Abstract
With climate change leading to poleward range expansion of species, populations are exposed to new daylength regimes along latitudinal gradients. Daylength is a major factor affecting insect life cycles and activity patterns, so a range shift leading to new daylength regimes is likely to affect population dynamics and species interactions; however, the impact of daylength in isolation on ecological communities has not been studied so far. Here, we tested for the direct and indirect effects of two different daylengths on the dynamics of experimental multitrophic insect communities. We compared the community dynamics under “southern” summer conditions of 14.5‐hr daylight to “northern” summer conditions of 22‐hr daylight. We show that food web dynamics indeed respond to daylength with one aphid species (Acyrthosiphon pisum) reaching much lower population sizes at the northern daylength regime compared to under southern conditions. In contrast, in the same communities, another aphid species (Megoura viciae) reached higher population densities under northern conditions. This effect at the aphid level was driven by an indirect effect of daylength causing a change in competitive interaction strengths, with the different aphid species being more competitive at different daylength regimes. Additionally, increasing daylength also increased growth rates in M. viciae making it more competitive under summer long days. As such, the shift in daylength affected aphid population sizes by both direct and indirect effects, propagating through species interactions. However, contrary to expectations, parasitoids were not affected by daylength. Our results demonstrate that range expansion of whole communities due to climate change can indeed change interaction strengths between species within ecological communities with consequences for community dynamics. This study provides the first evidence of daylength affecting community dynamics, which could not be predicted from studying single species separately.
Keywords: aphid, climate change, parasitoid, photoperiod, population dynamics, range expansion
1. INTRODUCTION
Climate change has led to an increase in global temperatures (Hansen, Sato, Ruedy, Schmidt, & Lo, 2016), which is predicted to continue, with a projected increase in the mean global surface air temperature of 3.0°C by the end of the 21st Century (2071–2100), relative to the period between 1961 and 1990 (Flato et al., 2013; Houghton et al., 2001). The increase in global temperatures is causing a change in species ranges; a meta‐analysis with data consisting of 1,367 species from a wide variety of taxa showed poleward range shifts and expansions of between 12.2 and 91.1 km per decade (Chen, Hill, Ohlemüller, Roy, & Thomas, 2011).
While a poleward range shift allows populations to track climatic conditions, it also causes organisms to be exposed to other environmental conditions that do not match those within the original range. A key example of this is the daylength regime, with a poleward shift extending both summer days and winter nights and increasing the rate of daylength change in spring and autumn. Photoperiod drives many aspects of life history and activity patterns of temperate organisms (Beck, 2012; Vaartaja, 1959; Withrow, 1959) and thereby has the potential to affect population dynamics and species interactions.
Insects use photoperiod to a great degree as a cue to induce seasonal changes, for example in the induction of diapause (Adkisson, Bell, & Wellso, 1963; Ruberson, Bush, & Kring, 1991), as well as its termination (Tauber & Tauber, 1976), with these reactions dependent on geographic location (Lankinen, 1986), and in interaction with temperature (Liefting, Cosijn, & Ellers, 2017; Saunders, 1973). Some species have been shown to use photoperiod to influence egg morphology (Wardhaugh, 1977) while others use it to determine number of molts (Ingram & Jenner, 1976). Daylength has also been shown to have an impact on insect growth rate (Kamm, 1972), as well as development rate (Fisher, Higley, & Foster, 2015), fecundity (Nissinen, Pinto‐Zevallos, Jauhiainen, & Vänninen, 2017), and the regulation of insect seasonal development in nature (Abrams, Leimar, Nylin, & Wiklund, 1996; Danilevskii, 1965). However, there is currently a lack of studies investigating how photoperiod affects communities.
All these factors are likely to affect the interactions between species that drive ecological and evolutionary processes in ecosystems (Thompson, 1999) and are important for ecosystem stability (de Ruiter, Neutel, & Moore, 1995; Thébault & Fontaine, 2010). As species are interconnected within networks of interactions (Bukovinszky, van Veen, Jongema, & Dicke, 2008; van Veen, Memmott, & Godfray, 2006), a perturbation affecting one single species can therefore lead to community‐wide impacts, see Rosenblatt and Schmitz (2016) for a conceptual framework of the direct and indirect effects of climate change on a food web. For example, the harvesting of a single parasitoid species led to a community‐wide extinction cascade in a recent experiment, an effect that was transmitted indirectly via competition at the herbivore level (Sanders, Kehoe, & van Veen, 2015). Similarly, removing predators from an intertidal system led to extinctions of algae species through indirect interactions (Donohue et al., 2017). This demonstrates the importance of indirect as well as direct interactions for community stability. Intriguingly, it has also been shown that photoperiod disruption from artificial light at night can alter multitrophic insect community dynamics (Sanders, Kehoe, Tiley, et al., 2015).
Aphids are sap‐feeding herbivorous insects. Many are major pest species, especially when acting as vectors for plant viruses, causing critical damage to agricultural crops (Dedryver, Le Ralec, & Fabre, 2010). Their population and community dynamics have been studied extensively, including in the context of indirect species interactions (Hassell, 2000; Kaiser‐Bunbury & Müller, 2009; Müller & Godfray, 1999; Sanders, Sutter, & Veen, 2013; Sanders, Kehoe, & van Veen, 2015; Snyder & Ives, 2001) as well as climate change (Forrest, 2016). Aphids and aphid parasitoids are therefore an ideal model system to study population dynamics and species interactions in a community context as the system is very tractable and the generation times are short (Sanders, Kehoe, Tiley, et al., 2015), allowing for the observation of parasitoid‐host interactions across a multigenerational time frame.
Here, we study for the first time, the effects of daylength on the dynamics of multitrophic communities, while keeping other factors such as temperature and the rate of change in daylength constant to test for the impact of short and long daylength in isolation. We focus in particular on the effects during summer conditions, when populations of aphids reach their greatest pest potential. In our experiments, we used a simple host‐parasitoid community consisting of two aphid species that compete for a single host plant species and a parasitoid that attacks one of the aphid species (Figure 1b). We hypothesized that longer daylength, associated with a poleward range shift, would increase the attack rate by the diurnal parasitoid and that this would (a) negatively affect the host aphid population and, through reduced interspecific competition, (b) positively affect the other aphid species. We show that while the host‐parasitoid interaction was not affected by daylength, we discovered that the competitive strength of the two aphid species changed with daylength resulting in higher Megoura viciae abundance under long days.
Figure 1.
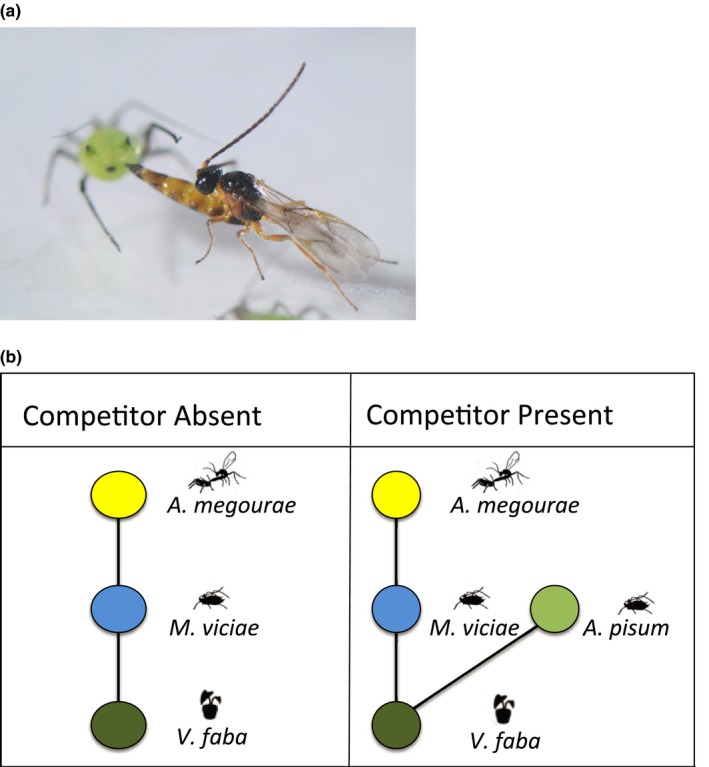
(a) Aphidius megourae attacking Megoura viciae. (b) Food web structure for experimental “Competitor Absent” and “Competitor Present” communities [Colour figure can be viewed at http://wileyonlinelibrary.com]
2. MATERIALS AND METHODS
2.1. Study system
The study system consisted of broad bean plants (Vicia faba, L., var. the Sutton), which supported two aphid species, M. viciae (Buckton) and Acyrthosiphon pisum (Haliday), and the parasitoid Aphidius megourae attacking the aphid M. viciae (Figure 1a).
2.2. Food web experiment
We used eight climate chambers (Percival Model 1‐30vl) programmed to constant 22°C and 75% humidity. The temperature was kept constant so as to enable the separation of daylength from any confounding impact of temperature, which has been shown to be linked to photoperiod, see Fischer et al. (2012). To test for the effect of daylength on aphid‐parasitoid communities, four chambers produced a day–night cycle of 14.5–9.5 hr (Southern) (depicting Marseille, France, 43°N, average daylength for the 9 weeks either side of the summer solstice), while the other four units produced a 22–2‐hr day–night cycle (Northern) (replicating Mosjoen, Norway, 65°N for the same time period). These locations were used to provide two distinct conditions for summer days, and daylength was kept constant in order to test for daylength per se and not the rate of daylength change. The intensity of the light within the incubators during “daylight” hours was recorded at 4,239 lux, equivalent to a typical overcast day (Gaston, Bennie, Davies, & Hopkins, 2013). We established two different communities with the aphid A. pisum either included “Competitor Present” or excluded “Competitor Absent.” This extended community allows for resource competition (Holt, 1977) between the two aphids and the potential for indirect interactions among the insects. Within each chamber were four cages, two consisting of the “Competitor Absent” community, with the other two consisting of the “Competitor Present” community, thus giving four treatments, each replicated eight times (see Figure 1). These cages were 35 cm × 24 cm × 20 cm and were constructed of untreated timber and thrip net with a mesh size of 0.29 mm × 0.8 mm, each with four 15 cm diameter pots containing a single broad bean plant in Melcourt All‐purpose Peat Free Compost.
All insects used in this experiment were taken from laboratory stock cultures, reared on broad bean plants at a temperature of 18°C and at a 16:8 day:night regime, for a number of years, and were kept at low insect densities. We tested for a difference in the growth rate of aphids under different daylength regimes from these stock cultures to those reared for three generations at the short daylength regime and found no difference for growth under short and long days (Supporting Information Figure S1). There was no impact of the origin of either species of aphids on their growth rate (M. viciae GLM Offspring number ~ Origin, mean = 13.371, T = 0.499, p = 0.621, A. pisum GLM Offspring number ~ Origin, mean = 18.361, T = −1.234, p = 0.226).
To establish the replicate insect communities, in week 1, five parthenogenetically reproducing adults of each aphid species (dependent on the treatment) were placed onto four 2‐week old broad bean plants and set into the climate chambers. At week 4, once aphid numbers had grown large enough to support an additional trophic level, two female, mated parasitoids of A. megourae were introduced to each cage with a further two added at week 5. This double introduction allowed for continuous production of parasitoids throughout the experiment. The numbers of both aphids and parasitoid mummies, the latter depicting a successful attack on aphids, were recorded. This count was repeated weekly over a 9‐week period, equivalent to 9–10 aphid generations. Plants were watered every second day throughout the experiment, with the oldest plants in each cage being replaced weekly with 2‐week‐old plants in order to ensure a continual food source for the aphids, while keeping all organisms in the cage. This method has been shown in Sanders et al. (2013). The cages were rotated within and between incubators of the same treatments weekly in a block design to account for a potential incubator bias.
2.3. Competition experiment
In order to explain the effects of the main experiment, we set up an additional competition experiment using three aphid combinations; A. pisum only, M. viciae only and a combination of two species. Two adult aphids of each species, depending on the species combination, were placed onto a 2‐week‐old broad bean plant over which a breathable bag was placed and secured with a rubber band. These were then placed into an incubator at photoperiods of either 14.5:9.5 or 22:2, at 22°C. The number of aphids was counted weekly for 3 weeks. Each treatment was replicated 10 times.
2.4. Statistical analysis
2.4.1. Food web experiment
Aspects of aphid and parasitoid population dynamics were analyzed using generalized linear models (GLM) with daylength treatment and community as explanatory variables. We used the following response variables:
Log‐transformed cumulative abundance (for each species, the total number of individuals for each cage over the length of the experiment), with Gaussian error structure.
Peak abundance. This is an ecologically important population measure for pest insects. This was measured as the maximum population size of each species at any point during the experiment and was analyzed using a GLM with Gaussian error structure. The data for M. viciae and A. pisum were normally distributed, whereas data for A. megourae were log transformed, to improve fit to a normal distribution.
Parasitism rate (proportion of hosts parasitised). This was analyzed using a GLM with a quasibinomial error structure. The response variable included the parasitized and nonparasitized aphid numbers per cage (using “cbind” in R).
Aphid population growth rate. This was analyzed using a GLM with a quasiPoisson error structure. Growth rate was calculated as daily increase in aphid number per cage between week 2 and week 4 (week 4 number − week 2 number, then divided by 14). These points were chosen as by that time there was no impact of parasitism on aphid numbers before week 4.
2.4.2. Competition experiment
The impact of treatment (four treatments: 14.5 single, 14.5 competitor present, 22 single, 22 competitor present) on aphid cumulative numbers were tested using linear models based on generalized least squares (errors are allowed to have unequal variances) provided by the nlme package (Pinheiro et al., 2017). We used VarIdent to account for variance heterogeneity in effect sizes between treatment groups. This test was replicated for both A. pisum and M. viciae. A Tukey's comparison was then used as a post hoc test for between treatment contrasts.
Throughout, best fitting models were chosen using AIC model selection (Akaike, 1998). Models for all analyses were visually checked for homoscedasticity and normality of the residuals, and all fulfilled the assumptions. All statistical analyses were computed using R version 3.2.1 (R Development Core Team, 2015).
3. RESULTS
3.1. Cumulative aphid abundance
The aphid M. viciae was not affected by daylength in the absence of the competitor A. pisum, but in its presence M. viciae densities were 32% higher in the Northern compared to the Southern treatment with A. pisum present (Figure 2, GLM Community × Daylength t = −2.09 (1, 28), p = 0.0495).
Figure 2.
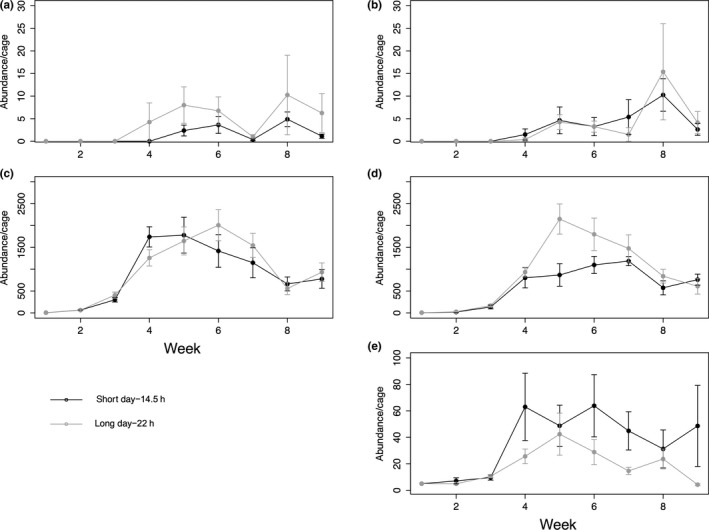
Population dynamics of all species. Sub plots (a and b) depict the parasitoid Aphidius megourae, (c and d) the aphid Megoura viciae and (e) the aphid Acyrthosiphon pisum. “a” and “c” show dynamics of the “Competitor Absent” community, while “b,” “d” and “e” depict the “Competitor Present” community. Black lines show Southern treatments, with gray lines showing Northern treatments. Error bars indicate standard error
Acyrthosiphon pisum was negatively affected by a longer daylength, with populations 50% smaller compared to the Southern treatment (t = 2.21 (1, 14), p = 0.03).
Neither community nor daylength affected the abundance of the parasitoid A. megourae (Daylength GLM t = −0.715 (1, 30), p = 0.481) (Community GLM t = 0.78 (1, 29) p = 0.44), see Figure 2.
3.2. Peak aphid abundance
Peak abundances of M. viciae were not affected by daylength in the absence of the competitor but in the presence of the competitor, the Northern treatment lead to 56% higher abundances than the Southern Treatment with A. pisum present (GLM Community × Daylength t = −3.32 (1, 28) p = 0.003). The peak densities of both the aphid A. pisum (Treatment GLM t = 1.52 (1, 16), p = 0.15) and the parasitoid A. megourae (Treatment t = −0.53 (1, 30), p = 0.6, community t = 1.12 (1, 29), p = 0.27) were not affected by daylength, see Figure 3.
Figure 3.
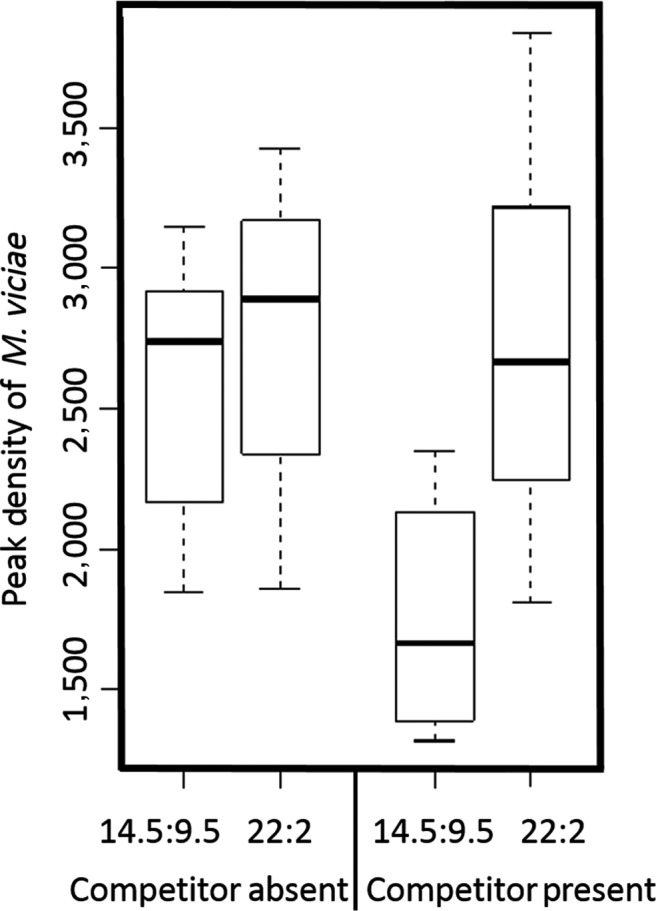
Peak density of Megoura viciae, divided into “Competitor Absent” community and “Competitor Present” community, and then subdivided into long and short daylength treatments
3.3. Parasitism rate
Parasitism rate of the aphid M. viciae by the parasitoid A. megourae was not affected by daylength or the presence of competitor (Treatment [Daylength, Community] GLM t = −0.56 (1, 30), p = 0.6).
3.4. Aphid population growth rate
The population growth rate of M. viciae was reduced by 72% by the presence of the competitor, A. pisum (t = −2.90 (1, 29) p = 0.007), with no effect of daylength (t = −0.85 (1, 30) p = 0.40), or interaction between daylength and community (t = 1.352 (1, 31), p = 0.187). Daylength regime did not affect the population growth rate of A. pisum (t = −1.51 (1, 14), p = 0.15).
3.5. Competition experiment
In an additional experiment, we tested whether aphid growth would be affected by the interplay between daylength treatment and competition between aphids in the absence of parasitoids. Megoura viciae numbers were indeed reduced when competing with A. pisum under short daylength but not long days (see Figure 4). Megoura viciae abundance declined by 86% in the presence of A. pisum under short day conditions (z = −5.35 p < 0.001). Interestingly, the opposite pattern was observed for A. pisum, with its densities being strongly negatively affected with a reduction by 81% in the presence of M. viciae under long day condition (see Figure 4, z = −2.65 p = 0.036). Megoura viciae densities were also higher under 22 than 14.5 daylength indicating that in isolation M. viciae grows better under longer (Northern) summer days (see Figure 4, t = 4.03 (1, 10), p = 0.002).
Figure 4.
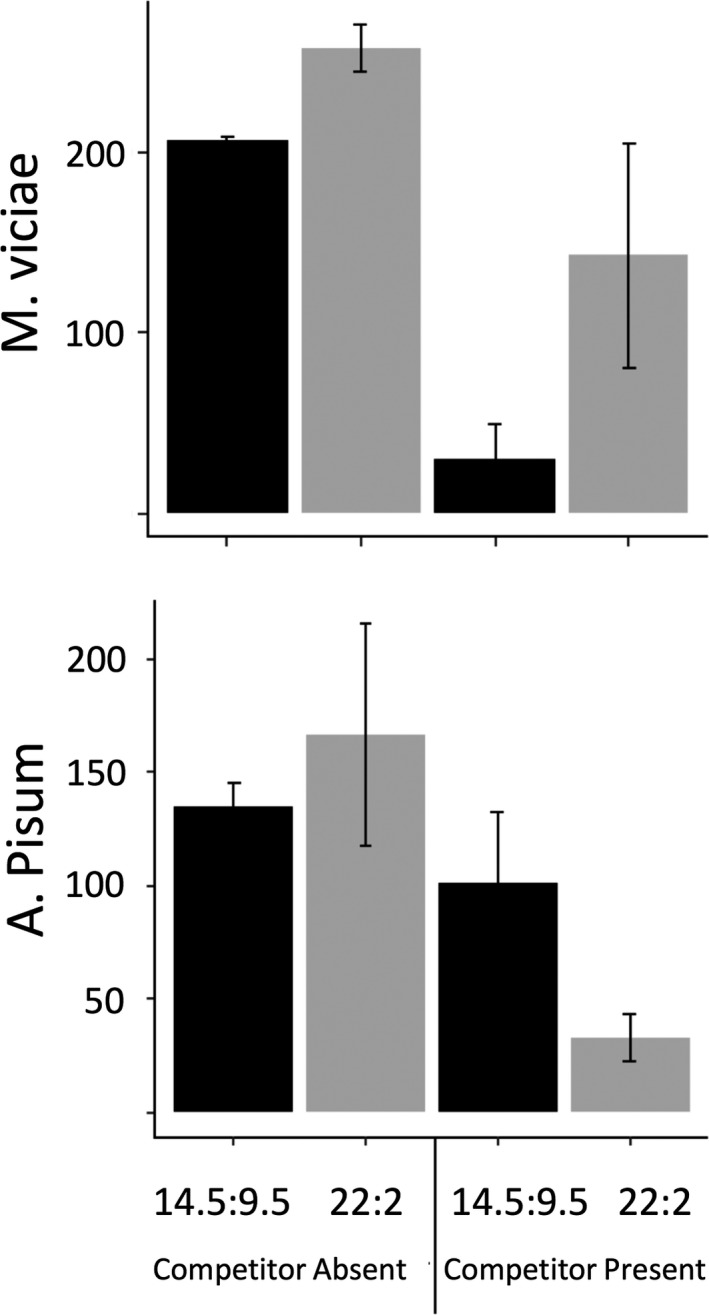
The mean cumulative density and standard error of (a) Megoura viciae and (b) Acyrthosiphon pisum in long (22:2) and short (14.5:9.5) daylengths, with and without a competitor. Black bars depict short daylengths, and gray long daylengths
4. DISCUSSION
We expected that longer daylength would increase parasitoid attack rate, which would in turn (a) negatively affect the host aphid population and, through reduced interspecific competition, (b) positively affect the other aphid species. We did not observe this but instead found a decrease in cumulative abundance of the aphid A. pisum under Northern conditions, coupled with an increase in cumulative and peak abundances of the aphid M. viciae. However, M. viciae did not respond to daylength when it was the only aphid species present in the food web experiment. This shows that an increase in summer daylength, associated with a poleward range shift, has an indirect positive impact on one pest species due to reduced competition from another that is negatively affected by increased daylength. Interestingly, the competition experiment demonstrated that the competitive dominance between the two aphids species switched with daylength. Megoura viciae is the dominant competitor under long days while it suffers more from competition with A. pisum under short days. This explains the outcome in the food web experiment, with M. viciae profiting from longer days under Northern conditions. This effect appears to be driven by different growth rates of M. viciae under the different daylength regimes as shown in the competition experiment. This effect was not visible in the food web experiment, maybe due to the more complex setting of the experiment.
One might expect that the population growth of, essentially sessile, sap‐feeding insects, such as aphids, will mostly be affected by changes in photosynthesis of their host plant which will determine resource availability, with aphid reproduction rate depending on the growth stage of its host plant (Watt, 1979), as well as the plant's degree of water stress (Simpson, Jackson, & Grace, 2012). Photosynthesis is highly dependent on photoperiod, with photosynthetic activity increasing with increasing photoperiod (Bauerle et al., 2012). The increase in growth rate for M. viciae in the competition experiment supports this effect in our experiments. However, this evidence that longer summer days had positive effects either on the early population growth rate of aphids was not found in the food web experiment. In fact, a negative effect was observed for A. pisum cumulative abundance, reflecting sustained differences between the treatments over at least three generations (Figure 2e). Photoperiod has been shown to affect individual growth rate and body size for a number of insects, a response that may or may not be adaptive (Gotthard, Nylin, & Wiklund, 1999; Margraf, Gotthard, & Rahier, 2003; Shama & Robinson, 2006) and we suggest that A. pisum and M. viciae were indeed affected by daylength but with very different outcomes, which is intriguing because the two species are ecologically and phylogenetically similar.
Our prediction that longer days would lead to increased top‐down control of aphids by parasitoids due to extended activity patterns had a number of underlying assumptions. First of these is that parasitoids are time‐limited rather than egg‐limited, or, in other words, that the number of hosts a female parasitoid parasitises is limited by the number of hosts that she encounters (Henri & Van Veen, 2011). It is not unlikely that in the confines of our experimental cages, with high densities of aphids, the female parasitoids encountered a sufficient number of hosts for all their eggs even in the shorter day. Further research is required under realistic field conditions in which host encounter rates will be lower to test the effect of changes in photoperiod on parasitoid efficiency. Our second assumption was that increased parasitoid attack rate would lead to increased parasitoid population growth and increased parasitism rate of the host aphid. It is, however, possible that higher attack rates lead to reduced parasitoid lifespan (Werner & Anholt, 1993) so that there is overall little net effect on the parasitoid population growth. It should also be noted that the parasitoid populations in the experiment remained relatively low despite the abundance of hosts. This indicates that larval survival of the parasitoids may have been low due to the competitive inferiority of parasitized aphids compared to unparasitised aphids under crowded conditions (Ives & Settle, 1996). This may have further weakened the effect of a change in attack rate on the numerical response of the aphid population. Again, this effect is likely to be less important under natural conditions because of nonuniform host distributions and therefore greater variation in intraspecific competition in the population.
Another mechanism by which the parasitoid A. megourae might have impacted upon the community is through their reluctance to parasitise aphids in unlit periods (Sanders, Kehoe, Cruse, van Veen, & Gaston, 2018), as well as the disruptive effect of nonhosts in the community reducing parasitism rate (Kehoe et al., 2016). Both of these mechanisms, however, do not explain the direction of the interaction, and as such we can conclude that these effects were overwhelmed by bottom‐up effects.
Understanding of how ecosystems do and will respond to climate change and associated range expansion of species needs to take into account that shifts in day–night regimes can trigger significant changes in species interactions. Our study was limited to summer conditions and it is likely that a year‐round perspective that includes key life‐cycle stages such as diapause would reveal further effects on insect community dynamics. The responses of agricultural pests to climate change remains one of the main unknown factors in the ability to predict crop productivity under future climate scenarios (Gornall et al., 2010; War, Taggar, War, & Hussain, 2016), although see (Gebauer, Hemerik, & Meyhöfer, 2015). With crop plants already grown outside of their natural range, the range expansion of any insects using them as a host plant will be instantaneous, as they do not require the expansion of their host plant range. As our study shows, species responses should not be studied in isolation but should be considered in the context of communities of interacting species, taking into account the change in abiotic factors such as photoperiod as well as evolutionary processes associated with poleward range shifts and expansions.
CONFLICT OF INTEREST
None declared.
AUTHORS’ CONTRIBUTIONS
FvV and KG conceived the ideas; FvV, KG, and DS designed methodology; DC collected the data; RK analyzed the data; RK, DS, and FvV led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
DATA ACCESSIBILITY
Data available from https://figshare.com/s/bdd2951343e341820014.
Supporting information
ACKNOWLEDGMENTS
The research leading to this paper was supported by NERC grant NE/N001672/1 and a studentship award to RK from the NERC GW4+ Doctoral Training Partnership.
Kehoe RC, Cruse D, Sanders D, Gaston KJ, van Veen FJF. Shifting daylength regimes associated with range shifts alter aphid‐parasitoid community dynamics. Ecol Evol. 2018;8:8761–8769. 10.1002/ece3.4401
REFERENCES
- Abrams, P. A. , Leimar, O. , Nylin, S. , & Wiklund, C. (1996). The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. The American Naturalist, 147, 381–395. 10.1086/285857 [DOI] [Google Scholar]
- Adkisson, P. L. , Bell, R. , & Wellso, S. (1963). Environmental factors controlling the induction of diapause in the pink bollworm, Pectinophora gossypiella (Saunders). Journal of Insect Physiology, 9, 299–310. 10.1016/0022-1910(63)90107-0 [DOI] [Google Scholar]
- Akaike, H. (1998). Information theory and an extension of the maximum likelihood principle Selected papers of Hirotugu Akaike (pp. 199–213). Springer; 10.1007/978-1-4612-1694-0 [DOI] [Google Scholar]
- Bauerle, W. L. , Oren, R. , Way, D. A. , Qian, S. S. , Stoy, P. C. , Thornton, P. E. , … Reynolds, R. F. (2012). Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proceedings of the National Academy of Sciences of the United States of America, 109, 8612–8617. 10.1073/pnas.1119131109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, S. D. (2012). Insect photoperiodism. Academic Press Inc (London) LDT: Elsevier. [Google Scholar]
- Bukovinszky, T. , van Veen, F. F. , Jongema, Y. , & Dicke, M. (2008). Direct and indirect effects of resource quality on food web structure. Science, 319, 804–807. 10.1126/science.1148310 [DOI] [PubMed] [Google Scholar]
- Chen, I.‐C. , Hill, J. K. , Ohlemüller, R. , Roy, D. B. , & Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science, 333, 1024–1026. 10.1126/science.1206432 [DOI] [PubMed] [Google Scholar]
- Danilevskii, A. S. (1965). Photoperiodism and seasonal development of insects. Edinburugh, c., Oliver Boyd Ltd: Edinburugh; [Google Scholar]
- de Ruiter, P. C. , Neutel, A.‐M. , & Moore, J. C. (1995). Energetics, patterns of interaction strengths, and stability in real ecosystems. Science, 269, 1257 10.1126/science.269.5228.1257 [DOI] [PubMed] [Google Scholar]
- Dedryver, C.‐A. , Le Ralec, A. , & Fabre, F. (2010). The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biologies, 333, 539–553. 10.1016/j.crvi.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Donohue, I. , Petchey, O. L. , Kéfi, S. , Génin, A. , Jackson, A. L. , Yang, Q. , & O'Connor, N. E. (2017). Loss of predator species, not intermediate consumers, triggers rapid and dramatic extinction cascades. Global Change Biology, 23, 2962–2972. 10.1111/gcb.13703 [DOI] [PubMed] [Google Scholar]
- Fischer, K. , Liniek, S. , Bauer, M. , Baumann, B. , Richter, S. , & Dierks, A. (2012). Phenotypic plasticity in temperature stress resistance is triggered by photoperiod in a fly. Evolutionary Ecology, 26, 1067–1083. 10.1007/s10682-011-9547-x [DOI] [Google Scholar]
- Fisher, M. , Higley, L. G. , & Foster, J. E. (2015). The influence of photoperiod on development rates of three species of forensically important blow flies. Journal of Insect Science, 15, 153 10.1093/jisesa/iev133 [DOI] [Google Scholar]
- Flato, G. , Marotzke, J. , Abiodun, B. , Braconnot, P. , Chou, S. C. , Collins, W. J. , … Eyring, V. (2013). Evaluation of climate models. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change, 5, 741–866. [Google Scholar]
- Forrest, J. R. (2016). Complex responses of insect phenology to climate change. Current Opinion in Insect Science, 17, 49–54. 10.1016/j.cois.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Gaston, K. J. , Bennie, J. , Davies, T. W. , & Hopkins, J. (2013). The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biological Reviews, 88, 912–927. 10.1111/brv.12036 [DOI] [PubMed] [Google Scholar]
- Gebauer, K. , Hemerik, L. , & Meyhöfer, R. (2015). Effects of climate change on pest‐parasitoid dynamics: Development of a simulation model and first results. Journal of Plant Diseases and Protection, 122, 28–35. 10.1007/BF03356527 [DOI] [Google Scholar]
- Gornall, J. , Betts, R. , Burke, E. , Clark, R. , Camp, J. , Willett, K. , & Wiltshire, A. (2010). Implications of climate change for agricultural productivity in the early twenty‐first century. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365, 2973–2989. 10.1098/rstb.2010.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard, K. , Nylin, S. , & Wiklund, C. (1999). Seasonal plasticity in two satyrine butterflies: State‐dependent decision making in relation to daylength. Oikos, 84, 453–462. 10.2307/3546424 [DOI] [Google Scholar]
- Hansen, J. , Sato, M. , Ruedy, R. , Schmidt, G. A. , & Lo, K. (2016). Global temperature in 2015. GISS, NASA, NY: URL: http://data.giss.nasa.gov [Google Scholar]
- Hassell, M. (2000). Host–parasitoid population dynamics. Journal of Animal Ecology, 69, 543–566. 10.1046/j.1365-2656.2000.00445.x [DOI] [PubMed] [Google Scholar]
- Henri, D. C. , & Van Veen, F. (2011). Body size, life history and the structure of host‐parasitoid networks. Advances in Ecological Research, 45, 135–180. 10.1016/B978-0-12-386475-8.00004-6 [DOI] [Google Scholar]
- Holt, R. D. (1977). Predation, apparent competition, and the structure of prey communities. Theoretical Population Biology, 12, 197–229. 10.1016/0040-5809(77)90042-9 [DOI] [PubMed] [Google Scholar]
- Houghton, J. T. , Ding, Y. , Griggs, D. J. , Noguer, M. , van der Linden, P. J. , Dai, X. , … Johnson, C. (2001). Climate change 2001: The scientific basis. Contribution of Working Group I to the third assessment report of the Intergovernmental Panel of Climate Change: 881.
- Ingram, B. R. , & Jenner, C. E. (1976). Influence of photoperiod and temperature on developmental time and number of molts in nymphs of two species of Odonata. Canadian Journal of Zoology, 54, 2033–2045. 10.1139/z76-237 [DOI] [Google Scholar]
- Ives, A. R. , & Settle, W. H. (1996). The failure of a parasitoid to persist with a superabundant host: The importance of the numerical response. Oikos, 75, 269–278. 10.2307/3546250 [DOI] [Google Scholar]
- Kaiser‐Bunbury, C. N. , & Müller, C. B. (2009). Indirect interactions between invasive and native plants via pollinators. Naturwissenschaften, 96, 339–346. 10.1007/s00114-008-0481-x [DOI] [PubMed] [Google Scholar]
- Kamm, J. A. (1972). Photoperiodic regulation of growth in an insect: Response to progressive changes in daylength. Journal of Insect Physiology, 18, 1745–1749. 10.1016/0022-1910(72)90105-9 [DOI] [Google Scholar]
- Kehoe, R. , Frago, E. , Barten, C. , Jecker, F. , Veen, F. , & Sanders, D. (2016). Nonhost diversity and density reduce the strength of parasitoid–host interactions. Ecology and Evolution, 6, 4041–4049. 10.1002/ece3.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen, P. (1986). Geographical variation in circadian eclosion rhythm and photoperiodic adult diapause in Drosophila littoralis . Journal of Comparative Physiology A, 159, 123–142. 10.1007/BF00612503 [DOI] [Google Scholar]
- Liefting, M. , Cosijn, J. , & Ellers, J. (2017). Synergistic effect of daily temperature fluctuations and matching light‐dark cycle enhances population growth and synchronizes oviposition behavior in a soil arthropod. Journal of Insect Physiology, 96, 108–114. 10.1016/j.jinsphys.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Margraf, N. , Gotthard, K. , & Rahier, M. (2003). The growth strategy of an alpine beetle: Maximization or individual growth adjustment in relation to seasonal time horizons? Functional Ecology, 17, 605–610. 10.1046/j.1365-2435.2003.00775.x [DOI] [Google Scholar]
- Müller, C. B. , & Godfray, H. C. J. (1999). Indirect interactions in aphid–parasitoid communities. Researches on Population Ecology, 41, 93–106. 10.1007/PL00011986 [DOI] [Google Scholar]
- Nissinen, A. I. , Pinto‐Zevallos, D. M. , Jauhiainen, L. , & Vänninen, I. (2017). The effect of photoperiod and light quality on Macrolophus pygmaeus Rambur (Hemiptera: Miridae) nymphal development, fecundity and longevity. Biological Control, 108, 30–39. 10.1016/j.biocontrol.2017.02.001 [DOI] [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , Sarkar, D. , Heisterkamp, S. , Van Willigen, B. , & Maintainer, R. (2017). Package ‘nlme’. Linear and nonlinear mixed effects models, 3, 1–117. [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2014). Available at: http://www.R-project.org/. (Accessed: 10th April 2014).
- Rosenblatt, A. E. , & Schmitz, O. J. (2016). Climate change, nutrition, and bottom‐up and top‐down food web processes. Trends in Ecology & Evolution, 31, 965–975. 10.1016/j.tree.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Ruberson, J. R. , Bush, L. , & Kring, T. J. (1991). Photoperiodic effect on diapause induction and development in the predator Orius insidiosus (Heteroptera: Anthocoridae). Environmental Entomology, 20, 786–789. 10.1093/ee/20.3.786 [DOI] [Google Scholar]
- Sanders, D. , Kehoe, R. , Cruse, D. , van Veen, F. J. F. , & Gaston, K. J. (2018). Low levels of artificial light at night strengthen top‐down control in insect food web. Current Biology, in press. [DOI] [PubMed] [Google Scholar]
- Sanders, D. , Kehoe, R. , Tiley, K. , Bennie, J. , Cruse, D. , Davies, T. W. , … Gaston, K. J. (2015). Artificial nighttime light changes aphid‐parasitoid population dynamics. Scientific Reports, 5, 15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D. , Kehoe, R. , & van Veen, F. F. (2015). Experimental evidence for the population‐dynamic mechanisms underlying extinction cascades of carnivores. Current Biology, 25, 3106–3109. 10.1016/j.cub.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Sanders, D. , Sutter, L. , & Veen, F. (2013). The loss of indirect interactions leads to cascading extinctions of carnivores. Ecology Letters, 16, 664–669. 10.1111/ele.12096 [DOI] [PubMed] [Google Scholar]
- Saunders, D. (1973). Thermoperiodic control of diapause in an insect: Theory of internal coincidence. Science, 181, 358–360. 10.1126/science.181.4097.358 [DOI] [PubMed] [Google Scholar]
- Shama, L. N. , & Robinson, C. T. (2006). Sex‐specific life‐history responses to seasonal time constraints in an alpine caddisfly. Evolutionary Ecology Research, 8, 169–180. [Google Scholar]
- Simpson, K. , Jackson, G. , & Grace, J. (2012). The response of aphids to plant water stress–the case of Myzus persicae and Brassica oleracea var. capitata . Entomologia Experimentalis et Applicata, 142, 191–202. 10.1111/j.1570-7458.2011.01216.x [DOI] [Google Scholar]
- Snyder, W. E. , & Ives, A. R. (2001). Generalist predators disrupt biological control by a specialist parasitoid. Ecology, 82, 705–716. 10.1890/0012-9658(2001)082[0705:GPDBCB]2.0.CO;2 [DOI] [Google Scholar]
- Tauber, M. J. , & Tauber, C. A. (1976). Insect seasonality: Diapause maintenance, termination, and postdiapause development. Annual Review of Entomology, 21, 81–107. 10.1146/annurev.en.21.010176.000501 [DOI] [Google Scholar]
- Thébault, E. , & Fontaine, C. (2010). Stability of ecological communities and the architecture of mutualistic and trophic networks. Science, 329, 853–856. 10.1126/science.1188321 [DOI] [PubMed] [Google Scholar]
- Thompson, J. N. (1999). The evolution of species interactions. Science, 284, 2116–2118. 10.1126/science.284.5423.2116 [DOI] [PubMed] [Google Scholar]
- Vaartaja, O. (1959). Evidence of photoperiodic ecotypes in trees. Ecological Monographs, 29, 91–111. 10.2307/1942199 [DOI] [Google Scholar]
- van Veen, F. F. , Memmott, J. , & Godfray, H. C. J. (2006). Indirect effects, apparent competition and biological control Trophic and guild in biological interactions control (pp. 145–169). Springer; 10.1007/1-4020-4767-3 Brodeur J., Boivin G., (eds) Springer, Dordrecht: [DOI] [Google Scholar]
- War, A. R. , Taggar, G. K. , War, M. Y. , & Hussain, B. (2016). Impact of climate change on insect pests, plant chemical ecology, tritrophic interactions and food production. International Journal of Clinical and Biological Sciences, 1, 16–29. [Google Scholar]
- Wardhaugh, K. (1977). The effects of temperature and photoperiod on the morphology of the egg‐pod of the Australian plague locust (Chortoicetes terminifera Walker, Orthoptera: Acrididae). Austral Ecology, 2, 81–88. 10.1111/j.1442-9993.1977.tb01129.x [DOI] [Google Scholar]
- Watt, A. (1979). The effect of cereal growth stages on the reproductive activity of Sitobion avenue and Metopolophium dirhodum . Annals of Applied Biology, 91, 147–157. 10.1111/j.1744-7348.1979.tb06485.x [DOI] [Google Scholar]
- Werner, E. E. , & Anholt, B. R. (1993). Ecological consequences of the trade‐off between growth and mortality rates mediated by foraging activity. The American Naturalist, 142, 242–272. 10.1086/285537 [DOI] [PubMed] [Google Scholar]
- Withrow, R. B. (1959). Photoperiodism and related phenomena in plants and animals. Washington 932. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from https://figshare.com/s/bdd2951343e341820014.


