Abstract
Objective
The composition of the extracellular matrix (ECM) impacts adipocyte function and might determine adipose tissue (AT) function and distribution. Cartilage oligomeric matrix protein (COMP), a matricellular protein usually studied in bone and cartilage, is highly differentially expressed between subcutaneous abdominal and gluteal AT. This study aimed to explore COMP's role in human subcutaneous abdominal and gluteal AT and preadipocyte biology.
Methods
COMP mRNA levels were measured in whole AT and immortalised preadipocytes via quantitative (q)-PCR. Tissue and cellular COMP protein were measured via Western blot and immunohistochemistry; plasma COMP was measured by ELISA. The effect of COMP on adipogenesis in immortalised preadipocytes was evaluated by qPCR of adipogenic markers and cellular triacylglycerol (TAG) accumulation.
Results
qPCR analysis of paired subcutaneous abdominal and gluteal AT biopsies (n = 190) across a range of BMI (20.7–45.5 kg/m2) indicated ∼3-fold higher COMP expression in gluteal AT (P = 1.7 × 10−31); protein levels mirrored this. Immunohistochemistry indicated COMP was abundant in gluteal AT ECM and co-localised with collagen-1. AT COMP mRNA levels and circulating COMP protein levels were positively associated with BMI/adiposity but unrelated to AT distribution. COMP expression changed dynamically during adipogenesis (time × depot, P = 0.01). Supplementation of adipogenic medium with exogenous COMP protein (500 ng/ml) increased PPARG2 expression ∼1.5-fold (P = 0.0003) and TAG accumulation ∼1.25-fold in abdominal and gluteal preadipocytes (P = 0.02).
Conclusions
We confirmed that COMP is an ECM protein which is differentially expressed between subcutaneous abdominal and gluteal AT. Despite its depot-specific expression pattern, however, AT COMP mRNA levels and plasma COMP concentration correlated positively with overall obesity but not body fat distribution. Exogenous COMP enhanced adipogenesis. These data identify COMP as a novel regulator of AT and highlight the importance of the ECM to AT biology.
Keywords: Adipose, Adipogenesis, Preadipocyte, Extracellular matrix
Abbreviations: Abd, abdominal; And/Gyn, android/gynoid fat mass ratio; ANOVA, analysis of variance; AT, adipose tissue; BMI, body mass index; CD36, cluster of differentiation 36; COMP, cartilage oligomeric matrix protein; ECM, extracellular matrix; ERK1/2, extracellular signal-regulated kinase-1/2; Glut, gluteal; HRP, horseradish peroxidase; OBB, Oxford Biobank; PPIA, peptidylprolyl isomerase A; PSACH, pseudoachondroplasia; PVDF, polyvinylidene fluoride; qPCR, quantitative polymerase chain reaction; TAG, triacylglycerol; THBS, thrombospondin
Highlights
-
•
COMP is a matricellular protein which is abundant in bone and cartilage.
-
•
COMP is differentially expressed between subcutaneous abdominal and gluteal AT.
-
•
COMP expression was positively correlated with overall AT mass but not distribution.
-
•
COMP promoted adipogenesis in subcutaneous abdominal and gluteal preadipocytes.
-
•
COMP represents a novel regulator of AT biology that resides in the ECM.
1. Introduction
Remodelling of the extracellular matrix (ECM) is crucial for adipogenesis [1], [2]. Mounting data indicate that altered expression of ECM components such as collagen [3] and hyaluranon [4] can modify adipose tissue (AT) function and thereby influence overall metabolic health. Adipocytes within AT are embedded in a three-dimensional ECM scaffold whose spatial arrangement is co-ordinated by matricellular proteins; these proteins physically interact with ECM components but do not directly contribute to the ECM's structural integrity [5]. Matricellular proteins are secreted modular molecules implicated in numerous processes ranging from proliferation, migration, and differentiation to angiogenesis and wound healing [6]. The thrombospondins comprise five calcium-binding matricellular proteins that regulate AT biology.
Thrombospondin-1 (THBS1) is an adipokine implicated in the development of obesity and metabolic disease [7]. The THBS1 gene is more highly expressed in visceral compared to subcutaneous abdominal AT [8] with mRNA levels in the latter reportedly correlating positively with BMI but correlating negatively with insulin sensitivity [9]. Further, circulating THBS1 protein levels were found to positively correlate with hypertension, hyperglycaemia, and central obesity in a Japanese cohort [10]. Thbs1 knock-out mice are protected from the deleterious effects of high-fat diet feeding [11], [12], [13]. Thbs2 has also been reported to exert anti-adipogenic effects in mice [14] although this finding has not been replicated [15].
THBS5 (also called cartilage oligomeric matrix protein; COMP) exhibits a striking depot-specific expression pattern between subcutaneous abdominal and gluteal AT [16]. Given that most differentially-expressed genes between white AT depots tend to be development-related transcriptional regulators such as HOX genes [16], [17], [18], regional variation in the expression of ECM components may be important in the context of depot-specific AT biology.
COMP has primarily been studied in the context of bone as mutations in THBS5 cause severe skeletal malformations, principally pseudoachondroplasia (PSACH) [19]. COMP coordinates collagen fibrillogenesis [20], but PSACH-causing mutations disrupt this process [21], typically by causing intracellular retention of mutant COMP protein which results in chondrocyte death [22]. It has been proposed that plasma COMP protein levels represent a biomarker of osteoarthritis progression [23]. With central obesity being associated with an increased risk of developing metabolic and cardiovascular disease as well as osteoarthritis [24], there is a growing appreciation of cross-talk between bone and AT via circulating factors in health and disease [25]. Based on its multi-functional properties and well-established role in cartilage/bone biology, COMP is an attractive candidate molecule to study in the context of AT. The aim of this study was to investigate COMP expression in human subcutaneous abdominal and gluteal AT and preadipocytes and relate this to AT distribution and function.
2. Methods
2.1. Immunohistochemistry
AT biopsies collected via gun biopsy under local anaesthetic (1% lignocaine) were fixed in 10% formaldehyde, embedded in paraffin wax, and cut into 5 μm sections. Sections were dewaxed, rehydrated (ethanol), and antigen retrieval was performed (heating in 1 mM sodium citrate). Endogenous peroxidase activity was blocked (0.3% H2O2 in methanol), and auto-fluorescence was quenched (1.5% glycine). Sections were blocked in 1/20 swine serum (Dako) and incubated with goat anti-COMP (1/100; AF3134; R&D Systems) and rabbit anti-collagen 1 (1/300; ab34710; Abcam) overnight at 4 °C. Sections were washed and incubated with horseradish peroxidase (HRP)-conjugated donkey anti-goat antibody (1/50; sc2020; Santa Cruz Biotechnology) to label COMP staining; the signal was amplified via tyramide amplification (0.3% H2O2 with 5% tyramide 488). Sections were then blocked in 1/20 goat serum (Dako) and incubated in goat anti-rabbit 568 (1/250; A11036; Life Technologies) to co-label collagen 1 staining. Sections were mounted and visualised using a Radiance 2100 laser scanning system confocal microscope (Bio-Rad); images were captured using Laser sharp software (Bio-Rad).
2.2. AT sample collection
Paired AT samples were taken from 97 females and 93 men in the Oxford Biobank (OBB) [26] by needle biopsy from the periumbilical (subcutaneous abdominal AT) and upper buttock (gluteo-femoral AT) areas under local anaesthetic (1% lignocaine) and immediately stored in RNAlater (ThermoFisher Scientific). Donors had a median age of 45 years (range 33–53 years) and median body mass index (BMI) of 25.6 kg/m2 (range 18.8–46.2 kg/m2). The taking of human AT samples was approved by the Oxfordshire Clinical Research Ethics Committee; all participants gave written informed consent.
2.3. Preadipocyte culture and differentiation
Immortalised subcutaneous abdominal and gluteal preadipocyte cell lines were generated, maintained and differentiated as recently described [27]. COMP (ACRO Biosystems) reconstituted as previously described [28] was added to the growth medium or adipogenic cocktail where indicated.
2.4. Gene expression analysis
Total RNA was extracted from AT biopsies [29] and preadipocytes [30]. cDNA was synthesised from total RNA using the High Capacity cDNA Reverse Transcription Kit (Life Technologies). Quantitative (q)-PCR was performed on cDNA diluted 1/20 in triplicate with Kapa Probe Fast Mastermix (Kapa Biosystems) in an 8 μl reaction. The following TaqMan Assays-on-Demand (Applied Biosystems) were used: COMP (Hs00164359_m1); PPARG2 (Hs01115510_m1); FASN (Hs00188012_m1); and PPIA (Hs99999904_m1). Data were captured on an ABI Prism 7900 HT. Relative transcript expression was calculated using the ΔΔCt relative quantification methods [31] where:
| ΔCt = Assay efficiency(minimum Ct−sample Ct) |
The ΔCt values of target genes were normalised to ΔCt of the reference transcript peptidylprolyl isomerase A (PPIA).
2.5. Western blotting
Whole AT biopsies lysates were prepared using an IKA homogeniser in ice-cold lysis buffer (8 M Urea; 1% SDS; 5% glycerol; 10 mM Tris–HCl; pH 6.8) and protease inhibitor cocktail (Complete EDTA-free; Roche). Whole cell lysates were processed as previously described [27]. Equal amounts of protein were loaded (50 μg/biopsy; 100 μg/cell lysate) and resolved by SDS-PAGE, transferred onto polyvinylidene (PVDF) membranes (Bio-Rad) and immunoblotted with: COMP (0.1 μg/ml; AF3134; R&D Systems); β-actin (1:2000; sc1616; Santa Cruz); and α-tubulin (1:2000; ab15246; Abcam) antibodies followed by an HRP-conjugated secondary antibody; goat anti-rabbit IgG (1:5000; 31460; ThermoFisher Scientific). Clarity enhanced chemiluminescence detection kit (Bio-Rad) was used for detection. Immunoblot images were captured on a Chemi-Doc XRS+ (Bio-Rad) and analysed using ImageJ (National Institute of Health, USA) software.
2.6. Measurement of cellular triacylglycerol content
Adipocyte triacylglycerol (TAG) content was measured in differentiated immortalised subcutaneous abdominal and gluteal preadipocytes on day 14 of adipogenesis using an ILAB 650 clinical analyser (Instrumentation Laboratory UK) according to the previously described method [27].
2.7. Quantification of plasma and cell media COMP concentrations
COMP concentration in preadipocyte-conditioned media and plasma was determined using ELISA (R&D Systems) in duplicate. Plasma samples were selected from participants in the OBB with distinctly different AT distribution phenotypes. Using dual X-ray absorptiometry-derived body composition data on 4900 participants, individuals from the top vs. lowest tertile of Android/Gynoid fat mass ratio were pair matched for total fat mass percentage, age, and sex (80 men; 72 women). Anthropometric characteristics for the study cohorts are presented in Supplementary Information Table 1. Depot-specific COMP release by AT into the bloodstream was measured using arterio-venous plasma samples (9 healthy lean women in the fasted state) obtained as previously described [32].
2.8. Data analysis and statistics
Data are presented as means ± SEM. Statistical analyses were performed in SPSS Statistics version 22 (IBM). Statistical tests used were as described in the results section. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. COMP is highly differentially expressed between subcutaneous abdominal and gluteal AT
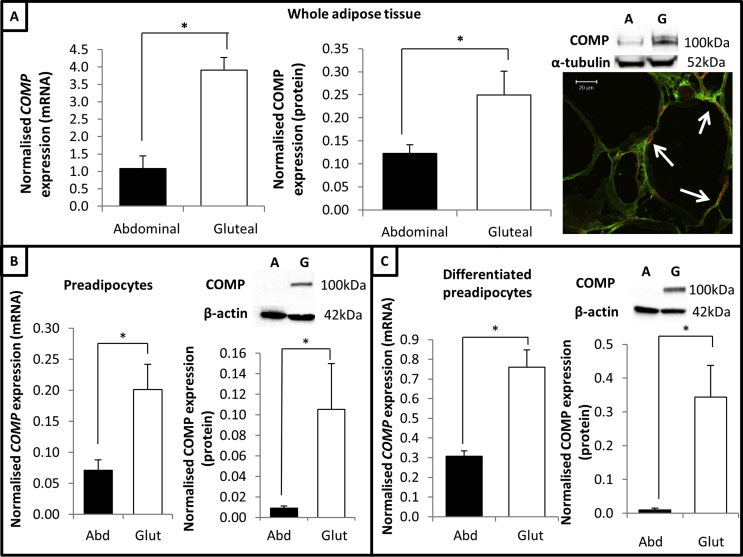
Our previous transcriptomic array data indicated that COMP was significantly more highly expressed in subcutaneous gluteal AT compared to abdominal AT [16]. qPCR analysis of unselected paired subcutaneous abdominal and gluteal AT biopsies (n = 190) across a range of BMI (20.7–45.5 kg/m2) validated the previous microarray data and showed that COMP expression was ∼3-fold higher in gluteal AT (P = 1.7 × 10−31) (Figure 1A). Consistent with the qPCR data, Western blot analysis of paired AT biopsies showed that COMP protein levels exhibited the same depot-specific expression pattern (Figure 1A). Immunohistochemistry analysis of (obese gluteal) AT highlighted that COMP protein was present throughout the ECM, including within the perivascular space, and co-localised with collagen-1 (Figure 1A).
Figure 1.
COMP is differentially expressed between abdominal and gluteal AT. (A) Left graph, COMP expression in abdominal and gluteal AT (corrected for age, sex and BMI). Right graph, COMP protein levels (normalised to α-tubulin) in paired AT biopsies (n = 6 healthy females) including representative blot. Data provided as mean ± SEM. Representative immunohistochemistry image of (obese gluteal) AT for COMP (green) and collagen 1 (red) staining; white arrows highlight co-localisation (yellow). (B) Left graph, COMP mRNA levels (n = 6) and right graph, COMP protein levels (n = 3) in proliferating immortalised subcutaneous abdominal and gluteal preadipocytes. (C) Left graph, COMP mRNA levels (n = 3) and right graph, COMP protein levels (n = 3) in differentiated immortalised subcutaneous abdominal and gluteal preadipocytes. COMP mRNA levels normalised to PPIA and protein levels normalised to β-actin. Data presented as mean ± SEM; analysed via paired T-test; *P < 0.05.
Analysis of COMP expression in immortalised preadipocytes showed that its depot-specific expression pattern was retained ex vivo at the level of transcription and translation. COMP mRNA and protein levels were significantly higher in proliferating gluteal preadipocytes compared to abdominal cells (Figure 1B); this depot-specific expression pattern was recapitulated in differentiated preadipocytes (Figure 1C). Collectively, these data confirm that COMP is highly differentially expressed between abdominal and gluteal AT and that this expression pattern was retained ex vivo.
3.2. COMP expression in subcutaneous AT is enhanced in obesity
It has been proposed that other members of the thrombospondin family, particularly THBS1, represent adipokines [9], [10]. Previous literature indicate that COMP is readily detectable in plasma [33] so we investigated whether COMP represents a novel adipokine by measuring the arterio-venous concentration difference across human subcutaneous AT using a previously described method [32]. To investigate this whilst accounting for the differential expression pattern of COMP, plasma samples taken across subcutaneous abdominal and gluteal AT (from 9 healthy, lean women in the fasted state) were analysed via ELISA to determine whether COMP protein was released by AT in a depot-specific manner. Although COMP was detectable in plasma, the arterio-venous difference in COMP protein concentration was not different from zero, thus indicating there was no measurable release of COMP from either abdominal or gluteal subcutaneous AT in these individuals (data not shown).
As the rate of COMP production and release by AT may be too slow to allow for detection using the arterio-venous difference technique (as for adiponectin [34]), we set out to determine whether circulating COMP levels could be used as an index of long-term COMP turnover in AT. We therefore hypothesised that plasma COMP levels would be higher in individuals with preferential lower body fat accumulation given the higher expression of COMP in gluteal AT. To test this, plasma COMP protein levels were measured in individuals exhibiting either predominantly abdominal or gluteo-femoral AT accumulation but matched for overall AT mass across a range of BMI. OBB data were sorted according to Android/Gynoid fat mass ratio (quantified via dual X-ray absorptiometry); individuals were selected from the top tertile (i.e. centrally-distributed AT) and matched to a corresponding individual from the lowest tertile (i.e. peripherally-distributed AT) with the same total fat mass percentage (+/−1%); individuals were also matched for age (+/−1 year) and sex. The anthropometric characteristics of the study participants are detailed in Supplementary Table 1.
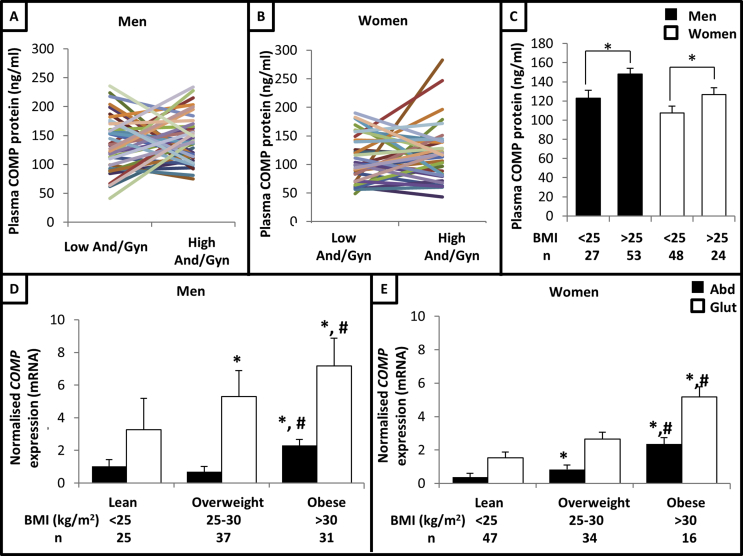
Plasma COMP protein levels were significantly higher in men than women (Mann–Whitney U-test, P = 0.0002), so the data were separated by sex for further analyses. Pairwise analysis of the 40 men (Figure 2A) and 36 women (Figure 2B) pairs highlighted there was no significant difference in circulating COMP protein levels between individuals with opposite body fat distribution phenotypes. However, when the data were sorted according to BMI, it was found that circulating COMP protein levels were significantly higher (Mann–Whitney U-test) in overweight/obese (BMI >25 kg/m2) men (P = 0.007) and women (P = 0.019) compared to their lean (BMI <25 kg/m2) counterparts (Figure 2C). Consistent with this, plasma COMP protein levels were positively correlated with total fat mass percentage in men (Spearman's rho = 0.352, P = 0.001) and women (Spearman's rho = 0.315, P = 0.007). Collectively, these data support the notion that COMP expression reflects overall adiposity. However, it was not possible to confirm whether COMP behaved as an adipokine in the sense that it is released from AT to act on a distant target tissue.
Figure 2.
COMP expression in AT is enhanced in obesity. Plasma COMP protein concentration in men (A) (n = 80) and women (B) (n = 72) matched to individual with same total fat mass % but opposite Android/Gynoid fat mass ratio (And/Gyn); each line connects a matched pair. (C) Plasma COMP protein concentration stratified by BMI (kg/m2). Data presented as mean ± SEM; data in A, B and C analysed via Mann–Whitney U-test; *P < 0.05. COMP mRNA levels (normalised to PPIA) in subcutaneous abdominal (Abd) and gluteal (Glut) AT in men (D) (n = 93) and women (E) (n = 97) stratified by BMI (kg/m2). Data presented as age-adjusted means ± SEM; data analysed via ANOVA (with post-hoc T-tests); *P < 0.05 compared to lean expression in same depot; #P < 0.05 compared to overweight expression in same depot.
Building on these data, we investigated whether COMP expression in AT was related to the degree of adiposity and/or regional AT distribution. This involved measuring COMP mRNA levels in paired AT biopsies obtained from 190 OBB participants and relating expression to various anthropometric parameters (DXA data were not available for these individuals). According to conventional BMI cut-offs, COMP mRNA levels were significantly higher in obese men (Figure 2D) and women (Figure 2E) compared to their overweight and lean counterparts in both abdominal and gluteal AT (analysis of variance - ANOVA - with post-hoc T-tests). The ratio of abdominal/gluteal AT COMP mRNA levels was not significantly different between BMI groups, thus indicating that COMP's depot-specific expression pattern was maintained across the range of adiposity (data not shown).
More detailed analyses (Partial Pearson's correlation with correction for age) showed a strong positive correlation between abdominal and gluteal AT COMP mRNA levels and BMI in both men and women (Table 1A). Similar positive correlations were detected between abdominal and gluteal AT COMP mRNA levels and waist and hip circumference as well as fasting plasma insulin (a reverse index of whole body insulin sensitivity) in men and women (Table 1A). However, these relationships were principally driven by BMI as most became non-significant after correcting for BMI barring female hip circumference and male fasting insulin which remained nominally significant (Table 1B).
Table 1.
A. Partial Pearson's correlation analysis (corrected for age) for (log-transformed) AT COMP mRNA levels (normalised to PPIA) with anthropometric and biochemical parameters in abdominal (Abd) and gluteal (Glut) AT. B. Partial Pearson's correlation analysis (corrected for age and BMI) for (log-transformed) AT COMP mRNA levels (normalised to PPIA) with anthropometric and biochemical parameters. Right-hand column - correlation analysis between (log-transformed) COMP mRNA levels (normalised to PPIA) and BMI (corrected for age and fasting plasma insulin).
| A – Age-corrected correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI |
Waist circumference |
Hip circumference |
Fasting Insulin |
||||||
| Pearson | P | Pearson | P | Pearson | P | Pearson | P | ||
| Men | Log Abd COMP | 0.400 | 7.0 × 10−5* | 0.350 | 0.001* | 0.338 | 0.001* | 0.484 | 2.0 × 10−6* |
| Log Glut COMP | 0.329 | 0.001* | 0.291 | 0.006* | 0.294 | 0.005* | 0.194 | 0.070 | |
| Women | Log Abd COMP | 0.529 | 3.0 × 10−8* | 0.463 | 2.0 × 10−6* | 0.370 | 2.1 × 10−4* | 0.398 | 6.0 × 10−5* |
| Log Glut COMP | 0.424 | 1.6 × 10−5* | 0.390 | 8.6 × 10−5* | 0.361 | 0.003* | 0.358 | 4.0 × 10−4* | |
| B – Age and BMI-corrected correlations | |||||||||
| Waist circumference |
Hip circumference |
Fasting Insulin |
BMI |
||||||
| Pearson | P | Pearson | P | Pearson | P | Pearson | P | ||
| Men | Log Abd COMP | −0.068 | 0.530 | −0.045 | 0.670 | 0.320 | 0.002* | 0.247 | 0.02* |
| Log Glut COMP | −0.052 | 0.630 | −0.008 | 0.940 | 0.002 | 0.990 | 0.299 | 0.005* | |
| Women | Log Abd COMP | −0.022 | 0.840 | −0.214 | 0.037* | 0.117 | 0.260 | 0.445 | 6.0 × 10−6* |
| Log Glut COMP | −0.029 | 0.780 | −0.017 | 0.870 | 0.146 | 0.160 | 0.310 | 0.02* | |
Note – BMI correlations are age- and insulin-corrected. *P < 0.05.
As these data suggested that insulin might exert an independent effect on COMP expression, we corrected the correlation between COMP mRNA levels and BMI for fasting plasma insulin but it remained statistically significant (Table 1B). Consistent with this, administration of insulin (100 nM) to proliferating immortalised subcutaneous abdominal or gluteal preadipocytes for 24 h did not increase COMP expression (data not shown). Overall, abdominal and gluteal AT COMP mRNA levels were strongly positively correlated with BMI in men and women, thus suggesting that AT COMP expression reflects overall adiposity.
3.3. COMP is a positive adipogenic regulator
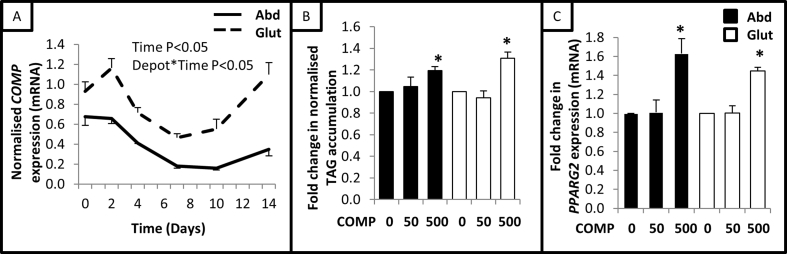
During a standard 14 day differentiation protocol, COMP mRNA levels were found to change dynamically in immortalised human subcutaneous abdominal and gluteal preadipocytes (Figure 3A). More specifically, COMP mRNA levels declined between days 2–10 before returning to the higher baseline levels toward the end of the differentiation protocol. While COMP mRNA levels fluctuated, its depot-specific expression pattern was always maintained.
Figure 3.
COMP is a positive adipogenic regulator. (A) COMP mRNA levels (normalised to PPIA) in immortalised abdominal (Abd) and gluteal (Glut) preadipocytes (n = 6) over 14 day adipogenic time-course (data analysed via repeated measures ANOVA). Day 14 TAG accumulation (B) (normalised to cellular protein) and PPARG2 expression (C) (normalised to PPIA) in immortalised preadipocytes cultured in adipogenic medium supplemented with exogenous COMP protein (n = 3). Data presented as mean ± SEM; ANOVA; *P < 0.05 compared to untreated cells from same depot.
To explore the potential role of COMP in adipogenesis, abdominal and gluteal preadipocytes were differentiated in adipogenic medium supplemented with exogenous COMP protein for the 14 day duration. According to analysis via ANOVA with post-hoc T-tests, no effect was observed at 50 ng/ml but treatment with 500 ng/ml COMP protein significantly increased final TAG accumulation by ∼1.25-fold (Figure 3B) and expression of the adipogenic marker PPARG2 by ∼1.5-fold (Figure 3C) in both abdominal and gluteal preadipocytes. On the contrary, culturing abdominal and gluteal preadipocytes in the presence of increasing doses of exogenous COMP protein (50 ng/ml or 500 ng/ml) for 48 h had no effect on the rate of cell proliferation (data not shown).
4. Discussion
COMP is a matricellular protein that plays a key role in bone and cartilage biology [35] as well as processes such as inflammation and angiogenesis [6]. The data presented in this study highlight that: COMP is expressed by AT in a depot-specific manner; COMP expression in AT and circulating COMP levels are positively correlated with BMI/overall adiposity; and exogenous COMP protein enhanced adipogenesis. These results identify COMP as a novel regulator of human AT biology and thereby highlight a previously unknown role for this multi-functional protein.
As most differentially-expressed genes between white AT depots tend to be development-related transcriptional regulators such as HOX genes [16], [17], [18], the identification of an ECM component exhibiting depot-specific expression in AT is somewhat novel and may have relevance to regional AT distribution, a strong determinant of metabolic health [34]. Despite being differentially expressed, however, neither subcutaneous abdominal or gluteal COMP AT mRNA levels nor circulating COMP protein levels were associated with regional AT accumulation. The data instead suggested that subcutaneous abdominal and gluteal AT COMP mRNA and plasma COMP protein levels reflect overall adiposity.
COMP protein was readily detectable in plasma and circulating COMP levels were significantly elevated in overweight/obese individuals compared to normal-weight controls. Furthermore, the observation that circulating COMP protein levels were positively correlated with BMI and total fat mass percentage in men and women is consistent with other reports that plasma COMP levels correlate positively with BMI [36] and decline upon weight loss [37]. Together these observations prompted the suggestion that COMP may represent a novel adipokine akin to THBS1 [10]. However, COMP release from AT in vivo could not be detected. This may be due to the slow turnover of COMP protein in AT and/or the nature of the plasma sample donors (i.e. healthy, normal weight women). It remains to be seen whether COMP release from AT would be detectable in overweight/obese men, individuals who would be predicted to have higher levels of AT COMP expression given the current data.
AT probably makes a contribution to the circulating pool of COMP (which likely increases in proportion to adiposity) although the majority likely derives from cartilage [37] and bone [19] with the vasculature [38], liver [39], and immune system [40] representing other potential sources. It is currently unclear what function circulating COMP serves. COMP may play an endocrine role that co-ordinates the activity of different tissues (e.g. bone and AT). Further studies are required to explore whether circulating COMP protein is a biomarker whose levels reflect not only adiposity but metabolic health parameters (e.g. fasting glucose and insulin levels) and/or the progression of obesity-related diseases such as osteoarthritis [37]; such a multi-faceted biomarker could inform diagnostic, prognostic and management decisions in a clinical setting.
Alternatively, plasma levels may reflect COMP turnover in the aforementioned tissue types and therefore provide insight into their ECM composition and turnover. Indeed, a recent report identified plasma COMP as a sensitive, non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis [41], consistent with the finding that exogenous COMP protein promotes collagen deposition in hepatic stellate cells [39]. As COMP co-ordinates collagen fibrillogenesis [20] and is involved in the tissue injury response [5], it would be worth exploring whether COMP mediates collagen deposition in AT (and/or other tissues). COMP-mediated collagen deposition might represent an important aspect of the ECM remodelling which supports adipogenesis and adipocyte function in developing and mature AT [42]. However, if this process occurs to an excessive level in obesity, potentially driven by increased COMP expression, it may promote AT fibrosis which can negatively affect adipocyte and AT function. If this were the case, it would implicate COMP in subcutaneous AT dysfunction and the development of obesity-associated insulin resistance and metabolic disease [43], [44].
With gene expression and immunohistochemistry analyses confirming that COMP is a highly differentially expressed matricellular protein in human AT, the importance of the ECM to AT biology and overall metabolic health should be acknowledged. The ECM plays a crucial role in determining the capacity of AT to expand [45] as well as the mechanism by which it expands [46]. Experimental data indicate there is regional variation in the mechanism by which human subcutaneous AT expands; abdominal AT reportedly expands via hypertrophy whereas gluteo-femoral AT expands via hyperplasia [47]. As adipocyte size represents an important index of AT function and metabolic health - in which smaller adipocyte size is associated with increased insulin sensitivity [48] - future studies could investigate the relationship between COMP expression (mRNA and protein) in subcutaneous abdominal and gluteal AT, adipocyte size, (regional) adiposity, and metabolic health.
Supplementation of adipogenic medium with exogenous COMP protein enhanced PPARG2 expression and TAG accumulation (but did not affect proliferation) of subcutaneous abdominal and gluteal preadipocytes. AT COMP expression was significantly increased under circumstances involving AT expansion (i.e. obesity), suggesting that COMP may act as a paracrine factor which enhances adipogenesis and/or TAG accumulation in adipocytes. Although the mechanism(s) employed by COMP to exert this effect remain unclear, they may involve physical interaction with ECM components such as collagens, fibronectin, growth factors (e.g. members of the transforming growth factor-β superfamily), and/or integrin [35].
Alternatively, COMP may activate signalling pathways which modulate adipogenesis. COMP protein has been reported to activate extracellular signal-regulated kinase (ERK)-1/2 signalling in rat hepatic stellate cells via cluster of differentiation (CD)-36 receptors [39]; ERK1/2 signalling is crucial for adipogenesis [49]. As CD36 expression increases in preadipocytes during adipogenesis [27], COMP's pro-adipogenic effect may involve activation of ERK1/2 signalling via CD36 during the latter phase of differentiation. It should be noted that while COMP is differentially expressed in subcutaneous AT, it exerted pro-adipogenic effects in both abdominal and gluteal preadipocytes. Although the biological significance of COMP's depot-specific pattern is currently unclear, this observation supports the notion that adipogenesis occurs in a depot-specific manner [34].
In summary, COMP is a matricellular protein that exhibits a striking depot-specific expression pattern in AT. COMP expression in abdominal and gluteal AT as well as circulating COMP levels were positively correlated with BMI/adiposity but not with regional AT distribution. Exogenous COMP protein promoted adipogenesis in subcutaneous abdominal and gluteal preadipocytes although the mechanism(s) employed remain unclear. These data identify COMP as an intriguing novel regulator of AT biology and also highlight the important yet poorly understood role of the ECM in AT.
Funding sources
This research was funded by the National Institute for Health Research, Oxford Biomedical Research Centre (BRC) and the British Heart Foundation [RG/17/1/32663]. The Oxford Biobank (www.oxfordbiobank.org.uk) was supported through the NIHR BRC Obesity and Nutrition theme and was critical for the human participants and samples used in this study.
Author contributions
ND, KEP, and FK designed the experiments. ND performed all in vitro experiments and analysed the plasma samples. ND wrote the paper with KEP and FK.
Acknowledgements
We would like to thank Sandy Humphreys and Marje Gilbert for assistance handling OBB AT and plasma samples, Dr Anne Clark for her immunohistochemistry expertise and the British Heart Foundation for their support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.07.005.
Contributor Information
Katherine E. Pinnick, Email: Katherine.Pinnick@ocdem.ox.ac.uk.
Fredrik Karpe, Email: Fredrik.Karpe@ocdem.ox.ac.uk.
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Nakajima I., Yamaguchi T., Ozutsumi K., Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation Research in Biological Diversity. 1998;63(4):193–200. doi: 10.1111/j.1432-0436.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima I., Muroya S., Tanabe R., Chikuni K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biology of the Cell. 2002;94(3):197–203. doi: 10.1016/s0248-4900(02)01189-9. [DOI] [PubMed] [Google Scholar]
- 3.Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and Cellular Biology. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Crewe C., Scherer P.E. Hyaluronan in adipose tissue: beyond dermal filler and therapeutic Carrier. Science Translational Medicine. 2016;8(323):323ps4. doi: 10.1126/scitranslmed.aad6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy-Ullrich J.E., Sage E.H. Revisiting the matricellular concept. Matrix Biology: Journal of the International Society for Matrix Biology. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein P., Sage E.H. Matricellular proteins: extracellular modulators of cell function. Current Opinion in Cell Biology. 2002;14(5):608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 7.Kong P., Cavalera M., Frangogiannis N.G. The role of thrombospondin (TSP)-1 in obesity and diabetes. Adipocyte. 2014;3(1):81–84. doi: 10.4161/adip.26990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramis J.M., Franssen-van Hal N.L.W., Kramer E., Llado I., Bouillaud F., Palou A. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cellular and Molecular Life Sciences: CMLS. 2002;59(11):1960–1971. doi: 10.1007/PL00012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma V., Yao-Borengasser A., Bodles A.M., Rasouli N., Phanavanh B., Nolen G.T. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57(2):432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo Y., Tanaka M., Yamakage H., Sasaki Y., Muranaka K., Hata H. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metabolism: Clinical and Experimental. 2015;64(11):1490–1499. doi: 10.1016/j.metabol.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Tong X., Rumala C., Clemons K., Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong P., Gonzalez-Quesada C., Li N., Cavalera M., Lee D.-W., Frangogiannis N.G. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. American Journal of Physiology - Endocrinology And Metabolism. 2013;305(3):E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M., Jiang Y., Barnes R.H., Tokunaga M., Martinez-Santibañez G., Geletka L. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154(12):4548–4559. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shitaye H.S., Terkhorn S.P., Combs J.A., Hankenson K.D. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biology: Journal of the International Society for Matrix Biology. 2010;29(6):549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hul M., Frederix L., Lijnen H.R. Role of thrombospondin-2 in murine adipose tissue angiogenesis and development. Obesity (Silver Spring, Md.) 2012;20(9):1757–1762. doi: 10.1038/oby.2011.260. [DOI] [PubMed] [Google Scholar]
- 16.Pinnick K.E., Nicholson G., Manolopoulos K.N., McQuaid S.E., Valet P., Frayn K.N. Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes. 2014;63(11):3785–3797. doi: 10.2337/db14-0385. [DOI] [PubMed] [Google Scholar]
- 17.Karastergiou K., Fried S.K., Xie H., Lee M.-J., Divoux A., Rosencrantz M.A. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. The Journal of Clinical Endocrinology and Metabolism. 2013;98(1):362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gesta S., Tseng Y.-H., Kahn C.R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Posey K.L., Alcorn J.L., Hecht J.T. Pseudoachondroplasia/COMP - translating from the bench to the bedside. Matrix Biology: Journal of the International Society for Matrix Biology. 2014;37:167–173. doi: 10.1016/j.matbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halász K., Kassner A., Mörgelin M., Heinegård D. COMP acts as a catalyst in collagen fibrillogenesis. The Journal of Biological Chemistry. 2007;282(43):31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 21.Hansen U., Platz N., Becker A., Bruckner P., Paulsson M., Zaucke F. A secreted variant of cartilage oligomeric matrix protein carrying a chondrodysplasia-causing mutation (p.H587R) disrupts collagen fibrillogenesis. Arthritis & Rheumatism. 2011;63(1):159–167. doi: 10.1002/art.30073. [DOI] [PubMed] [Google Scholar]
- 22.Hecht J.T., Makitie O., Hayes E., Haynes R., Susic M., Montufar-Solis D. Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2004;22(4):759–767. doi: 10.1016/j.orthres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Hoch J.M., Mattacola C.G., Medina McKeon J.M., Howard J.S., Lattermann C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2011;19(12):1396–1404. doi: 10.1016/j.joca.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Annals of Physical and Rehabilitation Medicine. 2016;59(3):157–160. doi: 10.1016/j.rehab.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Thijssen E., van Caam A., van der Kraan P.M. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford, England) 2015;54(4):588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 26.Karpe F., Vasan S.K., Humphreys S.M., Miller J., Cheeseman J., Louise Dennis A. Cohort profile: the Oxford Biobank. International Journal of Epidemiology. 2017 doi: 10.1093/ije/dyx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todorčević M., Hilton C., McNeil C., Christodoulides C., Hodson L., Karpe F. A cellular model for the investigation of depot specific human adipocyte biology. Adipocyte. 2017;6(1):40–55. doi: 10.1080/21623945.2016.1277052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H., Deere M., Hecht J.T., Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. The Journal of Biological Chemistry. 2000;275(34):26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- 29.Neville M.J., Collins J.M., Gloyn A.L., McCarthy M.I., Karpe F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity (Silver Spring, Md.) 2011;19(4):888–892. doi: 10.1038/oby.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins J.M., Neville M.J., Hoppa M.B., Frayn K.N. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. The Journal of Biological Chemistry. 2010;285(9):6044–6052. doi: 10.1074/jbc.M109.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuaid S.E., Manolopoulos K.N., Dennis A.L., Cheeseman J., Karpe F., Frayn K.N. Development of an arterio-venous difference method to study the metabolic physiology of the femoral adipose tissue depot. Obesity (Silver Spring, Md.) 2010;18(5):1055–1058. doi: 10.1038/oby.2009.486. [DOI] [PubMed] [Google Scholar]
- 33.Verma P., Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2013;31(7):999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- 34.Karpe F., Pinnick K.E. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nature Reviews Endocrinology. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 35.Acharya C., Yik J.H.N., Kishore A., Van Dinh V., Di Cesare P.E., Haudenschild D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biology: Journal of the International Society for Matrix Biology. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 36.El Defrawy A.O., Gheita T.A., Raslan H.M., El Ansary M.M., El Awar A.H. Serum and synovial cartilage oligomeric matrix protein levels in early and established rheumatoid arthritis. Zeitschrift Fur Rheumatologie. 2016;75(9):917–923. doi: 10.1007/s00393-015-1647-5. [DOI] [PubMed] [Google Scholar]
- 37.King L.K., Henneicke H., Seibel M.J., March L., Anandacoomarasmy A. Association of adipokines and joint biomarkers with cartilage-modifying effects of weight loss in obese subjects. Osteoarthritis and Cartilage. 2015;23(3):397–404. doi: 10.1016/j.joca.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Riessen R., Fenchel M., Chen H., Axel D.I., Karsch K.R., Lawler J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(1):47–54. doi: 10.1161/01.atv.21.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Magdaleno F., Arriazu E., Ruiz de Galarreta M., Chen Y., Ge X., Conde de la Rosa L. Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis. Journal of Hepatology. 2016;65(5):963–971. doi: 10.1016/j.jhep.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y., Gao C., Liang Y., Wang M., Huang Y., Ma W. Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circulation Research. 2016;119(2):261–276. doi: 10.1161/CIRCRESAHA.115.308021. [DOI] [PubMed] [Google Scholar]
- 41.Zachou K., Gabeta S., Shums Z., Gatselis N.K., Koukoulis G.K., Norman G.L. COMP serum levels: a new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. European Journal of Internal Medicine. 2017;38:83–88. doi: 10.1016/j.ejim.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Mariman E.C.M., Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cellular and Molecular Life Sciences: CMLS. 2010;67(8):1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Divoux A., Tordjman J., Lacasa D., Veyrie N., Hugol D., Aissat A. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams A.S., Kang L., Wasserman D.H. The extracellular matrix and insulin resistance. Trends in Endocrinology and Metabolism: TEM. 2015;26(7):357–366. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carobbio S., Pellegrinelli V., Vidal-Puig A. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Advances in Experimental Medicine & Biology. 2017;960:161–196. doi: 10.1007/978-3-319-48382-5_7. [DOI] [PubMed] [Google Scholar]
- 46.Crewe C., An Y.A., Scherer P.E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. The Journal of Clinical Investigation. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tchoukalova Y.D., Votruba S.B., Tchkonia T., Giorgadze N., Kirkland J.L., Jensen M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laforest S., Labrecque J., Michaud A., Cianflone K., Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Critical Reviews in Clinical Laboratory Sciences. 2015;52(6):301–313. doi: 10.3109/10408363.2015.1041582. [DOI] [PubMed] [Google Scholar]
- 49.Bost F., Aouadi M., Caron L., Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.