Abstract
Outbreaks of plague, a flea‐vectored bacterial disease, occur periodically in prairie dog populations in the western United States. In order to understand the conditions that are conducive to plague outbreaks and potentially predict spatial and temporal variations in risk, it is important to understand the factors associated with flea abundance and distribution that may lead to plague outbreaks. We collected and identified 20,041 fleas from 6,542 individual prairie dogs of four different species over a 4‐year period along a latitudinal gradient from Texas to Montana. We assessed local climate and other factors associated with flea prevalence and abundance, as well as the incidence of plague outbreaks. Oropsylla hirsuta, a prairie dog specialist flea, and Pulex simulans, a generalist flea species, were the most common fleas found on our pairs. High elevation pairs in Wyoming and Utah had distinct flea communities compared with the rest of the study pairs. The incidence of prairie dogs with Yersinia pestis detections in fleas was low (n = 64 prairie dogs with positive fleas out of 5,024 samples from 4,218 individual prairie dogs). The results of our regression models indicate that many factors are associated with the presence of fleas. In general, flea abundance (number of fleas on hosts) is higher during plague outbreaks, lower when prairie dogs are more abundant, and reaches peak levels when climate and weather variables are at intermediate levels. Changing climate conditions will likely affect aspects of both flea and host communities, including population densities and species composition, which may lead to changes in plague dynamics. Our results support the hypothesis that local conditions, including host, vector, and environmental factors, influence the likelihood of plague outbreaks, and that predicting changes to plague dynamics under climate change scenarios will have to consider both host and vector responses to local factors.
Keywords: climate, Cynomys spp., flea, plague, prairie dog, Yersinia pestis
1. INTRODUCTION
Sylvatic plague, caused by the bacteria Yersinia pestis, manifests in the western United States as episodic outbreaks in prairie dogs, squirrels, and small rodents, with occasional spillover to human populations (Gage, 1998). Prairie dogs (Cynomys spp.) are highly susceptible to plague and colonies often suffer >90% mortality during plague outbreaks (Cully & Williams, 2001). By decimating prairie dog populations, plague can have devastating effects on species such as black‐footed ferrets (Mustela nigripes), which depend on prairie dogs for their prey (Antolin et al., 2002). At present, the factors driving plague outbreaks are difficult to ascertain. A number of studies have indicated that small rodent composition (Stapp et al., 2009) and local weather conditions (Collinge et al., 2005; Hubbart, Jachowski, & Eads, 2011; Savage, Reich, Hartley, Stapp, & Antolin, 2011) may play a role in contributing to plague epizootics, potentially through their effects on flea distribution and abundance. However, these factors likely vary geographically in the strength of their effects. As the primary vector of plague, fleas play an important role in the dynamics of the disease (Bacot & Martin, 1914). In order to understand the conditions that are conducive to plague outbreaks and potentially predict spatial and temporal variations in risk, it is important to understand the factors associated with flea abundance, prevalence, and distribution that may lead to plague outbreaks.
Traditionally, the hypothesized mechanism for flea transmission of plague bacteria was the result of “blocking” (Bacot & Martin, 1914), whereby Y. pestis bacteria form a blockage in the midgut of fleas, causing them to increase the number of feeding attempts and regurgitate infectious material, increasing the likelihood of plague transmission. Lorange, Race, Sebbane, and Hinnebusch (2005) developed models that suggested, with this mechanism of transmission, high flea abundance was necessary for driving plague epizootics due to the low competence of fleas as vectors and the short lifespan of blocked fleas. Eisen et al. (2006) introduced the idea of early‐phase transmission to explain the rapid spread of plague through host populations, as an alternative hypothesis to blocking. Evidence of early‐phase transmission, which does not require blocking of fleas, was observed in Oropsylla montana (Eisen et al., 2006), and modeling has demonstrated that early‐phase transmission can drive plague epizootics (Buhnerkempe et al., 2011). Previous research has demonstrated that the ability of fleas to block decreases at higher temperatures (Cavanaugh, 1971; Hinnebusch, Fischer, & Schwan, 1998; Kartman, 1969), therefore potentially explaining the relationship between decreased temperature and frequency of plague outbreaks (Cavanaugh, 1971; Collinge et al., 2005; Enscore et al., 2002; Pollitzer, 1954). However, in contrast, early‐phase transmission in Xenopsylla cheopis was delayed or inhibited at cooler temperatures, but did not seem to be affected by warmer temperatures (Schotthoefer et al., 2011). These results indicate that local climate conditions may affect flea biology and the likelihood of plague outbreaks in prairie ecosystems.
Numerous factors likely affect on‐host flea prevalence (number of hosts with fleas) and abundance (number of fleas on hosts). Previous research has indicated that on‐host flea abundance has been associated with local weather conditions, although the relationships are seemingly complex. Increasing flea abundance has been associated with dry summers in a New Mexico population of black‐tailed prairie dogs (BTPD, C. ludovicianus; Eads, Biggins, Long, Gage, & Antolin, 2016), wetter springs and cooler summers on great gerbils (Rhombomys opimus) in Kazakhstan (Stenseth et al., 2006), and warmer autumns in the previous year in Mongolian gerbils (Meriones unguiculatus; Xu et al., 2015), while decreasing flea abundance was associated with increasing prior year growing season and winter precipitation in South Dakota BTPD (Eads & Hoogland, 2016).
Several studies have examined the relationship between the incidence of plague and climatic variables. Some studies have proposed that hot weather desiccates flea larvae and reduces overall flea abundance (Snäll, O'Hara, Ray, & Collinge, 2008), therefore, reducing the likelihood of plague, while others have proposed that drier weather leaves hosts in poorer condition resulting in increased flea abundance and increased potential for plague outbreaks (Eads et al., 2016). Precipitation also may affect the likelihood of plague, with increased precipitation leading to better forage conditions and increased host densities, which may create favorable conditions for plague outbreaks (Enscore et al., 2002). For example, Stenseth et al. (2006) found that the incidence of Y. pestis in great gerbils in Central Asia increased with warmer springs and wetter summers. Eads and Hoogland (2017) observed that flea abundance on Gunnison's prairie dogs (C. gunnisoni, GPD) in Arizona and white‐tailed prairie dogs (C. leucurus, WTPD) in Colorado varied inversely with increasing precipitation during the prior year growing season and suggested that this may explain increased risk of plague after drought. Enscore et al. (2002) found that the numbers of human plague cases in New Mexico and Arizona decreased with increasing summer temperatures and increased when spring precipitation in previous years was high. However, greater summer rainfall, but not previous year precipitation, was associated with plague events in BTPD in Colorado (Savage et al., 2011). At last, Eisen et al. (2012) suggested that flea diversity, which was positively related to rainfall and negatively related to temperature, may play a role in plague outbreaks in Uganda.
In addition to climate and weather variables, flea and host characteristics may influence flea abundance. Oropsylla hirsuta and O. tuberculata, flea species strongly associated with prairie dog colonies and plague outbreaks, exhibit seasonal trends (Mize & Britten, 2016). The abundance of O. tuberculata is often highest in early spring (Cully, Barnes, Quan, & Maupin, 1997; Salkeld & Stapp, 2008; Tripp, Gage, Montenieri, & Antolin, 2009), while O. hirsuta numbers tend to peak in mid or late summer (Salkeld & Stapp, 2008; Tripp et al., 2009). Eads et al. (2013) noted that overall prevalence of fleas also fluctuated seasonally. Flea abundance on BTPD has been found to be higher on adult males than on juvenile and/or adult female prairie dogs in Colorado (Tripp et al., 2009). However, in South Dakota, no difference in flea abundance was found on male and female BTPD, although juveniles had fewer fleas than adults (Eads & Hoogland, 2016).
In summary, the relationships between weather factors, flea abundance, and host characteristics (body condition or abundance) remain difficult to ascertain and may not be consistent between different host species, fleas, and locations. We collected fleas from four species of prairie dogs over a 4‐year period (2013–2016) on paired plots located along a latitudinal gradient from Montana to Texas and assessed local climate and other factors associated with flea prevalence and abundance (total fleas, O. hirsuta and P. simulans counts), as well as the incidence of plague outbreaks. Elucidating the relationship between flea species composition, abundance and distribution, the occurrence of plague, and environmental factors will provide information to management agencies responsible for controlling plague, as well as help predict how plague dynamics may change under future climate conditions.
2. METHODS
2.1. Study areas and design
We analyzed flea abundance and prevalence on prairie dogs from 23 paired plots that had been included in a large‐scale sylvatic plague vaccine (SPV) trial (see further details in Rocke et al., 2017). A pair consisted of one plot treated with SPV‐laden baits and one plot that received placebo baits. In brief, our study included 11 paired plots on BTPD colonies, one pair on a GPD colony, four pairs on WTPD, and seven pairs on Utah prairie dog (UPD, Cynomys parvidens) colonies, sampled over a 3‐year period, 2013–2015 (Figure 1). Fifteen of the pairs were resampled in 2016. For plots to be included in the study, we required that no pesticides had been used for at least one year prior to the beginning of the study. Most pairs had never been dusted; however, two pairs (4 plots) (Charles M Russell, Montana‐CMR) were dusted in 1997, and one pair (Wind Cave, South Dakota‐WCSD) was dusted in 2011. Tripp et al. (2016) observed a waning effect of dusting on flea abundance on sites in Colorado after 10–12 months.
Figure 1.
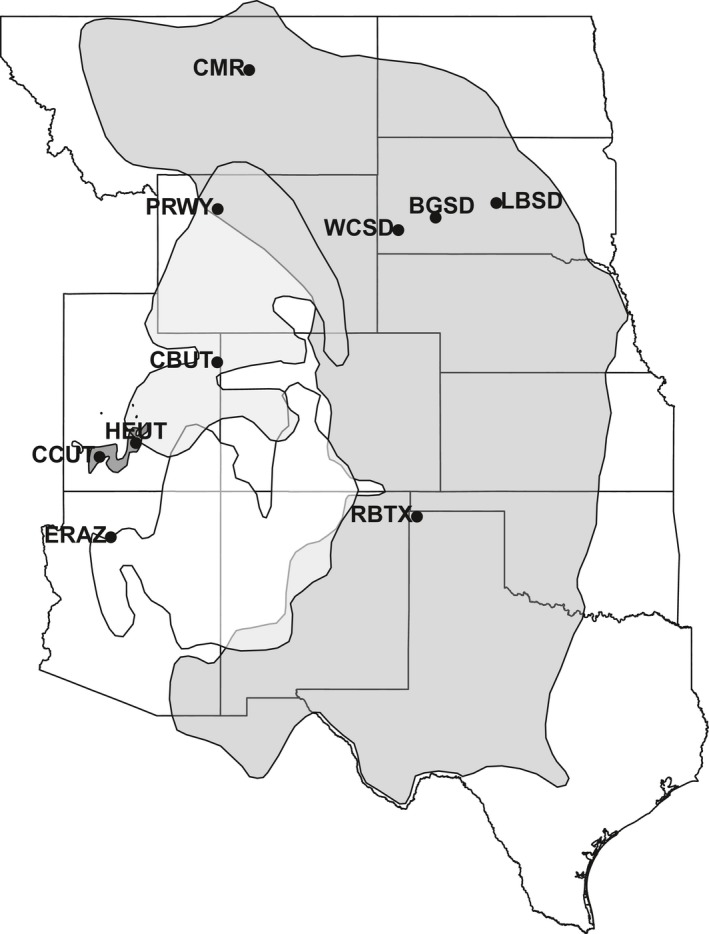
Map of study pairs. Polygons indicate the ranges of different prairie dog species; medium gray for black‐tailed prairie dogs (CMR: Charles M. Russell, Montana; WCSD: Wind Cave, South Dakota; BGSD: Buffalo Gap, South Dakota; LBSD: Lower Brule, South Dakota; RBTX: Rita Blanca, Texas), light gray for white‐tailed prairie dogs (PRWY: Pitchfork Ranch, Wyoming; CBUT: Coyote Basin, Utah), dark gray for Utah prairie dogs (CCUT: Cedar City, Utah; HEUT: High elevation, Utah), and white for Gunnison's prairie dogs (ERAZ: Espee Ranch, Arizona)
SPV‐laden baits were distributed on one randomly assigned plot in each pair and placebo baits were distributed on the other. Within 1–4 weeks, prairie dogs were trapped and sampled on the same days at both plots within a pair by the same personnel using Tomahawk live traps. Traps were baited with sweet feed and oats. Sex, age, weight, and foot length were recorded for each animal sampled, and fleas and other samples were collected from each animal upon first capture during a sampling period. Sampling dates varied by year, but occurred between June and November (Appendix 1), and a sampling period consisted of a minimum of 3 days of trapping (on consecutive days unless prevented by field conditions) (Appendix 2). During 2013, prairie dogs were sampled both pre‐ and post‐baiting, with approximately a month between sampling periods. In 2014–2015, each plot was sampled once (with the exception of the GPD pair and one of the WTPD pairs in Utah which were sampled twice, once in July and once in August).
For flea sampling, animals were anesthetized with isoflurane in an induction chamber and immediately combed for fleas that were collected into tubes of sterile saline or ethanol. After sample collection, animals were allowed to fully recover from anesthesia and then released at the location of capture (NWHC Animal Care and Use Committee, Protocol #EP130214). Fleas collected from live prairie dogs were stored at −20°C until identification. To remove surface contamination that might alter PCR results, fleas were rinsed with 70% ethanol containing 0.2% iodine and rinsed twice in sterile water (La Scola, Fournier, Brouqui, & Raoult, 2001; Raoult et al., 2001; Kernif et al., 2014). Fleas were then counted and identified to species using published taxonomic references (Furman & Catts, 1982; Hubbard, 1947; Stark, 1970), and pooled by species and sex, up to 10 individuals per pool from a single animal.
To determine plague status of our study plots, spleen and liver tissues from prairie dog carcasses, as well as flea pools collected from live and dead animals, were tested for the presence of Y. pestis using real‐time PCR (see Rocke et al., 2017 for details). In brief, tissue DNA was extracted using the Wizard SV Genomic DNA Purification System (Promega; Madison, WI), and PCR was performed for the caf1 gene sequence located on the Y. pestis pG8786 plasmid (Genbank accession NC_006323) and the pla gene located on the Y. pestis pPCP1 plasmid (Genbank accession AL109969). Tissues from carcasses were also cultured on blood agar plates, and suspect colonies were confirmed as Y. pestis using real‐time PCR. In the absence of positive cultures, DNA was only considered positive for Y. pestis if both the pla and caf1 genes were present. Flea DNA was extracted using the Zymo Quick gDNA Miniprep Kit (>1 flea) or Micro Kit (1 flea) (Zymo Research; Irvine, CA) depending on the number of fleas in each pool. DNA samples were then screened for the pim gene, which resides on the pPCP1 plasmid of Y. pestis, using real‐time PCR, as we found this gene to be more sensitive than the pla gene for fleas. Suspect positive fleas were then confirmed using caf1 gene as described above. A plot was classified as plague positive if at least one carcass or one flea pool from a live prairie dog tested positive for Y. pestis by culture or PCR. Once a plot was classified as “plague positive,” it was considered plague positive in subsequent years.
2.2. Statistical analyses
We do not formally account for detection error in our analyses; therefore, we have no repeated measures from which to estimate detection (see Eads et al., 2013 for methods with repeat detections). The amount of time spent combing for fleas was not standardized between pairs (although we assume effort between paired plots was similar); therefore, our counts should be considered relative indices. We used logistic regression and negative binomial regression with LASSO (least absolute shrinkage and selection operator; Tibshirani, 1996) priors in a Bayesian framework to assess factors related to total flea abundance, O. hirsuta abundance, and Pulex simulans abundance. This allowed us to examine factors related to both the number of fleas on a host (abundance) and the presence/absence of fleas on a host (prevalence). The LASSO allows for model selection and regularization to take place automatically, while improving predictive accuracy and providing interpretable parameter estimates (Tibshirani, 1996). Parameter estimates for variables that are not important predictors shrink to zero. We demonstrate fit of the data to a negative binomial distribution graphically (Appendix 3).
We evaluated factors associated with prevalence of fleas and overall flea abundance, including variables related to climate and seasonal weather patterns (NDVI [normalized difference vegetation index—a measure of vegetation greenness, see Table 1], number of days with a temperature over 85°F [Collinge et al., 2005], amount of precipitation during the prior year's growing season, winter precipitation amount, and day of sampling), plot‐level variables (catch per unit effort as an index of relative abundance of prairie dogs [see Rocke et al., 2017 for details], treatment [placebo vs. vaccine], plague status [plague detected vs. not detected], and elevation), and individual covariates (age, sex, body condition [=weight/foot length]) (Table 1). To incorporate effects of plot, we used a random effect for BTPD (n = 22 plots), UPD (n = 14), and WTPD (n = 8). Three of seven UPD pairs were located on the same prairie dog colony and significant movement was noted between the plots on these pairs, so treatment effects at these pairs may be compromised. GPD were collected at one pair of plots only, therefore, accounting for location was not necessary. For O. hirsuta and P. simulans abundance, we used the same covariates as described above; however, negative binomial regression was only appropriate for BTPD, due to the relatively few numbers of hosts with >1 flea observed for other species. Therefore, for all other species, we report results for logistic regression only.
Table 1.
Description of covariates used in statistical analyses of flea abundance
| Climate and weather variables | |
| Day of sampling | Day in the year (1–365) sampling took place |
| Winter precipitation | Centimeters of precipitation January‐April of current year (from NOAA Annual Climatological Survey, https://www.ncdc.noaa.gov/cdo-web/datatools/findstation) |
| Number of days > 85°F | Number of days in year with a temperature over 85 degrees F (from NOAA Annual Climatological Survey, https://www.ncdc.noaa.gov/cdo-web/datatools/findstation) |
| Prior year growing season precipitation | Centimeters of precipitation April‐August of previous year (from NOAA Annual Climatological Survey, https://www.ncdc.noaa.gov/cdo-web/datatools/findstation) |
| Normalized difference vegetation index (NDVI) | NDVI values from 7‐day Moderate Resolution Imaging Spectroradiometer (MODIS) composites that included the day of sampling for the center point of each plot were extracted for each plot (data available from the U.S. Geological Survey, https://lta.cr.usgs.gov/emodis; Jenkerson, Maiersperger, & Schmidt, 2010) |
| Plot‐level characteristics | |
| Elevation | Elevation in meters at center point of plot NED digital elevation map (from https://lta.cr.usgs.gov/NED, Archuleta et al., 2017) |
| Plague (detected, not detected) | Plague detected or plague not detected |
| Catch per unit effort (CPUE) | Number of individuals/number of trap days (see Rocke et al., 2017 for more details) |
| Treatment (vaccine, placebo) | Vaccine or placebo (see Rocke et al., 2017 for more details) |
| Flea species diversity | Number of flea species detected on a particular plot |
| Individual Characteristics | |
| Age (adult, juvenile) | Categorical variable adult = 1, juvenile = 0 |
| Body condition | Body condition: weight in g/foot length in mm |
| Sex (male, female) | Categorical variable male = 1, female = 0 |
We estimated factors associated with initial plague detection on placebo plots using logistic regression, including all plot‐level factors previously mentioned and flea species diversity as predictor variables. Data were summarized for each plot by year combination, and because we were interested in determining factors related to initial plague outbreaks (the first year plague was detected in fleas or carcasses), plot by year combinations with continuing plague outbreaks were excluded. We were interested in evaluating conditions that could predict plague outbreaks; therefore, we included catch per unit effort in the previous year. If we had instead related catch per unit effort in the year of initial plague detection rather than the year before, our models would inherently predict that low catch per unit effort is associated with plague outbreaks (i.e., the two variables are confounded). The inclusion of catch per unit effort from the previous year required us to exclude all 2013 data for this analysis, because we did not have prior year data (in any case, no plague was detected on any plots in 2013), leaving 55 plot by year combinations (including 8 initial plague detections on plots).
For all models, logistic and negative binomial, we calculated effect sizes by exponentiating the parameter estimates. Effects estimated from parameters of a negative binomial indicate the change in the expected number of fleas on a host given a 1 unit increase in the covariate (or a change in the covariate from one categorical level to another); we represent these effects as a percent change. Effects for coefficients from a logistic regression are odds ratios and indicate the odds of flea presence given a 1 unit increase in the covariate. Odds ratios of 1 indicate no change in the probability of presence given an associated increase in the covariate value, odds ratios<1 indicate that the probability of presence is less likely given an associated increase in the covariate value, and odds ratios >1 indicate an increase in the probability of presence.
All models were run in R (R Core Team, 2017). Models were run for a total of 60,000 iterations with a burn in of 20,000. All models were run in rjags (Plummer, 2013). We used Gelman–Rubin diagnostics to assess convergence (Gelman & Rubin, 1992). We used area under the curve statistics in the “pROC” package (Xavier et al., 2011) to evaluate goodness of fit for our logistic regression models. For negative binomial models, we used posterior predictive checks on the parameters of the negative binomial distribution by simulating 1,000 data sets from the estimated model parameters and comparing to the observed data (Gelman, Carlin, Stern, & Rubin, 2004). To display a visual representation of flea community structure, we used nonmetric multidimensional scaling (NMDS; an ordination technique) in R (package “vegan”; Oksanen et al., 2017). We assessed the fit of the NMDS by reporting a “stress” value (Oksanen et al., 2017).
3. RESULTS
In total, we identified 20,041 fleas from 6,542 prairie dogs (Table 2, Appendix 4) sampled between June and November over a 4‐year period, including 3,526 BTPD, 249 GPD, 1,590 UPD, and 1,177 WTPD. Four hundred and fifteen prairie dogs were sampled twice in the same year; however, samples were collected at least 30 days apart (at pre‐ and post‐bait intervals) and were therefore treated independently. We identified 18 species of fleas. The prairie dog specialist flea O. hirsuta was the most common species detected, comprising 59% of all fleas sampled and occurring on all pairs except UPD pairs located at high elevation (HEUT) and both WTPD pairs in Wyoming (PRWY). These pairs were also the most diverse, with 10 flea species detected at HEUT and 6 flea species at PRWY. The second most commonly detected flea species was Pulex simulans, a generalist flea species, accounting for 23% of fleas identified. All Pulex fleas were assumed to be P. simulans, because no male Pulex irritans were identified (Tripp et al., 2009). Pulex simulans was found mainly on BTPD pairs in Montana and Texas and WTPD pairs in Utah, with sporadic detections elsewhere. The ground squirrel flea Thrassis francisi comprised ~10% of detections and was most commonly detected on high elevation pairs in Utah. Three other Oropsylla species, O. tuberculata, O. labis, and O. idahoensis, comprised less than 10% of detections and were most commonly found on pairs where O. hirsuta was not detected. Two other species, Neopsylla inopina (a ground squirrel flea) and Aetheca wagneri (a common flea of Peromyscus spp.), were rarely encountered (<2% of detections). N. inopina was found only at PRWY, while only 6 A. wagneri were detected on BTPD and UPD (Appendix 5) during the entire study. Forty‐five fleas (<1%) of 10 additional species were identified (<20 of each) (Appendix 6).
Table 2.
Total number of fleas, prairie dogs sampled, and hosts with fleas. Flea prevalence = hosts with fleas/hosts sampled, flea intensity = total fleas/hosts with fleas, mean flea abundance = total fleas/hosts sampled. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), GPD is Gunnison's prairie dog (Cynomys gunnisoni), UPD is Utah prairie dog (Cynomys parvidens), and WTPD is white‐tailed prairie dog (Cynomys leucurus)
| Study area and species | Acronym | Total fleas | Prairie dogs sampled | Prairie dogs with fleas | Flea prevalence | Flea intensity | Mean Flea abundance |
|---|---|---|---|---|---|---|---|
| South Dakota BTPD | |||||||
| Buffalo Gap‐1 | BGSD‐1 | 792 | 467 | 246 | 52.68 | 3.22 | 1.70 |
| Buffalo Gap‐2 | BGSD‐2 | 682 | 397 | 228 | 57.43 | 2.99 | 1.72 |
| Lower Brule | LBSD‐1 | 2594 | 487 | 404 | 82.96 | 6.42 | 5.33 |
| Wind Cave | WCSD‐1 | 914 | 324 | 187 | 57.72 | 4.89 | 2.82 |
| Montana BTPD | |||||||
| Charles M. Russell‐1 | CMR‐1 | 912 | 574 | 351 | 61.15 | 2.60 | 1.59 |
| Charles M. Russell‐2 | CMR‐2 | 700 | 462 | 264 | 57.14 | 2.65 | 1.52 |
| Charles M. Russell‐3 | CMR‐3 | 1736 | 618 | 493 | 79.77 | 3.52 | 2.81 |
| Charles M. Russell‐4 | CMR‐4 | 1270 | 628 | 443 | 70.54 | 2.87 | 2.02 |
| Charles M. Russell‐5 | CMR‐5 | 1666 | 344 | 235 | 68.31 | 7.09 | 4.84 |
| Texas BTPD | |||||||
| Rita Blanca‐1 | RBTX‐1 | 1477 | 197 | 196 | 99.49 | 7.54 | 7.50 |
| Rita Blanca‐2 | RBTX‐2 | 1210 | 184 | 184 | 100.00 | 6.58 | 6.58 |
| Arizona GPD | |||||||
| Espee Ranch | ERAZ‐1 | 970 | 353 | 235 | 66.57 | 4.13 | 2.75 |
| Utah UPD | |||||||
| Cedar City‐1 | CCUT‐1 | 152 | 144 | 61 | 42.36 | 2.49 | 1.06 |
| Cedar City‐2 | CCUT‐2 | 332 | 232 | 149 | 64.22 | 2.23 | 1.43 |
| Cedar City‐3 | CCUT‐3 | 300 | 280 | 147 | 52.50 | 2.04 | 1.07 |
| High elevation‐1 | HEUT‐1 | 636 | 291 | 188 | 64.60 | 3.38 | 2.19 |
| High elevation‐2 | HEUT‐2 | 872 | 369 | 238 | 64.50 | 3.66 | 2.36 |
| High elevation‐3 | HEUT‐3 | 1464 | 336 | 240 | 71.43 | 6.10 | 4.36 |
| High elevation‐4 | HEUT‐4 | 284 | 214 | 92 | 42.99 | 3.09 | 1.33 |
| Utah WTPD | |||||||
| Coyote Basin‐1 | CBUT‐1 | 409 | 193 | 114 | 59.07 | 3.59 | 2.12 |
| Coyote Basin‐2 | CBUT‐2 | 423 | 236 | 114 | 48.31 | 3.71 | 1.79 |
| Wyoming WTPD | |||||||
| Pitchfork Ranch‐1 | PRWY‐1 | 207 | 475 | 115 | 24.21 | 1.80 | 0.44 |
| Pitchfork Ranch‐2 | PRWY‐2 | 213 | 443 | 105 | 23.70 | 2.03 | 0.48 |
3.1. Relationship of flea abundance and presence/absence with covariates
Results of our regression analyses on flea abundance and flea presence/absence for all fleas, O. hirsuta, and P. simulans indicate that multiple factors are associated with the abundance of fleas and that the relationship of some of these factors varies among prairie dog species (Figures 2, 3, 4, 5). Overall, area under the curve statistics for logistic regression models indicated an adequate fit for all fleas (0.72 BTPD, 0.61 GPD, 0.69 UPD, 0.77 WTPD), O. hirsuta (0.71 BTPD, 0.61 GPD, 0.94 UPD, 0.94 WTPD), and P. simulans (0.87 BTPD, 0.99 UPD, 0.97 WTPD; no P. simulans were detected on GPD). Posterior predictive checks on the parameters of the negative binomial distribution (mean and clustering parameter) also indicated adequate fits with p = 0.35–0.78 for comparisons of the observed mean and k for all prairie dog species to 1,000 simulated predictions from the model for total fleas (i.e., 35–78% percent of the time the values were above or below the true value; ideally 50% of the values are above and 50% below). For O. hirsuta and P. simulans, negative binomial regressions were only conducted for BTPD. Posterior predictive checks indicated adequate fits for these models as well with p‐values of 0.27 and 0.21 for the observed means and 0.61 and 0.88 for the observed clustering parameters for O. hirsuta and P. simulans, respectively.
Figure 2.
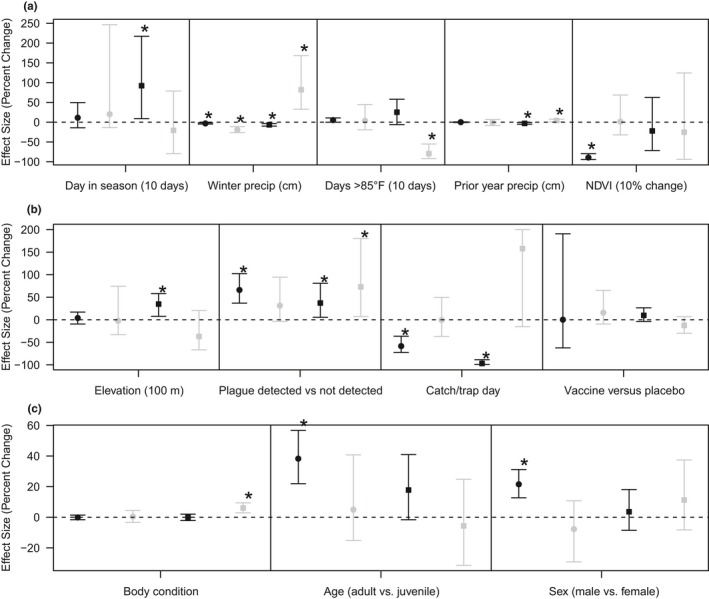
Effect sizes (represented as average percent change in flea abundance) as a function of (a) climate and environmental variables (precip=precipitation, see Table 1 for description of parameters), (b) nonplot‐level variables, and (c) characteristics of individual prairie dogs for black‐tailed prairie dog (Cynomys ludovicianus; black dots), Gunnison's prairie dog (Cynomys gunnisoni; gray dots), Utah prairie dog (Cynomys parvidens; black squares), white‐tailed prairie dog (Cynomys leucurus; gray squares). *Indicates effect sizes greater than zero
Figure 3.
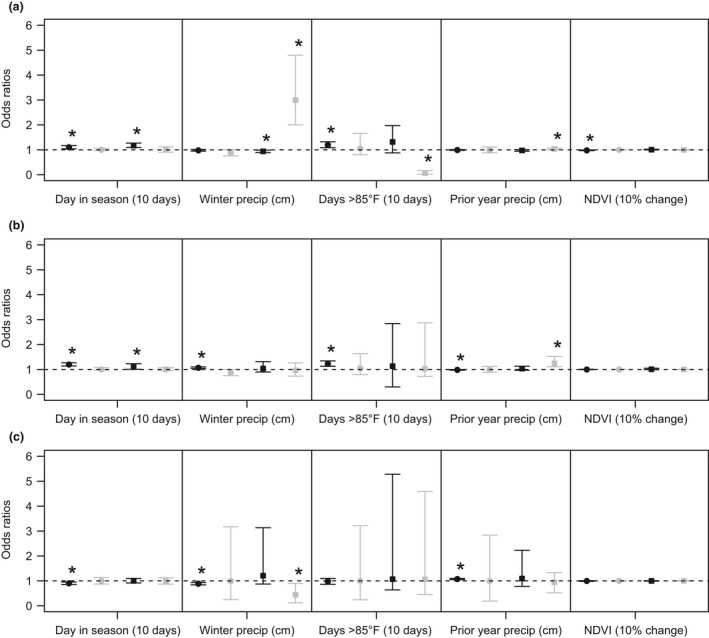
Odds ratios from results of logistic regression relating climate and weather variables (precip=precipitation, see Table 1 for description of parameters) to (a) flea presence (all flea species), (b) Oropsylla hirsuta presence, and c) Pulex simulans presence for black‐tailed prairie dog (Cynomys ludovicianus; black dots), Gunnison's prairie dog (Cynomys gunnisoni; gray dots), Utah prairie dog (Cynomys parvidens; black squares), white‐tailed prairie dog (Cynomys leucurus; gray squares). *Indicates odds ratios that do not overlap one
Figure 4.
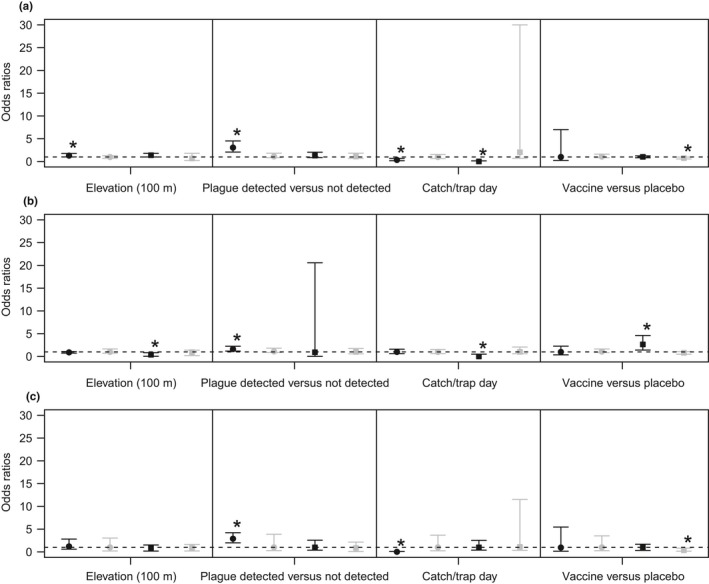
Odds ratios from results of logistic regression relating plot‐level variables (see Table 1 for description of parameters) to (a) flea presence (all flea species), (b) Oropsylla hirsuta presence, and (c) Pulex simulans presence for black‐tailed prairie dog (Cynomys ludovicianus; black dots), Gunnison's prairie dog (Cynomys gunnisoni; gray dots), Utah prairie dog (Cynomys parvidens; black squares), white‐tailed prairie dog (Cynomys leucurus; gray squares). * Indicates odds ratios that do not overlap one
Figure 5.
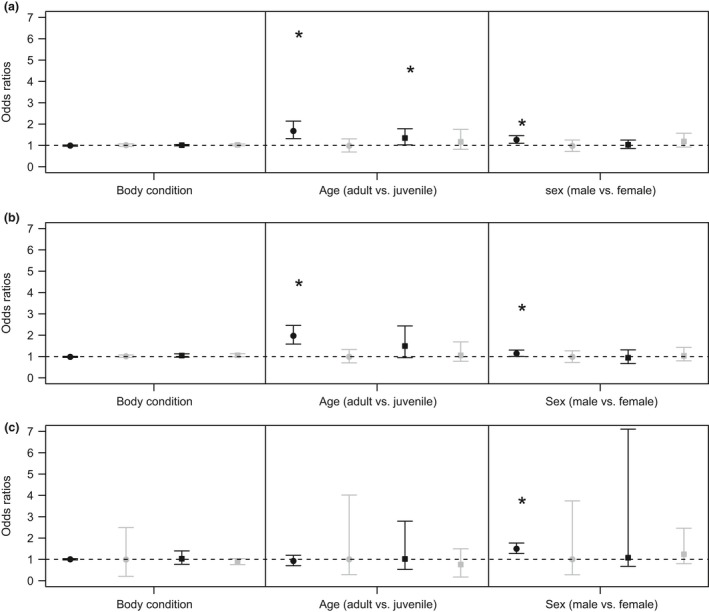
Odds ratios from results of logistic regression relating individual characteristics of prairie dogs (see Table 1 for description of parameters) to (a) total flea abundance, (b) Oropsylla hirsuta abundance, and (c) Pulex simulans abundance for black‐tailed prairie dog (Cynomys ludovicianus; black dots), Gunnison's prairie dog (Cynomys gunnisoni; gray dots), Utah prairie dog (Cynomys parvidens; black squares), white‐tailed prairie dog (Cynomys leucurus; gray squares). * Indicates odds ratios that do not overlap one
3.2. Climate and weather variables
Multiple weather and climate‐related variables were associated with flea abundance and presence/absence. Total flea abundance increased 92% (95% Credible Interval [C.I.] 9–217%) on UPD pairs over every 10‐day period (Figure 2). The odds of a prairie dog having at least one flea increased by 1.10 (95% C.I. 1.03–1.17) for BTPD and 1.16 (95% C.I. 1.09–1.26) for UPD as days in the season increased (Figure 3). In addition, on BTPD pairs, O. hirsuta abundance increased an average of 214% (95% C.I. 88–392) with every 10 days later in the year (Table 3), while P. simulans abundance decreased 66% (95% C.I. 50–78%) (Table 3). On UPD pairs, day of sampling was associated with an increase in the odds of O. hirsuta presence (1.01, 95% C.I. 1.12–1.23) but not P. simulans.
Table 3.
Effect sizes for results of negative binomial regressions estimating the associations between Oropsylla hirsuta and Pulex simulans abundance on BTPD. Effects are represented as a percent change. LCI: lower credible interval, UCI: upper credible interval
| Oropsylla hirsuta | Pulex simulans | |||||
|---|---|---|---|---|---|---|
| Median | 2.5% LCI | 97.5% UCI | Median | 2.5% LCI | 97.5% UCI | |
| Climate and weather variables | ||||||
| Day in season (10 days) | 214.36 | 88.17 | 392.51 | −66.66 | −77.98 | −50.55 |
| Winter precipitation (cm) | 0.51 | −1.59 | 2.66 | −7.33 | −10.4 | −4.26 |
| Number of days >85°F (10 days) | 21.75 | 13.82 | 30.54 | −1.99 | −10.09 | 6.75 |
| Prior year precipitation (cm) | −2.27 | −3.18 | −1.32 | 7.17 | 5.65 | 8.67 |
| NDVI (10% change) | −25.66 | −66.69 | 24.03 | −55.11 | −89.36 | 21.53 |
| Pair‐level characteristics | ||||||
| Elevation (100 m) | −11.09 | −27.75 | 3.9 | −5.37 | −48.72 | 88.84 |
| Plague detected vs no plague detected | 61.65 | 25.47 | 109.15 | 73.49 | 35.78 | 121.21 |
| Catch/trap days | −9.16 | −42.65 | 30.09 | −93.93 | −97.09 | −87.39 |
| Vaccine vs placebo | 4.51 | −46.62 | 133.21 | 2.98 | −80.3 | 1100.99 |
| Individual characteristics | ||||||
| Adult vs juvenile | 55.4 | 32.32 | 83.46 | −5.08 | −20.39 | 12.48 |
| Body condition (wt(g)/foot length (mm)) | −0.04 | −2.06 | 1.89 | 0.1 | −1.89 | 2.15 |
| Male vs female | 12.98 | 2.6 | 24.41 | 45.72 | 30.98 | 62.17 |
With a 10% increase in NDVI, total flea abundance decreased by 89% (95% C.I. 80–95%) on BTPD pairs, but these differences were not significant for individual flea species (Figures 2 and 3). For every 10 days with temperatures over 85°F, total flea abundance decreased by 80% (95% C.I. 55–92%) and the odds of flea presence decreased by 0.06 (95% C.I. 0.02–0.16) for WTPD; however, these results were not evident for individual flea species (Figure 3). For the same change in number of degree days, total flea abundance increased by 5% (95% C.I. 0–11%) and the odds of flea presence increased by 1.19 (95% C.I. 1.08–1.31) for BTPD; these differences were driven largely by changes in O. hirsuta with average increases in abundance of 21% (95% C.I. 14–20%; Table 3) and increases in odds of O. hirsuta presence of 1.23 (95% C.I. 1.13–1.34) (Figure 3).
Precipitation amounts also had varying effects on flea abundance (Figures 2 and 3). For every 1 cm increase in winter precipitation on WTPD pairs, total flea abundance increased by 83% (95% C.I. 33–165%) with odds of flea presence increasing by 2.99 (95% C.I. 2.00–4.80). However, odds of P. simulans presence declined with increasing precipitation (0.44, 95% C. I. 0.12–0.89) on these plots. On BTPD plots, flea abundance declined by 3% (95% C.I. 2–5%) and odds of flea presence decreased by 0.98 (95% C.I. 0.95–1.00) with increasing winter precipitation. These results were largely driven by declines in P. simulans with an average decline of 7% (95% C.I. 4–10%) with increasing winter precipitation. On GPD and UPD pairs, every 1 cm increase in winter precipitation led to odds of flea presence decreasing by 0.88 (95% C.I. 0.76–1.00) and 0.94 (95% C.I. 0.89–0.99), respectively, with corresponding decreases in total flea abundance of 19% (95% C.I. 11–27%) on GPD plots and 7% (95% C.I. 4–10%) on UPD plots. Odds of O. hirsuta presence also declined with increasing winter precipitation on GPD plots (0.87, 95% C.I. 0.75–1.00), but not UPD plots. Prior year precipitation was related to increasing odds of P. simulans presence (1.07, 95% C.I. 1.05–1.10) on BTPD plots and increasing odds of O. hirsuta presence (1.24, 95% C.I. 1.11–1.52) on WTPD plots. O. hirsuta flea abundance declined by an average of 2% (95% C.I. 1–3%), while P. simulans abundance increased an average of 7% (95% C.I. 6–9%) for every 1 cm increase in prior year precipitation on BTPD plots (Table 3).
3.3. Plot‐level characteristics
Plot‐level variables also indicated associations with flea abundance and presence/absence. The presence of plague was positively associated with flea abundance for all species except GPD (Figure 2). In addition, O. hirsuta and P. simulans abundance was associated with plague detection for BTPD (Table 3). Odds of flea presence increased for every 100 m increase in elevation on BTPD (1.29, 95% C.I. 1.01–1.77) and UPD (1.39, 95% C.I. 1.00–1.79) plots (Figure 4), and flea abundance increased by an average of 35% (95% C.I. 8–58%) on UPD plots as well (Figure 2). Odds of O. hirsuta presence, however, declined on UPD plots (0.37, 95% C.I. 0.04–0.83) with elevation increases. For every 1 unit increase in catch per unit effort, odds of flea presence was 0.02 (95% C.I. 0.00–0.14) for UPD and 0.35 (95% C.I. 0.17–0.70) for BTPD plots, while average flea abundance declined 97% (95% C.I. 89–99%) and 58% (95% C.I. 37–72%) on UPD and BTPD plots, respectively. These results were driven by declines in O. hirsuta on UPD plots and P. simulans on BTPD plots (Figure 4, Table 3). At last, on average, the odds of flea presence was lower on vaccine versus placebo plots for WTPD (0.74, 95% C.I. 0.56–0.97) only, and these results were reflected in declines in the odds of P. simulans presence (0.36, 95% C.I. 0.16–0.83). Increases in odds of O. hirsuta presence were detected on UPD vaccine versus placebo plots (2.65, 95% C.I. 1.41–4.65); however, O. hirsuta were only found on CCUT sites and not HEUT. The CCUT sites were small, <4 hectares in size, therefore the effects of the vaccine may have been swamped by movement on and off the site.
3.4. Individual characteristics of prairie dogs
Flea abundance increased by 6% (95% C.I. 3–9%) for every 1 unit increase in body condition (g/mm) for WTPD, but no other species (Figure 2). Odds of flea presence increased by 1.69 (95% C.I. 1.33–2.14) and 1.34 (95% C.I. 1.03–1.75) for adult versus juvenile BTPD and UPD respectively (Figure 5), and adult BTPD on average had 38% (95% C.I. 21–57%) more fleas than juveniles. Odds of O. hirsuta presence also increased on adult versus juvenile BTPD by 1.97 (95% C.I. 1.58–2.46) (Figure 5), and average O. hirsuta abundance was 55% (95% C.I. 32–83%) higher on adults. Male BTPD on average had 21% (95% C.I. 13–31%) more total fleas, 13% (95% C.I. 3–24%) more O. hirsuta, and 43% (95% C.I. 31–62%) more P. simulans than females.
3.5. Flea species composition
Results of our NMDS reflect results of our regression analyses (Figure 6). High elevation pairs in Wyoming and Utah (Appendix 7) had distinct flea communities compared with the rest of the study pairs. Communities dominated by O. hirsuta included lower elevation UPD pairs (CCUT) and WTPD pairs in Utah, BTPD pairs in South Dakota, and the one GPD pair in Arizona, while P. simulans was associated with BTPD pairs in Montana and Texas, as well as one WTPD pair in Utah. Stress values indicate adequate fit (0.98).
Figure 6.
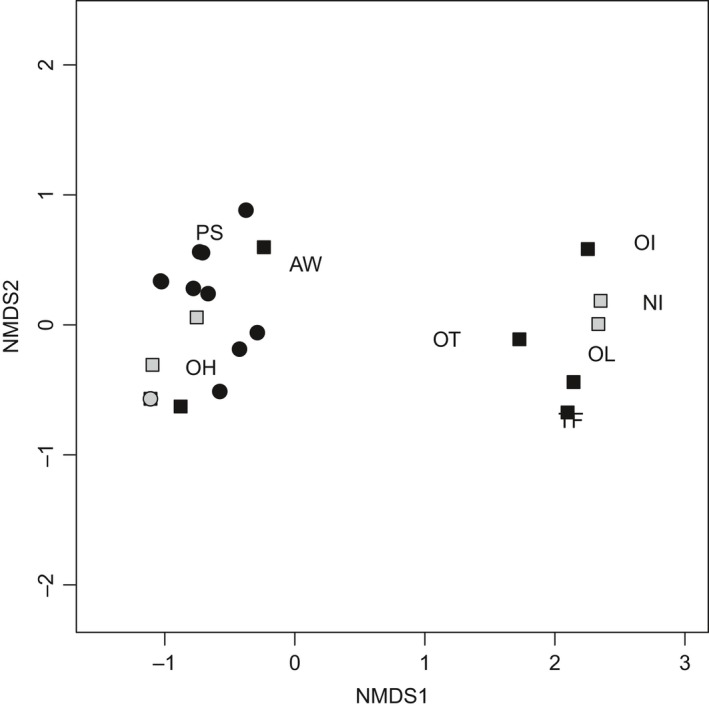
Nonmetric multidimensional scaling of flea communities, including black‐tailed prairie dog (Cynomys ludovicianus; black dots), Gunnison's prairie dog (Cynomys gunnisoni; gray dots), Utah prairie dog (Cynomys parvidens; black squares), white‐tailed prairie dog (Cynomys leucurus; gray squares). Flea species are indicated in large capital letters. OH: Oropsylla hirsuta; OT: O. tuberculata; OL: O. labis; OI: O. idahoensis; TF: Thrassis francisi; PS: Pulex simulans; AW: Aetheca wagneri; and NI: Neopsylla inopina
3.6. Plague detections
The incidence of prairie dogs with Y. pestis detections in fleas was low (n = 64 prairie dogs with positive fleas out of 5,024 prairie dogs sampled from 4,218 individual prairie dogs). Fleas positive for Y. pestis were found on BTPD plots in Montana (2016) and Texas (2014 and 2015), WTPD plots in Wyoming (2015 and 2016) and Utah (2015 and 2016), high elevation UPD plots (2014 and 2015), and the one GPD plot in Arizona (2014) (Appendix 4). Twenty‐five of the prairie dogs with Y. pestis positive flea pools were found on placebo plots and 39 on vaccine plots which represent less than 2% of all prairie dogs tested. Y. pestis positive fleas were found on juvenile and adult prairie dogs of both sexes and in flea pools of 7 different species including P. simulans, O. hirsuta, O. tuberculata, O. labis, O. idahoensis, Thrassis francisi, and Neopsylla inopina (Y. pestis was not detected in any A. wagneri pools). Thirty‐three plague‐positive carcasses were collected from seven placebo plots and five vaccine plots over the 4‐year period (Table 4, Appendix 4).
Table 4.
Number of plague‐positive carcasses collected from study plots. Carcasses were considered to be plague positive if Yersinia pestis was cultured from tissues or detected by PCR analysis
| Pair | Species | Treatment | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| CBUT‐2 | White‐tailed | Placebo | 1 | ||
| CCUT‐2 | Utah | Placebo | 2 | ||
| CMR‐1 | Black‐tailed | Placebo | 3 | ||
| CMR‐2 | Black‐tailed | Placebo | 1 | ||
| HEUT‐2 | Utah | Placebo | 3 | 4 | |
| HEUT‐3 | Utah | Placebo | 1 | ||
| PRWY‐1 | White‐tailed | Placebo | 1 | ||
| CBUT‐2 | White‐tailed | Vaccine | 4 | ||
| ERAZ‐1 | Gunnison's | Vaccine | 2 | ||
| HEUT‐2 | Utah | Vaccine | 5 | 2 | |
| PRWY‐1 | White‐tailed | Vaccine | 1 | ||
| PRWY‐2 | White‐tailed | Vaccine | 3 |
Flea abundance was higher on animals with PCR‐positive flea pools (mean = 11.7, SD = 14.1) than animals without PCR‐positive fleas (mean = 3.9, SD = 5.1). Plague detections in P. simulans only occurred at plots where plague was also detected in O. hirsuta. Plague‐positive fleas (T. francisi, O. labis, O. idahoensis, and O. tuberculata) were found on PRWY and HEUT plots, but no O. hirsuta were detected on these plots. Overall, plague was detected in carcasses or fleas at 18 (9 placebo plots and 9 vaccine plots, not all paired) of 46 plots (see Rocke et al., 2017 for more details). Logistic regression estimates indicated that no covariates were significantly associated with initial plague outbreaks on placebo plots.
4. DISCUSSION
Many factors were associated with flea abundance on prairie dogs, indicating the complexity of host‐vector‐disease dynamics in this system. Our results indicate that local weather factors influenced flea abundance, potentially by providing environmental conditions suitable for flea reproduction. As expected, however, environmental factors had different effects at different locations due to the wide range of environmental conditions and geographic locations of our study pairs. Differences in the direction of the relationship of environmental covariates with flea abundance for different prairie dog species is likely the result of differences in average local conditions and differences in the biology of the prairie dog and flea species in question. Abundance of fleas is likely more important for epizootic plague, versus the presence or absence of fleas on hosts at a location. High flea loads have been shown to be important for epizootic transmission via blocked fleas (Lorange et al., 2005). Abundant fleas are also likely to result in more efficient early‐phase transmission (Eisen et al., 2006), whereas flea presence merely indicates that at least one flea is present on the host. Therefore, a large number of hosts with low flea abundance (i.e., high flea prevalence) may not result in plague outbreaks. We did not find any relationship between local weather conditions and the likelihood of plague outbreaks. However, our sample sizes were small and geographically varied. It is likely that conditions under which plague epizootics occur are as locally‐specific as the conditions that result in high flea abundance.
Winter precipitation increases resulted in decreases in flea abundance for all species except WTPD, while overall flea abundance decreased with increasing number of days with temperatures above 85°F on WTPD plots but increased with warmer temperatures on BTPD plots. These results may reflect differences in species composition or local weather conditions. Salkeld and Stapp (2008) found increasing O. hirsuta and decreasing O. tuberculata populations in prairie dog burrows with increasing temperature, which may explain some of these differences. Soil type is an additional factor that may influence flea abundance. Savage et al. (2011) found that soils with increased soil moisture holding capacity were related to the incidence of plague epizootics; however, Salkeld and Stapp (2008) found no relationship between O. hirsuta abundance and soil type. Despite the overall increase in flea abundance on WTPD pairs, P. simulans abundance decreased with increasing winter precipitation on these plots (as well as BTPD plots), indicating that the increases in flea abundance on WTPD plots with winter precipitation are driven by other flea species. In addition, it is likely that flea abundance is optimal in a mid‐range of conditions. On WTPD plots, average winter precipitation was roughly 2 cm greater than on BTPD plots, indicating that greater precipitation on these wet plots led to decreases in flea abundance in contrast to plots that received lesser amounts of precipitation.
Several possible mechanisms may explain the effects of local weather on flea abundance and plague outbreaks. Local weather factors may affect flea abundance directly by influencing flea mortality or reproductive rates (Krasnov, Khokholova, Fielden, & Burdelova, 2001) or indirectly by changing forage availability and affecting host body condition and/or grooming behavior (Eads et al., 2016). Flea abundance on BTPD plots declined with increasing NDVI, supporting the hypothesis that better forage conditions on BTPD plots may lead to lower flea abundance. In our data set, body condition was not strongly associated with flea abundance of most species, except WTPD, and for this species, animals with better body condition tended to have more fleas, possibly due to their larger size. It is possible that body condition may increase in importance during drought conditions which were not observed on our plots during 2013–2016. One study noted that BTPD that survived plague outbreaks tended to have better body condition than prior to the die‐off, potentially due to less competition for forage (Pauli, Buskirk, Williams, & Edwards, 2006). This observation may explain the relationship we observed between flea abundance on plague‐positive plots and body condition in our study. We did not have direct before‐after comparisons; however, there was no difference in average body condition between animals captured on plots with plague detections (mean body condition = 14.0, SD = 4.4), compared to plots with no plague detection (mean body condition = 14.5, SD = 4.6).
Previous research has indicated that trends in flea abundance during a season are highly dependent on the flea community (Tripp et al., 2009). Different flea species have different seasonal peaks in abundance, with O. tuberculata tending to be more common in early spring, O. idahoensis in midsummer (Anderson & Williams, 1997), and O. hirsuta later in the summer (Tripp et al., 2009). Our study pairs were sampled between June and November over a short period of time, thus flea peaks in early spring were missed. In addition, different pairs were sampled at different times of the year (Appendix 1), with pairs in Wyoming sampled the earliest on average and pairs in Texas surveyed the latest, an average of 125 days later. Therefore, our result that flea abundance increased over time may be reflective of seasonal differences in the timing of sampling between pairs and/or the location of the pair along the latitudinal gradient. Our data indicated that total flea abundance (specifically O. hirsuta) tended to increase during summer months for UPD and BTPD, while abundance of P. simulans declined on BTPD plots. Most of our BTPD colonies were located at the more northern range of our study pairs; therefore, optimal conditions for flea growth may have occurred later in the season at these pairs.
Flea community composition and diversity also varied by location. The highest elevation pairs (2 WTPD pairs in Wyoming, PRWY, and 4 UPD pairs in Utah, HEUT), with elevation >2000 m, (Appendix 7) had the highest diversity of flea species, although they were the only pairs where O. hirsuta was not found. Instead, two other Oropsylla species, O. tuberculata and O. labis, were commonly found, while O. idahoensis was commonly found on both Wyoming pairs but only the highest elevation pair in Utah (HEUT‐4). In addition, HEUT pairs were the only ones where T. francisi was commonly found. These results may indicate that at high elevation, different flea species associated with different small mammal communities play a role in the maintenance of plague in the system.
On UPD and BTPD plots, increasing catch per unit effort was associated with decreasing flea abundance, potentially indicating that larger prairie dog populations resulting from improved forage conditions lead to lower flea abundance. However, this relationship may also be driven by plague dynamics. Higher flea abundance on plague‐affected plots (which also have lower catch per unit effort) may be the result of fleas congregating on surviving animals (Tripp et al., 2009). It is also possible that the combination of high flea abundance and high prairie dog abundance leads directly to plague epizootics and is therefore rarely observed (i.e., once these conditions are met there is a plague outbreak which drives prairie dog densities lower). Lastly, the combination of high densities of animals in summers that are drier than average could lead to poorer condition of animals overall, leading to higher susceptibility to plague the following summer, particularly if forage availability (as indicated by NDVI) is low.
Changing climate conditions will likely affect aspects of both flea and host communities, including population densities and species composition, and lead to changes in plague dynamics. We expected that plague outbreaks would be less likely when temperatures were high (Snäll et al., 2008); however, we did not observe this relationship in our data set. High temperatures have been observed to interfere with transmission of Y. pestis in rat fleas (Cavanaugh, 1971) and may lead to decreases in plague outbreaks with warming temperatures. In an alternative manner, hosts and plague vectors may expand their range northward, thus shifting the range of Y. pestis outbreaks (Nakazawa et al., 2007). A meta‐analysis recently provided evidence for the effects of local factors on disease outbreaks, including the specific composition of the host and vector community (Salkeld, Padgett, & Jones, 2013). Our results support the hypothesis that local conditions, including host, vector, and environmental factors, influence the likelihood of plague outbreaks, and that predicting changes to plague dynamics under climate change scenarios will require consideration of both host and vector responses to local factors.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
T.R., D.T., and R.R. conceived the ideas and design of the study; R.R. and T.R. designed the methodology; R.A. and D.T. collected the data; R.R. analyzed the data and R.R., T.R., D.T., and R.A. contributed to analysis interpretations; R.R. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
DATA ACCESSIBILITY
Data are available at https://doi.org/10.5066/f7tm79ck.
ACKNOWLEDGMENTS
Our work was supported by the U.S. Geological Survey, U.S. Fish and Wildlife Service, National Park Service, U.S. Forest Service, U.S. Department of Agriculture Wildlife Services, Bureau of Land Management, Colorado Parks and Wildlife, Colorado's Species Conservation Trust Fund, Utah Division of Wildlife, Arizona Game and Fish, Wyoming Game and Fish Department, Lower Brule Sioux Tribe, World Wildlife Fund, the Western Association of Fish and Wildlife Agencies and the U.S. Department of Defense. Thanks to a large contingent of field personnel and volunteers for trapping and sampling prairie dogs. Thanks also to S. Green for laboratory assistance. Rebecca Cole provided critical review of the manuscript. Any use of trade, product, or firm names does not imply endorsement by the U.S. Government.
APPENDIX 1.
Sampling times for study sites. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (Cynomys leucurus), UPD is Utah prairie dog (Cynomys parvidens), and GPD is Gunnison's prairie dog (Cynomys gunnisoni). NS: not sampled
| Study area and species | 2013 | 2014 | 2015 | 2016 | ||
|---|---|---|---|---|---|---|
| Prebait | Postbait | Postbait | Postbait | Prebait | Postbait | |
| South Dakota BTPD | ||||||
| Buffalo Gap‐1 | June | July | July | July | NS | NS |
| Buffalo Gap‐2 | June | July | July | July | NS | NS |
| Lower Brule | July | August | August | August | NS | August |
| Wind Cave | June | July | July | July | NS | NS |
| Montana BTPD | ||||||
| Charles M. Russell‐1 | June | July | July | July | June | July |
| Charles M. Russell‐2 | July | July | July | July | June | July |
| Charles M. Russell‐3 | NS | August | July | July | June | August |
| Charles M. Russell‐4 | June | July | July | July | June | August |
| Charles M. Russell‐5 | July | August | August | August | NS | NS |
| Texas BTPD | ||||||
| Rita Blanca‐1 | August | October | October | October | NS | NS |
| Rita Blanca‐2 | August | November | October | October | NS | NS |
| Arizona GPD | ||||||
| Espee Ranch | June | July | July; August | July | NS | July |
| Utah UPD | ||||||
| Cedar City‐1 | June | July‐August | September | NS | NS | NS |
| Cedar City‐2 | July | August | July | July | June | NS |
| Cedar City‐3 | July | August | September | August | NS | September |
| High Elevation‐1 | NS | June | July‐August | July | July | NS |
| High Elevation‐2 | NS | June‐July | July‐August | July | July | NS |
| High Elevation‐3 | NS | July | July‐August | July | July‐August | NS |
| High Elevation‐4 | NS | July‐August | August | August | August | NS |
| Utah WTPD | ||||||
| Coyote Basin‐1 | June | July | July | July; August | NS | July |
| Coyote Basin‐2 | June | July‐August | July | July | NS | July |
| Wyoming WTPD | ||||||
| Pitchfork Ranch‐1 | June | July | June | June | NS | June |
| Pitchfork Ranch‐2 | June | July | July | July | NS | July |
APPENDIX 2.
Number of trap days. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (Cynomys leucurus), UPD is Utah prairie dog (Cynomys parvidens), and GPD is Gunnison's prairie dog (Cynomys gunnisoni). NS: not sampled
| Plot | Treatment | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| South Dakota BTPD | |||||
| Buffalo Gap‐1 | Placebo | 330 | 237 | 339 | NS |
| Vaccine | 255 | 214 | 210 | NS | |
| Buffalo Gap‐2 | Placebo | 330 | 248 | 190 | NS |
| Vaccine | 240 | 234 | 230 | NS | |
| Lower Brule‐1 | Placebo | 1,446 | 999 | 868 | 1,200 |
| Vaccine | 1,513 | 968 | 972 | 1,200 | |
| Wind Cave‐1 | Placebo | 353 | 390 | 379 | NS |
| Vaccine | 315 | 363 | 252 | NS | |
| Montana BTPD | |||||
| Charles M. Russell‐1 | Placebo | 332 | 194 | 176 | 960 |
| Vaccine | 342 | 206 | 190 | 960 | |
| Charles M. Russell‐2 | Placebo | 228 | 222 | 190 | 960 |
| Vaccine | 212 | 197 | 184 | 961 | |
| Charles M. Russell‐3 | Placebo | 216 | 226 | 201 | 1,200 |
| Vaccine | 220 | 202 | 199 | 1,200 | |
| Charles M. Russell‐4 | Placebo | 217 | 213 | 204 | 1,200 |
| Vaccine | 213 | 205 | 176 | 1,200 | |
| Charles M. Russell‐5 | Placebo | 188 | 191 | 149 | NS |
| Vaccine | 190 | 201 | 146 | NS | |
| Texas BTPD | |||||
| Rita Blanca‐1 | Placebo | 119 | 89 | 90 | NS |
| Vaccine | 117 | 89 | 89 | NS | |
| Rita Blanca‐2 | Placebo | 120 | 90 | 90 | NS |
| Vaccine | 120 | 90 | 90 | NS | |
| Arizona GPD | |||||
| Espee Ranch | Placebo | 399 | 627 | 434 | 450 |
| Vaccine | 402 | 642 | 449 | 531 | |
| Utah UPD | |||||
| Cedar City‐1 | Placebo | 222 | 225 | NS | NS |
| Vaccine | 225 | 228 | NS | NS | |
| Cedar City‐2 | Placebo | 225 | 225 | 225 | 4 |
| Vaccine | 219 | 225 | 225 | 1 | |
| Cedar City‐3 | Placebo | 189 | 225 | 225 | 225 |
| Vaccine | 225 | 225 | 225 | 225 | |
| High Elevation‐1 | Placebo | 504 | 623 | 498 | 336 |
| Vaccine | 477 | 619 | 484 | 390 | |
| High Elevation‐2 | Placebo | 560 | 712 | 347 | 420 |
| Vaccine | 560 | 730 | 360 | 420 | |
| High Elevation‐3 | Placebo | 375 | 506 | 281 | 480 |
| Vaccine | 350 | 449 | 298 | 480 | |
| High Elevation‐4 | Placebo | 504 | 264 | 286 | 192 |
| Vaccine | 630 | 242 | 268 | 384 | |
| Utah WTPD | |||||
| Coyote Basin‐1 | Placebo | 396 | 297 | 487 | 150 |
| Vaccine | 388 | 272 | 351 | 150 | |
| Coyote Basin‐2 | Placebo | 520 | 287 | 176 | 120 |
| Vaccine | 270 | 387 | 551 | 180 | |
| Wyoming WTPD | |||||
| Pitchfork Ranch‐1 | Placebo | 762 | 1,249 | 1,216 | 960 |
| Vaccine | 742 | 1,264 | 1,153 | 1,280 | |
| Pitchfork Ranch‐2 | Placebo | 1,553 | 1,262 | 1,186 | 1,280 |
| Vaccine | 1,486 | 1,261 | 1,184 | 960 | |
APPENDIX 3.
Observed data compared to fitted distributions for (a) negative binomial (observed‐white bars, theoretical‐black bars) and (b) poisson. Empirical (solid gray line) and theoretical (negative binomial‐solid black line, poisson is dotted gray line) cumulative distribution functions (CDF).
APPENDIX 4.
Number of prairie dogs sampled (number of unique animals if different from number sampled), number of prairie dogs with plague‐positive flea pools (indicated by +), number of plague‐positive carcasses (boldface). BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (Cynomys leucurus), UPD is Utah prairie dog (Cynomys parvidens), and GPD is Gunnison's prairie dog (Cynomys gunnisoni). NS: not sampled
| Study area and species | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|
| South Dakota BTPD | ||||
| Buffalo Gap‐1 | 142 (130) | 210 | 115 | NS |
| Buffalo Gap‐2 | 124 (121) | 169 | 104 | NS |
| Lower Brule | 175 (152) | 106 | 100 | 106 (105) |
| Wind Cave | 122 (106) | 100 | 102 (100) | NS |
| Montana BTPD | ||||
| Charles M. Russell‐1 | 102 (97) | 106 | 99 | 267 (227) 5+ 1 |
| Charles M. Russell‐2 | 111 (101) | 113 | 124 | 114 (87) 11+ 1 |
| Charles M. Russell‐3 | 101 | 128 | 127 | 262 (203) |
| Charles M. Russell‐4 | 131 (119) | 108 | 109 | 280 (236) |
| Charles M. Russell‐5 | 119 (106) | 112 | 113 | NS |
| Texas BTPD | ||||
| Rita Blanca‐1 | 120 (113) | 58 1+ | 19 10+ | NS |
| Rita Blanca‐2 | 93 (82) | 38 | 53 | NS |
| Arizona GPD | ||||
| Espee Ranch | 107 (100) | 77 (50) 6+ 2 | 70 (68) | 96 (92) |
| Utah UPD | ||||
| Cedar City‐1 | 86 (77) | 58 | NS | NS |
| Cedar City‐2 | 89 (79) | 68 | 70 | NS |
| Cedar City‐3 | 106 (90) | 52 | 94 | 28 |
| High Elevation‐1 | 113 | 92 (87) | 39 | 47 (42) |
| High Elevation‐2 | 123 | 182 (181) 2+ 8 | 34 4+ 4 | 30 |
| High Elevation‐3 | 108 | 96 (95) | 84 8+ | 48 |
| High Elevation‐4 | 62 | 37 | 95 | 20 |
| Utah WTPD | ||||
| Coyote Basin‐1 | 56 (53) | 108 (107) | 28 3+ 2 | 1 1+ |
| Coyote Basin‐2 | 73 (70) | 95 (94) | 65 6+ | 3 |
| Wyoming WTPD | ||||
| Pitchfork Ranch‐1 | 151 (141) | 132 | 119 2+ | 73 3+ 2 |
| Pitchfork Ranch‐2 | 140 (131) | 121 (118) | 121 | 61 2+ 3 |
APPENDIX 5.
Summary of mean flea abundance per prairie dog by flea species and location of prairie dog colony. Total number of fleas is in parentheses; if no total is listed, no fleas of that species were found. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (Cynomys leucurus), UPD is Utah prairie dog (Cynomys parvidens), and GPD is Gunnison's prairie dog (Cynomys gunnisoni). OH: Oropsylla hirsuta; OT: O. tuberculata; OL: O. labis; OI: O. idahoensis; TF: Thrassis francisi; PS: Pulex simulans; AW: Aetheca wagneri; NI: Neopsylla inopina. Sampling periods and sample sizes are listed in Appendices S1 and S4, respectively
| Study area and species | Year | OH | OT | OL | OI | TF | PS | AW | NI |
|---|---|---|---|---|---|---|---|---|---|
| South Dakota BTPD | |||||||||
| Buffalo Gap‐1 | 2013 | 3.15 (448) | 0.09 (13) | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 (1) | 0.00 |
| 2014 | 0.75 (158) | 0.00 (1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 1.48 (170) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Buffalo Gap‐2 | 2013 | 1.56 (194) | 0.04 (5) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 1.83 (309) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 1.67 (174) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Lower Brule | 2013 | 7.49 (1310) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 3.63 (385) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 6.63 (663) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2016 | 2.21 (234) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Wind Cave | 2013 | 4.54 (554) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 0.89 (89) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 2.61 (266) | 0.01 (1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 (1) | 0.00 | |
| Montana BTPD | |||||||||
| Charles M. Russell‐1 | 2013 | 0.69 (70) | 0.08 (8) | 0.00 | 0.00 | 0.00 | 1.39 (142) | 0.00 | 0.00 |
| 2014 | 0.31 (33) | 0.01 (1) | 0.00 | 0.00 | 0.00 | 0.09 (10) | 0.00 | 0.00 | |
| 2015 | 1.02 (101) | 0.03 (3) | 0.00 | 0.00 | 0.00 | 0.34 (34) | 0.00 | 0.00 | |
| 2016 | 1.17 (312) | 0.01 (2) | 0.00 | 0.00 | 0.00 | 0.73 (196) | 0.00 | 0.00 | |
| Charles M. Russell‐2 | 2013 | 0.62 (69) | 0.00 | 0.00 | 0.00 | 0.00 | 0.49 (54) | 0.00 | 0.00 |
| 2014 | 0.37 (42) | 0.00 | 0.00 | 0.00 | 0.00 | 0.87 (98) | 0.00 | 0.00 | |
| 2015 | 0.61 (76) | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 (25) | 0.00 | 0.00 | |
| 2016 | 1.69 (193) | 0.01 (1) | 0.00 | 0.00 | 0.00 | 1.25 (142) | 0.00 | 0.00 | |
| Charles M. Russell‐3 | 2013 | 1.96 (198) | 0.00 | 0.00 | 0.00 | 0.00 | 1.46 (147) | 0.00 | 0.00 |
| 2014 | 0.45 (58) | 0.00 | 0.00 | 0.00 | 0.00 | 3.59 (460) | 0.02 (2) | 0.00 | |
| 2015 | 1.95 (248) | 0.00 | 0.00 | 0.00 | 0.00 | 1.28 (162) | 0.00 | 0.00 | |
| 2016 | 0.92 (240) | 0.01 (3) | 0.00 | 0.00 | 0.00 | 0.83 (218) | 0.00 | 0.00 | |
| Charles M. Russell‐4 | 2013 | 0.65 (85) | 0.01 (1) | 0.00 | 0.00 | 0.00 | 0.99 (130) | 0.00 | 0.00 |
| 2014 | 0.62 (67) | 0.00 | 0.01 (1) | 0.00 | 0.00 | 2.94 (318) | 0.00 | 0.00 | |
| 2015 | 1.34 (146) | 0.00 | 0.00 | 0.00 | 0.00 | 1.47 (160) | 0.00 | 0.00 | |
| 2016 | 0.95 (266) | 0.00 (1) | 0.00 | 0.00 | 0.00 | 0.33 (91) | 0.00 | 0.00 | |
| Charles M. Russell‐5 | 2013 | 0.30 (36) | 0.01 (1) | 0.00 | 0.00 | 0.00 | 0.62 (74) | 0.00 | 0.00 |
| 2014 | 2.76 (309) | 0.01 (1) | 0.01 (1) | 0.00 | 0.00 | 4.47 (501) | 0.00 | 0.00 | |
| 2015 | 3.64 (411) | 0.02 (2) | 0.00 | 0.00 | 0.00 | 2.92 (330) | 0.00 | 0.00 | |
| Texas BTPD | |||||||||
| Rita Blanca‐1 | 2013 | 5.10 (612) | 0.00 | 0.00 | 0.00 | 0.00 | 2.44 (293) | 0.00 | 0.00 |
| 2014 | 2.67 (155) | 0.00 | 0.00 | 0.00 | 0.00 | 3.90 (226) | 0.00 | 0.00 | |
| 2015 | 1.95 (37) | 0.00 | 0.00 | 0.00 | 0.00 | 8.11 (154) | 0.00 | 0.00 | |
| Rita Blanca‐2 | 2013 | 3.83 (356) | 0.00 | 0.00 | 0.00 | 0.00 | 3.27 (304) | 0.00 | 0.00 |
| 2014 | 3.58 (136) | 0.00 | 0.00 | 0.00 | 0.00 | 2.26 (86) | 0.00 | 0.00 | |
| 2015 | 2.36 (125) | 0.00 | 0.00 | 0.00 | 0.00 | 3.83 (203) | 0.00 | 0.00 | |
| Arizona GPD | |||||||||
| Espee Ranch | 2013 | 1.43 (153) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 6.14 (473) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 1.24 (87) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2016 | 2.56 (253) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Utah UPD | |||||||||
| Cedar City‐1 | 2013 | 1.29 (111) | 0.00 | 0.00 | 0.00 | 0.01 (1) | 0.00 | 0.00 | 0.00 |
| 2014 | 0.69 (40) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Cedar City‐2 | 2013 | 1.82 (162) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 1.37 (93) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 1.00 (70) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2016 | 1.40 (7) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Cedar City‐3 | 2013 | 0.94 (100) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2014 | 1.52 (79) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.70 (66) | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 (3) | 0.00 | 0.00 | |
| 2016 | 1.71 (48) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 (1) | 0.00 | |
| High elevation‐1 | 2013 | 0.00 | 0.05 (6) | 0.08 (9) | 0.00 | 0.85 (96) | 0.00 | 0.00 | 0.00 |
| 2014 | 0.00 | 0.08 (7) | 0.41 (38) | 0.00 | 2.86 (263) | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.00 | 0.00 | 0.49 (19) | 0.00 | 2.69 (105) | 0.00 | 0.00 | 0.00 | |
| 2016 | 0.00 | 0.30 (14) | 0.32 (15) | 0.00 | 1.09 (51) | 0.00 | 0.00 | 0.00 | |
| High elevation‐2 | 2013 | 0.00 | 0.64 (79) | 0.03 (4) | 0.00 | 0.65 (80) | 0.00 | 0.00 | 0.00 |
| 2014 | 0.00 | 0.43 (78) | 0.08 (15) | 0.01 (1) | 1.04 (189) | 0.00 | 0.01 (1) | 0.00 | |
| 2015 | 0.00 | 4.12 (140) | 1.38 (47) | 0.00 | 1.53 (52) | 0.00 | 0.00 | 0.00 | |
| 2016 | 0.00 | 3.97 (119) | 0.17 (5) | 0.00 | 0.63 (19) | 0.00 | 0.00 | 0.00 | |
| High elevation‐3 | 2013 | 0.00 | 0.34 (37) | 0.08 (9) | 0.00 | 1.13 (122) | 0.00 | 0.00 | 0.00 |
| 2014 | 0.00 | 0.30 (29) | 0.34 (33) | 0.01 (1) | 4.42 (424) | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.00 | 0.44 (37) | 0.26 (22) | 0.00 | 3.45 (290) | 0.00 | 0.00 | 0.00 | |
| 2016 | 0.00 | 1.75 (84) | 0.29 (14) | 0.02 (1) | 5.77 (277) | 0.00 | 0.00 | 0.00 | |
| High elevation‐4 | 2013 | 0.00 | 0.03 (2) | 0.02 (1) | 0.27 (17) | 0.05 (3) | 0.00 | 0.00 | 0.00 |
| 2014 | 0.00 | 0.00 | 0.11 (4) | 3.84 (142) | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.00 | 0.00 | 0.00 | 0.73 (69) | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2016 | 0.00 | 0.35 (7) | 0.05 (1) | 1.00 (20) | 0.35 (7) | 0.00 | 0.00 | 0.00 | |
| Utah WTPD | |||||||||
| Coyote Basin‐1 | 2013 | 0.36 (20) | 0.04 (2) | 0.00 | 0.00 | 0.00 | 0.05 (3) | 0.00 | 0.00 |
| 2014 | 0.83 (90) | 0.00 | 0.00 | 0.00 | 0.00 | 0.53 (57) | 0.00 | 0.00 | |
| 2015 | 4.86 (136) | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 (28) | 0.00 | 0.00 | |
| 2016 | 70.00 (70) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Coyote Basin‐2 | 2013 | 0.36 (26) | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 (1) | 0.00 | 0.00 |
| 2014 | 0.63 (60) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 4.92 (320) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2016 | 5.33 (16) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Wyoming WTPD | |||||||||
| Pitchfork Ranch‐1 | 2013 | 0.00 | 0.00 | 0.05 (7) | 0.05 (8) | 0.00 | 0.00 | 0.00 | 0.03 (4) |
| 2014 | 0.00 | 0.04 (5) | 0.01 (1) | 0.08 (10) | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.00 | 0.06 (7) | 0.15 (18) | 0.33 (39) | 0.00 | 0.00 | 0.00 | 0.06 (7) | |
| 2016 | 0.00 | 0.41 (30) | 0.47 (34) | 0.42 (31) | 0.00 | 0.00 | 0.00 | 0.07 (5) | |
| Pitchfork Ranch‐2 | 2013 | 0.00 | 0.01 (1) | 0.04 (6) | 0.21 (29) | 0.00 | 0.00 | 0.00 | 0.01 (1) |
| 2014 | 0.00 | 0.00 | 0.01 (1) | 0.05 (6) | 0.00 | 0.00 | 0.00 | 0.00 | |
| 2015 | 0.00 | 0.02 (3) | 0.26 (31) | 0.50 (60) | 0.00 | 0.00 | 0.00 | 0.01 (1) | |
| 2016 | 0.00 | 0.08 (5) | 0.28 (17) | 0.72 (44) | 0.00 | 0.00 | 0.00 | 0.10 (6) | |
APPENDIX 6.
Uncommon flea species identified on prairie dogs. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (C. leucurus), UPD is Utah prairie dog (C. parvidens), and GPD is Gunnison's prairie dog (C. gunnisoni). See Hubbard, 1947 for references to typical hosts
| Flea species | Typical host | Number of fleas | Site | Prairie dog species hosting flea |
|---|---|---|---|---|
| Catallagia sp. | Mouse | 1 | HEUT | UPD |
| Eumolpianus eumolpi | Chipmunk | 7 | HEUT | UPD |
| Foxella ignota | Pocket gopher | 1 | CCUT | UPD |
| Hoplopsyllus affinis | Rabbit | 3 | ERAZ | GPD |
| Hoplopsyllus anomalus | Ground squirrel | 6 | CCUT; HEUT; CBUT | UPD, WTPD |
| Hystrichopsylla dippiei | Mouse | 1 | PRWY | WTPD |
| Oropsylla bruneri | Ground squirrel | 3 | CMR | BTPD |
| Oropsylla montana | Ground squirrel | 1 | BGSD | BTPD |
| Rhadinopsylla sectilis goodi | Mouse, ground squirrel | 15 | HEUT; PRWY | UPD, WTPD |
| Thrassis stanfordi | Marmot, ground squirrel | 7 | HEUT | UPD |
APPENDIX 7.
Summary statistics of environmental factors associated with on‐host flea abundance on prairie dogs. Mean values of covariates by area. Standard deviations are in parentheses. BTPD is black‐tailed prairie dog (Cynomys ludovicianus), WTPD is white‐tailed prairie dog (C. leucurus), UPD is Utah prairie dog (C. parvidens), and GPD is Gunnison's prairie dog (C. gunnisoni). NDVI: Normalized difference vegetation index (see Table 1 for explanation).
| Study area and species | Flea load | NDVI | Catch per unit effort (animal/100 trap days) | Winter precipitation (cm) | Prior growing season precipitation (cm) | Days over 85°F | Elevation (m) |
|---|---|---|---|---|---|---|---|
| South Dakota BTPD | |||||||
| Buffalo Gap‐1 | 1.70 (3.12) | 5317.54 (584.82) | 31.63 (16.72) | 6.27 (4.15) | 28.49 (2.73) | 42.00 (6.99) | 827 |
| Buffalo Gap‐2 | 1.72 (2.96) | 5107.97 (956.53) | 30.07 (12.78) | 6.27 (4.15) | 28.49 (2.73) | 42.00 (6.99) | 822 |
| Lower Brule | 5.33 (8.06) | 4406.52 (471.8) | 13.3 (5.89) | 8.22 (4.64) | 33.06 (7.43) | 45.00 (11.74) | 545 |
| Wind Cave | 2.82 (6.05) | 5514.27 (582.37) | 19.79 (4.56) | 9.6 (1.92) | 37.35 (7.8) | 30.33 (6.28) | 1150 |
| Montana BTPD | |||||||
| Charles M. Russell‐1 | 1.59 (2.19) | 4083.27 (488.76) | 22.6 (11.00) | 5.23 (2.1) | 26.57 (7.82) | 43.25 (8.15) | 832 |
| Charles M. Russell‐2 | 1.52 (2.3) | 4038.79 (814.46) | 32.33 (22.16) | 5.23 (2.1) | 26.57 (7.82) | 43.25 (8.15) | 860 |
| Charles M. Russell‐3 | 2.81 (3.4) | 2627.61 (494.11) | 35.48 (13.54) | 5.23 (2.1) | 26.57 (7.82) | 43.25 (8.15) | 725 |
| Charles M. Russell‐4 | 2.02 (2.6) | 4442.67 (623.66) | 31.18 (9.39) | 5.23 (2.1) | 26.57 (7.82) | 43.25 (8.15) | 720 |
| Charles M. Russell‐5 | 4.84 (7.19) | 3252.76 (782.12) | 43.28 (15.36) | 4.19 (1.00) | 29.70 (6.21) | 45.67 (8.07) | 706 |
| Texas BTPD | |||||||
| Rita Blanca‐1 | 7.50 (5.9) | 2763.77 (225.61) | 29.05 (14.77) | 2.38 (1.23) | 19.30 (5.86) | 80.67 (9.48) | 1325 |
| Rita Blanca‐2 | 6.58 (3.46) | 2954.04 (566.05) | 28.56 (6.82) | 7.82 (3.59) | 28.10 (5.19) | 105.00 (4.1) | 1220 |
| Arizona GPD | |||||||
| Espee Ranch | 2.75 (4.38) | 1975.38 (397.05) | 10.34 (5.34) | 6.01 (2.33) | 16.45 (2.58) | 50.75 (3.81) | 1693 |
| Utah UPD | |||||||
| Cedar City‐1 | 1.06 (2.1) | 3672.56 (761.01) | 13.99 (5.54) | 7.34 (2.43) | 18.78 (3.09) | 70.00 (1.15) | 1741 |
| Cedar City‐2 | 1.43 (1.65) | 2440.6 (380.76) | 33.16 (35.78) | 8.59 (2.1) | 15.51 (4.53) | 71.25 (1.91) | 1805 |
| Cedar City‐3 | 1.07 (1.43) | 3799.53 (898.5) | 13.24 (9.33) | 6.81 (3.21) | 15.11 (2.54) | 40.00 (6.97) | 1940 |
| High elevation‐1 | 2.19 (6.34) | 2220.44 (472.26) | 7.48 (3.58) | 11.07 (3.59) | 19.66 (5.52) | 0 (0) | 2575 |
| High elevation‐2 | 2.36 (3.61) | 3259.92 (747.25) | 8.52 (4.71) | 18.57 (1.99) | 28.91 (3.04) | 0 (0) | 2797 |
| High elevation‐3 | 4.36 (6.84) | 3054.9 (412.94) | 11.57 (7.69) | 18.57 (1.99) | 28.91 (3.04) | 0 (0) | 2772 |
| High elevation‐4 | 1.33 (2.89) | 4045.04 (685.55) | 8.94 (6.00) | 18.57 (1.99) | 28.91 (3.04) | 0 (0) | 2864 |
| Utah WTPD | |||||||
| Coyote Basin‐1 | 2.12 (5.88) | 1989.68 (241.82) | 8.17 (7.77) | 7.62 (3.42) | 11.4 (7.07) | 67.43 (11.62) | 1627 |
| Coyote Basin‐2 | 1.79 (6.53) | 1729.71 (243.86) | 7.59 (5.93) | 8.38 (3.83) | 12.92 (7.84) | 68 (10.88) | 1672 |
| Wyoming WTPD | |||||||
| Pitchfork Ranch‐1 | 0.44 (1.10) | 3412.5 (626.96) | 14.33 (10.1) | 9.53 (1.19) | 22.45 (8.44) | 3.75 (2.66) | 2130 |
| Pitchfork Ranch‐2 | 0.48 (1.25) | 2921.04 (504.49) | 8.29 (5.63) | 9.53 (1.19) | 22.45 (8.44) | 3.75 (2.66) | 2087 |
Russell RE, Abbott RC, Tripp DW, Rocke TE. Local factors associated with on‐host flea distributions on prairie dog colonies. Ecol Evol. 2018;8:8951–8972. 10.1002/ece3.4390
REFERENCES
- Anderson, S. H. , & Williams, E. S. (1997). Plague in a complex of white‐tailed prairie dogs and associated small mammals in Wyoming. Journal of Wildlife Diseases, 33, 720–732. 10.7589/0090-3558-33.4.720 [DOI] [PubMed] [Google Scholar]
- Antolin, M. F. , Gober, P. , Luce, B. , Biggins, D. E. , Van Pelt, W. E. , Seery, D. B. , & Ball, M. L. (2002). The influence of sylvatic plague on North American wildlife at the landscape level, with special emphasis on black‐footed ferret and prairie dog conservation. Transactions of the North American Wildlife and Natural Resources Conference, 67, 104–127. [Google Scholar]
- Archuleta, C.M. , Constance, E.W. , Arundel, S.T. , Lowe, A. J. , Mantey, K. S. , & Phillips, L. A. (2017). The National Map seamless digital elevation model specifications: U.S. Geological Survey Techniques and Methods. 10.3133/tm11b9 [DOI]
- Bacot, A. W. , & Martin, C. J. (1914). LXVII. Observations on the mechanism of the transmission of plague by fleas. Journal of Hygiene, 13, 423–439. [PMC free article] [PubMed] [Google Scholar]
- Buhnerkempe, M. G. , Eisen, R. J. , Goodell, B. , Gage, K. L. , Antolin, M. F. , & Webb, C. T. (2011). Transmission shifts underlie variability in population responses to Yersinia pestis infection. PLoS ONE, 6, e22498 10.1371/journal.pone.0022498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh, D. C. (1971). Specific effect of temperature upon transmission of the plague bacillus by the Oriental rat flea, Xenopsylla cheopis . American Journal of Tropical Medicine and Hygiene, 20, 264–273. 10.4269/ajtmh.1971.20.264 [DOI] [PubMed] [Google Scholar]
- Collinge, S. K. , Johnson, W. C. , Ray, C. , Matchett, R. , Grensten, J. , Cully, J. F. Jr , … Martin, A. P. (2005). Landscape structure and plague occurrence in black‐tailed prairie dogs on grasslands of the Western USA. Landscape Ecology, 20, 941–955. 10.1007/s10980-005-4617-5 [DOI] [Google Scholar]
- Cully, J. F. , Barnes, A. M. , Quan, T. J. , & Maupin, G. (1997). Dynamics of plague in a Gunnison's prairie dog colony complex from New Mexico. Journal of Wildlife Diseases, 33, 706–719. 10.7589/0090-3558-33.4.706 [DOI] [PubMed] [Google Scholar]
- Cully, J. F. , & Williams, E. S. (2001). Interspecific comparisons of sylvatic plague in prairie dogs. Journal of Mammalogy, 82, 894–905. [DOI] [Google Scholar]
- Eads, D. A. , Biggins, D. E. , Doherty, P. F. Jr , Gage, K. L. , Huyvaert, K. P. , Long, D. H. , & Antolin, M. F. (2013). Using occupancy models to investigate the prevalence of ectoparasitic vectors on hosts: An example with fleas on prairie dogs. International Journal for Parasitology: Parasites and Wildlife, 2, 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads, D. A. , Biggins, D. E. , Long, D. H. , Gage, K. L. , & Antolin, M. F. (2016). Droughts may increase susceptibility of prairie dogs to fleas: Incongruity with hypothesized mechanisms of plague cycles in rodents. Journal of Mammalogy, 97, 1044–1053. 10.1093/jmammal/gyw035 [DOI] [Google Scholar]
- Eads, D. A. , & Hoogland, J. L. (2016). Factors that affect parasitism of black‐tailed prairie dogs by fleas. Ecosphere, 7, e01372 10.1002/ecs2.1372 [DOI] [Google Scholar]
- Eads, D. A. , & Hoogland, J. L. (2017). Precipitation, climate change, and parasitism of prairie dogs by fleas that transmit plague. Journal of Parasitology, 103, 309–319. 10.1645/16-195 [DOI] [PubMed] [Google Scholar]
- Eisen, R. J. , Bearden, S. W. , Wilder, A. P. , Montenieri, J. A. , Antolin, M. F. , & Gage, K. L. (2006). Early‐phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proceedings of the National Academy of Sciences, USA, 103, 15380–15385. 10.1073/pnas.0606831103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen, R. J. , Borchert, J. N. , Mpanga, J. T. , Atiku, L. A. , MacMillan, K. , Boegler, K. A. , & Gage, K. L. (2012). Flea diversity as an element for persistence of plague bacteria in an East African plague focus. PLoS ONE, 7, e35598 10.1371/journal.pone.0035598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enscore, R. E. , Biggerstaff, B. J. , Brown, T. L. , Fulgham, R. G. , Reynolds, P. J. , Engelthaler, D. M. , … Gage, K. L. (2002). Modeling relationship between climate and the frequency of human plague cases in the southwestern United States, 1960‐1977. American Journal Tropical Medical Hygiene, 66, 186–196. 10.4269/ajtmh.2002.66.186 [DOI] [PubMed] [Google Scholar]
- Furman, D. P. , & Catts, E. P. (1982). Order Siphonaptera Manual of medical entomology, 4th ed. (pp. 138–157). New York, NY: Cambridge University Press. [Google Scholar]
- Gage, K. L. (1998). Plague In Collier L., Balows A., & Sussman M. (Eds.), Topley and Wilson's microbiology and microbial infections, 9th ed. (pp. 885–906). New York, NY: Oxford University Press. [Google Scholar]
- Gelman, A. , Carlin, J. B. , Stern, H. S. , & Rubin, D. B. (2004). Bayesian data analysis. New York, NY: Chapman and Hall/CRC. [Google Scholar]
- Gelman, A. , & Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Statistical Science, 7, 457–511. 10.1214/ss/1177011136 [DOI] [Google Scholar]
- Hinnebusch, B. J. , Fischer, E. R. , & Schwan, T. G. (1998). Evaluation of the role of the Yersinia pestis plasminogen activator and other plasmid‐encoded factors in temperature‐dependent blockage of the flea. Journal of Infectious Diseases, 178, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Hubbard, C. A. (1947). The fleas of western North America. Ames, IA: Iowa State College Press. [Google Scholar]
- Hubbart, J. A. , Jachowski, D. S. , & Eads, D. A. (2011). Seasonal and among‐site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). Journal of Vector Ecology, 36, 117–123. 10.1111/j.1948-7134.2011.00148.x [DOI] [PubMed] [Google Scholar]
- Jenkerson, C. B. , Maiersperger, T. , & Schmidt, G. (2010). eMODIS: a user‐friendly data source. U.S. Geological Survey Open‐File Report 2010‐1055.
- Kartman, L. (1969). Effect of differences in ambient temperature upon the fate of Pasteurella pestis in Xenopsylla cheopis . Transactions of the Royal Society of Tropical Medicine and Hygiene, 63, 71–75. 10.1016/0035-9203(69)90068-6 [DOI] [PubMed] [Google Scholar]
- Kernif, T. , Leulmi, H. , Socolovschi, C. , Berenger, J. M. , Lepidi, H. , Bitam, I. , … Parola, P. (2014). Acquisition and excretion of Bartonella quintana by the cat flea, Ctenocephalides felis felis . Molecular Ecology, 23, 1204–1212. 10.1111/mec.12663 [DOI] [PubMed] [Google Scholar]
- Krasnov, B. R. , Khokholova, I. S. , Fielden, L. J. , & Burdelova, N. V. (2001). Effect of air temperature and humidity on the survival of pre‐imaginal stages of two flea species (Siphonaptera: Pulicidae). Journal of Medical Entomology, 38, 629–637. 10.1603/0022-2585-38.5.629 [DOI] [PubMed] [Google Scholar]
- La Scola, B. , Fournier, P. E. , Brouqui, P. , & Raoult, D. (2001). Detection and culture of Bartonella quintana, Serratia marcescens, and Acinetobacter spp. from decontaminated human body lice. Journal of Clinical Microbiology, 39, 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorange, E. A. , Race, B. L. , Sebbane, F. , & Hinnebusch, B. J. (2005). Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis . Journal of Infectious Diseases, 191, 1907–1912. 10.1086/429931 [DOI] [PubMed] [Google Scholar]
- Mize, E. L. , & Britten, H. B. (2016). Detections of Yersinia pestis east of the known distribution of active plague in the United States. Vector‐borne and Zoonotic Diseases, 16, 88–95. 10.1089/vbz.2015.1825 [DOI] [PubMed] [Google Scholar]
- Nakazawa, Y. , Williams, R. , Peterson, A. T. , Mead, P. , Staples, E. , & Gage, K. L. (2007). Climate change effects on plague and tularemia in the United States. Vector‐Borne and Zoonotic Diseases, 7, 529–540. 10.1089/vbz.2007.0125 [DOI] [PubMed] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Friendly, M. , Kindt, R. , Legendre, P. , McGlinn, D. , … Wagner, H. (2017). vegan: Community Ecology Package. R package version 2.4‐4. https://CRAN.R-project.org/package=vegan.
- Pauli, J. , Buskirk, S. , Williams, E. , & Edwards, W. (2006). A plague epizootic in the black‐tailed prairie dog (Cynomys ludovicianus). Journal of Wildlife Diseases, 42, 74–80. 10.7589/0090-3558-42.1.74 [DOI] [PubMed] [Google Scholar]
- Plummer, M. (2013). rjags: Bayesian graphical models using MCMC. R package version 3‐10. https://CRAN.R-project.org/package=rjags
- Pollitzer, R. (1954). Plague (p. 698). Geneva: World Health Organization. [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Raoult, D. , La Scola, B. , Enea, M. , Fournier, P. E. , Roux, V. , Fenollar, F. , … de Lamballerie, X. (2001). A flea‐associated Rickettsia pathogenic for humans. Emerging Infectious Diseases, 7, 73–81. 10.3201/eid0701.010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocke, T. E. , Tripp, D. W. , Russell, R. E. , Abbott, R. C. , Richgels, K. L. D. , Matchett, M. R. , … Miller, M. W. (2017). Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials. EcoHealth, 14, 438–450. 10.1007/s10393-017-1253-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld, D. J. , Padgett, K. A. , & Jones, J. H. (2013). A meta‐analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecology Letters, 5, 679–686. 10.1111/ele.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld, D. J. , & Stapp, P. (2008). Prevalence and abundance of fleas in black‐tailed prairie dog burrows: Implications for the transmission of plague (Yersinia pestis). Journal of Parasitology, 94, 616–621. 10.1645/GE-1368R.1 [DOI] [PubMed] [Google Scholar]
- Savage, L. T. , Reich, R. M. , Hartley, L. M. , Stapp, P. , & Antolin, M. F. (2011). Climate, soils, and connectivity predict plague epizootics in black‐tailed prairie dogs (Cynomys ludovicianus). Ecological Applications, 21, 2933–2943. 10.1890/10-1946.1 [DOI] [Google Scholar]
- Schotthoefer, A. M. , Bearden, S. W. , Vetter, S. M. , Holmes, J. , Montenieri, J. A. , Graham, C. B. , … Gage, K. L. (2011). Effects of temperature on early‐phase transmission of Yersinia pestis by the flea, Xenopsylla cheopis . Journal of Medical Entomology, 48, 411–417. 10.1603/ME10155 [DOI] [PubMed] [Google Scholar]
- Snäll, T. , O'Hara, R. B. , Ray, C. , & Collinge, S. K. (2008). Climate‐driven spatial dynamics of plague among prairie dog colonies. American Naturalist, 171, 238–248. 10.1086/525051 [DOI] [PubMed] [Google Scholar]
- Stapp, P. , Salkeld, D. J. , Franklin, H. A. , Kraft, J. P. , Tripp, D. W. , Antolin, M. F. , & Gage, K. L. (2009). Evidence for the involvement of an alternate rodent host in the dynamics of introduced plague in prairie dogs. Journal of Animal Ecology, 78, 807–817. 10.1111/j.1365-2656.2009.01541.x [DOI] [PubMed] [Google Scholar]
- Stark, H.E. (1970). A revision of the flea genus Thrassis Jordan, 1933 (Siphonaptera: Ceratophyllidae), with observations on the ecology and relationship to plague. University of California Publications in Entomology, 53, l–184. [Google Scholar]
- Stenseth, N. C. , Samia, N. I. , Viljugrein, H. , Kausrud, K. L. , Begon, M. , Davis, S. , … Chan, K. (2006). Plague dynamics are driven by climate variation. Proceedings of the National Academy of Science, 103, 13110–13115. 10.1073/pnas.0602447103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani, R. (1996). Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society, Series B (Methodological), 58, 267–288. [Google Scholar]
- Tripp, D. W. , Gage, K. L. , Montenieri, J. A. , & Antolin, M. F. (2009). Flea abundance on black‐tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics. Vector‐Borne and Zoonotic Diseases, 9, 313–321. 10.1089/vbz.2008.0194 [DOI] [PubMed] [Google Scholar]
- Tripp, D. W. , Streich, S. P. , Sack, D. A. , Martin, D. J. , Griffin, K. A. , & Miller, M. W. (2016). Season of deltamethrin application affects flea and plague control in white‐tailed prairie dog (Cynomys leucurus) colonies, Colorado, USA. Journal of Wildlife Diseases, 52, 553–561. 10.7589/2015-10-290 [DOI] [PubMed] [Google Scholar]
- Xavier, R. , Turck, N. , Hainard, A. , Tiberti, N. , Lisacek, F. , Sanchez, J. C. , & Müller, M. (2011). pROC: An open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics, 7, 77 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Schmid, B. V. , Liu, J. , Si, X. , Stenseth, N. C. , & Zhang, Z. (2015). The trophic responses of two different rodent–vector–plague systems to climate change. Proceedings Royal Society B, 282, 20141846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at https://doi.org/10.5066/f7tm79ck.


