Abstract
Over the past decade, major technological and analytical advancements have propelled efforts toward identifying the molecular mechanisms that govern human adaptation to high altitude. Despite remarkable progress with respect to the identification of adaptive genomic signals that are strongly associated with the “hypoxia-tolerant” physiological characteristics of high-altitude populations, many questions regarding the fundamental biological processes underlying human adaptation remain unanswered. Vital to address these enduring questions will be determining the role of epigenetic processes, or non-sequence-based features of the genome, that are not only critical for the regulation of transcriptional responses to hypoxia but heritable across generations. This review proposes that epigenomic processes are involved in shaping patterns of adaptation to high altitude by influencing adaptive potential and phenotypic variability under conditions of limited oxygen supply. Improved understanding of the interaction between genetic, epigenetic, and environmental factors holds great promise to provide deeper insight into the mechanisms underlying human adaptive potential, and clarify its implications for biomedical research.
Keywords: adaptation, hypoxia, epigenetics, DNA methylation, high altitude
exposure to the environmental hypoxia of high altitude profoundly challenges oxygen homeostasis, one of the most fundamental processes required for survival. Yet, human populations have occupied highland regions of the Qinghai-Tibetan Plateau, the Andean Altiplano, and the Semien Plateau of Ethiopia for thousands of years, in some cases establishing communities at altitudes exceeding 5,000 m (1, 76, 83). These highlanders exhibit unique “hypoxia-tolerant” physiological characteristics that are paralleled by strong genetic signals of recent positive selection (e.g., 6, 12, 13, 30, 57, 83, 86, 94, 110), some of which are in close proximity to genes that are essential for the regulation of transcriptional responses to hypoxia. Recent attention, therefore, has predominantly been directed toward identifying the genetic mechanisms that govern human adaptation to the high-altitude environment. It is of fundamental importance to emphasize, however, that phenotypes, the objects on which selective pressures act, are rarely determined by genetic variation alone. Rather, phenotypes more often arise through the complex interaction of multiple genes, gene-environment interaction, and the functional interaction of the genome with a wide variety of epigenetic marks, or non-sequence-based features of the genome, that are critical for coordinating transcriptional responses to environmental and developmental stimuli.
Here, we discuss the possibility that epigenomic processes are involved in shaping patterns of human adaptation to high altitude by influencing adaptive potential and phenotypes that improve reproductive fitness and survival under conditions of chronic hypoxia. Several distinct epigenetic mechanisms exist, including DNA methylation, histone modification, histone variants, and RNA-based mechanisms. This review focuses on cytosine methylation, defined by the addition of a methyl group to the C-5 position of cytosine residues within CpG dinucleotides, given its prominent role for transcriptional regulation, silencing of repetitive DNA elements, and genomic imprinting (41, 79). Across the human genome, the vast majority of CpG sites are methylated (31, 82). Interspersed among these, however, are regions containing a high density of CpGs in sequence (“CpG islands”) that are typically devoid of methylation, a feature that most often promotes transcription factor binding and active gene transcription. In contrast, hypermethylation of CpG islands often prohibits transcription factor binding, thereby establishing a quiescent chromatin state (22). However non-canonical epigenetic strategies also exist (20, 39, 40, 49, 109). This review begins with a discussion of the central role of epigenetic processes in human adaptability, the involvement of epigenetic processes for transcriptional and developmental responses to hypoxia, and epigenetic inheritance. Using two high-altitude phenotypes that are paralleled by strong genetic signals of recent positive selection, the possibility that adaptive genetic variants affect the capacity for the epigenetic regulation of gene transcription under hypoxic conditions is then considered. Also highlighted are important conceptual issues, outstanding questions and promising avenues of research, emphasizing the importance of understanding the role of genome-epigenome interactions.
Epigenetics, the Epicenter of Adaptation
Human adaptability: a dynamic process.
Models of human adaptability typically divide the adaptive process into discrete elements that vary with respect to the timescale, durability, and mode of acquisition for a given adaptation. On the most transient end of the adaptive spectrum are physiological changes that serve to maintain homeostasis under rapidly shifting environmental conditions. Acute hypoxic exposure, for instance, transiently increases arterial chemoreceptor activity, thereby raising alveolar ventilation (i.e., the hypoxic ventilatory response) and, in turn, the partial pressure of oxygen in the arterial blood. Environmental exposures during critical developmental periods such as perinatal life, on the other hand, can induce durable physiological changes that are retained into adulthood, a process that is commonly known as developmental programming or developmental plasticity. Chronic hyperoxia during the postnatal period, for example, modifies carotid body function and, as a result, impairs the hypoxic ventilatory response in ways that continue into adulthood (21, 100). On the most durable and gradual end of the adaptive spectrum lies natural selection, the process by which genetic variants producing phenotypes that improve survival and reproductive success in a given environment gradually increase in frequency within the population, thereby propagating adaptive features.
An alternative, yet complementary, view is that human adaptation is dynamic, requiring the interaction of the various modes of adaptation. In this model, epigenomic processes may be viewed as the central hub that connects environmental, physiological, and genomic input (Fig. 1). First, epigenetic events are essential for the regulation of transcriptional physiological responses to environmental cues including hypoxia (27, 50). However, evidence suggests that physiological responses can also influence epigenetic patterns. In mice, for instance, corticosterone induces anxiety-like behavior that subsequently alters DNA methylation marks in hippocampal and hypothalamic tissue (51). Second, epigenomic processes play a central role in the determination of cellular identity and are implicated in the effect of early life exposures to modify the developmental trajectory of numerous organ systems. During embryonic development and organogenesis, for example, epigenetic processes govern the differentiation of pluripotent cells, each with an identical genetic code, into hundreds of distinct cell types (48, 77). Third, although epigenomic marks exert a powerful influence on the way hardwired genomic sequences are translated into durable phenotypic traits (15, 22), genetic background can influence the probability of epigenetic modification (9, 24, 63, 66, 112). Finally, epigenetic marks can promote changes to genetic sequence via various means (e.g., chromosomal recombination and cytosine to tyrosine transitions resulting from the deamination of methylated CpG motifs) (18, 36, 91). From this vantage point, elements of the adaptive process are highly interdigitated. Prior papers have speculated that epigenetic processes may be important for human acclimatization and adaptation to high altitude (16) and the developmental programming of hypoxia-associated pulmonary vascular dysfunction (84). However, only two publications have reported site-specific methylation data in human high-altitude populations (2, 43) and none, to my knowledge, have integrated genetic and epigenetic mapping to explore the molecular mechanisms underlying human acclimatization to the high-altitude environment or the “hypoxia-tolerant” phenotypes characteristic of indigenous highland populations.
Fig. 1.
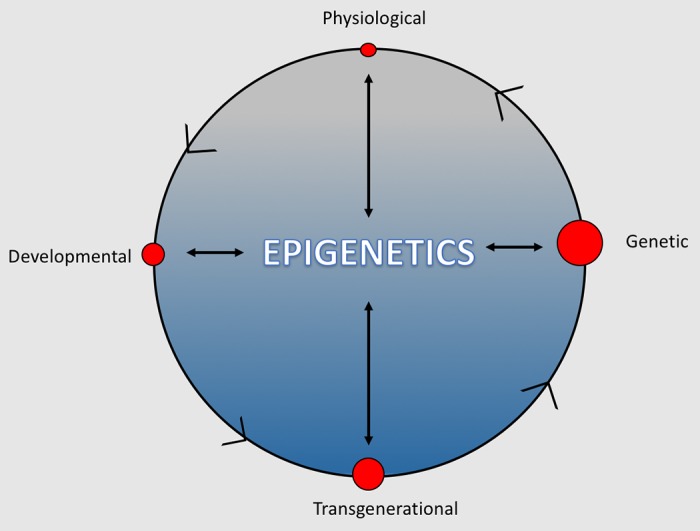
The interactive model of adaptation. Models of human adaptability typically divide the adaptive process into discrete elements that vary with respect to the timescale, durability, and mode of acquisition for a given adaptation. The outer ring depicts these traditional modes of adaptation—physiological, developmental, transgenerational, and genetic—as separate entities. The size of the adjacent dots represents the relative time needed to acquire such adaptations and their durability once acquired. An alternative, yet complementary, view is that human adaptation is dynamic, requiring the interaction of the various modes of adaptation. In this case, epigenomic processes may be viewed as the central hub that connects environmental, physiological, and genomic elements of the adaptive process.
Importance of DNA methylation for transcriptional responses to hypoxia.
Epigenetic events and hypoxia-inducible transcription factors (HIF) work in coordination to elicit a robust transcriptional response to hypoxia (85). HIF consists of two α-subunits (HIF1α and HIF2α) and a constitutively expressed β-subunit. Under normoxic conditions, prolylhydroxylases (PHDs), which are oxygen-dependent negative regulators of HIF, facilitate the hydroxylation of proline residues on the HIF1/2α subunits (17) which promotes the binding of von Hippel–Lindau tumor suppressor (vHL) protein and, subsequently, the proteasomal degradation of HIF1/2α (34, 35). Under hypoxic conditions, PHD does not hydroxylate HIF1/2α, allowing these subunits to escape recognition by vHL, bind with hypoxia responsive elements and associated cofactors, and initiate HIF-regulated gene transcription and cellular adaptation to hypoxia (62).
Epigenetic silencing of genes involved in HIF stabilization, including vHL and endothelial PAS Domain Protein 1 (EPAS1), the gene encoding HIF2α, are integral for regulating the HIF transcriptional program (27, 50). For instance, DNA methyltransferase 3a induces the de novo methylation of CpG motifs located within the EPAS1 promoter and thereby prohibits HIF2α-mediated gene expression and, in turn, the proliferation of benign, differentiated cells under hypoxic conditions (50). In contrast, defective DNA methyltransferase 3a prevents the epigenetic silencing of EPAS1 in cancer cells, resulting in the unscheduled activation of EPAS1 and malignant tumor growth (50). Further illustrating the importance of epigenetic processes for the transcriptional responses to hypoxia, the hypoxic induction of erythropoietin, which is considered to be the master regulator of red blood cell production at high altitude, is mediated through DNA methylation events in the erythropoietin promoter and 5′-untranslated region (111). Moreover, histone acetyltransferases and demethylases, enzymes that modify the epigenetic state of histones and cytosine residues, respectively, are regulated in part by hypoxia and are involved in determining whether chromatin conformation within and around HIF-binding sites is transcriptionally active or inactive (46, 98, 99). Interruption of these epigenetic processes could plausibly compromise, or even perhaps refine, transcriptional responses that are integral to sustain tissue metabolism and oxygenation under the chronic hypoxia of high altitude.
Epigenetics and the developmental programming of physiological responses to hypoxia.
During early life, the epigenome is particularly vulnerable to environmental insults and appears to be central for the effect of environmental exposures occurring during critical developmental periods to influence physiological responses to hypoxia in adulthood. In an experimental murine model, for instance, intrauterine hypoxia results in the hypermethylation of CpG motifs in the promoter region of protein kinase C epsilon, which encodes a protein that improves cardiovascular hemodynamics in ischemia-reperfusion injury; this reduces cardiac protein kinase C epsilon expression and, ultimately, enhances susceptibility to ischemia-reperfusion injury during adulthood (55, 70, 106). Similarly, in rats, intermittent hypoxia during the neonatal period evokes DNA methylation changes that augment hypoxic sensitivity of the carotid bodies and adrenal chromaffin cells in adulthood (68). In support of the involvement of epigenetic factors in the fetal programming of hypoxia-induced pulmonary vascular dysfunction in mice, maternal undernutrition during pregnancy exaggerates pulmonary vascular dysfunction in response to hypoxia and modifies global DNA methylation levels in the lung of affected offspring (78). In this restrictive diet model, treating affected progeny with epigenetic modifiers (histone deacetylase inhibitors) not only normalized pulmonary DNA methylation levels but also pulmonary vascular function (78). Hypoxia-related exposures during the perinatal period also appear to influence adaptive capacity of the pulmonary vascular system to the hypoxia of high altitude in humans. The earliest paper to test this hypothesis showed that lowland residents who experienced transient perinatal hypoxic pulmonary hypertension during perinatal life had a nearly 34% greater rise in pulmonary artery pressure in response to short-term, high-altitude exposure (4,559 m) during adulthood compared with controls (81). Recent studies of Andean highlanders (3,600–4,100 m) further demonstrate that adverse perinatal oxygenation raises the risk of excessive erythrocytosis (EE), a preclinical form of chronic mountain sickness, and attendant pulmonary vascular dysfunction in early adulthood 6.4-fold (42). Basal pulmonary artery pressure is also higher in individuals born to women with preeclampsia (a hypoxia-related, hypertensive disorder of pregnancy) vs. normotensive women at high altitude (37). Indicating that such effects may be due to epigenetic modification of gene expression, offspring of preeclamptic women who went on to develop modest pulmonary vascular dysfunction at high altitude show distinct methylation-expression relationships within several vascular-related genes (43). Taken together, such studies support the premise that adverse oxygenation during critical developmental periods induces epigenetic modifications that influence physiological responses to hypoxia in later life, yet much work remains to be done in this area.
Inheritance of epigenetic diversity.
To be relevant for human adaptation on an evolutionary scale, epigenetic marks must be inherited. In humans and other mammals, the heritability of epigenetic marks remains a subject of debate, mainly because nonimprinted genes undergo vast, but incomplete, reprogramming before implantation (88). However, environmentally induced methylation changes have been shown to extend to subsequent generations (3, 78, 80) and increasing evidence indicates that DNA methylation marks can be transmitted through both germline and somatic pathways (28). For example, constitutional epialleles, or epigenetic marks originating from the parental germ line or early embryo, appear to be stable across meiotic division and thereby provide one avenue for pure transgenerational epigenetic inheritance (28). Recent work also provides compelling evidence that somatic epigenetic modifications may not need to be conveyed through the gamete intact, but may instead be transferred via epigenetic modifying RNA species (19). Therefore, heritable DNA methylation marks may completely avoid the epigenetic reprogramming that occurs early in development. Genetic variation at specific loci [e.g., single-nucleotide polymorphisms (SNPs)] also heavily influence DNA methylation status (9, 24, 63, 66, 112). A recent report suggests that more than 80% of genetic variants that disrupt a CpG motifs modify DNA methylation status, not only at the CpG-disrupting SNP site itself but also extending to CpG motifs located up to 10 kb distant (113). These observations raise new questions about how genetic factors orchestrate a system of traits at high altitude and indicate that putatively adaptive genetic variants that modify CpG motifs should spark particular interest.
Genetic Variants Associated with Adaptive High-Altitude Phenotypes
Several of the genomic signals showing evidence of recent positive selection in high-altitude populations are associated with physiological characteristics such as reduced hemoglobin levels and the protection of fetal growth that are considered beneficial for reproductive fitness at high altitude (e.g., 6, 13, 57, 83, 86, 93, 108, 110). Under conditions of chronic hypoxia, acclimatized lowlanders and Andeans have notably higher hemoglobin concentrations than Tibetans and Ethiopians, who retain values within the range of sea-level normal despite residing at altitudes upwards of 3,500 m (5, 7, 8, 23, 53, 97, 102). Modest increases of red blood cell mass raise arterial oxygen content under conditions of ambient hypoxia. However, excessive red blood cell production, such as that observed in chronic mountain sickness (CMS), is considered to be maladaptive given that the resultant increase in blood viscosity impairs blood flow and oxygen delivery to tissues (74, 75). CMS is defined by elevated hemoglobin levels (≥19 g/dl for women, 21 g/dl for men) accompanied by at least three of the following symptoms: dyspnea, palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, or tinnitus occurring in the absence of underlying cardiopulmonary disease (54). Complications of the pulmonary circulation attendant to CMS raise the risk of severe disability and mortality from pulmonary hypertension and right heart failure (61, 71, 95). Compared with Andeans, Han Chinese, and acclimatized lowlanders, Tibetans are comparatively protected against CMS despite living at similar or greater altitudes (67, 69, 96, 103, 104). Given the association between CMS and increased mortality, genomic regions showing evidence of recent positive selection that are associated with lower hemoglobin levels are of particular interest. For example, EPAS1, egl-9 family hypoxia-inducible factor 1 (EGLN1), peroxisome proliferator activator receptor alpha (PPARA), hypoxia upregulated 1 (HYOU1), hydroxymethylbilane synthase (HMBS), and thyroid hormone receptor beta (THRB) variants are associated with lower hemoglobin in Tibetan (EPAS1, PPARA, EGLN1) (6, 86, 93, 110), Sherpa (EPAS 1, HYOU1, HMBS) (38), or Amhara Ethiopian (THRB, EPAS1, and PPARA) (83) residents of high altitude. Implicating the functional importance of EGLN1 for unique phenotypes of native high-altitude populations, a high-frequency, protein-coding EGLN1 variant (Asp4Glu/Cys127Ser) identified in Tibetans reduces erythroid progenitor proliferation, erythropoietin sensitivity, and hemoglobinization under hypoxic conditions (57). Further experimental studies indicate that this specific Tibetan-associated EGLN1 variant impairs PHD2-mediated downregulation of the HIF pathway in cultured HEK293 FT cells (89). In the aggregate, these studies establish that select, high-frequency EGLN1 genetic variants observed in highland Tibetans may contribute to unique cellular and molecular responses to hypoxia.
Highland ancestry also confers protection against the increased incidence of intrauterine growth restriction observed at high altitude (44, 47). As a consequence, compared with European infants born at high altitude (3,100–3,600 m), Andean infants have a fivefold lower risk of intrauterine growth restriction (44). Intrauterine growth restriction raises the risk of perinatal mortality 8- to 20-fold and often occurs in tandem with preeclampsia, a hypoxia-related complication of pregnancy that accounts for 40% of fetal and 18% of maternal deaths, and increases the risk of premature cardiovascular disease in affected mothers 2- to 10-fold (4, 10, 25, 32, 58, 87, 101). For this reason, physiological attributes that protect fetal growth and maternal vascular adaptation to pregnancy under conditions of ambient hypoxia should be subject to intense selective pressure at high altitude. In Andeans, this effect appears to stem, in part, from greater uteroplacental blood flow and oxygen delivery during pregnancy at high altitude in women of Andean vs. European ancestry (45). Genomic regions showing evidence of natural selection in Andeans are associated with greater birth weight [adaptive protein kinase, AMP-activated, alpha 1 (PRKAA1) and endothelin receptor alpha (EDNRA)] and larger uterine artery diameter (PRKAA1) at high altitude (13). Strongly suggesting the involvement of gene-by-environment interaction for the protection of fetal growth and uteroplacental vascular responses during high-altitude pregnancy, enhanced uteroplacental blood flow, greater birth weight, and the association of PRKAA1 and EDNRA with birth weight identified in Andeans are confined to high altitude (45). This raises the possibility that greater phenotypic flexibility under hypoxic conditions may provide a selective advantage.
Genomic studies, such as those described above, are poised to unravel important functional links between adaptive genetic signals and prominent high-altitude phenotypes. Equally important clues, however, likely reside in CpG-rich regions of the genome, particularly because the vast majority of genetic variants identified by genomewide association studies do not affect protein-coding sequence.
Are Epigenomic Processes Involved in Shaping Patterns of Human Adaptation to High Altitude?
As reviewed above, substantial evidence demonstrates that SNPs, particularly those located at CpG sites, are important for the regulation of genome-epigenome interactions that, in turn, govern transcriptional and developmental responses to hypoxia. Here, we consider the possibility that genetic variants showing evidence of natural selection in high-altitude populations may affect the capacity for the epigenetic regulation of gene transcription in ways that increase reproductive fitness under hypoxic conditions. To accomplish this aim, putatively adaptive EPAS1 SNPs available in published literature were first examined to determine whether these SNPs resulted in the loss or gain of CpG motifs and, if so, whether these SNPs were located in or near transcriptionally active regions of the genome. EPAS1 was chosen as an illustration for several reasons. First, EPAS1 is epigenetically regulated under hypoxic conditions and its promoter is fully enveloped within a CpG island (50). Second, because EPAS1 encodes HIF-2α, the potential for its functional involvement for adaptation to hypoxia is high. Third, adaptive genomic signals near EPAS1 are evident in multiple high-altitude populations, and independent research groups have reported functional associations between putatively adaptive variants and reduced hemoglobin concentrations at high altitude.
Also considered is the possibility that epigenetic processes may be involved in maladaptive phenotypes at high altitude, namely, increased susceptibility to chronic mountain sickness in Andeans. The primary goal of this exercise was to test for differentially methylated regions in Andean high-altitude residents with a preclinical form of CMS (i.e., excessive erythrocytosis or “EE”) and to determine their proximity to genetic variants showing evidence of recent selection in highland populations. If the differentially methylated regions identified neighbored such genomic regions, this would raise an important and provocative question: Do select genomic variants exert their effect, in part, by altering the capacity to epigenetically regulate genes that are important for adaptation to chronic hypoxia of high altitude?
Do adaptive EPAS1 SNPs modify CpG motifs?
Sixty-six EPAS1 SNPs enumerated in three publications were queried (6, 72, 105). Of the 38 EPAS1 SNPs identified in Tibetans residing between 4,200 and 4,300 m (6), 14 (37%) resulted in the loss or gain of CpG sites (Table 1). Several of these 14 CpG modifying SNPs were located within transcriptionally active chromatin regions, as determined by strong enrichment for promoter-associated histone marks via chromatin immunoprecipitation-sequencing assays and areas of open chromatin via DNase hypersensitivity assays (112), suggesting their potential importance for the regulation of gene expression. Furthermore, numerous SNPs (e.g., rs2121266, rs4953353, rs6756667, rs3768729) also overlapped transcription factor binding sites (Table 1). Eleven (44%) of the 25 EPAS1 SNPs identified by Xu et al. (105), and one (33%) of three EPAS1 tag SNPs reported by Peng et al. (72) also modified CpG content (Table 1). In total, nearly 40% of the SNPs reported modified CpG content. Given that such SNPs can influence local and distant (~10 kb) methylation status (113) these heritable differences in CpG density may provide greater flexibility with respect to the epigenetic regulation of gene transcription in response to the environmental hypoxia of high altitude. These observations highlight the need for the integrative analyses of genomic and epigenomic variability to determine whether putatively adaptive genomic regions or individual loci showing evidence of natural selection in highland populations influence the epigenetic regulation of gene transcription in ways that are beneficial for reproductive fitness under hypoxic conditions.
Table 1.
Effect of adaptive EPAS1 SNPs on CpG content
| SNP | Allele Frequencies (dbSNP, UCSC) | Effect of SNP on CpG Motif | Ref. No. |
|---|---|---|---|
| rs2121266 | A/C, 58.3/41.7 | AAG || ACG | 6 |
| rs17034950 | G/A, 72.3/27.7 | CGT || CAT | 6 |
| rs11689011 | T/C, 59.4/40.6 | ATG || ACG | 6 |
| rs10193827 | C/T, 79.5/20.5 | CTG || CCG | 6 |
| rs13419896 | G/A, 81.8/18.2 | CAC || CGC | 6, 88, 89 |
| rs4953353 | G/T, 60.5/39.5 | CGT || CTT | 6 |
| rs6756667 | G/A, 72.3/27.7 | CAT || CGT | 6, 89 |
| rs7583554 | C/T, 50.8/49.2 | CTG || CCG | 6 |
| rs3768729 | C/T, 51.7/48.3 | GTG || GCG | 6 |
| rs10206434 | G/A, 89.0/11.0 | CAT || CGT | 6 |
| rs1374749 | A/G, 58.9/41.1 | CGT || CAT | 6 |
| rs1992846 | C/T, 50.6/49.4 | ACG || ATG | 6 |
| rs7557402 | C/G, 59.3/40.7 | CGC || CCC | 6 |
| rs7571218 | A/G, 50.6/49.4 | CGG || CAG | 6 |
| rs6544888 | C/A, 70/30.0 | TAG || TCG | 89 |
| rs7589621 | G/A: 56.0/44.0 | CAT || CGT | 89 |
| rs6755594 | A/G, 53.0/47.0 | CAG || CGG | 89 |
| rs1374749 | A/G, 58.8/41.1 | CAT || CGT | 89 |
| rs11675232 | T/C, 71.5/28.6 | TCG || TTG | 89 |
| rs7571218 | A/G, 50.6/49.4 | CAG || CGG | 89 |
| rs1109285 | G/C, 73.5/26.5 | CCG || CGG | 89 |
| rs1447563 | A/C, 73.1/26.9 | AAG || ACG | 89 |
| rs7582701 | C/G, 33.5/66.5 | GGC || GCG | 89 |
Allele frequencies for each SNP and the effect of the SNP on CpG content are shown. For example, for rs2121266 the C allele increases CpG content (AAG > ACG).
EE-associated differentially methylated regions.
Genomewide methylation studies were performed in peripheral blood mononuclear cells (PBMCs) obtained from young Andean men residing in La Paz-El Alto, Bolivia (3,600–4,100 m) with EE and healthy, age- and altitude-matched controls (n = 6, each). PBMCs were chosen for use because erythropoiesis and erythroid differentiation depend, in part, on cytokines produced by PBMCs (e.g., 26, 59) as well as epigenetic and transcriptional changes within erythroid precursors themselves. Using the Comprehensive High-Throughput Relative Methylation method (33), several EE-associated differentially methylated regions were identified at base-pair resolution, the most notable of which was a hypermethylated region within EGLN1, the gene encoding PHD2, an enzyme that is critical for the proteasomal degradation of HIF1/2α and, in turn, the inhibition of HIF-regulated gene transcription. The hypermethylation of EGLN1 observed in EE would be expected to reduce PHD2 expression and promote the transcription of HIF-regulated genes, including erythropoietin. Given that inactivation of EGLN1 in mice leads to polycythemia resulting from overproduction of erythropoietin and EGLN1 mutations have been linked to familial polycythemia in humans (65, 73, 92), it is enticing to propose that the hypermethylation of EGLN1 observed contributes to the excessive production of red blood cells that defines EE. Further work is of course required to validate these initial findings and to determine the functional importance of the differentially methylated regions identified.
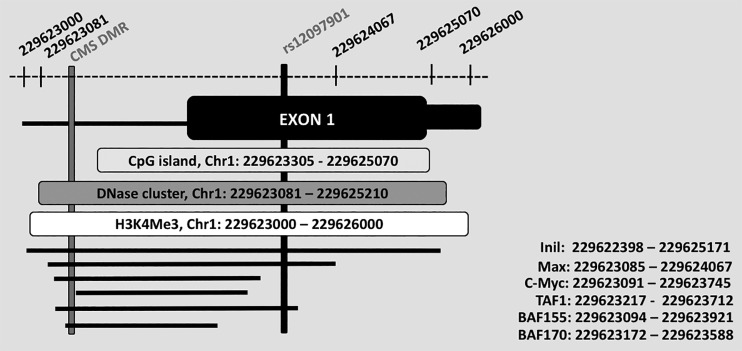
As illustrated in Fig. 2, the differentially methylated region identified for EGLN1 is located within 700 bp of an EGLN1 SNP (rs12097901) that shows strong evidence of selection in Tibetans and lies within the CpG island that encompasses the dominant EGLN1 promoter. Notably, the EGLN1 promoter contains HIF-1 specific binding site (64), and overlaps several transcriptional factor binding sites and regions of DNase hypersensitivity, further emphasizing the potential regulatory importance of rs12097901. In Tibetans, the rs12097901 SNP is strongly associated with reduced hemoglobin concentration and appears to influence PHD2 binding, although the nature of the latter remains the subject of some debate (14). The EGLN1 rs12097901 SNP is also present in Andeans, albeit at a lower frequency, suggesting that adaptive benefits or functional effects associated with this particular SNP may also be apparent in select individuals belonging to other high-altitude populations.
Fig. 2.
A differentially methylated region within EGLN1 identified in Andeans with excessive erythrocytosis (EE). As shown, the EE-associated differentially methylated region identified in Andean high-altitude residents (gray vertical bar; Chr1: 229,623,151–229,623,259) is located only 46 bp from the CpG island (Chr1: 229,623,305–229,625,070) that encompasses the EGLN1 promoter and <700 bp from rs12097901 (black vertical bar, Chr1: 229623259), suggesting its potential functional importance. Further supporting the possibility that this differentially methylated region is functionally important, it overlaps with a DNase cluster (dark gray horizontal bar), a promoter-associated H3K4Me3 (white horizontal bar), and several regulatory elements (e.g., transcriptional binding sites; black horizontal lines). The specific regulatory elements shown and their genomic location are noted.
Although the investigation of the functional effects of locus-specific methylation (or demethylation) events remains difficult, recent advancements in genome-editing technologies using CRISPR/Cas-9 based approaches provide the opportunity to induce targeted CpG methylation and demethylation events both in vitro and in vivo (56). Other genome-editing techniques such as TALENs have also been used to target locus-specific CpG methylation sites (11, 60). Using these strategies, future experimental animal studies could be developed to determine whether hypermethylation of specific CpG sites, or CpG sites comprising a differentially methylated region of interest such as that described above, affects molecular and ultimately physiological function.
One challenge of epigenomic studies using primary human specimens is that epigenetic marks are often cell specific, yet it is frequently not possible to obtain purified cell populations from the tissue of interest. Although cell specificity is not a limitation of genotyping studies, it is a constraint that is shared across many types of molecular studies (e.g., gene and protein expression). As such, epigenomic studies should carefully consider the selection of surrogate cells or tissues and acknowledge that findings in such cases may not mirror epigenomic patterns in the target organ. Specimens containing mixed cell populations, such as blood, present another challenge. For methylation studies involving blood samples, one can either obtain cell differentials at the time of collection and, if cell proportions differ between comparison groups, include these values as covariates for analysis. In cases where cell differentials are not available, one can also estimate proportions of mononuclear cell populations from DNA methylation data (29) and, coupled with statistical approaches, determine methylation changes that are independent of differences in cellular composition of the samples used for analysis (107). Given the sensitivity of the epigenome to environmental exposures, it is also important to account for individual level factors (e.g., age, tobacco use) and community or geographic level exposures (e.g., ultraviolet radiation, nutritional scarcity) to the extent possible. Strategies to account for such factors include subject matching and statistical approaches, such as principal components analysis to identify observable batch effects or covariates within the data set that explain variation (52) and probabilistic estimation of expression residuals (90) to account for unknown or “hidden” confounders that may influence epigenomic patterns.
Summary
Expanding knowledge of the interactive nature of genetic, epigenetic, and environmental factors for human variation and disease, coupled with advances in sequencing technologies and analytical capabilities, reinforces the timeliness and need to identify the mechanisms underlying human adaptive potential. This review proposes that the epigenome may harbor important clues regarding the molecular mechanisms underlying human adaptation (or maladaptation) to high altitude. As described, the epigenome serves as an important interface between the genome and the environment, and is vital for “translating” genetic sequence into physiological responses that are appropriate for a given environment. Although the vast majority of the literature has focused on the transient nature of these epigenetic effects, recent work has also been directed toward mechanisms for inheritance of epigenomic marks, and the interactive nature of epigenomic and genomic information. Taken together, these published studies suggest that epigenetic processes may provide an avenue to rapidly acquire heritable, adaptive features. Such phenotypic flexibility could confer a selective advantage in a rapidly changing environment or during periods of the lifespan, such as pregnancy, that require extensive physiological adaptations over a short period of time. Although it is certainly premature to make assertions, this concept is provocative and warrants further investigation. Such studies will provide deeper insight into the mechanisms underlying human adaptive potential and, ultimately, may clarify its implications for biomedical research and human evolutionary history.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
C.G.J. conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
REFERENCES
- 1.Aldenderfer M. Peopling the Tibetan plateau: insights from archaeology. High Alt Med Biol : 141–147, 2011. doi: 10.1089/ham.2010.1094. [DOI] [PubMed] [Google Scholar]
- 2.Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet : e1003110, 2012. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science : 1466–1469, 2005. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels DB, Kreienbrock L, Dammann O, Wenzlaff P, Poets CF. Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed : F53–F59, 2005. doi: 10.1136/adc.2004.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol : 385–400, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA : 11459–11464, 2010. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA : 17215–17218, 2002. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beall CM, Reichsman AB. Hemoglobin levels in a Himalayan high altitude population. Am J Phys Anthropol : 301–306, 1984. doi: 10.1002/ajpa.1330630306. [DOI] [PubMed] [Google Scholar]
- 9.Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, Gilad Y, Pritchard JK. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol : R10, 2011. doi: 10.1186/gb-2011-12-1-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ : 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein DL, Le Lay JE, Ruano EG, Kaestner KH. TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts. J Clin Invest : 1998–2006, 2015. doi: 10.1172/JCI77321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, López Herráez D, Brutsaert T, Parra EJ, Moore LG, Shriver MD. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet : e1001116, 2010. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics : 687–697, 2014. doi: 10.1152/physiolgenomics.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigham AW, Lee FS. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev : 2189–2204, 2014. doi: 10.1101/gad.250167.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science : 612–616, 2010. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CJ, Rupert JL. Hypoxia and environmental epigenetics. High Alt Med Biol : 323–330, 2014. doi: 10.1089/ham.2014.1016. [DOI] [PubMed] [Google Scholar]
- 17.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science : 1337–1340, 2001. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 18.Carbone L, Harris RA, Vessere GM, Mootnick AR, Humphray S, Rogers J, Kim SK, Wall JD, Martin D, Jurka J, Milosavljevic A, de Jong PJ. Evolutionary breakpoints in the gibbon suggest association between cytosine methylation and karyotype evolution. PLoS Genet : e1000538, 2009. doi: 10.1371/journal.pgen.1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet : 733–743, 2016. doi: 10.1038/nrg.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, Miller J, Schlaeger T, Daley GQ, Feinberg AP. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet : 1350–1353, 2009. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly DF, Kim I, Carle C, Carroll JL. Perinatal hyperoxia for 14 days increases nerve conduction time and the acute unitary response to hypoxia of rat carotid body chemoreceptors. J Appl Physiol (1985) : 114–119, 2005. doi: 10.1152/japplphysiol.01009.2004. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature : 433–440, 2007. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 23.Garruto RM, Dutt JS. Lack of prominent compensatory polycythemia in traditional native Andeans living at 4,200 meters. Am J Phys Anthropol : 355–366, 1983. doi: 10.1002/ajpa.1330610310. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs JR, van der Brug MP, Hernandez DG, Traynor BJ, Nalls MA, Lai SL, Arepalli S, Dillman A, Rafferty IP, Troncoso J, Johnson R, Zielke HR, Ferrucci L, Longo DL, Cookson MR, Singleton AB. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet : e1000952, 2010. doi: 10.1371/journal.pgen.1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol : 1596–1599, 2003. doi: 10.1067/mob.2003.384. [DOI] [PubMed] [Google Scholar]
- 26.Goicoechea M, Martin J, de Sequera P, Quiroga JA, Ortiz A, Carreño V, Caramelo C. Role of cytokines in the response to erythropoietin in hemodialysis patients. Kidney Int : 1337–1343, 1998. doi: 10.1046/j.1523-1755.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 27.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA : 9700–9704, 1994. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hitchins MP, Ward RL. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet : 793–802, 2009. doi: 10.1136/jmg.2009.068122. [DOI] [PubMed] [Google Scholar]
- 29.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics : 86, 2012. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu H, Petousi N, Glusman G, Yu Y, Bohlender R, Tashi T, Downie JM, Roach JC, Cole AM, Lorenzo FR, Rogers AR, Brunkow ME, Cavalleri G, Hood L, Alpatty SM, Prchal JT, Jorde LB, Robbins PA, Simonson TS, Huff CD. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet : e1006675, 2017. doi: 10.1371/journal.pgen.1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Illingworth RS, Gruenewald-Schneider U, Webb S, Kerr AR, James KD, Turner DJ, Smith C, Harrison DJ, Andrews R, Bird AP. Orphan CpG islands identify numerous conserved promoters in the mammalian genome. PLoS Genet : e1001134, 2010. doi: 10.1371/journal.pgen.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. Pre-eclampsia and cardiovascular disease later in life: who is at risk? BMJ : 1213–1217, 2001. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irizarry RA, Ladd-Acosta C, Carvalho B, Wu H, Brandenburg SA, Jeddeloh JA, Wen B, Feinberg AP. Comprehensive high-throughput arrays for relative methylation (CHARM). Genome Res : 780–790, 2008. doi: 10.1101/gr.7301508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science : 464–468, 2001. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 35.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science : 468–472, 2001. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 36.Janion C. Influence of methionine on the mutation frequency in Salmonella typhimurium. Mutat Res : 331–338, 1982. doi: 10.1016/0027-5107(82)90295-0. [DOI] [PubMed] [Google Scholar]
- 37.Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation : 488–494, 2010. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 38.Jeong C, Alkorta-Aranburu G, Basnyat B, Neupane M, Witonsky DB, Pritchard JK, Beall CM, Di Rienzo A. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat Commun : 3281, 2014. doi: 10.1038/ncomms4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, Rossi DJ, Inlay MA, Serwold T, Karsunky H, Ho L, Daley GQ, Weissman IL, Feinberg AP. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature : 338–342, 2010. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet : 484–492, 2012. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 41.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet : 415–428, 2002. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 42.Julian CG, Gonzales M, Rodriguez A, Bellido D, Salmon CS, Ladenburger A, Reardon L, Vargas E, Moore LG. Perinatal hypoxia increases susceptibility to high-altitude polycythemia and attendant pulmonary vascular dysfunction. Am J Physiol Heart Circ Physiol : H565–H573, 2015. doi: 10.1152/ajpheart.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Julian CG, Pedersen BS, Salmon CS, Yang IV, Gonzales M, Vargas E, Moore LG, Schwartz DA. Unique DNA methylation patterns in offspring of hypertensive pregnancy. Clin Transl Sci : 740–745, 2015. doi: 10.1111/cts.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed : F372–F377, 2007. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol : R1564–R1575, 2009. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kallio PJ, Okamoto K, O’Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J : 6573–6586, 1998. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res : 20–25, 2003. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 48.Khavari DA, Sen GL, Rinn JL. DNA methylation and epigenetic control of cellular differentiation. Cell Cycle : 3880–3883, 2010. doi: 10.4161/cc.9.19.13385. [DOI] [PubMed] [Google Scholar]
- 49.Kulis M, Heath S, Bibikova M, Queirós AC, Navarro A, Clot G, Martínez-Trillos A, Castellano G, Brun-Heath I, Pinyol M, Barberán-Soler S, Papasaikas P, Jares P, Beà S, Rico D, Ecker S, Rubio M, Royo R, Ho V, Klotzle B, Hernández L, Conde L, López-Guerra M, Colomer D, Villamor N, Aymerich M, Rozman M, Bayes M, Gut M, Gelpí JL, Orozco M, Fan JB, Quesada V, Puente XS, Pisano DG, Valencia A, López-Guillermo A, Gut I, López-Otín C, Campo E, Martín-Subero JI. Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat Genet : 1236–1242, 2012. doi: 10.1038/ng.2443. [DOI] [PubMed] [Google Scholar]
- 50.Lachance G, Uniacke J, Audas TE, Holterman CE, Franovic A, Payette J, Lee S. DNMT3a epigenetic program regulates the HIF-2α oxygen-sensing pathway and the cellular response to hypoxia. Proc Natl Acad Sci USA : 7783–7788, 2014. doi: 10.1073/pnas.1322909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, Huo Y, Rongione M, Wand GS, Potash JB. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology : 4332–4343, 2010. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leek JT, Scharpf RB, Bravo HC, Simcha D, Langmead B, Johnson WE, Geman D, Baggerly K, Irizarry RA. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet : 733–739, 2010. doi: 10.1038/nrg2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.León-Velarde F, Gamboa A, Chuquiza JA, Esteba WA, Rivera-Chira M, Monge CC. Hematological parameters in high altitude residents living at 4,355, 4,660, and 5,500 meters above sea level. High Alt Med Biol : 97–104, 2000. doi: 10.1089/15270290050074233. [DOI] [PubMed] [Google Scholar]
- 54.León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol : 147–157, 2005. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- 55.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig : 265–274, 2003. doi: 10.1016/S1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 56.Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA methylation in the mammalian genome. Cell : 233–247.e17, 2016. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet : 951–956, 2014. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension : 944–951, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 59.Macdougall IC. Poor response to erythropoietin: practical guidelines on investigation and management. Nephrol Dial Transplant : 607–614, 1995. [PubMed] [Google Scholar]
- 60.Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, Ho QH, Sander JD, Reyon D, Bernstein BE, Costello JF, Wilkinson MF, Joung JK. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol : 1137–1142, 2013. doi: 10.1038/nbt.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maignan M, Rivera-Ch M, Privat C, Leòn-Velarde F, Richalet JP, Pham I. Pulmonary pressure and cardiac function in chronic mountain sickness patients. Chest : 499–504, 2009. doi: 10.1378/chest.08-1094. [DOI] [PubMed] [Google Scholar]
- 62.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell : 294–309, 2010. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClay JL, Shabalin AA, Dozmorov MG, Adkins DE, Kumar G, Nerella S, Clark SL, Bergen SE, Hultman CM, Magnusson PK, Sullivan PF, Aberg KA, van den Oord EJ; Swedish Schizophrenia Consortium . High density methylation QTL analysis in human blood via next-generation sequencing of the methylated genomic DNA fraction. Genome Biol : 291, 2015. doi: 10.1186/s13059-015-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metzen E, Stiehl DP, Doege K, Marxsen JH, Hellwig-Bürgel T, Jelkmann W. Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: identification of a functional hypoxia-responsive element. Biochem J : 711–717, 2005. doi: 10.1042/BJ20041736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG Jr. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood : 3236–3244, 2008. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moen EL, Zhang X, Mu W, Delaney SM, Wing C, McQuade J, Myers J, Godley LA, Dolan ME, Zhang W. Genome-wide variation of cytosine modifications between European and African populations and the implications for complex traits. Genetics : 987–996, 2013. doi: 10.1534/genetics.113.151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monge-C C, Arregui A, León-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med , Suppl 1: S79–S81, 1992. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- 68.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA : 2515–2520, 2012. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niermeyer S, Andrade-M MP, Vargas E, Moore LG. Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude (2013 Grover Conference series). Pulm Circ : 48–62, 2015. doi: 10.1086/679719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCepsilon gene repression in rat hearts. Circ Res : 365–373, 2010. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pei T, Li X, Tao F, Xu H, You H, Zhou L, Liu Y, Gao Y. Burden of disease resulting from chronic mountain sickness among young Chinese male immigrants in Tibet. BMC Public Health : 401, 2012. doi: 10.1186/1471-2458-12-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu, Basang, Ciwangsangbu, Danzengduojie, Chen H, Shi H, Su B. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol : 1075–1081, 2011. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- 73.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci USA : 654–659, 2006. doi: 10.1073/pnas.0508423103. [DOI] [PubMed] [Google Scholar]
- 74.Prchal JT. Production of erythrocytes. In: Williams’ Hematology (8th ed), edited by Kaushansky K, Lichtman M. New York: McGraw-Hill, 2010, chapt. 31, p. 435–448. [Google Scholar]
- 75.Prchal JT. Primary and secondary polycythemia (erythrocytosis) In: Williams’ Hematology (8th ed), edited by Kaushansky K, Lichtman M. New York: McGraw-Hill, 2010, chapt. 56, p. 823–838. [Google Scholar]
- 76.Rademaker K, Hodgins G, Moore K, Zarrillo S, Miller C, Bromley GR, Leach P, Reid DA, Álvarez WY, Sandweiss DH. Paleoindian settlement of the high-altitude Peruvian Andes. Science : 466–469, 2014. doi: 10.1126/science.1258260. [DOI] [PubMed] [Google Scholar]
- 77.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature : 425–432, 2007. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 78.Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, Mathieu C, Tolsa JF, Nicod P, Scherrer U, Sartori C. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol : H247–H252, 2011. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 79.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene : 3139–3155, 2001. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 80.Roemer I, Reik W, Dean W, Klose J. Epigenetic inheritance in the mouse. Curr Biol : 277–280, 1997. doi: 10.1016/S0960-9822(06)00124-2. [DOI] [PubMed] [Google Scholar]
- 81.Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet : 2205–2207, 1999. doi: 10.1016/S0140-6736(98)08352-4. [DOI] [PubMed] [Google Scholar]
- 82.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA : 1412–1417, 2006. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scheinfeldt LB, Soi S, Thompson S, Ranciaro A, Woldemeskel D, Beggs W, Lambert C, Jarvis JP, Abate D, Belay G, Tishkoff SA. Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol : R1, 2012. doi: 10.1186/gb-2012-13-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scherrer U, Allemann Y, Rexhaj E, Rimoldi SF, Sartori C. Mechanisms and drug therapy of pulmonary hypertension at high altitude. High Alt Med Biol : 126–133, 2013. doi: 10.1089/ham.2013.1006. [DOI] [PubMed] [Google Scholar]
- 85.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med : 537–547, 2011. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 86.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science : 72–75, 2010. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 87.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet : 2002–2006, 2001. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 88.Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, Eggan K, Meissner A. DNA methylation dynamics of the human preimplantation embryo. Nature : 611–615, 2014. doi: 10.1038/nature13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR, Lee FS. Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem : 14656–14665, 2014. doi: 10.1074/jbc.M113.541227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stegle O, Parts L, Durbin R, Winn J. A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLOS Comput Biol : e1000770, 2010. doi: 10.1371/journal.pcbi.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sved J, Bird A. The expected equilibrium of the CpG dinucleotide in vertebrate genomes under a mutation model. Proc Natl Acad Sci USA : 4692–4696, 1990. doi: 10.1073/pnas.87.12.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood : 3229–3235, 2008. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tashi T, Scott Reading N, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, Koul P, Guchhait P, Wittwer CT, Julian CG, Shah B, Huff CD, Gordeuk VR, Prchal JT, Ge R. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J Mol Med (Berl) : 665–670, 2017. doi: 10.1007/s00109-017-1519-3. [DOI] [PubMed] [Google Scholar]
- 94.Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Claydon VE, Hainsworth R, Gamboa JL, Zibenigus M, Zenebe G, Xue J, Liu S, Frazer KA, Li Y, Bafna V, Haddad GG. Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol : R36, 2014. doi: 10.1186/gb-2014-15-2-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vargas E, Spielvogel H. Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol : 138–149, 2006. doi: 10.1089/ham.2006.7.138. [DOI] [PubMed] [Google Scholar]
- 96.Vargas E, Villena M, Salinas C, Rodriguez A, Spielvogel H, Tellez M, Bellido D. Excessive polycythemia occurs in young high altitude (3600 m) residents in the absence of lung disease. In: Heath & Height: Proceedings of the Fifth World Congress on Mountain Medicine and High Altitude Physiology. Barcelona, Spain: University of Barcelona, 2002. [Google Scholar]
- 97.Vásquez R, Villena M. Normal hematological values for healthy persons living at 4000 meters in Bolivia. High Alt Med Biol : 361–367, 2001. doi: 10.1089/15270290152608534. [DOI] [PubMed] [Google Scholar]
- 98.Watson JA, Watson CJ, McCrohan AM, Woodfine K, Tosetto M, McDaid J, Gallagher E, Betts D, Baugh J, O’Sullivan J, Murrell A, Watson RW, McCann A. Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum Mol Genet : 3594–3604, 2009. doi: 10.1093/hmg/ddp307. [DOI] [PubMed] [Google Scholar]
- 99.Wellmann S, Bettkober M, Zelmer A, Seeger K, Faigle M, Eltzschig HK, Bührer C. Hypoxia upregulates the histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun : 892–897, 2008. doi: 10.1016/j.bbrc.2008.05.150. [DOI] [PubMed] [Google Scholar]
- 100.Wenninger JM, Olson EB, Wang Z, Keith IM, Mitchell GS, Bisgard GE. Carotid sinus nerve responses and ventilatory acclimatization to hypoxia in adult rats following 2 weeks of postnatal hyperoxia. Respir Physiol Neurobiol : 155–164, 2006. doi: 10.1016/j.resp.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 101.Wikström AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG : 1486–1491, 2005. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 102.Winslow RM, Chapman KW, Gibson CC, Samaja M, Monge CC, Goldwasser E, Sherpa M, Blume FD, Santolaya R. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol (1985) : 1561–1569, 1989. [DOI] [PubMed] [Google Scholar]
- 103.Wu TY. [An epidemiological study on high altitude disease at Qinghai-Xizang (Tibet) plateau]. Zhonghua Liu Xing Bing Xue Za Zhi : 65–69, 1987. [PubMed] [Google Scholar]
- 104.Xie C, Pei S. Some physiological data of sojourners and native highlanders at three different altitudes in Xizhang. In: Geological and Ecological Studies of Qinghai-Xizang Plateau, edited by Liu D. New York: Gordon & Breach, 1981, p. 1449–1552. [Google Scholar]
- 105.Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H, Jin L. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol : 1003–1011, 2011. doi: 10.1093/molbev/msq277. [DOI] [PubMed] [Google Scholar]
- 106.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther : 624–632, 2009. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang IV, Pedersen BS, Liu A, O’Connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SJ, Gill MA, Calatroni A, David G, Hennessy CE, Davidson EJ, Zhang W, Gergen P, Togias A, Busse WW, Schwartz DA. DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol : 69–80, 2015. doi: 10.1016/j.jaci.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang J, Jin ZB, Chen J, Huang XF, Li XM, Liang YB, Mao JY, Chen X, Zheng Z, Bakshi A, Zheng DD, Zheng MQ, Wray NR, Visscher PM, Lu F, Qu J. Genetic signatures of high-altitude adaptation in Tibetans. Proc Natl Acad Sci USA : 4189–4194, 2017. doi: 10.1073/pnas.1617042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell : 577–590, 2014. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science : 75–78, 2010. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yin H, Blanchard KL. DNA methylation represses the expression of the human erythropoietin gene by two different mechanisms. Blood : 111–119, 2000. [PubMed] [Google Scholar]
- 112.Zhang X, Moen EL, Liu C, Mu W, Gamazon ER, Delaney SM, Wing C, Godley LA, Dolan ME, Zhang W. Linking the genetic architecture of cytosine modifications with human complex traits. Hum Mol Genet : 5893–5905, 2014. doi: 10.1093/hmg/ddu313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhi D, Aslibekyan S, Irvin MR, Claas SA, Borecki IB, Ordovas JM, Absher DM, Arnett DK. SNPs located at CpG sites modulate genome-epigenome interaction. Epigenetics : 802–806, 2013. doi: 10.4161/epi.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]