Abstract
The underlying cause of systolic heart failure is the inability of the adult mammalian heart to regenerate damaged myocardium. In contrast, some vertebrate species and immature mammals are capable of full cardiac regeneration following multiple types of injury through cardiomyocyte proliferation. Little is known about what distinguishes proliferative cardiomyocytes from terminally differentiated, nonproliferative cardiomyocytes. Recently, several reports have suggested that oxygen metabolism and oxidative stress play a pivotal role in regulating the proliferative capacity of mammalian cardiomyocytes. Moreover, reducing oxygen metabolism in the adult mammalian heart can induce cardiomyocyte cell cycle reentry through blunting oxidative damage, which is sufficient for functional improvement following myocardial infarction. Here we concisely summarize recent findings that highlight the role of oxygen metabolism and oxidative stress in cardiomyocyte cell cycle regulation, and discuss future therapeutic approaches targeting oxidative metabolism to induce cardiac regeneration.
Keywords: cardiac regeneration, cardiomyocyte proliferation, hypoxia, oxygen metabolism
the adult heart is one of the least regenerative organs in mammals, and therefore significant cardiac injury often results in heart failure. In sharp contrast to adult mammals, some vertebrate species, including zebrafish (64) and urodele amphibians (9, 55, 56), have a remarkable cardiac regeneration capacity throughout their lifetime (reviewed in 11, 43). In addition, recent reports demonstrate that mammals can regenerate their heart during embryogenesis (16, 70), and for a short period of time after birth (4, 8, 13, 42, 53, 61–63, 76, 77). Genetic lineage tracing demonstrated that the major source of newly formed cardiomyocytes is preexisting cardiomyocytes both in neonatal mice (62, 63, 76) and in zebrafish (29, 32). As expected, the limited postnatal time window of cardiac regeneration in neonatal mammals coincides with the cell cycle withdrawal and binucleation in a majority of cardiomyocytes (62, 63, 69). This temporal correlation between cardiomyocyte cell cycle withdrawal and diminishing cardiac regenerative capacity is also reported in humans (47). Although multiple studies reported genes and proteins that regulate postnatal polyploidy and binucleation of cardiomyocytes in mammals (1, 38, 54, 73, 79), upstream environmental cues that trigger the postnatal shift in regenerative capacity remain poorly understood.
Oxidative Metabolism and Postnatal Cardiomyocyte Cell Cycle Arrest
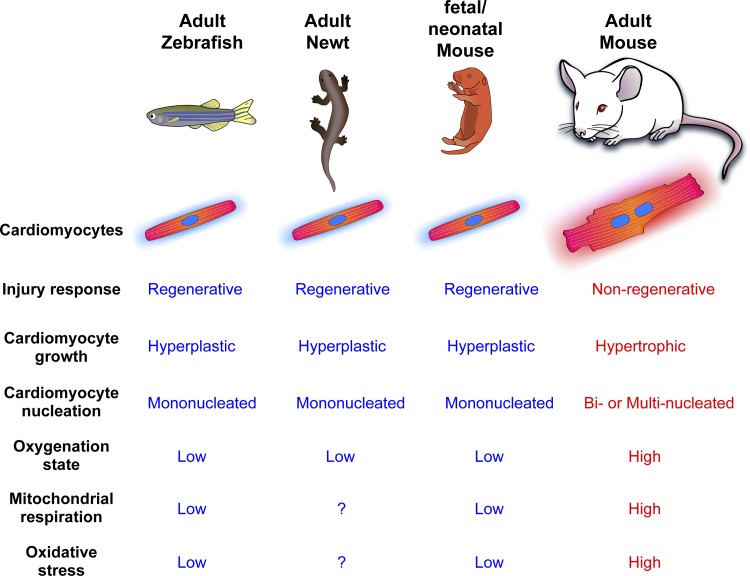
It is especially intriguing that across vertebrate species, there is a strong correlation between oxidative metabolism and proliferative capacity of cardiomyocytes (Fig. 1) given that in several tissue specific and pluripotent stem cells, mitochondrial metabolism and ROS level are critical regulators of proliferative capacity and the cell cycle (28, 33, 80). For example, fish and amphibians have two-chambered and three-chambered hearts, respectively, which result in the mixing of arterial and venous blood (20, 22). Similarly, the developing mammalian circulation carries relatively hypoxic blood, with arterial of 25–35 mmHg, primarily due to arteriovenous mixing in the shunt-dependent fetal circulation (12, 15, 17, 35, 36, 57). However, the rises rapidly to ~100 mmHg with the first breath due to shunt closure (71). Consequently, a drastic metabolic transition occurs in neonatal cardiomyocytes. Early physiological and biochemical studies showed that embryonic/neonatal cardiomyocytes rely primarily on glycolysis as an energy source, whereas postnatal cardiomyocytes utilize mitochondrial respiration as a main source of energy production to meet their high metabolic demands (3, 39–41). We recently examined enzymes related to glycolytic and mitochondrial metabolism in the early postnatal period by utilizing quantitative mass-spectrometry and showed that mitochondrial Krebs cycle enzymes and fatty acid oxidation enzymes are upregulated within 7 days after birth, with a synchronized downregulation of anaerobic glycolysis (66). This is consistent with previous findings demonstrating increased mitochondrial biogenesis in postnatal cardiomyocytes (27, 45, 58–60). As a result of the steep rise in mitochondrial energy production in the postnatal heart, there is an increase in ROS production and oxidative nuclear DNA damage in cardiomyocytes. We showed that activation of Wee1 kinase, a component of the DNA damage response pathway, directly triggers cell cycle arrest in postnatal cardiomyocytes (66). It is intriguing that mitochondrial DNA damage is also elevated in the postnatal heart (58), which may also play an important role in cardiomyocyte cell cycle regulation.
Fig. 1.
Comparison of cardiac regeneration and oxidative metabolism across vertebrate species.
Hypoxic Proliferative Cardiomyocytes in the Adult Heart
Recent advances in in vivo lineage tracing have shown that adult mouse cardiomyocytes are renewed at a slow rate, which is mainly mediated by cell division of preexisting cardiomyocytes (2, 5, 6, 44, 68). This indicates that a limited number of cardiomyocytes are still capable of proliferation in the adult heart. But if all cardiomyocytes experience increased oxidative stress after birth, how are some cardiomyocytes able to retain proliferative competency within the adult heart? We postulated that proliferative cardiomyocytes in the adult heart are less oxidative, not unlike those in embryos or zebrafish, and thus protected from oxidative DNA damage. To test this hypothesis, we sought to identify, and trace the lineage of hypoxic cardiomyocytes in the adult heart utilizing protein expression of hypoxia inducible factor 1 alpha (Hif-1α), a master regulator of cellular hypoxic response. Hif-1α protein is stabilized during hypoxia, resulting in transcriptional activation of multiple target genes involved in glycolytic and mitochondrial metabolism, antioxidant enzymes, cell cycle regulators, etc. (30, 65). In fact, Hif-1α protein is stabilized in hypoxic stem cells and required for the maintenance of their quiescent state (72), and in addition, is required for cardiac regeneration after injury in zebrafish (29) and cardiac development in fetal hypoxic cardiomyocytes (17) through regulation of cell cycle, stress response pathways and cellular metabolism (7, 23, 46). As a result of Hif-1α stabilization-based lineage tracing, we identified a rare cardiomyocyte population that retains embryonic/neonatal features, such as smaller cell size, mononucleation, lower levels of oxidative DNA damage, and contribution to cardiomyocyte renewal (34). In addition to having a lower capillary density, RNA sequencing analysis of isolated hypoxic proliferative cardiomyocytes suggests the existence of an intrinsic mechanism that mediates the expression of Hif-1α such as an upregulation of Hif-1α mRNA and downregulation of prolyl hydroxylases (34). Therefore, our results suggest that some adult proliferative cardiomyocytes are protected from oxidative stress, either by inhabiting a hypoxic microenvironment, or activating an innate safeguard mechanism to stabilize Hif-1α or both.
Hypoxia Induces Cardiac Regeneration in the Adult Heart
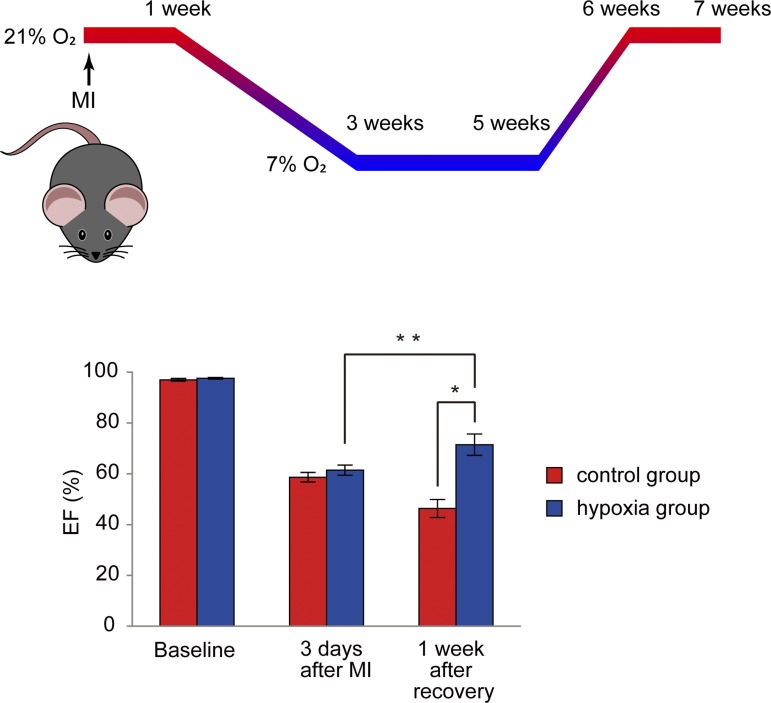
As we have outlined earlier, postnatal mitochondrial ROS production inhibits cardiomyocyte proliferation (34, 66). Therefore, we reasoned that long-term systemic hypoxemia might decrease mitochondrial respiration and mitochondrial ROS production, and thereby activate cell cycle reentry through reduction in oxidative DNA damage. To test this hypothesis, we exposed adult mice to hypoxia (7% oxygen, equivalent to summit of Mt. Everest) for 2 wk following a gradual decrease in oxygen tension by 1% per day from 20.9% ambient oxygen to 7% over 14 days. Given that change in the fraction of inspired oxygen does not necessarily correlate with a change in oxidative metabolism (21), it is critical to directly assess mitochondrial metabolism. Following hypoxia, we observed a reduction in mitochondrial metabolism as evidenced by a decreased mitochondrial DNA copy number, cristae density, and the protein expression of Krebs cycle and fatty acid beta-oxidation enzymes (51). In parallel, we observed reduced oxidative DNA damage in cardiomyocyte nuclei and cell cycle reentry in differentiated cardiomyocytes (51). Remarkably, chronic hypoxia also induced cardiomyocyte proliferation following myocardial infarction (MI), which was accompanied by significant recovery of LV systolic function (Fig. 2). It is important to note here that the effect of hypoxia on cardiomyocyte proliferation was almost completely abolished upon injection of the ROS generator diquat, indicating that the reduction in ROS and oxidative DNA damage plays a causative role in cardiomyocyte cell cycle reentry (51). There was no increase in mortality either in the MI group, or the sham group during 2 wk of exposure to 7% O2 following a gradual decrease in oxygen concentration by 1% per day (51). However, mortality in both MI group and in sham-operated group increased during 3 wk of 7% oxygen exposure, suggesting that prolonged exposure to severe hypoxia is poorly tolerated. Even so, the surviving MI mice showed a significant improvement in LV systolic function following recovery from hypoxia to ambient oxygen (51). Moreover, we tested whether exposure to more moderate hypoxia (10% oxygen, in combination with the administration of mitochondrial-ROS scavenger mitoTEMPO) induces an improvement in LV function in MI mice. We found that this intervention resulted in prevention of LV remodeling, which suggests that there may be some beneficial effects to moderate degrees of hypoxia, although no significant cardiomyocyte proliferation was observed. It is noteworthy here that chronic hypoxia also enhanced vascular supply as evidenced by elevated coronary collateral formation and vasodilation in cardiac capillaries, consistent with previous observations (14). In agreement, we observed an increased cell division in nonmyocyte lineages, suggesting the contribution of nonmyocyte proliferation to improvement of cardiac function (51). This may contribute to the favorable effect on cardiac remodeling following exposure to hypoxia (51). These findings may seem counterintuitive given that myocardial ischemia causes cardiomyopathy through cardiomyocyte death (reviewed in 10, 18). However, the effect of gradual prolonged systemic hypoxemia, which can reduce mitochondria-derived oxidative damage in cardiomyocytes, has not been tested, and represents a fundamentally different scenario compared with acute ischemia or hypoxia where adaptive changes do not occur. Our findings indicate that hypoxia and targeting hypoxia signaling may represent novel therapeutic strategies for heart regeneration. It is important to note here that a forced-activation of hypoxia signaling by means of genetic ablations of genes encoding prolyl hydroxylases (PHD2, -3) (50) or von Hippel Lindau (VHL) (37) in cardiomyocytes resulted in some deleterious effects including heart failure or cardiomyopathy. This is potentially due to either dysregulation of metabolic state (37, 52) or the activation of Hif-independent pathways (78). Thus it is critical to better understand the precise signaling cascades of hypoxia-induced cardiomyocyte proliferation in order to develop new therapeutic approaches.
Fig. 2.
Long-term hypoxia induced functional recovery in the infarcted heart. *P < 0.05, **P < 0.01. [Modified from Nakada et al. (51) with permission.]
Clinical Implications and Conclusion
An important question with a view to the future clinical application is how beneficial, or detrimental, hypoxia is for patients with cardiomyopathy (2a). Epidemiological data indicate significantly lower incidence of coronary artery diseases in high altitude in the United States (48, 74), although another study showed no correlation between altitude and coronary heart diseases (49). A study in Switzerland with a relatively homogeneous population in terms of ethnicity and culture revealed a protective effect of altitude in cardiovascular mortality (19). In agreement with previous studies including our own, coronary vasodilation is also found in humans at high altitude (31), which may contribute to the inverse correlation between coronary disease incidence and altitude. Nevertheless, given a few studies that have assessed the safety of exposure of patients with cardiovascular diseases to low oxygen were mainly in the setting of mountain climb or trekking (24, 26, 67, 75) which is fundamentally different from a therapeutic setting, further studies are required to evaluate the effect of hypoxia on cardiovascular disease patients.
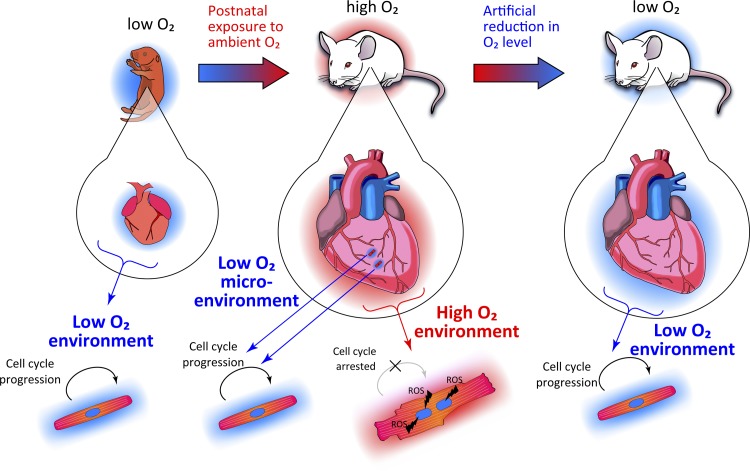
In conclusion, mounting evidence suggests that oxidative metabolism is a key regulator of proliferative competency of mammalian cardiomyocytes (Fig. 3). Although further studies are required for evaluation of safety and efficacy, hypoxia may be a new, albeit counterintuitive, strategy for treatment of cardiomyopathy. From a mechanistic standpoint, molecular mechanisms underlying environmental oxygen-dependent metabolic switch in cardiomyocytes remain poorly understood. In particular, upstream signaling pathways, transcription factors, and epigenetic modifiers involved in modulating the metabolic shift from an energy-demanding nonproliferative state to a regenerative state are important future therapeutic targets.
Fig. 3.
Oxygenation state and cardiac regeneration in mammals.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.K., Y.N., and H.A.S. prepared figures; W.K., Y.N., and H.A.S. drafted manuscript; W.K., Y.N., and H.A.S. edited and revised manuscript; W.K., Y.N., and H.A.S. approved final version of manuscript.
REFERENCES
- 1.Aix E, Gutiérrez-Gutiérrez Ó, Sánchez-Ferrer C, Aguado T, Flores I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J Cell Biol 213: 571–583, 2016. doi: 10.1083/jcb.201510091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci USA 111: 8850–8855, 2014. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Anderson JD, Honigman B. The effect of altitude-induced hypoxia on heart disease: do acute, intermittent, and chronic exposures provide cardioprotection? High Alt Med Biol 12: 45–55, 2011. doi: 10.1089/ham.2010.1021. [DOI] [PubMed] [Google Scholar]
- 3.Ascuitto RJ, Ross-Ascuitto NT, Chen V, Downing SE. Ventricular function and fatty acid metabolism in neonatal piglet heart. Am J Physiol Heart Circ Physiol 256: H9–H15, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 124: 1382–1392, 2014. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of cell generation and turnover in the human heart. Cell 161: 1566–1575, 2015. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Breckenridge RA, Piotrowska I, Ng KE, Ragan TJ, West JA, Kotecha S, Towers N, Bennett M, Kienesberger PC, Smolenski RT, Siddall HK, Offer JL, Mocanu MM, Yelon DM, Dyck JR, Griffin JL, Abramov AY, Gould AP, Mohun TJ. Hypoxic regulation of hand1 controls the fetal-neonatal switch in cardiac metabolism. PLoS Biol 11: e1001666, 2013. doi: 10.1371/journal.pbio.1001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol 79: 315–318, 2015. doi: 10.1016/j.yjmcc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano-Martínez A, Vargas-González A, Guarner-Lans V, Prado-Zayago E, León-Oleda M, Nieto-Lima B. Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch Cardiol Mex 80: 79–86, 2010. [PubMed] [Google Scholar]
- 10.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2: e244, 2011. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi WY, Poss KD. Cardiac regeneration. Curr Top Dev Biol 100: 319–344, 2012. doi: 10.1016/B978-0-12-387786-4.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross KW, Dawes GS, Mott JC. Anoxia, oxygen consumption and cardiac output in new-born lambs and adult sheep. J Physiol 146: 316–343, 1959. doi: 10.1113/jphysiol.1959.sp006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darehzereshki A, Rubin N, Gamba L, Kim J, Fraser J, Huang Y, Billings J, Mohammadzadeh R, Wood J, Warburton D, Kaartinen V, Lien CL. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev Biol 399: 91–99, 2015. doi: 10.1016/j.ydbio.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Günther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 15.Dawes GS, Mott JC, Widdicombe JG. The foetal circulation in the lamb. J Physiol 126: 563–587, 1954. doi: 10.1113/jphysiol.1954.sp005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell 15: 521–533, 2008. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell 17: 755–773, 2009. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 17: 1391–1401, 2011. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faeh D, Gutzwiller F, Bopp M; Swiss National Cohort Study Group . Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation 120: 495–501, 2009. doi: 10.1161/CIRCULATIONAHA.108.819250. [DOI] [PubMed] [Google Scholar]
- 20.Glass ML, Rantin FT. Gas exchange and control of respiration in air-breathing teleost fish. In: Cardio-Repiratory Control in Vertebrates, edited by Glass M, Wood S. Heidelberg, Germany: Springer, 2009, p. 99–119, doi: 10.1007/978-3-540-93985-6_5. [DOI] [Google Scholar]
- 21.Gleason CA, Hamm C, Jones MD Jr. Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol Heart Circ Physiol 258: H1064–H1069, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Graham JB, Wegner NC. Breathing air in water and in air: the air-breathing fishes. In: Respiratory Physiology of Vertebrates: Life With and Without Oxygen, edited by Nilsson GE. Cambridge, UK: Cambridge Univ. Press, 2010, p. 174–221. doi: 10.1017/CBO9780511845178.007. [DOI] [Google Scholar]
- 23.Guimarães-Camboa N, Stowe J, Aneas I, Sakabe N, Cattaneo P, Henderson L, Kilberg MS, Johnson RS, Chen J, McCulloch AD, Nobrega MA, Evans SM, Zambon AC. HIF1α represses cell stress pathways to allow proliferation of hypoxic fetal cardiomyocytes. Dev Cell 33: 507–521, 2015. doi: 10.1016/j.devcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J 159: 25–32, 2010. doi: 10.1016/j.ahj.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Hultgren HN. The safety of trekking at high altitude after coronary bypass surgery. JAMA 260: 2218–2219, 1988. doi: 10.1001/jama.1988.03410150066024. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, Mizushima N, Mihara K, Ishihara N. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol 35: 211–223, 2015. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15: 243–256, 2014. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464: 606–609, 2010. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaelin WG., Jr The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun 338: 627–638, 2005. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann BA, Bernheim AM, Kiencke S, Fischler M, Sklenar J, Mairbäurl H, Maggiorini M, Brunner-La Rocca HP. Evidence supportive of impaired myocardial blood flow reserve at high altitude in subjects developing high-altitude pulmonary edema. Am J Physiol Heart Circ Physiol 294: H1651–H1657, 2008. doi: 10.1152/ajpheart.00760.2007. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464: 601–605, 2010. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura W, Muralidhar S, Canseco DC, Puente B, Zhang CC, Xiao F, Abderrahman YH, Sadek HA. Redox signaling in cardiac renewal. Antioxid Redox Signal 21: 1660–1673, 2014. doi: 10.1089/ars.2014.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 523: 226–230, 2015. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan J, Ahuja P, Bodenmann S, Knapik D, Perriard E, Krek W, Perriard JC. Essential role of developmentally activated hypoxia-inducible factor 1alpha for cardiac morphogenesis and function. Circ Res 103: 1139–1146, 2008. doi: 10.1161/01.RES.0000338613.89841.c1. [DOI] [PubMed] [Google Scholar]
- 36.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 220: 175–186, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol 28: 3790–3803, 2008. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Yue S, Chen X, Kubin T, Braun T. Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ Res 106: 1498–1506, 2010. doi: 10.1161/CIRCRESAHA.109.211888. [DOI] [PubMed] [Google Scholar]
- 39.Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res 26: 1172–1180, 1992. doi: 10.1093/cvr/26.12.1172. [DOI] [PubMed] [Google Scholar]
- 40.Lopaschuk GD, Spafford MA. Energy substrate utilization by isolated working hearts from newborn rabbits. Am J Physiol Heart Circ Physiol 258: H1274–H1280, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol Heart Circ Physiol 261: H1698–H1705, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, Burns CE, Burns CG, MacRae CA, Poss KD, Lee RT. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell 34: 387–399, 2015. doi: 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoud AI, Porrello ER. Turning back the cardiac regenerative clock: lessons from the neonate. Trends Cardiovasc Med 22: 128–133, 2012. doi: 10.1016/j.tcm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 5: 191–209, 2013. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Heart mitochondrial DNA and enzyme changes during early human development. Mol Cell Biochem 210: 47–52, 2000. doi: 10.1023/A:1007031919298. [DOI] [PubMed] [Google Scholar]
- 46.Menendez-Montes I, Escobar B, Palacios B, Gómez MJ, Izquierdo-Garcia JL, Flores L, Jiménez-Borreguero LJ, Aragones J, Ruiz-Cabello J, Torres M, Martin-Puig S. Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Dev Cell 39: 724–739, 2016. doi: 10.1016/j.devcel.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kühn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA 110: 1446–1451, 2013. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortimer EA Jr, Monson RR, MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N Engl J Med 296: 581–585, 1977. doi: 10.1056/NEJM197703172961101. [DOI] [PubMed] [Google Scholar]
- 49.Morton WE, Davids DJ, Lichty JA. Mortality from heart disease at high altitude. the effect of high altitude on mortality from arteriosclerotic and hypertensive heart disease. Arch Environ Health 9: 21–24, 1964. doi: 10.1080/00039896.1964.10663788. [DOI] [PubMed] [Google Scholar]
- 50.Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, Signoretti S, Liao R, Kaelin WG Jr. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation 122: 1004–1016, 2010. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature 541: 222–227, 2017. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 52.Neary MT, Ng KE, Ludtmann MH, Hall AR, Piotrowska I, Ong SB, Hausenloy DJ, Mohun TJ, Abramov AY, Breckenridge RA. Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J Mol Cell Cardiol 74: 340–352, 2014. doi: 10.1016/j.yjmcc.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Meara CC, Wamstad JA, Gladstone RA, Fomovsky GM, Butty VL, Shrikumar A, Gannon JB, Boyer LA, Lee RT. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ Res 116: 804–815, 2015. doi: 10.1161/CIRCRESAHA.116.304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill TJ IV, Mack CP, Taylor JM. Germline deletion of FAK-related non-kinase delays post-natal cardiomyocyte mitotic arrest. J Mol Cell Cardiol 53: 156–164, 2012. doi: 10.1016/j.yjmcc.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool 187: 249–253, 1974. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 56.Oberpriller JO, Oberpriller JC, Arefyeva AM, Mitashov VI, Carlson BM. Nuclear characteristics of cardiac myocytes following the proliferative response to mincing of the myocardium in the adult newt, Notophthalmus viridescens. Cell Tissue Res 253: 619–624, 1988. doi: 10.1007/BF00219752. [DOI] [PubMed] [Google Scholar]
- 57.Okazaki K, Maltepe E. Oxygen, epigenetics and stem cell fate. Regen Med 1: 71–83, 2006. doi: 10.2217/17460751.1.1.71. [DOI] [PubMed] [Google Scholar]
- 58.Pohjoismäki JL, Boettger T, Liu Z, Goffart S, Szibor M, Braun T. Oxidative stress during mitochondrial biogenesis compromises mtDNA integrity in growing hearts and induces a global DNA repair response. Nucleic Acids Res 40: 6595–6607, 2012. doi: 10.1093/nar/gks301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pohjoismäki JL, Goffart S, Taylor RW, Turnbull DM, Suomalainen A, Jacobs HT, Karhunen PJ. Developmental and pathological changes in the human cardiac muscle mitochondrial DNA organization, replication and copy number. PLoS One 5: e10426, 2010. doi: 10.1371/journal.pone.0010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pohjoismäki JL, Krüger M, Al-Furoukh N, Lagerstedt A, Karhunen PJ, Braun T. Postnatal cardiomyocyte growth and mitochondrial reorganization cause multiple changes in the proteome of human cardiomyocytes. Mol Biosyst 9: 1210–1219, 2013. doi: 10.1039/c3mb25556e. [DOI] [PubMed] [Google Scholar]
- 61.Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennett DG, dos Remedios CG, Haubner BJ, Penninger JM, Kühn B. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci Transl Med 7: 281ra45, 2015. doi: 10.1126/scitranslmed.aaa5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080, 2011. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA 110: 187–192, 2013. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science 298: 2188–2190, 2002. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 65.Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology (Bethesda) 30: 340–348, 2015. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157: 565–579, 2014. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rimoldi SF, Sartori C, Seiler C, Delacrétaz E, Mattle HP, Scherrer U, Allemann Y. High-altitude exposure in patients with cardiovascular disease: risk assessment and practical recommendations. Prog Cardiovasc Dis 52: 512–524, 2010. doi: 10.1016/j.pcad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436, 2013. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol Heart Circ Physiol 271: H2183–H2189, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Sturzu AC, Rajarajan K, Passer D, Plonowska K, Riley A, Tan TC, Sharma A, Xu AF, Engels MC, Feistritzer R, Li G, Selig MK, Geissler R, Robertson KD, Scherrer-Crosbie M, Domian IJ, Wu SM. Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation 132: 109–121, 2015. doi: 10.1161/CIRCULATIONAHA.114.011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thiriet M. Pathogenesis of cardiac diseases. In: Diseases of the Cardiac Pump. Cham, Switzerland: Springer International, 2015, p. 1–98. [Google Scholar]
- 72.Takubo K, Suda T. Roles of the hypoxia response system in hematopoietic and leukemic stem cells. Int J Hematol 95: 478–483, 2012. doi: 10.1007/s12185-012-1071-4. [DOI] [PubMed] [Google Scholar]
- 73.Tane S, Okayama H, Ikenishi A, Amemiya Y, Nakayama KI, Takeuchi T. Two inhibitory systems and CKIs regulate cell cycle exit of mammalian cardiomyocytes after birth. Biochem Biophys Res Commun 466: 147–154, 2015. doi: 10.1016/j.bbrc.2015.08.102. [DOI] [PubMed] [Google Scholar]
- 74.Voors AW, Johnson WD. Altitude and arteriosclerotic heart disease mortality in white residents of 99 of the 100 largest cities in the United States. J Chronic Dis 32: 157–162, 1979. doi: 10.1016/0021-9681(79)90044-4. [DOI] [PubMed] [Google Scholar]
- 75.Wyss CA, Koepfli P, Fretz G, Seebauer M, Schirlo C, Kaufmann PA. Influence of altitude exposure on coronary flow reserve. Circulation 108: 1202–1207, 2003. doi: 10.1161/01.CIR.0000087432.63671.2E. [DOI] [PubMed] [Google Scholar]
- 76.Xiao Q, Zhang G, Wang H, Chen L, Lu S, Pan D, Liu G, Yang Z. A p53-based genetic tracing system to follow postnatal cardiomyocyte expansion in heart regeneration. Development 144: 580–589, 2017. doi: 10.1242/dev.147827. [DOI] [PubMed] [Google Scholar]
- 77.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA 110: 13839–13844, 2013. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang M, Su H, Soga T, Kranc KR, Pollard PJ. Prolyl hydroxylase domain enzymes: important regulators of cancer metabolism. Hypoxia (Auckl) 2: 127–142, 2014. doi: 10.2147/HP.S47968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zebrowski DC, Vergarajauregui S, Wu CC, Piatkowski T, Becker R, Leone M, Hirth S, Ricciardi F, Falk N, Giessl A, Just S, Braun T, Weidinger G, Engel FB. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. eLife 4: 4, 2015. doi: 10.7554/eLife.05563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang CC, Sadek HA. Hypoxia and metabolic properties of hematopoietic stem cells. Antioxid Redox Signal 20: 1891–1901, 2014. doi: 10.1089/ars.2012.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]