Abstract
Microdialysis is a minimally invasive technique often paired with laser Doppler flowmetry to examine cutaneous microvascular function, yet presents with several challenges, including incompatibility with perfusion of highly lipophilic compounds. The present study addresses this methodological concern, with an emphasis on the independent effects of commonly used vehicle dialysis solutions to improve solubility of pharmacological agents with otherwise low aqueous solubility. Four microdialysis fibers were placed in the ventral forearm of eight subjects (4 men, 4 women; 25 ± 1 yr) with sites randomized to serve as 1) control (lactated Ringer’s), 2) Sodium carbonate-bicarbonate buffer administered at physiological pH [SCB-HCl; pH 7.4, achieved via addition of hydrochloric acid (HCl)], 3) 0.02% ethanol, and 4) 2% dimethyl sulfoxide (DMSO). After baseline (34°C), vehicle solutions were administered throughout a standardized local heating protocol to 42°C. Laser Doppler flowmetry provided an index of blood flow. Cutaneous vascular conductance was calculated and normalized to maximum (%CVCmax, sodium nitroprusside and 43°C local heat). The SCB-HCl solution increased baseline %CVCmax (control: 9.7 ± 0.8; SCB-HCl: 21.5 ± 3.5%CVCmax; P = 0.03), but no effects were observed during heating or maximal vasodilation. There were no differences with perfusion of ethanol or DMSO at any stage of the protocol (P > 0.05). These data demonstrate the potential confounding effects of some vehicle dialysis solutions on cutaneous vascular function. Notably, this study provides evidence that 2% DMSO and 0.02% ethanol are acceptable vehicles with no confounding local vascular effects to a standardized local heating protocol at the concentrations presented.
NEW & NOTEWORTHY This study examined the independent effects of common vehicle solutions on cutaneous vascular responses. A basic buffer (SCB-HCl) caused baseline vasodilation; 2% DMSO and 0.02% ethanol had no effects. This highlights the need for considering potential confounding effects of solubilizing solutions when combined with low aqueous soluble pharmacological agents. Importantly, DMSO and ethanol do not appear to influence cutaneous vascular function during baseline or local heating at the concentrations studied, allowing their use without confounding effects.
Keywords: microdialysis, solubility, laser Doppler flowmetry, vehicle solution, skin blood flow
the cutaneous circulation is an accessible microvascular bed that allows for in vivo assessment of cutaneous vascular function and elucidation of mechanisms of vascular control. Microdialysis is a minimally invasive technique that is commonly paired with laser Doppler flowmetry, as an index of skin blood flow (SkBF), to deliver specific pharmacological agents directly to the intradermal space without exerting any systemic effects (10). This approach has been successfully utilized to pharmaco-dissect a variety of signaling pathways involved in cutaneous vascular control. Multiple disease groups, including, primary aging (25), hypertension (8), hypercholesterolemia (34), Type 2 diabetes (38), and heart failure (23), have been studied, helping advance our knowledge of underlying vascular dysfunction in these groups. This approach has been widely adopted because alterations in cutaneous microvascular function mirror and often precede alterations in microvascular function in other organs and tissues in both mechanism and magnitude (13, 35).
The development and availability of pharmacological agents utilized for pharmaco-dissection of signaling pathways involved in cutaneous microvascular control has grown rapidly with this area of study. To facilitate delivery to the cutaneous circulation via intradermal microdialysis, pharmacological agents are mixed with a vehicle solution and perfused through microdialysis fibers. Lactated Ringer’s, a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water, is the most common vehicle used for delivery of water-soluble pharmacological agents via intradermal microdialysis and often serves as a control solution when perfused alone. However, the low aqueous solubility of some pharmacologics presents a significant obstacle in effective drug delivery, necessitating the use of other vehicle additives, such as alcohols, acids, and bases, to improve solubility. Investigators must therefore carefully consider the independent effects of such vehicle solutions, the drug concentration achievable, and the use of appropriate control sites and data normalization strategies when appropriate.
The most commonly utilized solubilizing agents include ethanol, dimethyl sulfoxide (DMSO), glycerol, and propylene glycol, with varying concentrations depending on the specific drug of interest. The two most widely used vehicle solutions for nonaqueous pharmacologics delivered via cutaneous microdialysis are ethanol and DMSO, yet limited data have been presented on the independent effects of these vehicles. A small number of studies report pilot experiment data suggesting these vehicles cause baseline vasodilation, but their effects during experimental protocols have largely not been considered. In particular, DMSO has consistently been reported to cause baseline vasodilation, but the use of concentrations ranging from 0.1 to 5% has caused varying degrees of baseline shift (31, 33, 36).
Whereas some pharmacologics have poor solubility in aqueous solutions, others require preparation in a basic buffer solution to ensure stability and are returned to physiological pH immediately before delivery [i.e., the prostacyclin I2 (PGI2) agonist epoprostenol sodium is prepared and stored at pH >10.2, and hydrogen sulfide donors (Na2S, NaHS, pH > 11)]. Sodium carbonate-bicarbonate (SCB) buffers are simple to prepare, relatively inexpensive, and provide good stability, making them an ideal and commonly used buffer for pharmalogics requiring a basic solution. To avoid painful delivery into the cutaneous circulation and local pH shifts, hydrochloric acid is used to reduce the vehicle pH before administration. Despite the increasing utilization of drug delivery via intradermal microdialysis, the independent effects of such agents on cutaneous vascular function have not been determined. This raises an important concern in studies that utilize solubilizing and stabilizing agents, which may potentially confound SkBF responses if the effects of the vehicle solution are not taking into consideration. Accordingly, the primary aim of this study was to determine the independent effects of three widely used vehicle solutions on cutaneous microvascular function during a commonly utilized (5, 18, 24, 46) standard local heating (LH) protocol to 42°C. Based on observations reported by other authors and preliminary data from our laboratory (Unpublished data; Clarke MM and Smith CJ 2016; Kutz JL and Alexander LM 2016; Craighead DH; Alexander LM 2015), we hypothesized that the SCB buffer with hydrochloric acid (pH 7.4) and the 2% DMSO would cause vasodilation during both baseline and the LH plateau, while 0.02% ethanol would not significantly affect SkBF at any point during the protocol.
METHODS
The study was approved by the institutional review board at The Pennsylvania State University and conformed to the guidelines set forth in the Declaration of Helsinki. Written and verbal consent was voluntarily obtained from all subjects before participation in the study. The experiment was conducted in a group of eight young, healthy participants who were nonsmokers, not pregnant or breastfeeding, and were free from any known cardiovascular or metabolic diseases. Women were tested regardless of menstrual cycle phase or use of birth control. Participants were not taking any other medications that are known to alter metabolic or vascular function.
All experiments took place in a thermoneutral laboratory with subjects in a semisupine position. Subjects refrained from consuming alcohol and caffeine, and from exercise, for 12 h before the study. On arrival at the laboratory, subjects completed a medical history and underwent measurements of anthropometrics, blood pressure, and heart rate. All women participants had a negative urine pregnancy test on the morning of the experiment. After screening, four intradermal microdialysis fibers (30 kDa cutoff; MD 2000, LM-10, Bioanalytical Systems) were placed in the skin of the ventral surface of the forearm as previously described (40). After microdialysis fiber placement, lactated Ringer’s solution was perfused through all sites at a rate of 2 μl/min (BASi Bee Hive controller and Baby Bee syringe drive) for 60–90 min to allow full resolution of hyperemia due to fiber insertion trauma.
After subsidence of hyperemia, LH units (Moor Instruments, UK) were placed over all microdialysis sites and skin temperature was clamped at 34°C to maintain thermoneutral baseline skin temperature. Each LH unit housed a laser Doppler flowmeter probe (Moor Instruments) for the continuous measurement of red cell flux, providing an index of SkBF. Subjects were outfitted with an automated blood pressure cuff (Cardiocap 5, General Electric), and blood pressure was measured via brachial auscultation every 5 min throughout the protocol.
Baseline data were obtained for 10 min (baseline 1) before the solution vehicles were randomly assigned to each microdialysis site, including 1) lactated Ringer’s to serve as control (Owens and Minor), 2) 0.02% ethanol, 3) 2% dimethyl sulfoxide (DMSO), and 4) sodium carbonate/sodium bicarbonate + hydrochloric acid [SCB-HCl: a sodium carbonate/sodium bicarbonate buffer solution (pH 10.4) was mixed and then returned to physiological pH with hydrochloric acid]. All solution vehicles were perfused at a rate of 2 μl/min for a minimum of 20 min before obtaining a second “vehicle baseline” (baseline 2), ensuring a stable plateau in SkBF was achieved. A standardized LH protocol was employed in which the temperature of the local heating units were raised to 42°C at a rate of 0.5°C/5 s. This protocol is known to induce vasodilation that is predominantly dependent on nitric oxide (NO) produced via endothelial NO synthase (eNOS) (5, 30, 32) and is commonly utilized to assess cutaneous microvascular function (1, 15, 21, 24, 39, 43). Local skin temperature (Tsk) was clamped at 42°C, and SkBF measured until a stable “local heating plateau” in SkBF was achieved (~40 min), after which the local heaters were increased to 43°C, and 28 mM sodium nitroprusside (SNP) was perfused through each fiber at a rate of 4 μl/min to elicit maximum cutaneous vasodilation (5, 26).
In conjunction with our in vivo functional analysis, the ionic compositions of all vehicle solutions were analyzed with an automated electrolyte analyzer (Diamond Diagnostics SmartLyte Electrolyte Analyzer) to determine sodium, potassium, and chloride concentrations. After completion of in vivo experiments, vehicle samples were prepared in the exact same manner as for the functional experiments and samples were analyzed in triplicate.
Data and statistical analysis.
Red cell flux data were sampled at 40 Hz with WinDaq data acquisition software (DATAQ Instruments) and stored offline for later analysis. Red cell flux was normalized to cutaneous vascular conductance (CVC: flux per mean arterial pressure) and expressed as a percentage of site specific maximum (%CVCmax) obtained from perfusion of SNP and 43°C heat. Presentation and interpretation of data as a percentage of maximum dilation is standard when evaluating cutaneous microvascular function since it reduces variability due to the heterogeneous distribution of microvessels within skin tissue (3, 4).
All data were analyzed with SAS version 9.3 software. One-way ANOVAs with Dunnett’s test for multiple comparisons was used to compare between site differences relative to control (lactated Ringer’s) at each phase of the protocol. An a priori power analysis was conducted where we determined that seven subjects would be sufficient to detect a 10% differences in %CVCmax, between treatment sites with a standard deviation of the difference at 8% (determined from our previously published data) (5, 37, 45), a β = 0.80, and ɑ = 0.05. The interday reproducibility of SkBF measured using LDF is considered acceptable when normalized to maximal values (%CVCmax); however, due to the recognized variability in baseline SkBF compared with the LH plateau, a 10% difference in %CVCmax was determined as a physiologically meaningful difference (45). Effect size (Cohen’s d) is reported for mean differences. Interpretation of effect size followed the convention of Cohen: 0.2, 0.5, and 0.8 corresponding to a “small,” “medium,” and “large” effect size, respectively (9).
RESULTS
Eight (4 men, 4 women) young, healthy subjects (age: 25 ± 1 yr) participated in this study, all of whom were nonobese (BMI: 25.1 ± 1.4 kg/m−2) and normotensive (MAP: 85 ± 3 mmHg).
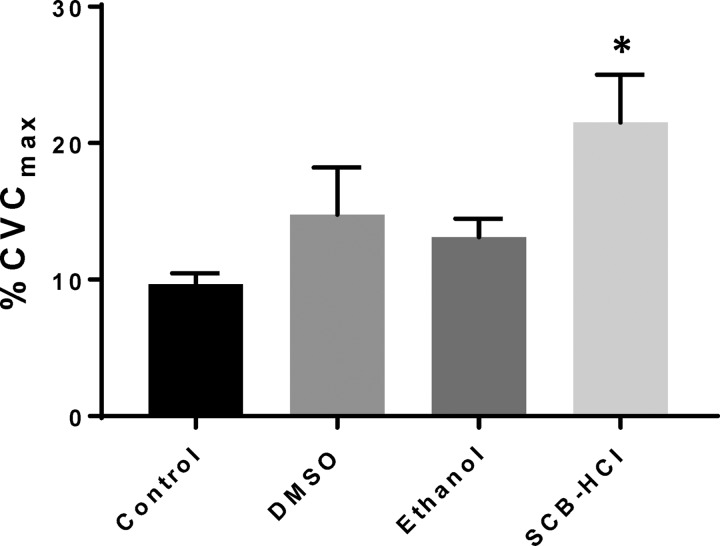
As expected, no differences were observed between sites at baseline 1 (lactated Ringer’s at all sites, P = 0.14; data not shown). Figure 1 illustrates perfusion of solution vehicles at baseline 2 (local temperature clamped at 34°C). Cutaneous vascular conductance (%CVCmax) was greater at the SCB-HCl site relative to control [lactated Ringers: 9.67 (95% confidence interval: 7.81, 11.53), SCB-HCl: 21.52 (13.27, 29.76) %CVCmax; P = 0.03] and the effect size was large (1.66). %CVCmax was similar between control and both the ethanol site [13.12 (9.91, 16.32) %CVCmax; P = 0.11] and DMSO site [14.75 (6.56, 22.95) %CVCmax; P = 0.31].
Fig. 1.
Baseline vasodilation (means ± SE) during perfusion of investigational substances. Vasodilation was significantly greater with perfusion of sodium carbonate-bicarbonate–hydrochloric acid (SCB-HCl) compared with control; n = 8 subjects; *P < 0.05 compared with control site. Cutaneous vascular conductance (CVC: flux per mean arterial pressure) expressed as a percentage of site specific maximum (%CVCmax).
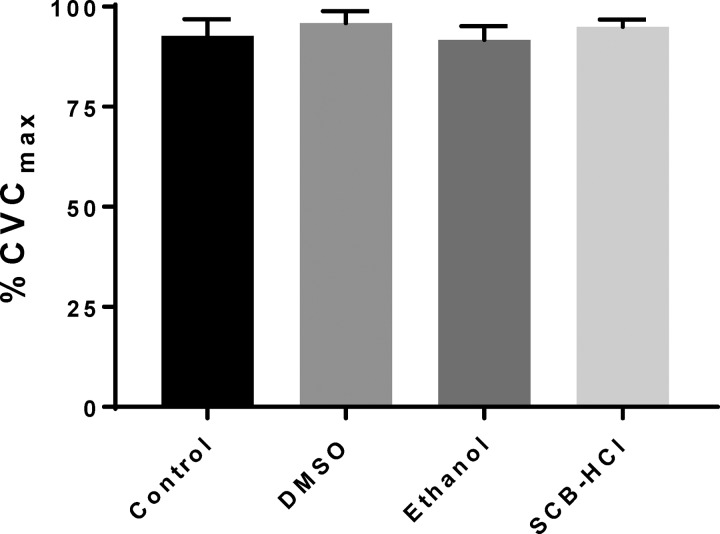
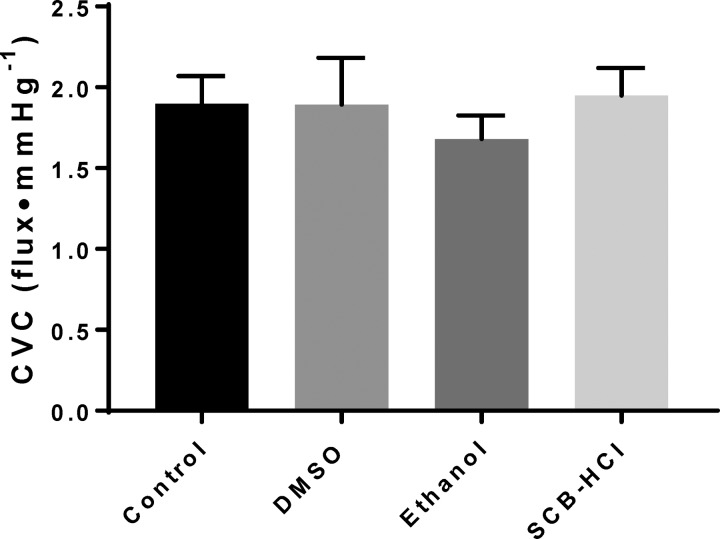
During the standardized LH protocol to 42°C, the plateau in SkBF was similar between all sites (Fig. 2, all P > 0.05). Figure 3 depicts absolute maximal vasodilation (expressed as CVC: flux/mmHg) obtained during perfusion of SNP and simultaneous 43°C local heat, which did not differ between sites (all P > 0.05). The solution vehicles were analyzed for ion composition (Table 1) with the only notable difference being the higher Na+ and Cl− concentrations in the SCB-HCl solution.
Fig. 2.
Vasodilation (means ± SE) during local heating to 42°C. Vasodilation was not different from control with any solubilizing agent; n = 8 subjects.
Fig. 3.
Maximum vasodilation (expressed as absolute CVC; means ± SE) at each site. Maximum vasodilation was not different from control with any solubilizing agent; n = 8 subjects.
Table 1.
Vehicle ionic composition
| Vehicle | Sodium, mmol/l | Potassium, mmol/l | Chloride, mmol/l |
|---|---|---|---|
| Lactated Ringer’s | 123.0 ± 0.0 | 3.8 ± 0.0 | 104.0 ± 0.0 |
| DMSO | 132.0 ± 0.0 | 4.1 ± 0.0 | 104.0 ± 0.0 |
| Ethanol | 123.3 ± 0.3 | 3.8 ± 0.0 | 104.0 ± 0.0 |
| SCB-HCl | 244.0 ± 0.0 | 3.6 ± 0.0 | 155.3 ± 0.3 |
Values are means ± SE. SCB-HCl, sodium carbonate-bicarbonate–hydrochloric acid.
DISCUSSION
The main findings from the present study were that 1) a SCB buffer perfused at physiological pH (following addition of HCl) increased cutaneous blood flow at baseline relative to lactated Ringer’s (control), whereas 2% DMSO and 0.02% ethanol did not; and 2) perfusion of the vehicle solutions SCB-HCl (pH 7.4), 2% DMSO, or 0.02% ethanol did not alter SkBF responses vs. the control site during 42°C LH or maximal vasodilation. These findings are methodologically important for delivering pharmacological agents with low aqueous solubility via microdialysis by using these solubilizing agents during investigation of cutaneous vascular function.
In the present study, the independent effects of three vehicle solutions on SkBF responses were determined during a standard LH protocol. Vehicle solutions were selected based on their common utility as solubilizing agents for effective delivery of pharmacologics with low aqueous solubility or stability. Addition of HCl for delivery of buffer solutions at physiological pH is important for subject comfort and safety, and to avoid independent effects of local pH shifts on vascular function (27). Baseline vasodilation was observed with perfusion of SCB-HCl, but not 2% DMSO or 0.02% ethanol through the skin of young, healthy men and women. This baseline vasodilation is an important finding with relevance to data interpretation and normalization. If laser Doppler flux data are normalized to baseline, as they are in many vasoconstriction data, the differences in baseline can potentially confound the interpretation of the data. Additionally, if the dilation from this buffer and a pharmacological agonist (such as endothelin receptor antagonists including sulfisoxazole and PD-156707) are additive, differences in vasodilation between a buffered and a nonbuffered microdialysis site may not be mediated entirely by the selected pharmacological agonist, but in part from the presence of the buffer. A “vehicle control” site, with continuous perfusion of the vehicle solution (drug free), should therefore be included in experimental protocols to assess the independent effects at all stages of the protocol.
A small number of studies have considered the independent effects of specific vehicle solutions on vascular function, yet none have directly compared the effects of different vehicles. In contrast to the present data, DMSO has been shown to cause mild cutaneous vasodilation at thermoneutral baseline (33°C), which is likely due to the use of concentrations >2% (7, 31, 36, 48). Animal studies using rat aortas exposed to increasing concentrations of DMSO (0.1–3%) demonstrated increased cGMP and significant increases in vasodilation with 3% DMSO only (28). DMSO-induced vasodilation was inhibited with addition of the nonspecific NOS inhibitor nitro-l-arginine methyl ester (l-NAME) and with removal of the rat aorta endothelium, collectively indicating DMSO to act via endothelium-derived NO-dependent mechanisms in a rat model (28). The use of 2% DMSO, as in the present study, has shown no significant effect on SkBF (33), yet concentrations of 3–5% DMSO have consistently shown minor vasodilatory properties when delivered in vivo via microdialysis in the human cutaneous circulation (7, 31, 36). Kellogg and colleagues (31) included a 5% DMSO (vehicle control) site throughout all experimental protocols and observed significant vasodilation at normothermic baseline (10 ± 2%CVCmax), but no significant differences vs. LR during whole body cold (3 min, 18°C water perfused suit) or heat stress conditions (48°C water perfused suit, 1°C∆ core temperature). Similar results have been observed by other laboratories using 5% DMSO (7) and 3% DMSO (36), suggesting ≤5% DMSO may cause baseline cutaneous vasodilation but no alteration of thermoregulatory responses. Notably, during a separate LH protocol, Kellogg et al. (31) observed no significant differences in SkBF between 5% DMSO and LR sites at baseline (Tsk 34°C; DMSO 28 ± 5% vs. LR 27 ± 5%CVCmax) or a standard LH protocol to 41°C (DMSO 88 ± 7% vs. 83 ± 9%CVCmax). The 34°C baseline Tsk may have masked the small vasodilatory effects of 5% DMSO observed at thermoneutral baseline (Tsk 33°C), which highlights the need for careful consideration of baseline conditions in experimental design. Alternatively, a lower concentration of a drug of interest may be dissolved in a lower concentration of DMSO (33) or in an alternative vehicles that does not elicit vasodilation, provided the required physiological effect is achieved. For example, following preliminary studies utilizing 5% DMSO, Wong and Fieger (48) selected 20 mM capsazepine dissolved in 90% propylene glycol and 10% LR solution rather than pursuing higher concentrations of capsazepine requiring DMSO.
In the present study, unlike baseline CVC, no change in LH-induced vasodilation or maximal vasodilation was observed with any of the vehicle solutions. These findings are consistent with other studies using higher concentrations of DMSO (3–5%) during similar protocols (7, 31, 36). The similarity in local thermal hyperemia between sites suggests that the SCB-HCl buffer augments only baseline dilation and has no significant effect when SkBF is elevated by an agonist. As hypothesized, 0.02% ethanol had no significant effect on SkBF at any stage of the protocol and is therefore a suitable solubilizing agent at such concentrations. Ethanol is a water-soluble organic solvent commonly used as a solubilizing agent, but has shown mixed effects on vascular function. Ethanol has been shown to inhibit transient receptor potential channel 8 (TRPM8) in animal models and human cell cultures (2, 47), while causing arterial vasodilation via TRPV1 receptors in both human and animal isolated vessel studies (20). In human in vivo studies, Craighead et al. (11) observed an attenuated menthol-mediated dilation when dissolved in a vehicle solution containing ethanol vs. LR (preliminary data), supporting the potential inhibitory effects of ethanol on cold-menthol TRPM8 receptors and alterations in cutaneous vascular function. Efficacious administration was subsequently achieved using a heating-stirring plate to aid menthol solubility, and may be considered as an alternative approach to solubilizing agents when feasible. Overall, these findings indicate that these vehicle solutions, at the concentrations utilized in this study, do not interfere with endothelial function in the cutaneous circulation and may be utilized without fear of alterations in microvascular function obscuring results.
The similarity in maximal vasodilation observed between all sites is important, but not unexpected. Maximum vascular conductance (or minimum vascular resistance) represents the maximum ability of the vasculature to dilate, and is an index of vascular structure (8, 12, 17, 42, 44). It is extremely unlikely that the acute use of a solubilizing agent would induce any alteration in microvascular structure over the short duration of a single microdialysis experiment. As anticipated, we observed no increase in maximum CVC with any of the solubilizing agent used. Solubilizing agents could theoretically cause a functional impairment that would attenuate maximum CVC. We observed no difference between our solubilizing agents and control, indicating that no functional decrements occurred. It is important that maximum CVC was not altered by any of the utilized solubilizing agents, as maximum CVC is used for data normalization and alteration of this value would affect all the measures of %CVCmax within a microdialysis site.
Analysis of the ionic composition of the vehicle solutions indicated a higher concentration of sodium and chloride in the SCB-HCl solution vs. all other vehicle solutions, but no difference in potassium. One possible mechanism mediating the augmented baseline SkBF with SCB-HCl perfusion may be an alteration in the cell membrane potential resulting from increased interstitial sodium. During LH, the intensity of the stimulus may have overridden any baseline effect of sodium on cell membrane potential, resulting in no significant impact on SkBF during heating. Furthermore, chloride ion concentration was slightly higher in the SCB-HCl vehicle solution, which has the potential to bring the membrane potential closer to resting state. Overall, the effect of the higher Na+ and Cl− concentrations appear to have affected only baseline vascular function. Notably, microdialysis perfusion of a hypertonic saline (3% NaCl) has been observed to elicit acute microvascular dysfunction when compared with normal saline, reducing the initial peak and LH plateau during the same standardized LH protocol as the present study (14). When co-infused with the nonspecific NOS inhibitor l-NAME, a reduced NO contribution to the LH plateau was observed at the hypertonic vs. normal saline site, suggesting a role of sodium in the attenuated cutaneous vascular response, potentiality mediated via reduced NO production or availability. The high sodium chloride concentration used by DuPont and colleagues (14) may explain the disparity in results compared with the present data, but does provide some evidence that altering interstitial sodium concentration may alter vascular responses.
Our data, demonstrating no differences between vehicle solutions in maximal CVC, are important with relevance to data interpretation using these techniques. Data obtained via laser Doppler flowmetry are subject to multiple possible data normalization schemes. It is standard to normalize the arbitrary red cell flux units measured via laser Doppler flowmetry to mean arterial pressure to account for changes in driving pressure. When cutaneous vasodilation is induced, either through pharmacological (acetylcholine, SNP) or physiological (local or whole body heating) stimuli, the data are generally expressed as a percentage of maximum conductance (%CVCmax) elicited by high local temperature (≥43°C) (22, 41), a high dose of SNP (≥28 mM) (6, 19, 29), or a combination of the two (1, 15, 16, 21). Normalizing to maximum conductance helps to account for variance in local vessel density within the relatively small field of view (~1 mm2) of individual laser Doppler probes (4) and for comparing between different measurement sites.
Recommendations for the use of solubilizing agents for delivery of pharmacologics via cutaneous microdialysis.
Investigators should consider the following points for experimental design and data analysis when vehicle solutions other than LR are used: 1) when possible, identify pharmacologics with high aqueous solubility for delivery with LR, low molecular weight, and high stability at physiological pH; 2) consider using a heating-stirring plate to improve solubility of a drug as an alternative to a solubilizing agent if efficacious delivery is possible, 3) ideally, a vehicle control site should be used throughout the protocol to confirm the effects of the solubilizing agent at all stages, 4) if a vehicle control site is not methodologically possible, vehicle solutions should be perfused at baseline before drug delivery to determine the independent effects of the vehicle/solubilizing agent; and 5) investigators must acknowledge baseline shift and consider appropriate normalization strategies.
Limitations.
A limitation to this study is that we examined a group of young, healthy subjects. In this population, the SkBF response to LH is likely limited by a ceiling effect as CVC reaches near maximum values. Therefore augmentation of conductance by any of the employed solubilizing agents may not be observable in this healthy population. It remains to be seen whether SCB-HCl (or any of the other agents) alters the SkBF response to LH in older or clinical populations that exhibit impaired endothelial function and attenuation of SkBF or during other protocols. Additionally, all subjects adhered to the usual conditions for experimental testing (i.e., no alcohol or caffeine for 12 h before testing); however, menstrual cycle phase was not controlled for during the present study. A further consideration is the limited generalizability of the present data based on the type of microdialysis probe used, molecular weight cutoff (MWCO), and the perfusion rates employed. In the present study, a polyacrylonitrile (PAN) probe membrane with a 30-kDa MWCO and perfusion rates of 2 μl/min were utilized. Notably, the delivery characteristics may be altered when using different probe membrane materials, for example, cuprophane (regenerated cellulose), polycarbonate, or polyethersulfone, differing MWCO (noting that the effective MWCO is typically only 20–30% of the reported MWCO), varying probe lengths, and perfusion rates. This reinforces the importance of including a vehicle control site throughout an experimental protocol. Finally, a power calculation was performed to detect a physiologically meaningful difference of 10%CVCmax between vehicles sites; however, we were underpowered to detect smaller differences.
Conclusions.
The present study identified that a basic buffer solution administered at physiological pH (SCB-HCl), increased baseline CVC compared with lactated Ringer’s solution. However, no change in baseline conductance was observed with DMSO (2%) or ethanol (0.02%). Neither local thermal hyperemia nor maximum CVC were altered by the use of any of the solubilizing agents. These data suggest that baseline shifts in CVC when interpreting data must be accounted for when buffer solutions are used to improve microdialysis perfusate solubility. Importantly, our data indicate that DMSO and ethanol, at the concentrations used in the present study, did not independently alter cutaneous vascular function during a commonly utilized LH protocol. These findings further highlight the utility of intradermal microdialysis, coupled with laser Doppler flowmetry, as an effective, reproducible, and minimally invasive technique to assess cutaneous microvascular responses.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.J.S. and L.M.A. conceived and designed research; C.J.S. and D.H.C. performed experiments; C.J.S. and D.H.C. analyzed data; C.J.S., D.H.C., and L.M.A. interpreted results of experiments; C.J.S. and D.H.C. drafted manuscript; C.J.S., D.H.C., and L.M.A. edited and revised manuscript; C.J.S., D.H.C., and L.M.A. approved final version of manuscript; D.H.C. prepared figures.
ACKNOWLEDGMENTS
The authors thank Sue Slimak for participant recruitment, Jane Pierzga for IRB preparation, and Megan Clarke and Ashlee Snyder for technical assistance throughout the study. Finally, we thank the participants for providing their valuable time.
REFERENCES
- 1.Alba BK, Stanhewicz AE, Kenney WL, Alexander LM. Acute dairy milk ingestion does not improve nitric oxide-dependent vasodilation in the cutaneous microcirculation. Br J Nutr 116: 204–210, 2016. doi: 10.1017/S0007114516001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedikt J, Teisinger J, Vyklicky L, Vlachova V. Ethanol inhibits cold-menthol receptor TRPM8 by modulating its interaction with membrane phosphatidylinositol 4,5-bisphosphate. J Neurochem 100: 211–224, 2007. doi: 10.1111/j.1471-4159.2006.04192.x. [DOI] [PubMed] [Google Scholar]
- 3.Braverman IM. The cutaneous microcirculation: ultrastructure and microanatomical organization. Microcirculation 4: 329–340, 1997. 9329009. [DOI] [PubMed] [Google Scholar]
- 4.Brothers RM, Wingo JE, Hubing KA, Crandall CG. Methodological assessment of skin and limb blood flows in the human forearm during thermal and baroreceptor provocations. J Appl Physiol (1985) 109: 895–900, 2010. doi: 10.1152/japplphysiol.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt VE, Fujii N, Minson CT. No independent, but an interactive, role of calcium-activated potassium channels in human cutaneous active vasodilation. J Appl Physiol (1985) 115: 1290–1296, 2013. doi: 10.1152/japplphysiol.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension 20: 349–355, 1992. doi: 10.1161/01.HYP.20.3.349. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates, 1988, p. xxi. [Google Scholar]
- 10.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res 110: 43–47, 2017. doi: 10.1016/j.mvr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craighead DH, Smith CJ, and Alexander LM. Blood pressure normalization via pharmacotherapy improves cutaneous microvascular function through NO-dependent and -independent mechanisms. Microcirculation 24: 2017. doi: 10.1111/micc.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debbabi H, Bonnin P, Ducluzeau PH, Lefthériotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens 23: 541–546, 2010. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 14.DuPont JJ, Farquhar WB, Edwards DG. Intradermal microdialysis of hypertonic saline attenuates cutaneous vasodilatation in response to local heating. Exp Physiol 96: 674–680, 2011. doi: 10.1113/expphysiol.2011.058404. [DOI] [PubMed] [Google Scholar]
- 15.DuPont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or l-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol (1985) 111: 1561–1567, 2011. doi: 10.1152/japplphysiol.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 17.Folkow B, Griyby G, Thulesius O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand 44: 255–272, 1958. doi: 10.1111/j.1748-1716.1958.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujii N, Brunt VE, Minson CT. Tempol improves cutaneous thermal hyperemia through increasing nitric oxide bioavailability in young smokers. Am J Physiol Heart Circ Physiol 306: H1507–H1511, 2014. doi: 10.1152/ajpheart.00886.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii N, Meade RD, Minson CT, Brunt VE, Boulay P, Sigal RJ, Kenny GP. Cutaneous blood flow during intradermal NO administration in young and older adults: roles for calcium-activated potassium channels and cyclooxygenase? Am J Physiol Regul Integr Comp Physiol 310: R1081–R1087, 2016. doi: 10.1152/ajpregu.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzieri D, Trevisani M, Tarantini F, Bechi P, Masotti G, Gensini GF, Castellani S, Marchionni N, Geppetti P, Harrison S. Ethanol dilates coronary arteries and increases coronary flow via transient receptor potential vanilloid 1 and calcitonin gene-related peptide. Cardiovasc Res 70: 589–599, 2006. doi: 10.1016/j.cardiores.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greaney JL, Stanhewicz AE, Proctor DN, Alexander LM, Kenney WL. Impairments in central cardiovascular function contribute to attenuated reflex vasodilation in aged skin. J Appl Physiol (1985) 119: 1411–1420, 2015. doi: 10.1152/japplphysiol.00729.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DJ, Maiorana AJ, Siong JH, Burke V, Erickson M, Minson CT, Bilsborough W, O’Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J 27: 338–343, 2006. doi: 10.1093/eurheartj/ehi655. [DOI] [PubMed] [Google Scholar]
- 24.Hodges GJ, Del Pozzi AT, McGarr GW, Mallette MM, Cheung SS. The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating. Eur J Appl Physiol 115: 2091–2098, 2015. doi: 10.1007/s00421-015-3188-7. [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed) 15: 718–739, 2010. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivic I, Solymar M, Pakai E, Rumbus Z, Pinter E, Koller A, Garami A. Transient receptor potential vanilloid-1 channels contribute to the regulation of acid- and base-induced vasomotor responses. J Vasc Res 53: 279–290, 2016. doi: 10.1159/000452414. [DOI] [PubMed] [Google Scholar]
- 28.Kaneda T, Sasaki N, Urakawa N, Shimizu K. Endothelium-dependent and -independent vasodilator effects of dimethyl sulfoxide in rat aorta. Pharmacology 97: 171–176, 2016. doi: 10.1159/000443894. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985) 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellogg DL Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. doi: 10.1113/jphysiol.2007.144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellogg DL Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol (1985) 107: 1438–1444, 2009. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellogg DL Jr, Zhao JL, Wu Y, Johnson JM. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J Appl Physiol (1985) 110: 1406–1413, 2011. doi: 10.1152/japplphysiol.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenney WL, Cannon JG, Alexander LM. Cutaneous microvascular dysfunction correlates with serum LDL and sLOX-1 receptor concentrations. Microvasc Res 85: 112–117, 2013. doi: 10.1016/j.mvr.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 115: 295–300, 2008. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- 36.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol 282: H232–H236, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with Type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 292: E314–E318, 2007. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- 39.Stanhewicz AE, Alba BK, Kenney WL, Alexander LM. Dairy cheese consumption ameliorates single-meal sodium-induced cutaneous microvascular dysfunction by reducing ascorbate-sensitive oxidants in healthy older adults. Br J Nutr 116: 658–665, 2016. doi: 10.1017/S0007114516002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol (1985) 112: 791–797, 2012. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanhewicz AE, Greaney JL, Alexander LM, Kenney WL. Blunted increases in skin sympathetic nerve activity are related to attenuated reflex vasodilation in aged human skin. J Appl Physiol (1985) 121: 1354–1362, 2016. doi: 10.1152/japplphysiol.00730.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stead EA Jr, Kunkel P. Nature of peripheral resistance in arterial hypertension. J Clin Invest 19: 25–33, 1940. doi: 10.1172/JCI101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JM, Taneja I, Raghunath N, Clarke D, Medow MS. Intradermal angiotensin II administration attenuates the local cutaneous vasodilator heating response. Am J Physiol Heart Circ Physiol 295: H327–H334, 2008. doi: 10.1152/ajpheart.00126.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita A, Mark AL. Decreased vasodilator capacity of forearm resistance vessels in borderline hypertension. Hypertension 2: 610–616, 1980. doi: 10.1161/01.HYP.2.5.610. [DOI] [PubMed] [Google Scholar]
- 45.Tew GA, Klonizakis M, Moss J, Ruddock AD, Saxton JM, Hodges GJ. Reproducibility of cutaneous thermal hyperaemia assessed by laser Doppler flowmetry in young and older adults. Microvasc Res 81: 177–182, 2011. doi: 10.1016/j.mvr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Van Duijnhoven NT, Janssen TW, Green DJ, Minson CT, Hopman MT, Thijssen DH. Effect of functional electrostimulation on impaired skin vasodilator responses to local heating in spinal cord injury. J Appl Physiol (1985) 106: 1065–1071, 2009. doi: 10.1152/japplphysiol.91611.2008. [DOI] [PubMed] [Google Scholar]
- 47.Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol Pharmacol 68: 518–527, 2005. doi: 10.1124/mol.105.012146. [DOI] [PubMed] [Google Scholar]
- 48.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]