Abstract
The 2016 Colorado Trail Race (CTR) was an ultra-endurance mountain bike race in which competitors cycled for up to 24 h/day between altitudes of 1,675 and 4,025 m to complete 800 km and 21,000 m of elevation gain. In one athlete, we had the unique opportunity to characterize skeletal muscle protein synthesis and mitochondrial respiration in response to a normal activity control period (CON) and the CTR. We hypothesized that mitochondrial protein synthesis would be elevated and mitochondrial respiration would be maintained during the extreme stresses of the CTR. Titrated and bolus doses of ADP were provided to determine substrate-specific oxidative phosphorylation (OXPHOS) and electron transport system (ETS) capacities in permeabilized muscle fibers via high-resolution respirometry. Protein synthetic rates were determined by daily oral consumption of deuterium oxide (2H2O). The endurance athlete had OXPHOS (226 pmol·s−1·mg tissue−1) and ETS (231 pmol·s−1·mg tissue−1) capacities that rank among the highest published to date in humans. Mitochondrial (3.2-fold), cytoplasmic (2.3-fold), and myofibrillar (1.5-fold) protein synthesis rates were greater during CTR compared with CON. With titrated ADP doses, the apparent Km of ADP, OXPHOS, and ETS increased after the CTR. With provision of ADP boluses after the CTR, the addition of fatty acids (−12 and −14%) mitigated the decline in OXPHOS and ETS capacity during carbohydrate-supported respiration (−26 and −31%). In the face of extreme stresses during the CTR, elevated rates of mitochondrial protein synthesis may contribute to rapid adaptations in mitochondrial bioenergetics.
NEW & NOTEWORTHY The mechanisms that maintain skeletal muscle function during extreme stresses remain incompletely understood. In the current study, greater rates of mitochondrial protein synthesis during the energetic demands of ultra-endurance exercise may contribute to rapid adaptations in mitochondrial bioenergetics. The endurance athlete herein achieved mitochondrial respiratory capacities among the highest published for humans. Greater mitochondrial protein synthesis during ultra-endurance exercise may contribute to improved mitochondrial respiration and serve as a mechanism to resist cellular energetic stresses.
Keywords: mitochondrial bioenergetics, endurance exercise, proteostasis, stress resistance, deuterium oxide
INTRODUCTION
The cellular physiology required to resist the extreme stress of ultra-endurance exercise (stress resistance) and sustain physical function remains incompletely understood. Maintaining stress resistance during ultra-endurance events requires balancing competing energetic needs, such as that needed for physical work and cellular remodeling. To date, stress resistance has been primarily studied in vitro (23) with limited in vivo inquiry.
Previous studies have shown increased rates of myofibrillar and mitochondrial skeletal muscle protein synthesis “after” acute endurance exercise (3, 8, 9, 27, 35). However, no previous studies have measured the cumulative mitochondrial protein synthesis during sustained stresses of multiday, ultra-endurance racing or during an acute bout of aerobic exercise. Measuring protein synthesis after an acute bout of exercise or endurance training period provides different information than measuring cumulative protein synthesis during an event. The latter provides information about what processes can be maintained during the stress, rather than once the stress is removed. Skeletal muscle protein synthesis is an energetically costly process competing with other physiological systems for a finite energy pool to maintain homeostasis (30). During long-term energetic stress in animal models (life-long caloric restriction, 12 wk of rapamycin, and 8 wk of exercise), skeletal muscle mitochondrial protein synthesis is preferentially maintained, while the synthesis of cytosolic and myofibrillar proteins is decreased (5, 18, 20, 21). Maintenance of high mitochondrial protein synthesis rates may serve as a proteostatic mechanism to preserve mitochondrial energy production and resist the extreme energetic stress of ultra-endurance racing, as previously indicated in Alaskan Husky sled dogs (22).
In the current study, we had a rare opportunity to determine skeletal muscle (vastus lateralis) protein synthesis rates and mitochondrial respiratory capacities of a highly trained endurance athlete in response to the stresses of the 2016 Colorado Trail Race (CTR). The 2016 CTR was a self-supported, multi-day, mountain bike race at which competitors cycled for up to 24 h/day between altitudes of 1,675 and 4,025 m to complete 800 km and 21,000 m of elevation gain. The purpose of this study was to test the hypothesis that in response to the sustained stresses (energetic, hypoxic, sleep deprivation) of the CTR 1) mitochondrial protein synthesis would be preferentially increased, while cytoplasmic and myofibrillar protein synthesis would be decreased, and 2) increased mitochondrial protein synthesis would be accompanied by the maintenance of submaximal and maximal mitochondrial respiration.
METHODS
This study was approved by the Institutional Review Board at Colorado State University (16–6751HH). The potential risks and benefits of the laboratory procedures were reviewed, and the participant provided written informed consent.
Experimental Design
The study consisted of a period of measurement around the CTR and a normal activity control (CON) (Fig. 1). The CON occurred at a later time point to match the length of the CTR testing period, since the time required to complete the CTR could not be predicted a priori. The athlete had 20+ years of endurance training and cycling competitions. Before the CTR, the athlete completed several months of endurance training, including bouts of cycling exceeding 7 h and altitudes over 3,700 m. During the 5 days of the CTR, the participant consumed three meals in the mountain towns of Copper Mountain (day 2), Leadville (day 3), and Buena Vista (day 4). The remaining caloric intake was in the form of supplemental nutrition (trail mix, gummies, energy bars, chocolate, nuts, and electrolyte drinks). The participant slept ~5 h per night during the CTR. The temperature during the CTR ranged from ~3°C to 35°C, with an estimated mean temperature of 18°C and low humidity (<40%). During the 5 days of CON, the participant commuted daily via bicycle (16-km round trip) and performed 2 days of 2–2.5 h of cycling at ~1,500 m of altitude. During the 45 days between the CTR and CON, the participant spent several days removed from a bicycle, including travel to lower altitude (≤300 m) but was still active before resuming normal activity. Before the CTR and CON, the athlete arrived to the laboratory fasted overnight, ≥36 h after the last exercise bout to complete a dual-energy X-ray absorptiometry scan, obtain a skeletal muscle biopsy, and perform an oral glucose (75 g) tolerance test (OGTT). These procedures were repeated after the CTR and CON.
Fig. 1.
After an overnight fast and ≥36 h after the last exercise, the participant arrived for a dual energy X-ray absorptiometry (DEXA) scan, oral glucose tolerance test (OGTT), and skeletal muscle biopsy for each condition. Prestudy days occurred 5 days before the beginning of the Colorado Trail Race (CTR) and the control period (CON). The participant stopped competing at the CTR at 7:00 PM, was transported to Colorado State University, and was studied 36 h after exercise at 7:00 AM. Pre-CON was conducted 45 days after Post-CTR. The 2H2O labeling started 1 day before the CTR and CON by consuming 2 × 50 ml/day of 2H2O followed by 6 days of 1 × 50 ml/day of 2H2O consumed each morning. This labeling scheme required the participant to carry 6 days of 2H2O during the CTR.
Aerobic Capacity
Maximal aerobic capacity (V̇o2max) was determined on a stationary cycle ergometer (Velotron, RacerMate), as previously performed (13, 27, 29). Briefly, the workload began at 100 W and was increased 50 W every 2 min. Respiratory gases were measured using a ParvoMedics Metabolic System. V̇o2max was expressed relative to body weight (ml·kg−1·min−1).
Skeletal Muscle Biopsy
Skeletal muscle biopsy samples were obtained from the vastus lateralis. POST time points were taken proximally from PRE, and opposite legs were used for the CTR and CON. Muscle bundles for high-resolution respirometry were immediately placed in ice-cold BIOPS buffer (25) or embedded in tragacanth gum and frozen in liquid nitrogen-cooled isopentane for histological evaluation of muscle cross sections. All remaining muscle was immediately frozen in liquid nitrogen, stored at −80°C, and processed as previously described for Western blot analysis (5) and GC-MS analysis (1, 10, 19).
Deuterium Oxide Labeling
The day before the CTR, the participant consumed 2 × 50 ml doses of 99% 2H2O (Sigma Aldrich) once in the morning and once in the afternoon. The participant carried 99% 2H2O during CTR (5 days) and consumed an oral dose (~50 ml) each morning. This labeling scheme was repeated for CON. Plasma and skeletal muscle samples collected 36 h after CTR and CON were analyzed for deuterium enrichment, as previously described (15, 29, 31).
Oral Glucose Tolerance Test
A venous catheter was inserted to collect serial blood samples before (−10 min) and after (0, 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, and 120 min) consumption of 75 g of glucose. Blood chemistries were assessed from fasted blood samples (Piccolo Blood Chemistry Analyzer). Blood glucose was analyzed by an automated glucose analyzer (2300 STAT; Yellow Springs Instruments, Yellow Springs, CO). Plasma insulin was analyzed using a human insulin ELISA kit (Crystal Chem) in select time points. A modified Matsuda calculation was used to estimate insulin sensitivity (17).
Histology
Cross sections (10 µm) were stained with hematoxylin and eosin (H&E) to identify all nuclei and myofiber structure. Images were captured at ×20 magnification using an Olympus SC30 microscope using Olympus cellSens (Olympus, Waltham, MA) software. Five to seven images were analyzed per time point. Myofibers (PRE CON: 215 fibers; POST CON: 484 fibers; PRE CTR: 159 fibers; POST CTR: 732 fibers) were manually traced using ImageJ (1.50i; National Institutes of Health) to evaluate size [cross-sectional area (CSA)] and an index of morphology (circularity). Nuclei were quantified using the Analyze Particles function of ImageJ and were expressed relative to the number of myofibers in a given field of view. Nuclei within myofibers that did not have contact with the sarcolemma were considered centralized, which is one indication of skeletal muscle remodeling and regeneration (16). Tissue consistent with fibrosis or extracellular matrix was also manually traced and expressed as a percentage of the total area of the field of view.
Mitochondrial Respiration
Permeabilization of muscle bundles was performed as previously described (22). Blebbistatin, a myosin II-specific inhibitor, was added to BIOPS and MiR06 (12.5 µM) to prevent contraction of muscle fibers, similar to published methods (24). Technical duplicates were used for each of the three protocols unless otherwise noted. Data files were collected and coded by two members of the research team, while a separate member of the research team analyzed the data in a blinded fashion. The coefficient of variation (CV) between technical duplicates for this study was 4–9% for oxidative phosphorylation (OXPHOS) and 4–6% for ETS. Respiration rates were normalized to muscle tissue wet weight (pmol·s−1·mg tissue−1).
High-resolution respirometry was performed using three different substrate-uncoupler-inhibitor-titration (SUIT) protocols. SUIT1 examined fatty acid [0.5 mM malate (M), 0.2 mM octanylcarnitine] supported leak respiration (FAOL) and maximal fatty acid oxidation (FAOP) with an ADP bolus (5 mM). Then, the sequential addition of complex I- [5 mM pyruvate (P), 10 mM glutamate (G); CI&FAOP] and complex II-linked substrates [10 mM succinate (S), CI+CII&FAOP] determined oxidative phosphorylation (OXPHOS) and FCCP (0.5-µM titrations) mediated uncoupled electron transport system (ETS) capacity (CI+CII&FAOE). SUIT2 investigated carbohydrate supported leak respiration (PGM; CIL) followed by an ADP bolus to stimulate maximal CI-linked (PGM; CIP) and CI+II-linked (S; CI+IIP) OXPHOS and uncoupled ETS capacity (CI+IIE) without fat supply. For SUIT1 and SUIT2, rotenone (5 µM) was added to inhibit CI-linked respiration and FAO, with the remaining respiration representing CIIE. The third protocol (ADP titration) provided CI-linked substrates (PGM) followed by an ADP titration designed to measure submaximal and maximal CI-linked respiration and derive ADP sensitivity (apparent Km) and Vmax. Following the ADP titration, sequential addition of S and FCCP was used to determine maximal OXPHOS (CI+IIP) and ETS capacity (CI+IIE), respectively. For each protocol, cytochrome c (5 mM) was added before succinate, and a ≤10% increase in respiration was observed, suggesting intact mitochondrial membranes. Indices of mitochondrial efficiency [OXPHOS/Leak (P/L) and OXPHOS/ETS (P/E)] were also evaluated.
Western Blot Analyses
Western blot analyses was performed on the cytoplasmic muscle fraction, as previously described (5, 15, 31). Samples were run in duplicate unless otherwise noted, and equal protein loading was verified using Ponceau S staining. Antibodies from Cell Signaling Technologies [Boston, MA; no. 2531S Phospho-AMPKα (Thr-172), no. 2532 AMPKα, no. 4858 Phopsho-S6 ribosomal protein (rpS6) (Ser-235/236), no. 2217 rpS6, no. 4108 LC3A/B] or Santa Cruz Biotechnology [Santa Cruz, CA; sc-14495 pyruvate dehydrogenase kinase 4 (PDK4), sc-13067 peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α), sc1616 actin] were diluted 1:1,000. Because of limited sample availability, data are unavailable after CON for select proteins.
Data Analysis and Interpretation
To ensure qualitatively reliable and quantitatively robust data, we have performed the following analyses and interpretation. Data are presented in all figures as means ± SE of the technical duplicate unless otherwise stated. Skeletal muscle protein synthesis rates during the CON and CTR were compared. For mitochondrial respiration, we conservatively considered any change outside of the CV for technical duplicates (i.e., 9%) as meaningful differences. Because no differences in mitochondrial respiration were observed between Pre-CON vs. Pre-CTR, Pre vs. Post comparisons are presented for CON and CTR. No asterisks or P values are provided, as statistical analyses were not performed for one subject.
RESULTS
Subject Characteristics
The physical and metabolic characteristics for the athlete are provided in Table 1. The 43-yr-old male endurance athlete obtained a V̇o2max of 59 ml·kg−1·min−1 on a cycle ergometer at an altitude of 1,500 m. After 5 days and ~580 km of the CTR, the athlete stopped competing due to an inability to consume sufficient calories because of suppressed appetite at an altitude above 3,000 m. The athlete completed ~66% of the elevation gain of the CTR. Before and after CON and CTR, there were no differences in body weight or composition (Table 1). Postprandial glucose and insulin AUC were lower before and after CTR vs. CON (Table 1), while an estimate of whole body insulin sensitivity was ~80% greater before and after the CTR vs. CON (Table 1). There were no substantial differences in response to the CTR or the CON (Pre vs. Post).
Table 1.
Physical and metabolic characteristics of the athlete
| CON |
CTR |
|||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
| Age, yr | 43 | |||
| V̇o2max, ml·kg−1·min−1 | 59 | |||
| Body weight, kg | 68.9 | 68.6 | 69.8 | 69.8 |
| FFM, kg | 55.0 | 55.7 | 56.6 | 56.3 |
| Fat mass, kg | 11.3 | 10.4 | 10.6 | 10.9 |
| Fat, % | 16.3 | 15.1 | 15.1 | 15.6 |
| Leg FFM, kg | 19.1 | 19.3 | 18.7 | 19.6 |
| Leg fat mass, kg | 3.6 | 3.3 | 3.0 | 2.1 |
| HOMA-IR | 0.19 | 0.20 | 0.18 | 0.17 |
| Glucose AUC | 14301 | 13106 | 11488 | 10574 |
| Insulin AUC | 1246 | 1809 | 1064 | 904 |
| Matsuda index | 16.2 | 12.6 | 26.9 | 26.2 |
| Na, mmol/l | 134.0 | 134.0 | 139.0 | 139.0 |
| K, mmol/l | 3.9 | 3.9 | 4.1 | 3.3* |
| tCO2, mmol/l | 25.0 | 25.0 | 26.0 | 23.0 |
| Cl, mmol/l | 102.0 | 103.0 | 106.0 | 107.0 |
| Ca, mg/dl | 8.8 | 9.0 | 9.0 | 8.2 |
| BUN, mg/dl | 15.0 | 14.0 | 14.0 | 15.0 |
| CRE, mg/dl | 0.6 | 1.0 | 0.9 | 0.5* |
| ALP, U/l | 54.0 | 50.0 | 49.0 | 46.0 |
| ALT, U/l | 16.0 | 16.0 | 18.0 | 31.0 |
| AST, U/l | 26.0 | 24.0 | 24.0 | 44.0* |
| TBIL, mg/dl | 2.3 | 3.0 | 2.2 | 1.9* |
| ALB, g/dl | 3.6 | 3.8 | 3.6 | 3.2* |
| TP, g/dl | 6.1 | 6.4 | 6.4 | 5.6* |
FFM, fat free mass; BUN, blood urea nitrogen; CRE, creatinine; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; HOMA-IR, homeostatic model assessment of insulin resistance; tCO2, total CO2; TBIL, total bilirubin; ALB, albumin; TP, total protein.
Outside normal reference ranges.
Several blood values were outside normal reference ranges after the CTR, including low levels of potassium, creatinine, albumin, and total protein and high levels of aspartate aminotransferase, and total bilirubin (Table 1).
Histology
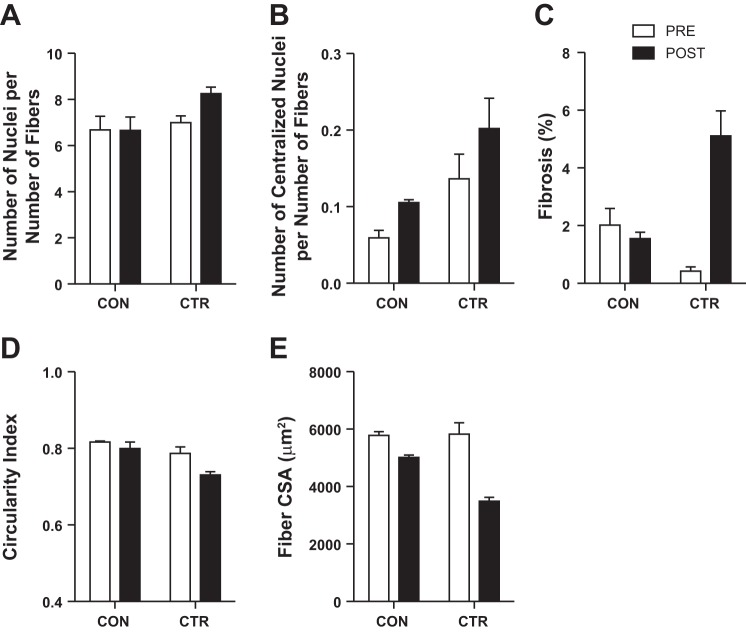
Using H&E staining, we evaluated the number of total and centralized nuclei and myofiber circularity and size (Fig. 2, A–E). The number of nuclei per number of muscle fibers was 18% greater after CTR (6.9 ± 0.3 vs. 8.3 ± 0.3) but did not change with CON (6.7 ± 0.6 vs. 6.7 ± 0.6). Overall, the most centralized nuclei per number of muscle fibers were apparent after the CTR (0.20 ± 0.04) with very few centralized nuclei detected before the CTR (0.13 ± 0.03) or before (0.06 ± 0.01) and after (0.10 ± 0.04) the CON. We also observed a greater percentage of tissue that was consistent with fibrosis or deposition of extracellular matrix after the CTR (0.4 ± 0.2 vs. 5.1 ± 0.9%) but not after the CON (2.1 ± 0.6 vs. 1.6 ± 0.2%). There was a subtle 7% decrease in myofiber circularity after the CTR without a noticeable difference after the CON (2%). Myofiber CSA decreased 40% after the CTR and 13% after the CON. From these histological assessments, there appears to be more profound differences after the CTR compared with after the CON.
Fig. 2.
Skeletal muscle histology before (PRE) and after (POST) CON and CTR. The number of nuclei (A) and centralized nuclei (B) are expressed per number of muscle fibers. Tissue consistent with fibrosis or extracellular matrix deposition (C) was expressed as a percentage of the total area. Skeletal muscle fibers were manually assessed for morphology (circularity index; D) and size [cross-sectional area (CSA); E]. Images were obtained at ×20 magnification. Data are expressed as means ± SE of 5–7 cross sections.
Protein Synthesis
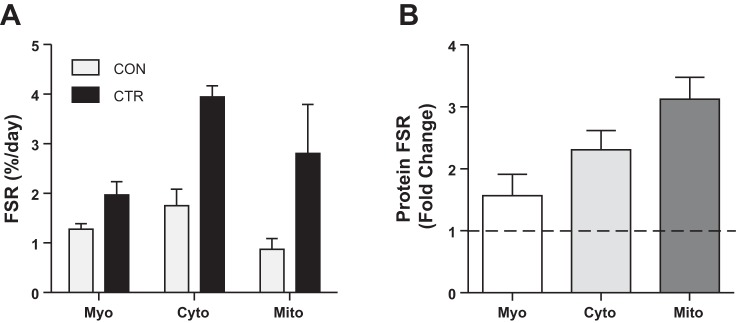
The rates of mitochondrial, cytoplasmic, and myofibrillar protein synthesis were greater during CTR than CON (Fig. 3A). The higher protein synthesis rates shown in Fig. 3B were most striking for mitochondrial proteins (3.2-fold) followed by cytoplasmic (2.3-fold) and myofibrillar (1.5-fold).
Fig. 3.
Skeletal muscle protein synthesis rates during the CTR and CON (A) and the fold change in skeletal muscle protein synthesis rates during the CTR vs. CON (B). Myo, myofibrillar; Cyto, cytoplasmic; Mito, mitochondrial protein fraction. Data are expressed as means ± SE of technical duplicates.
Mitochondrial Respiration
ADP titration.
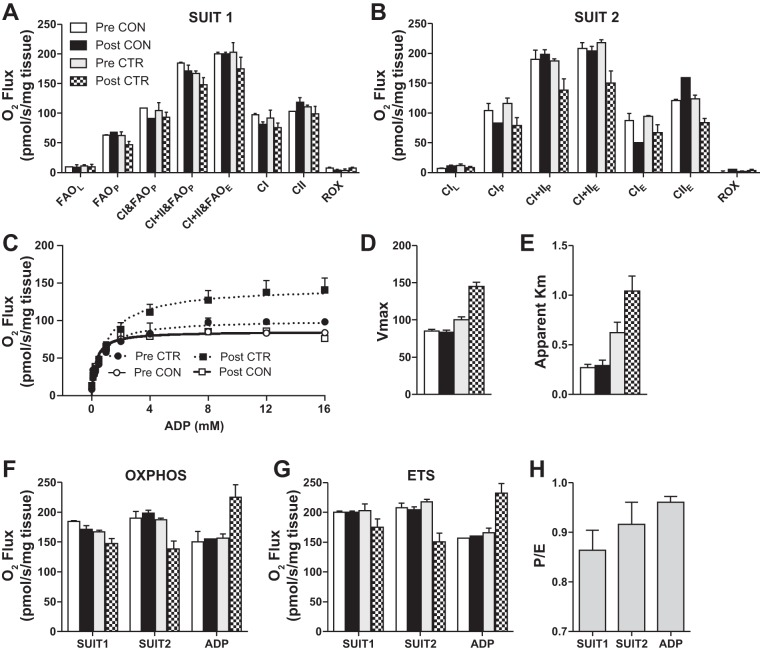
Submaximal CI-linked respiration was 13–34% greater after the CTR beginning at 0.25 mM and continuing to 4 mM ADP, while maximal CI-linked respiration (8–16 mM of ADP) was 30–43% greater after the CTR. After the CTR, the apparent Km of ADP was increased 67% and accompanied with greater Vmax (44%), OXPHOS (43%), and ETS (40%) capacity (Fig. 4, D–G). No appreciable changes for any variable (−8–7%) occurred during the CON.
Fig. 4.
Skeletal muscle mitochondrial respiration during SUIT1 (A), SUIT2 (B), and ADP titration protocol (C). Vmax (D) and apparent Km of ADP (E) were calculated from the ADP titration curve. Comparison of OXPHOS (F) and ETS (G) between SUIT1, SUIT2, and ADP titration (ADP) protocols before (Pre) and after (Post) CON and CTR. An average of the P/E ratio for each protocol (SUIT1, SUIT2, and ADP) (H) are presented with no differences between study days. Data are expressed as means ± SE of technical duplicates. Because of a limited sample, the Post-CON for the ADP titration was not run in duplicate.
SUIT1.
Fatty acid and carbohydrate-supported respiration during ADP (5 mM) stimulated OXPHOS (CI+CII&FAO)P and ETS flux (CI+CII&FAO)E were slightly lower after the CTR (−12 and −14%). After the CON, there were no differences in OXPHOS (−7%) or ETS (0%).
SUIT2.
Carbohydrate supported OXPHOS (CI+IIP) and ETS (CI+IIE) flux were decreased (−26 and −31%) after the CTR (Fig. 4B). The observed decline in OXPHOS and ETS after the CTR in SUIT2 (−26 and −31%) was more than double SUIT1 (−12 and −14%). After the CON, there were no differences in OXPHOS (4%) or ETS (−2%).
In all three protocols, ETS flux reached values ≥200 pmol·s−1·mg tissue−1 (200–231 pmol·s−1·mg tissue−1), with the highest values recorded during the ADP titration protocol. The greatest OXPHOS values per protocol were 184, 198, and 226 pmol·s−1·mg tissue−1 for SUIT1, SUIT2, and ADP titration, respectively. No differences in mitochondrial efficiency indices, P/L (SUIT1: 2%; SUIT2: 8%; ADP: −4%) or P/E (SUIT1: 3%; SUIT2: 6%; ADP: 3%) were observed after the CTR. However, the P/E ratio (0.93) was the highest during the ADP titration protocol (Fig. 4H).
Protein Content and Intracellular Signaling
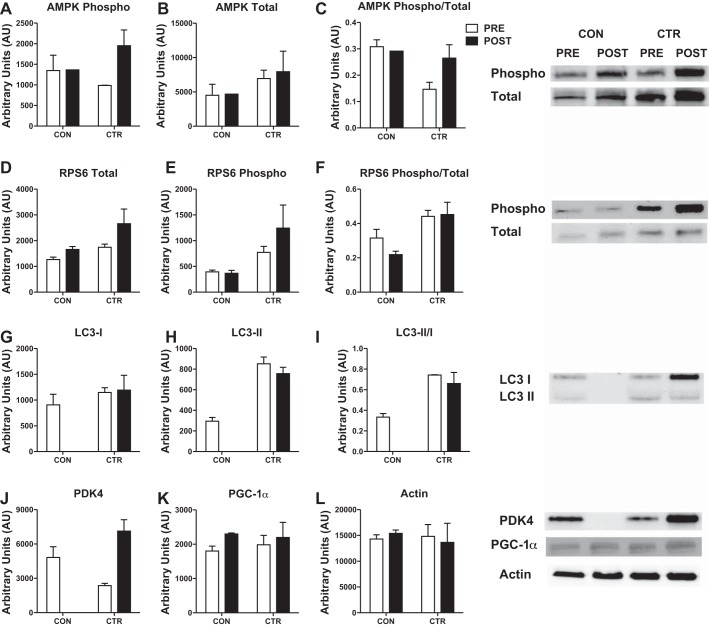
We evaluated select proteins involved in energetic signaling and skeletal muscle remodeling. After the CTR, phosphorylated and total AMPK, an index of cellular energetic stress, were increased 24% and 13%, respectively, while the ratio of phosphorylated to total AMPK was not different (Fig. 5, A–C). The content of phosphorylated and total rpS6K, a component of the translational machinery, were increased 60% and 52% after CTR, while the ratio of phosphorylated to total was not altered (Fig. 5, D–F). The ratio of phosphorylated to total rpS6K was greater before and after the CTR compared with CON. The ratio of LC3-II/I was ~110% greater before and after the CTR, primarily due to greater LC3-II, which is the lipidated LC3 isoform (Fig. 5, G–I). The protein content of PDK4 was increased 200% after CTR (Fig. 5J). Although mitochondrial biogenesis was increased more than three-fold greater during the CTR, the protein content of PGC-1α was not different between CON or CTR (Fig. 5K). Actin was unaltered with CTR or CON and was not different between conditions (Fig. 5L).
Fig. 5.
Phosphorylated and/or total protein content of AMPK (A–C), rpS6K (D–F), LC3 (G–I), PDK4 (J), PGC-1α (K), and actin (L) with representative Western blot images. Data are expressed as means ± SE of technical replicates. Because of limited sample, some proteins for Post-CON are not run in duplicate (AMPK) or are missing completely (LC3, PDK4).
DISCUSSION
In this study, we evaluated whether mitochondrial remodeling could be maintained during a physically and energetically challenging event to facilitate energetic stress resistance. Mitochondrial protein synthesis rates were greater (three-fold) during the CTR and accompanied with increased Vmax, OXPHOS, and ETS capacities during the provision of titrated ADP doses. The high degree of mitochondrial remodeling may have promoted a change in the capacity for substrate-supported energy production, as was evident by decreased carbohydrate-supported respiration (SUIT2) that was attenuated when fatty acids were also provided (SUIT1). These data suggest that mitochondrial protein remodeling can be enhanced in the face of an extreme physical and energetic stress.
Skeletal Muscle Protein Synthesis
One unique aspect of the current study was the use of 2H2O to isotopically label new proteins and determine integrated synthesis rates during the CTR and CON. Commonly, the intravenous infusion of labeled amino acid isotopes is used to determine acute snapshots of muscle protein synthesis (i.e., hours) after a perturbation (8, 9, 27). However, these procedures are not appropriate to measure protein synthesis rates during a multiday ultra-endurance event.
Skeletal muscle protein synthesis rates increase after acute (8, 9, 35) and chronic endurance exercise (28, 32). This is the first study to show greater skeletal muscle protein synthesis rates during the sustained energetic stress of a multiple-day ultra-endurance race, with the most profound difference observed in the mitochondrial protein fraction. Our previous assessments of skeletal muscle protein synthesis in animals challenged with a long-term energetic stress (5, 20, 21) showed a maintenance of mitochondrial protein synthesis, while other protein fractions decreased. In the current study, the greater synthesis rates in all protein fractions (mitochondrial, cytoplasmic, and myofibrillar) occurred with elevated rpS6, which is a downstream target of mechanistic target of rapamycin (mTOR) involved in the initiation of global protein translation. However, in our studies, static readouts of mTOR signaling have not been consistent with dynamic measurements of protein synthesis (4, 5, 20, 21). mTOR signaling has been shown to stimulate PGC-1α activity, the master transcriptional regulator of mitochondrial biogenesis, and increase OXPHOS genes and respiration (2). However, PGC-1α protein content did not increase in accordance with mitochondrial protein synthesis (i.e., biogenesis) and is consistent with our previous observations (5, 31). These findings and others (28) suggest that posttranscriptional mechanisms (e.g., translation, posttranslational, location, activity) may be primary regulators of mitochondrial protein turnover after exercise. The greater rates of skeletal muscle protein synthesis during the CTR occurred with a greater LC3-II/I ratio, which is one marker that has been associated with increased macroautophagy flux (14). Macroautophagy includes the degradation and removal of damaged cellular constituents, including mitochondria, to maintain cellular homeostasis during energetic stress. Collectively, select proteins involved in translation and autophagic flux may contribute to the greater rates of skeletal muscle protein synthesis during the extreme stress of the CTR.
Skeletal Muscle Histology
In addition to substantial differences in protein synthesis, a greater number of total nuclei per number of muscle fibers were present after the CTR and appeared to colocalize to tissue consistent with fibrosis or extracellular matrix deposition. These observations may suggest that inflammatory and other cells assisted in the regeneration of fibrotic or damaged skeletal muscle; however, a limitation of the current staining approach is that we were unable to quantitatively confirm the deposition of fibrotic tissue. In support of this notion, we also found 65% more centralized nuclei after the CTR, which is indicative of cellular regeneration after damage. Accordingly, after the CTR, we observed crude markers that suggest increased skeletal muscle regeneration, which may be related to the greater rates of skeletal muscle protein synthesis. Surprisingly, we found decreased (40%) muscle fiber CSA after the CTR. We speculate that the decreased CSA could pertain to glycogen depletion and the associated shifts in muscle water from the intracellular to extracellular space. Storage of glycogen is associated with intracellular water (estimates of 2.7 g of water bound per gram of glycogen) and muscle size. Previous evidence suggests a 30% reduction in skeletal muscle glycogen 2 days after marathon racing (1), which is congruent with the change in muscle fiber CSA 36 h after 5 days of an ultra-endurance race.
Mitochondrial Respiration
We hypothesized that mitochondrial remodeling would promote stress resistance after the CTR by maintaining or increasing mitochondrial respiration. Consistent with a stress-resistant phenotype, we observed an increased apparent Km for ADP and maximal respiration rates (Vmax, OXPHOS, and ETS) during a titrated supply of ADP. Previously, when highly trained runners were exposed to intermittent hypoxic training (two of the five sessions/week at simulated altitude of 3,000 m), the apparent Km for ADP was increased without alterations in Vmax (26). However, the results in the current study are in line with the link between elevated Km for ADP and OXPHOS in elite athletes and after exercise training (33, 34, 36) and suggest the athlete was able to rapidly adapt to the extreme stresses of the CTR.
When using similar SUIT protocols as the current study, Jacobs et al. (10, 12) showed decreased OXPHOS and ETS capacity in individuals exposed to high altitude for 9–11 days (>4,000 m) or 28 days (>3,000 m). Although these studies did not investigate ultra-endurance exercise, this may suggest that hypoxia partially mediated the decrease in OXPHOS and ETS after the CTR in the SUIT protocols. Both hypoxia and energetic stress are known activators of PDK4, which can inhibit pyruvate dehydrogenase and the conversion of carbohydrate-derived pyruvate to acetyl-CoA. Increased PDK4 after the CTR may contribute to decreased NADH-linked OXPHOS and ETS capacity during the SUIT protocols that was more profound during carbohydrate-supported respiration (SUIT2) and attenuated by the addition of fatty acids (SUIT1). These findings are in line with sparing carbohydrate fuel stores and preferentially utilizing fatty acids for energy production in endurance-trained humans. Immediately after 24 h of ultra-endurance exercise, fatty acid supported OXPHOS capacity was increased in isolated mitochondria while fatty acid + carbohydrate supported OXPHOS was maintained (6). Collectively, these findings may suggest a preferential increase or maintenance of mitochondrial fatty acid oxidation during the energetic stress of ultra-endurance exercise.
Although it is unclear why OXPHOS and ETS capacity were increased during the ADP titration protocol but decreased during SUIT protocols after the CTR, we hypothesize that the ADP bolus (5 mM) was no longer a saturating dose after the CTR. This concept is supported by results from the ADP titration protocol that indicate the apparent Km of ADP doubled after the CTR. As a result, mitochondrial respiration did not plateau until ~8 mM after the CTR vs. ~4 mM at each of the other study time points. Because previous studies have not utilized multiple protocols to assess mitochondrial respiration, these data provide valuable insight into designing future studies to assess mitochondrial respiration after the sustained stress of ultra-endurance exercise.
Comparison of Human and Alaskan Husky Endurance Athletes
We used similar SUIT protocols to allow for comparisons of ETS capacities in the human and canine (22) endurance athletes before and after a multiday, ultra-endurance race (e.g., CTR and the Iditarod). In the control conditions, the maximal ETS capacity was greater in the Alaskan Husky (AH; 254 ± 42) than the human (200 ± 2) when fatty acids were added (SUIT1), but similar (204 ± 27 vs. 208 ± 7) during carbohydrate supported respiration (SUIT2). In response to stresses of ultra-endurance competitions, ETS increased in the AH but decreased in the human athlete, with the most robust changes occurring in carbohydrate-supported respiration (SUIT2; 254 ± 27 vs. 150 ± 15) (22). It is important to note that the different ADP bolus for AH (6.2 mM) and the human (5 mM) may influence the response to their respective ultra-endurance race. Despite these subtle protocol differences, the divergent results are consistent with our previous studies in AH that showed the primary adaptations of substrate use with exercise was an increased capacity for carbohydrate oxidation (19, 22). Conversely, human endurance athletes have an elevated capacity for fatty acid oxidation (11), which can be further increased immediately after 24 h of ultra-endurance exercise (6).
In addition to mitochondrial respiration, we also compared mitochondrial protein synthesis rates in AH and human endurance athletes. In the CON conditions, the human endurance athlete had similar protein synthesis rates to individuals after an exercise training program (31) but half that of the AH (20). The AH maintained constitutively high rates of mitochondrial protein synthesis during the onset of an exercise training program, while the human endurance athlete had greater rates of mitochondrial protein synthesis during the CTR. The mitochondrial protein synthesis rates during the CTR surpassed the rates observed in AH (20). We interpret that the maintenance or increase of constitutively high rates of mitochondrial protein synthesis may serve as mechanisms for rapid mitochondrial adaptations to resist the extreme stress of ultra-endurance exercise.
Limitations
We attempted to provide a rigorous study design by including sample collection before and after the CTR and CON. While an n = 1 provides inherent study limitations, we included standard error of technical replicates to help frame any change with the CTR or CON. A potential limitation of this study was that caloric intake was not recorded during the CTR and that the training regimen for the CTR could have contributed to the high rates of mitochondrial protein synthesis measured during the CTR. Since this was a field study, we were unable to obtain tissue samples immediately following the CTR. The athlete was transported back to Colorado State University where blood and muscle samples were obtained 36 h after the CTR. Therefore, one day of noncompetition was included in the cumulative protein synthesis measurement, as well as using a precursor enrichment value that could have been different from that during the race. Because of the high rate of body water flux during the race, there could have been a decrease in body water enrichment. To calculate fractional synthesis rates, the product enrichment (muscle protein) is divided by the precursor enrichment (free alanine enrichment, which is in equilibrium with the body water pool). Therefore, a lower body water enrichment during the race would underestimate the actual synthesis rates compared with the value taken one day later in the laboratory.
Conclusions
The CTR provides robust physiological stresses to evaluate skeletal muscle remodeling. This study is unique in that we have determined skeletal muscle protein synthesis rates during a multiday, ultra-endurance race, extending our knowledge from previous studies that have captured the protein synthetic response in the immediate hours after acute endurance exercise (8, 9, 27). Here, we show that skeletal muscle mitochondrial protein synthesis rates were three-fold greater during the CTR than CON. These dramatic differences in protein synthesis rates during the CTR may permit enhanced mitochondrial bioenergetics. The highly trained endurance athlete had mitochondrial respiratory capacities among the highest published to date in human skeletal muscle (7). After the CTR, we observed increased Km for ADP, Vmax, OXPHOS, and ETS capacity during provision of titrated ADP doses and decreased reliance on carbohydrates for energy production during a bolus of ADP, which are collectively consistent with adaptations to endurance exercise training. These findings indicate that mitochondrial remodeling was greater during a profound energetic and physical challenge and may contribute to rapid adaptations in mitochondrial bioenergetics to resist the stresses of an ultra-endurance race.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.K., C.A.W., K.L.H., and B.F.M. conceived and designed research; A.R.K., W.M.C., C.A.W., R.V.M., J.J.R., and J.L.L. performed experiments; A.R.K., W.M.C., R.V.M., J.J.R., and Z.J.V. analyzed data; A.R.K., W.M.C., C.A.W., R.V.M., J.J.R., Z.J.V., K.L.H., and B.F.M. interpreted results of experiments; A.R.K. and R.V.M. prepared figures; A.R.K. drafted manuscript; A.R.K., W.M.C., C.A.W., R.V.M., J.J.R., Z.J.V., K.L.H., and B.F.M. edited and revised manuscript; A.R.K., W.M.C., C.A.W., R.V.M., J.J.R., J.L.L., Z.J.V., K.L.H., and B.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the dedication of the athlete. We thank Frederick Peelor III for the GCMS technical assistance. We also acknowledge the contributions of Bobbette Hickson and Susie Miller for transporting the athlete back to Colorado State University after the Colorado Trail Race.
REFERENCES
- 1.Asp S, Rohde T, Richter EA. Impaired muscle glycogen resynthesis after a marathon is not caused by decreased muscle GLUT-4 content. J Appl Physiol (1985) 83: 1482–1485, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450: 736–740, 2007. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 3.Di Donato DM, West DWD, Churchward-Venne TA, Breen L, Baker SK, Phillips SM. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am J Physiol Endocrinol Metab 306: E1025–E1032, 2014. doi: 10.1152/ajpendo.00487.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake JC, Bruns DR, Peelor FF III, Biela LM, Miller RA, Miller BF, Hamilton KL. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell 14: 474–482, 2015. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake JC, Peelor FF III, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci 68: 1493–1501, 2013. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernström M, Bakkman L, Tonkonogi M, Shabalina IG, Rozhdestvenskaya Z, Mattsson CM, Enqvist JK, Ekblom B, Sahlin K. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24-h ultraendurance exercise. J Appl Physiol (1985) 102: 1844–1849, 2007. doi: 10.1152/japplphysiol.01173.2006. [DOI] [PubMed] [Google Scholar]
- 7.Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41: 1837–1845, 2009. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 9.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010. doi: 10.1152/ajpregu.00348.2010. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs RA, Boushel R, Wright-Paradis C, Calbet JAL, Robach P, Gnaiger E, Lundby C. Mitochondrial function in human skeletal muscle following high-altitude exposure. Exp Physiol 98: 245–255, 2013. doi: 10.1113/expphysiol.2012.066092. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 114: 344–350, 2013. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs RA, Siebenmann C, Hug M, Toigo M, Meinild A-K, Lundby C. Twenty-eight days at 3,454-m altitude diminishes respiratory capacity but enhances efficiency in human skeletal muscle mitochondria. FASEB J 26: 5192–5200, 2012. doi: 10.1096/fj.12-218206. [DOI] [PubMed] [Google Scholar]
- 13.Jordan LY, Melanson EL, Melby CL, Hickey MS, Miller BF. Nitrogen balance in older individuals in energy balance depends on timing of protein intake. J Gerontol A Biol Sci Med Sci 65A: 1068–1076, 2010. doi: 10.1093/gerona/glq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki M, Karim MR. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol 452: 199–213, 2009. doi: 10.1016/S0076-6879(08)03613-6. [DOI] [PubMed] [Google Scholar]
- 15.Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF III, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience 39: 175–186, 2017. doi: 10.1007/s11357-017-9968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Miller BF, Drake JC, Naylor B, Price JC, Hamilton KL. The measurement of protein synthesis for assessing proteostasis in studies of slowed aging. Ageing Res Rev 18: 106–111, 2014. doi: 10.1016/j.arr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller BF, Drake JC, Peelor FF III, Biela LM, Geor R, Hinchcliff K, Davis M, Hamilton KL. Participation in a 1,000-mile race increases the oxidation of carbohydrate in Alaskan sled dogs. J Appl Physiol (1985) 118: 1502–1509, 2015. doi: 10.1152/japplphysiol.00588.2014. [DOI] [PubMed] [Google Scholar]
- 20.Miller BF, Ehrlicher SE, Drake JC, Peelor FF III, Biela LM, Pratt-Phillips S, Davis M, Hamilton KL. Assessment of protein synthesis in highly aerobic canine species at the onset and during exercise training. J Appl Physiol (1985) 118: 811–817, 2015. doi: 10.1152/japplphysiol.00982.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell 11: 150–161, 2012. doi: 10.1111/j.1474-9726.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller B, Hamilton K, Boushel R, Williamson K, Laner V, Gnaiger E, Davis M. Mitochondrial respiration in highly aerobic canines in the non-raced state and after a 1,600-km sled dog race. PLoS One 12: e0174874, 2017. doi: 10.1371/journal.pone.0174874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci 64A: 179–182, 2009. doi: 10.1093/gerona/gln072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry CGR, Kane DA, Lin C-T, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J 437: 215–222, 2011. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 26.Ponsot E, Dufour SP, Zoll J, Doutrelau S, N’Guessan B, Geny B, Hoppeler H, Lampert E, Mettauer B, Ventura-Clapier R, Richard R. Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol (1985) 100: 1249–1257, 2006. doi: 10.1152/japplphysiol.00361.2005. [DOI] [PubMed] [Google Scholar]
- 27.Robinson MM, Bell C, Peelor FF III, Miller BF. β-Adrenergic receptor blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in humans. Am J Physiol Regul Integr Comp Physiol 301: R327–R334, 2011. doi: 10.1152/ajpregu.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011. doi: 10.1096/fj.11-186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997. [DOI] [PubMed] [Google Scholar]
- 30a.Rüegg M, Meinen S. Histopathology in hematoxylin and eosin-stained muscle sections [Online]. 2011. www.treat-nmd.eu/downloads/file/sops/cmd/MDC1A_M.1.2.004.pdf.
- 31.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HLR, Szallar SE, Wood LM, Peelor FF III, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- 32.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286: E92–E101, 2004. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 33.Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: effects of prolonged exercise. J Physiol 510: 279–286, 1998. doi: 10.1111/j.1469-7793.1998.279bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch 442: 420–425, 2001. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoll J, Sanchez H, N’Guessan B, Ribera F, Lampert E, Bigard X, Serrurier B, Fortin D, Geny B, Veksler V, Ventura-Clapier R, Mettauer B. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 543: 191–200, 2002. doi: 10.1113/jphysiol.2002.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]