to an aversive stimulus by Hudson in 1950, termed “defensive burying”. This burying behavior was determined to be an innate response of rodents (including rats, mice, hamsters, and ground squirrels) to threats in their burrows (see De Boer and Koolhaas, 2003). Additionally, it was found that rats that are allowed to bury a shock-associated stimulus have less HPA axis activation than rats who were prevented from burying and forced to adopt a passive (immobile) response, suggesting that defensive burying is more adaptive compared with passive responses (De Boer et al., 1990 ; Korte et al., 1992 ; Bondi et al., 2007 ). Originally described by Pinel and Treit in 1978, we have adopted the shock-probe defensive burying test in our lab to model central components of stress-related psychiatric illness, i.e., preference for passive, avoidant coping strategies versus adaptive, active coping strategies. Using the protocol described here, we have shown that chronic unpredictable stress induces a shift from active to passive coping in adult Sprague-Dawley rats in this test ( Jett et al., 2015 ; Hatherall et al., 2017 ; Fucich et al., 2016 and 2018), modeling the maladaptive, avoidant coping strategies adopted by patients across many stress-related psychiatric illnesses ( Koolhaas et al., 1999 ; Bondi et al., 2007 ). We have previously described the validity of this test as a measure of anxiety-like responding (see Lapiz- Bluhm et al., 2008 ), and have more recently shown the efficacy of novel antidepressant drugs as well as behavioral therapy in reversing chronic stress-induced shifts in coping style choice ( Jett et al., 2015 ; Hatherall et al., 2017 ; Fucich et al., 2016 and 2018). Thus, measuring coping style and aversive stimulus reactivity with the shock-probe defensive burying test in rats is useful in characterizing animal models of stress-related psychiatric illnesses like depression and posttraumatic stress disorder as well as testing novel therapeutic interventions and their mechanisms. Variations of the shock-probe defensive burying test have been used to test mice (e.g., Sluyter et al., 1996 and 1999; López- Rubalcava et al., 2000 ; Paez- Martinez et al., 2003 ) as well as various rat strains (as reviewed in De Boer et al., 2003 ), therefore the protocol described here may feasibly be adapted to any rodent model which displays innate defensive burying.
Materials and Reagents
Certified Sani Chips, identical to rat’s home cage bedding material (Envigo, Harlan, Teklad, catalog number: 7090C)
Polyethylene tubing, PE-50 (Scientific Commodities, catalog number: BB31695-PE/1)
Industrial grade adhesive (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: 24006-0000)
Abrasive sanding sheets (3MTM WetordryTM Abrasive Sheets, aluminum oxide, grade P400) (3M, catalog number: 213Q)
Paper towels
Numbered index cards to identify animals for blind scoring
Masking tape
Permanent marker
Rat (Sprague-Dawley)
Distilled water
10% ethanol
Equipment
Plastic “shoebox” rat cage with plastic barrier lid (ours are 42 cm x 20 cm x 20 cm, identical to our home cages; e.g., Allentown, catalog number: PC10198)
Uninsulated copper wiring, 1 mm diameter (Fisher Scientific, catalog number: 15-545-1B)Manufacturer: Arcor Electronics, catalog number: BARE18GA.
Glass stirring rods, 5 mm diameter x 200 mm length (Fisher Scientific, catalog number: S63449)
Ring stand (Eisco, catalog number: CH0653E1RD4)
Three prong extension clamp (Fisher Scientific, catalog number: 05-769-7Q), attached to ring stand
Alligator clips (Eisco, catalog number: PH1053B), cut and soldered, one to each lead of the shock output cable for the shock generator
Uninsulated wire-wrapped shock probe (Custom fabrication, see Step A1 of Procedure)
Modified test cage and lid (Custom fabrication, see Step A2 of Procedure)
Electric drill
Utility knife
Wire cutters
Shock generator (e.g., Coulbourn Instruments, model: H13-15)
Video camera
Tripod
Computer
Timer
Software
Video recording software, e.g., QuickTime (Apple)
Statistical analysis software, e.g., Statistica (TIBCO Software)
Procedure
-
Fabricating custom equipment
-
Uninsulated, wire-wrapped probes
Cut two segments of copper wire and two segments of polyethylene tubing per glass stirring rod, each approximately 1 m in length.
Glue ~1 cm of the end of one piece of copper wire at an angle near the end of the glass stirring rod, such that the remainder of the copper wire can be coiled around the length of the stirring rod once the glue is dry (see Figure 1).
Glue 1 cm of the end of a polyethylene tubing segment to the rod, adjacent to the wire; glue second piece of copper wire adjacent to tubing, and glue second segment of tubing adjacent to second segment of copper wire. Let the glue dry completely.
Wrap each wire and piece of tubing, in parallel and snugly adjacent to each other, alternating wire/tubing/wire/tubing, around 15 cm of the rod, leaving the last 5 cm of rod bare (mark the 15 cm point on the rod). Wrap 5 loops per cm. A shock will be delivered when the circuit is closed between the two uninsulated wires by the rat touching the probe. The tubing is sandwiched between the two wires along the length of the rod to ensure that the wires do not come into contact with one another and short the circuit. Carefully inspect the wires as they are wrapped to ensure the two copper wires do not make contact with one another.
Glue the four segments again at the 15 cm mark on the glass rod. Once dry, clip the ends of the tubing flush against the probe. Clip the ends of the copper wiring such that ~5 cm of each protrude from the 15 cm mark; these ends should be oriented in opposite directions (ensuring no contact made between the two wires), and will serve as the point of contact for the alligator clips that will connect the probe to the shock generator.
Again, inspect the probe carefully to ensure the copper wires are separated by the tubing along the entire length of the probe. Use sandpaper to remove any excess glue covering the wires at the end of the probe (where the rats will make contact). You can test the probes for short circuits by connecting them to the shock generator (see Step B9 of Procedure).
-
Modified test cage and lid
Measure 5 cm from the bottom of several points around the plastic test cage and mark with a permanent marker. Use a straight edge to create a dashed line on the outside of the test cage at the 5 cm mark, and use this as a “fill line” when adding fresh bedding for testing (see Figure 2).
On the short end of the plastic cage, mark a point that is 7 cm from the bottom of the cage and centered horizontally. Drill a 2 cm diameter hole at this point, which is where the shock-probe will be inserted into the cage. Sand the inside edges of the hole to be smooth.
Remove the fabric barrier from the cage lid, and cut away any plastic ribs or sheeting that cover the center of the lid. Leave the perimeter of the lid intact, as this will prevent the rat from attempting to jump out of the cage during testing. You want to be able to place the rat into the cage without removing the lid, and be able to see the rat clearly in all parts of the cage from a camera mounted directly above the cage while the lid is in place. Sand the edges of any cut plastic on the lid to be smooth. If animals are not housed with a barrier lid over any wire bar lids as ours are, we do not suggest modifying the wire bar lid for testing but rather recommend purchasing a barrier lid as referenced in the materials list. Additional tools would be required to cut a hole into the wire bars and smooth the cut edges, and the animal would be able to climb out of a modified wire bar lid unless it is raised.
-
-
Setting up and testing equipment
-
Set up equipment as in Figure 3A
Place test cage on the floor beneath a tripod-mounted camera (providing a complete view of the test cage). Mark the position of the cage on the floor with tape or marker to ensure consistent cage placement. In locating the cage and tripod, it is important to avoid shadows or bright spots which can influence the rat’s behavior or can interfere with clear vision while scoring.
Place a ring stand on the floor, such that a shock-probe may be clamped to it and inserted into the 2 cm hole on the short side of the test cage. Also, mark the position of the ring stand on the floor with tape.
Place a partition or curtain behind the ring stand to block the rat’s view of the experimenter during testing.
Create experimenter’s workstation behind the partition, to include a desk containing a computer with video recording software (e.g., QuickTime) connected to the tripod-mounted camera, and a shock generator within reach of the experimenter that can connect to the shock-probe when clamped to the ring stand.
Turn on tripod-mounted camera, computer, and shock generator.
Open a new recording in video recording program (e.g., for QuickTime: Open QuickTime. Select “File”→”New Movie”).
Ensure the camera is connected to the computer, feed is detected by video recording software, and field of view incorporates the entire overhead view of the test cage when the cage is placed beneath the camera. Also, ensure destination folder for video recordings has ample memory space for new recordings.
Fill the test cage with 5 cm of new Sani Chips bedding and position cage (with lid on) on the floor beneath the camera, with the aid of the floor markings for consistent placement.
Lightly brush the surface of the wire on the probe with fine abrasive paper and wipe with a dry paper towel. This removes any biofilm that may coat the wire over time and compromise shock delivery upon contact with the probe and also removes any debris that may potentially short the circuit between the two wires.
Clamp the wire-free end of the rod and attach it to the ring stand. Move the ring stand into position to insert the probe into 2 cm hole in the end of the test cage. The probe should be positioned 2 cm above the surface of the 5 cm-deep layer of Sani Chips bedding material, and it should protrude 6 cm into the test cage.
Connect the 2-pole output of the shock generator to the probe by clamping one alligator clip to one length of uninsulated copper wire and the other alligator clip to the other length of uninsulated copper wire (see Figures 3B and 3C).
-
Set shock generator to deliver 2 mA (see Figures 4A and 4B)
Flip “METER RANGE” toggle to “HIGH”.
Flip “SHOCK ROUTING” toggle to “SET/TEST”.
Hold down “OPERATE” toggle on “MANUAL” while dialing the “SET SHOCK” knob on the “MANUAL” panel until the meter reads 2 mA. Manually check current reading to ensure no shorting of the circuit by flipping the “SHOCK ROUTING” toggle to “SUBJECT” and holding down the “OPERATE” toggle. The current reading should be < 0.2 mA when setting up before testing. If it is higher, remove the probe and clean again with sandpaper and a dry paper towel, and visually inspect the probe for a short circuit (contact between the wires). If the manual current reading remains high, switch to a different probe, clean the first probe with distilled water and dry with a paper towel. Allow the first probe to air dry thoroughly before using again.
Bring subjects (in home cages) into the testing room and allow them to acclimate to the testing room for 60 min (see Note 1). Rats in our experiments were adult Sprague-Dawley rats, housed in a 12 h/12 h light/dark cycle and tested during the lights-on phase in white light. We have never tested animals during the dark (lights-off) phase, but anticipate testing during this time could increase the animals overall motor activity in the test and some reports indicate increases in burying in rats tested in darkness vs. light (De Boer et al., 1991 ; Pinel et al., 1994 ). Animals were singly housed and handled regularly for a minimum of two weeks before behavioral testing, during which time they received chronic stress or control handling treatment.
-
-
Conducting the shock-probe defensive burying test
Start video recording (“Record” in QuickTime) and place numbered blind-card under camera briefly to identify the test subject. Keep a separate record of what experimental condition corresponds to the card number in order to break the blind after videos have been scored.
Place rat gently into the far end of the test cage (with the lid on), facing away from the probe (see Figure 5).
Press the “manual” toggle on the shock generator until the rat approaches the probe and makes contact.
After the rat is shocked and visibly reacts and withdraws from the probe, release toggle and start a 15 min timer. Sometimes the rat will get shocked twice in rapid succession before withdrawing. If it is unclear whether the rat was shocked, allow the rat to continue exploring the probe until clearly shocked (see Note 2).
After 15 min of recording the rat’s behavioral response to the shock, stop the recording, save the video, and return the rat to its home cage (see Note 3).
Between subjects, replace the bedding in the test cage and clean the shock-probe (resuming from Step B5 of Procedure).
At the end of a test session, dispose of used bedding, clean shock-probes, and wipe down empty test cages with distilled water followed by 10% ethanol.
-
Scoring behavior from video recordings
An experimenter blind to the experimental conditions will score the recorded videos.
When scoring, make a note of the timestamp on the video corresponding to when the rat visibly reacts to receiving a shock from the probe. Behavior will then be scored during the 15 min of video beginning at this time point.
Dependent measures scored during the 15 min following the initial shock and withdrawal from the probe (see Figure 6) include: a) the amount of time the rat spends immobile in the distal half of the test cage, away from the shock-probe. We define immobility as all 4 paws on the bedding, displaying only slight movements associated with breathing, slight postural shifts, ear twitches, sniffing and slight scanning movements of the head. Grooming is not immobility. Turning the torso or lifting a limb is not immobility; b) the amount of time the rat spends burying, which is defined as any displacement of bedding material, including burrowing, flicking, pushing, plowing, or kicking the bedding. The latency to begin burying is another measure that may be recorded (from time of shock to time of first burying activity).
Figure 1. Fabricating the wire-wrapped probe.
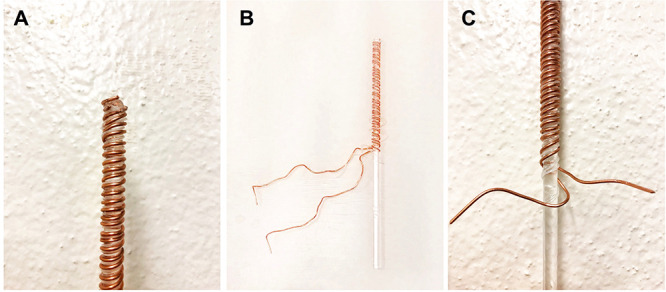
A. Four alternating segments of copper wiring and polyethylene tubing glued to the end of a glass stirring rod at an angle. B. Wires are wrapped around the rod up to the 15 cm mark, with tubing separating the two copper wires. C. Segments of copper wire extend from rod, such that they may be connected to the two-pole socket of a shock generator with alligator clips.
Figure 2. Modified test cage and lid.
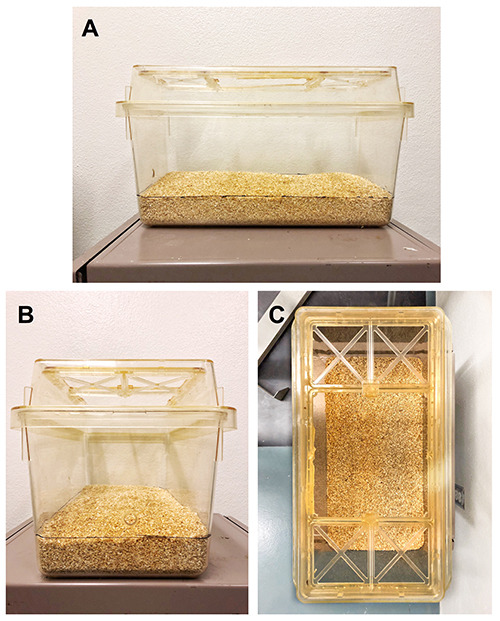
A. “Fill line” is drawn around the cage 5 cm from the cage bottom. B. A 2 cm diameter hole is drilled into the short end of the cage, centered 7 cm from the bottom, for insertion of the shock-probe. C. Plastic is removed from the center of the lid to allow visualization and access to rat in cage during testing.
Figure 3. Testing area.
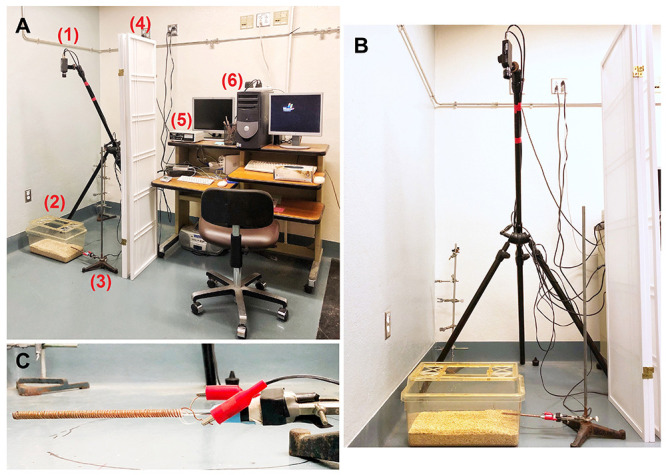
A. Equipment set-up: (1) tripod and camera; (2) cage; (3) ring stand; (4) partition; (5) shock generator; (6) computer; B. Cage positioned under camera, with shock-probe connected to shock generator, clamped to ring stand, and inserted into test cage. C. Close-up of probe clamped to a ring stand, with alligator clips attached.
Figure 4. Shock generator and operation.
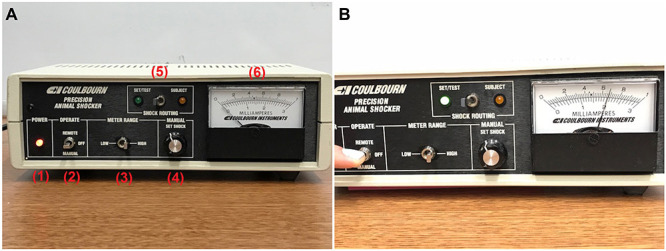
A. Shock generator: (1) POWER indicator (bulb is lit when power is on, power switch on back of shock generator); (2) OPERATE panel with toggle to select between “REMOTE” operation and “MANUAL” operation (manual operation is used for this test, toggle must be held down by experimenter); (3) METER RANGE panel with toggle to select between “LOW” and “HIGH” milliamp setting, corresponding to the top and bottom scales on the milliamps meter, respectively (must be set to “HIGH” for this protocol in order to achieve a setting of 2mA); (4) MANUAL panel with “SET SHOCK” knob that can be dialed to adjust shock setting; (5) SHOCK ROUTING panel with toggle to select between “SET/TEST”, used when manually setting the shock output during setting up, and “SUBJECT”, used when testing the probe for short circuits and when delivering shock to the subject while conducting the shock-probe defensive burying test; (6) milliamps meter, with the lower scale depicting the milliamps readout when the METER RANGE is set to “HIGH”; B. Setting the shock generator to deliver 2 mA. With SHOCK ROUTING toggle set to “SET/TEST” and METER RANGE toggle set to “HIGH”, experimenter holds down OPERATE toggle to “MANUAL” and dials “SET SHOCK” knob on MANUAL panel until needle on milliamps meter reads 2 mA, as depicted. The corresponding bulb on the SHOCK ROUTING panel will light up when OPERATE toggle is flipped to “MANUAL”.
Figure 5. Conducting the shock-probe defensive burying test.
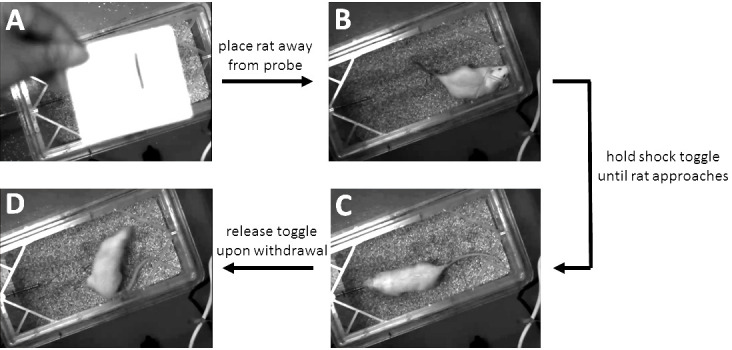
A. Blind-identification card is displayed to the camera before testing. B. Rat is placed into the end of the cage opposite the probe, facing away from the probe. C. Rat approaches the probe and receives a shock. D. After withdrawing from shock, the current is turned off and the 15 min scoring period begins, during which the rat may display immobility, displace bedding, i.e., bury, or engage in “other” behavior, e.g., exploration, grooming, etc., which is not scored (see Figure 6).
Figure 6. Representative images of different behaviors displayed during 15 min test period.
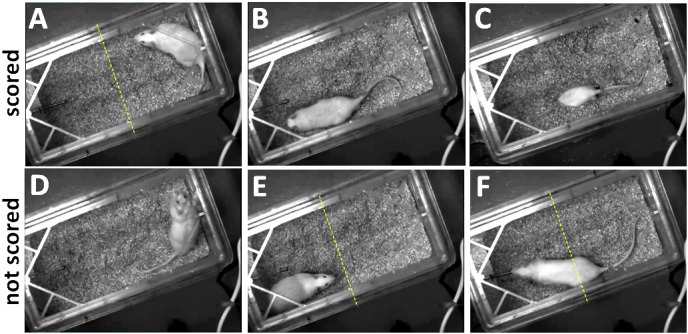
A. Immobility; B. Burying–the rat is pushing bedding toward the probe with the forepaws. C. Burrowing into the bedding (scored as burying). This is often accompanied by flicking or pushing bedding toward the probe. D. Exploratory behavior (not scored); E. Immobility near probe (not scored); F. Immobility while attending to the probe (not scored). Dashed line indicates the midline of the testing area, which serves as the cutoff for where immobility may be scored (i.e., in testing area away from the probe).
Data analysis
Immobility time (in seconds) and burying time (in seconds) are compared between subjects, typically via ANOVA to determine interactions and main effects (e.g., stress or therapeutic intervention) and a suitable test for pairwise comparisons, e.g., Newman-Keuls test. Changes in immobility and burying are interpreted as changes in passive and active coping, respectively, and increases or decreases in both measures are interpreted as increases or decreases in overall behavioral reactivity.
When a new investigator begins using the test, inter-rater reliability is assessed by conducting a correlation analysis of the immobility and burying scores generated by the new investigator and an experienced investigator scoring the same videos. A correlation coefficient of > 0.95 is required for a new investigator to begin scoring the shock-probe defensive burying test independently.
-
The bury ratio is used as a proportional measure of preferred response, with a bury ratio above 0.5 indicating a preference for active coping, a bury ratio below 0.5 indicating a preference for passive coping, and a bury ratio equal to 0.5 indicating no preference. The bury ratio (but see Note 4) is calculated as:
Thus, changes in bury ratio between groups can reveal shifts in coping style. For example, we typically see a shift from active to passive coping in chronically stressed rats compared to unstressed control rats. Bury ratios are analyzed similarly to immobility time and burying time, typically by ANOVA and, e.g., Newman-Keuls test for pairwise comparisons.
Latency to bury (in seconds) and pile height (in centimeters, measured at the time of testing) can also be analyzed in this way, with decreases in bury latency and increases in pile height corresponding to a more active coping style.
Notes
On the behavioral testing day, notes were made to animal care staff not to change cages/water/food. The experimenter would perform these care tasks only after behavioral testing was complete, to avoid variability in motor activity in the shock-probe test due to different housing conditions between subjects on the day of testing.
It has been well-established that rats faced with a novel object in their environment will contact it. Rats will typically make contact with the probe within 30 sec ( Fucich et al., 2018 ). In the rare case, the rat will not approach the probe to receive a shock within 15 min of beginning the test, discontinue testing and exclude the subject from the experiment, noting the exclusion.
As an alternative measure to time spent burying, the “pile height” may also be measured after returning the rat to its home cage (i.e., the height in cm of the highest point of bedding in the test cage after the 15 min test session; Note that this measure cannot be obtained from video recordings). This measure can be used as an index of “amount of burying” in addition to the “time spent burying” scored from the videos (Procedure C).
We have found that not all rats display burying behavior, sometimes up to approximately 20% of the rats in a study. If this results in non-normality, it may be necessary to apply non-parametric statistics to analyze the bury score. More importantly, this can artificially skew the bury ratio to zero (as the numerator will be 0), even though differing amounts of immobility are displayed even by animals that do not exhibit burying. Thus, to avoid generating misleading bury ratio scores, to calculate the bury ratio, any bury score of 0 has 1 sec added to it. In such cases, the bury ratio is then calculated as (burying time + 1 sec)/(burying time + 1 sec + immobility time), thereby maintaining the information conveyed by the differing immobility scores.
Unpublished findings from our lab suggest that surgical disruption of medial prefrontal cortex with chronic cannula implants can abolish burying behavior. Thus, when we have tested the role of this brain region in defensive burying, we have used angled approaches to target the medial prefrontal cortex while minimizing tissue damage to this region with our implants (see Fucich et al., 2016 and 2018).
Acknowledgments
This work was supported by National Institute of Mental Health Research Grants MH072672 and MH053851, National Institutes of Health T32 Training Grant NS082145, National Center for Advancing Translational Sciences Fellowship Grant TL1 TR001119, U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program Merit Award 1I01BX003512, and William and Ella Owens Medical Research Foundation. The contents of this paper do not represent the views of the Department of Veterans Affairs or the U.S. Government. This protocol was adapted from procedures published in Fucich et al. (2018), Roth et al. (2012), and Lapiz-Bluhm et al. (2008).
DA Morilak receives research funding from Alkermes and in-kind research support from H. Lundbeck A/S. These activities have no relation to any of the work presented in this paper.
Competing interests
The authors declare no conflict of interest.
Ethics
All procedures were in accordance with NIH guidelines and approved by the UTHSCSA institutional animal care and use committee.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Bondi C. O., Barrera G., Lapiz M. D., Bedard T., Mahan A. and Morilak D. A.(2007). Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry 31(2): 482-495. [DOI] [PubMed] [Google Scholar]
- 2. De Boer S. F. and Koolhaas J. M.(2003). Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol 463(1-3): 145-161. [DOI] [PubMed] [Google Scholar]
- 3. De Boer S. F., Slangen J. L. and Van der Gugten J.(1990). Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav 47(6): 1089-1098. [DOI] [PubMed] [Google Scholar]
- 4. De Boer S. F., van der Gugten J. and Slangen J. L.(1991). Behavioural and hormonal indices of anxiolytic and anxiogenic drug action in the shock prod defensive burying/avoidance paradigm. Animal models in psychopharmacology pp: 81-96. Birkhäuser, Basel. [Google Scholar]
- 5. Fucich E. A., Paredes D. and Morilak D. A.(2016). Therapeutic effects of extinction learning as a model of exposure therapy in rats. Neuropsychopharmacology 41(13): 3092-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fucich E. A., Paredes D., Saunders M. O. and Morilak D. A.(2018). Activity in the ventral medial prefrontal cortex is necessary for the therapeutic effects of extinction in rats. J Neurosci 38(6): 1408-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatherall L., Sánchez C. and Morilak D. A.(2017). Chronic vortioxetine treatment reduces exaggerated expression of conditioned fear memory and restores active coping behavior in chronically stressed rats. Int J Neuropsychopharmacol 20(4): 316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hudson B. B.(1950). One-trial learning in the domestic rat. Genet Psychol Monogr 41(First Half): 99-145. [PubMed] [Google Scholar]
- 9. Jett J. D., Boley A. M., Girotti M., Shah A., Lodge D. J. and Morilak D. A.(2015). Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology(Berl) 232(17): 3123-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. and Blokhuis H. J.(1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23(7): 925-935. [DOI] [PubMed] [Google Scholar]
- 11. Korte S. M., Buwalda B., Bouws G. A., Koolhaas J. M., Maes F. W. and Bohus B.(1992). Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav 51(4): 815-822. [DOI] [PubMed] [Google Scholar]
- 12. Lapiz-Bluhm M. D., Bondi C. O., Doyen J., Rodriguez G. A., Bedard-Arana T. and Morilak D. A.(2008). Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol 20(10): 1115-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Rubalcava C., Hen R. and Cruz S. L.(2000). Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behav Brain Res 115(1): 85-94. [DOI] [PubMed] [Google Scholar]
- 14. Paez-Martinez N., Cruz S. L. and Lopez-Rubalcava C.(2003). Comparative study of the effects of toluene, benzene, 1,1,1-trichloroethane, diethyl ether, and flurothyl on anxiety and nociception in mice. Toxicol Appl Pharmacol 193(1): 9-16. [DOI] [PubMed] [Google Scholar]
- 15. Pinel J. P., Mumby D. G., Dastur F. N. and Pinel J. G., (1994). Rat(Rattus norvegicus) defensive behavior in total darkness: Risk-assessment function of defensive burying. J Comp Psychol 108(2): 140-147. [DOI] [PubMed] [Google Scholar]
- 16. Pinel J. P. and Treit D.(1978). Burying as a defensive response in rats. J Comp Physiol Psychol 92(4): 708-712. [Google Scholar]
- 17. Roth M. K., Bingham B., Shah A., Joshi A., Frazer A., Strong R. and Morilak D. A.(2012). Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology 63(6): 1118-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sluyter F., Korte S. M., Bohus B. and Van Oortmerssen G. A.(1996). Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav 54(1): 113-116. [DOI] [PubMed] [Google Scholar]
- 19. Sluyter F., Korte S. M., Van Baal G. C., De Ruiter A. J. and Van Oortmerssen G. A.(1999). Y chromosomal and sex effects on the behavioral stress response in the defensive burying test in wild house mice. Physiol Behav 67(4): 579-585. [DOI] [PubMed] [Google Scholar]


