Abstract
The ability to induce reversible transitions between homogeneous solutions and biphasic systems is of paramount relevance in separation processes. In this context, pH-triggered aqueous biphasic systems composed of ionic liquids and salts are here disclosed as switchable mono/biphasic systems, and their potential application further demonstrated through an intregated aproach comprising both the production and separation of hydroxymethylfurfural from fructose.
Liquid-liquid extraction processes are of technological simplicity and of low cost, and are frequently a preferred strategy in separation processes engineering.1 Nevertheless, the use of molecular and volatile organic solvents in separation routes presents major drawbacks due to their high volatility and recurrent toxicity.2 In this context, and in addition to their tailoring ability, ionic liquids (ILs) represent a viable alternative as a result of their non-volatile nature at ambient conditions.3, 4 The research in liquid–liquid extractions from aqueous media using ILs has been focused on two main approaches: (i) the direct use of non-water miscible hydrophobic ILs; and (ii) the use of aqueous biphasic systems (ABS) composed of ILs and organic/inorganic salts. Within the two types of systems, ABS constitute a “greener” and more benign option since they are mainly composed of water (up to 70 wt% in the overall system).5–8 Moreover, a more delicate tuning of the phases polarities can be achieved with IL-based ABS, resulting in systems that usually allow the complete extraction and purification of a wide variety of compounds.5–8
More recently, there has been a large interest on the use/applications of dynamic and reversible mono/biphasic systems formed by ILs.9–11 It was demonstrated that phase transitions in IL-containing mixtures can be induced by a temperature-driven phenomenon or by CO2/N2 flushing.12–15 Both upper critical solution temperature (UCST)16, 17 and lower critical solution temperature (LCST)18–20 phase behaviours have been reported for IL-solvent mixtures, whereas these temperature-dependent phase transitions have shown to be advantageous in the selective separation of proteins21 and metals.22 Reversible liquid-liquid systems have been also gathered with molecular solvents that react with CO2 forming salts and/or ILs, and applied in the separation of aliphatic and aromatic amines12–14 and in the synthesis/separation of gold (Au) porous films.15 However, common UCST and LCST behaviours in systems involving ILs typically occur at temperatures well above room temperature and a high energy input is required to trigger their phase switch, while the CO2/N2-dependent reversibility pattern requires the use of specific equipment. Furthermore, these systems are usually composed of an IL-rich phase (typically with hydrophobic characteristics) and a more hydrophilic molecular-solvent-rich phase.17–22 These systems are thus restricted in polarity differences amongst the coexisting phases, hindering improved extraction and selectivity performances to be obtained when dealing with the separation/fractionation of complex matrices. In this line, IL-based ABS can be seen as promising options, although the investigation on their use as reversible systems lagged behind. To the best of our knowledge, only temperature-dependent reversible behaviours of IL-based ABS have been reported.23 Contrarily to most IL-based ABS, which display a weak dependence on temperature, it was recently found23 that ABS formed by protic ILs and polymers are highly temperature dependent, allowing therefore to trigger reversible phase separations by small changes in temperature.
Given the potential of applications of switchable (aqueous-rich) biphasic systems discussed above, in this work we demonstrate the reversibility of IL-based ABS attained by a pH-driven phenomenon and their use as integrated platforms, comprising both the production and separation of 5-hydroxymethylfurfural (HMF) from fructose.
Aiming at evaluating the possibility of moving from monophasic to biphasic regimes (and vice-versa) by a proper tailoring of the pH of the aqueous media, ABS formed by a wide range of chloride-based ILs and potassium citrate were investigated. ILs with no speciation capability were chosen, and as such, the pH-triggered reversible phenomenon is a result of the salt (potassium citrate) speciation as a function of the pH. Citric acid presents 4 pKa values, namely 3.05, 4.67, 5.39 and 13.92.24 The speciation curves of citric acid are depicted in the ESI†. At pH values lower than 3.05, the non-charged citric acid is the dominant species present, whereas at pH values above 3.07, 4.67 and 5.39, there is the prevalence of the monovalent, divalent and trivalent charged dihydrogencitrate, hydrogencitrate and citrate anions, respectively. All these citrate-based species can be ranked within the Hofmeister series,6 in which higher charge density and higher charge valence anions are better hydrated and stronger salting-out species, being thus more favourable to the creation of IL-based ABS. In fact, previous works have been published on the pH effect towards the formation of IL-based ABS,25–27 although no investigations on their reversible behaviour have been carried out hitherto. To be able to change the pH in the investigated ABS and to trigger the phase transition while avoiding the introduction of new species into the overall system, citric acid (C6H8O7) and potassium hydroxide (KOH) were used. The pair potassium citrate/citric acid also was selected due to their biodegradable and non-toxic characteristics.28 Fig. 1 sketches the principle of the reversible process under investigation, where the transitions between the monophasic and the biphasic regimes could be attained by the alternate addition of an acidic or an alkaline species to the overall aqueous system. For mixture compositions above each solubility curve there is the formation of a two-phase system at given conditions, while mixture compositions below the same curve result in the formation of a homogeneous solution (no phase-separation).
Fig. 1.
Ternary phase diagrams of an IL-based ABS at 25 °C, at pH ≈9 (▲) and pH ≈5 (◆). In all the investigated ABS, the top phase corresponds to the IL-rich phase while the bottom phase is mainly composed of salt and water.
We firstly determined the liquid-liquid ternary phase diagrams at different pH values of ABS composed of ILs + K3C6H5O7/C6H8O7 + water. These were determined at pH values ranging between 5 and 9 for the following hydrophilic ILs: [C4C1im]Cl, [C4C1C1im]Cl, [C4C1pip]Cl, [C4C1py]Cl, [C4C1im]Br and [P4444]Cl. The definition of the ILs acronyms is provided as a footnote‡; details on their purity are given in the ESI†. Further details on the experimental procedure adopted are provided in the ESI†.
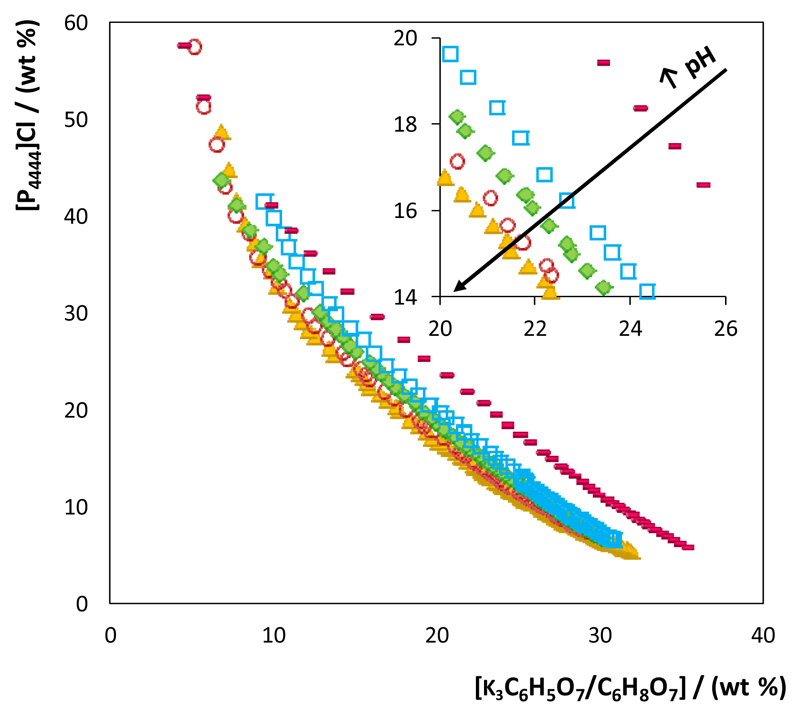
The ternary phase diagrams at 25 °C for the ABS composed of [P4444]Cl at different pH values are illustrated in Fig. 2. The depicted ABS phase diagrams at the most acidic pH values also denote the limit in compositions and pH values for which ABS no longer form. The experimental weight fraction data, as well as the representation of the phase diagrams for the remaining ILs, are given in the ESI†. All the phase diagrams were further characterized by the determination of several tie-lines to infer on the phases compositions for given mixture compositions (cf. the ESI†). In general, and for all ILs, there is a decrease on the ability for ABS formation with the pH decrease - the higher the pH of the aqueous medium the larger is the biphasic region, and the lower the IL/salt amounts required to induce phase separation. Given that ILs with no speciation capability have been chosen, the observed dependency of the ABS formation on the pH is related to the speciation performance of potassium citrate. As discussed before, at pH values lower than 3.05 and 4.07, the non-charged citric acid and monovalent dihydrogencitrate anion are, respectively, the prevalent species, and none of these species is able to form ABS with the ILs investigated. At pH values above 4.67 and 5.39 there is the main presence of the divalent and trivalent charged hydrogencitrate and citrate anions, respectively. IL-based ABS are only formed at pH values higher than 5, meaning that only the divalent and trivalent citrate-based anions induce phase separation. This behaviour is in agreement with the Hofmeister series6 since higher charge density and higher charge valence anions are stronger salting-out species able to induce the salting-out of the IL, further resulting in the creation of IL-based ABS.
Fig. 2.
Phase diagrams of ABS composed of [P4444]Cl + water + K3C6H5O7/C6H8O7 at pH ≈ 9 (▲), pH ≈ 8 (○), pH ≈ 7 (◆), pH ≈ 6 (□) and pH ≈ 5 (▬).
At a fixed pH, the IL ability to form ABS follows the order: [P4444]Cl > [C4C1im]Br > [C4C1py]Cl ≈ [C4C1pip]Cl > [C4C1C1im]Cl ≈ [C4C1im]Cl (cf. the ESI† with all the phase diagrams). More hydrophobic ILs, resulting either from cations with longer and more aliphatic moieties or from anions of lower hydrogen-bond basicity29, 30, are more capable of forming ABS, i.e. are able to form two-phase systems at lower pH values or require lower amounts of the phase-forming components for phase separation. Examples of this are seen for [P4444]Cl and [C4mim]Br, the only two ILs that form ABS at pH 5. On the other hand, ILs such as [C4C1im]Cl and [C4C1C1im]Cl, which are amongst the most hydrophilic ILs investigated, do not form ABS even at a pH of 6, being 7 the minimum pH value required for ABS formation with these ILs. This pattern also mirrors the salting-out effect of the citrate-based salt over the IL.5–8 A summary of the overall results according to the ability of each IL to form ABS at all studied pH values is given in the ESI†.
After establishing the ternary phase diagrams, which describe the mixture compositions and pH values for which the liquid-liquid demixing occurs, the reversible IL-based ABS behaviour was further appraised by the alternate addition of citric acid and potassium hydroxide. For this purpose, an initial ternary mixture at the biphasic region (IL at 25 wt% + K3C6H5O7 at 35 wt% + water at 40 wt%) was prepared and allowed for phase separation at 25 °C. At this initial mixture composition the pH values of the aqueous media of all IL-containing systems are ca. 9. An aqueous solution of citric acid at 50 wt% was added dropwise, under constant agitation, until the mixture became homogeneous (monophasic). Then, an aqueous solution of potassium hydroxide at 50 wt% was added, under agitation, to recover the initial pH value of 9 and the biphasic system. During these manipulations the pH of the aqueous solutions was experimentally controlled. Fig. 1 depicts the procedure carried out to attain the reversible process under investigation. It should be remarked that the differences in the phases’ compositions are indeed negligible since a small amount of each aqueous solution (± 0.6 wt%) is enough to trigger the phase transition. The pH-driven reversibility was experimentally confirmed with all the ILs investigated, at least 3 times, with no significant changes in the phases’ composition.
In summary, it is possible to prepare reversible IL-based ABS playing around with the speciation behaviour of the organic salt. This phase reversibility takes place at room temperature (25 °C) and is achieved with cheap compounds already present in the original mixture and with no need of sophisticated equipment. Hence, IL-based ABS can be envisaged as potential alternatives to the more complex reversible systems that require additional energy inputs or the addition of gases and more specific equipment. Their reversibility is however a major advantage in liquid-liquid extractions to attain selective separations, and as demonstrated hereinafter.
We investigated the possibility of using the proposed systems for the production of hydroxymethylfurfural (HMF) through fructose dehydration at acidic pH, and then to proceed with an integrated separation step of HMF from the unreacted precursor by an increase in the pH and consequent formation of two-phase systems. The dehydration of carbohydrates (e.g. cellulose, glucose, fructose, sucrose, inulin, and cellobiose) to produce HMF has attracted large attention in the past few years.31, 32 In fact, HMF is nowadays considered a key biomass-derived building block relevant in bio-based industries.31, 33 The current applications of HMF are widespread and comprise the production of solvents34, biofuels (dimethylfuran),35 polymeric materials (mainly based on its 2,5-furandicarboxylic acid derivative),36 fine chemicals37, among others. Recently, a short-time synthesis of the active pharmaceutical ingredient ranitidine (Zantac) also was reported making use of HMF.38 Typically, HMF is produced from fructose via a dehydration reaction, generally catalysed by acids (pH values ranging between 1.5 and 3).39 However, high temperatures (>200 °C), reduced pressure and long reaction times are usually required.39 More recently, IL-acid mixtures or acidic ILs have been used for sugars dehydration and production of HMF in high yields, obtained at lower temperatures (<120 °C) and within short reaction times.40 Although good results using ILs for the fructose dehydration have been described, the development of a cost-effective, integrated and more sustainable production-separation process is still in crucial demand. In this line, we propose herein the discussed switchable ABS as an integrated and more benign platform for the production and separation of HMF.
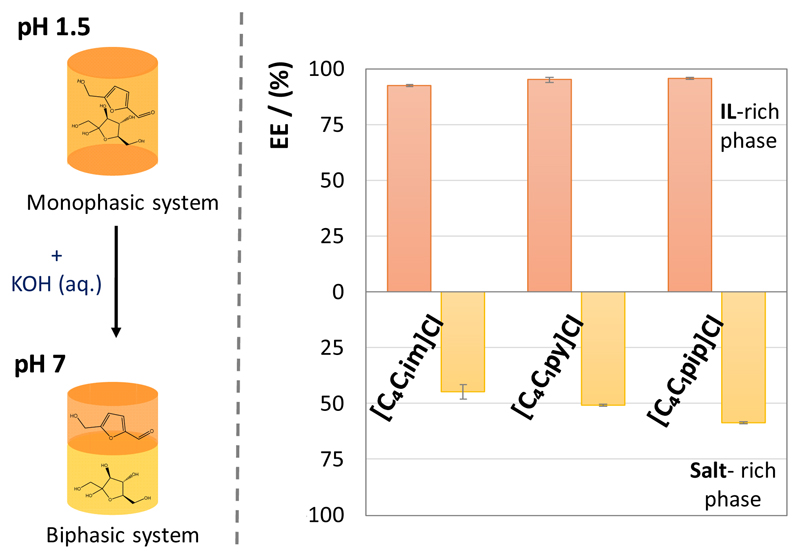
The production of HMF was carried out through fructose dehydration in acidic medium (pH ca. 1.5), at 80 °C for 80 min, in the IL-based ABS monophasic regions. After reaction, an aqueous solution of KOH was added to stop the reaction and to move the system into the biphasic region by a pH increase – Fig. 1. The creation of two-phase systems is intended for the selective separation (migration for opposite phases) of HMF and unreacted fructose. Further experimental details are given in the ESI†.
Fig. 3 depicts the results on the production of HMF in citric acid aqueous solutions at the monophasic region (corresponding to a mixture composition of 40 wt% of IL + 30 wt% of citric acid). The respective reactions were also carried out in the absence of ILs, as well as in the absence of citric acid, for comparison purposes. As shown, the use of an acidic medium is required for the production of HMF through the dehydration of fructose – when no acid was used a maximum yield of 0.00281 g.L-1 of HMF was obtained. On the other hand, without IL, a maximum yield of HMF of 2.28 g.L-1 was found. However, when ILs are used in acidic medium, HMF yields ranging between 4.61and 6.37 g.L-1 have been observed, supporting the need of the presence of both the IL and the acidic medium to maximise the HMF production yield. These results are consistent with previous studies describing the requirement of the addition of ILs and acid catalysts to obtain high HMF yields.40, 41
Fig. 3.
Production of HMF through fructose dehydration in presence (or not) of citric acid and ILs, at 80ºC for 80 min.
After the HMF production, the reaction was stopped and the two-phase systems simultaneously formed by the addition of aqueous KOH (up to pH 7). Fig. 4 depicts the results regarding the selective separation of HMF from fructose using IL-based ABS. The results are given in extraction efficiencies of both HMF and fructose (EEHMF% and EEFructose%) for opposite phases. The extraction efficiencies are defined as the percentage ratio between the total weight of HMF or fructose in one of the phases to that in the total mixture (detailed results are given in the ESI†). The results in partition coefficients values, defined as the concentration of HMF between the IL-rich phase and the salt-rich phase, as well as selectivity of the systems to HMF, also are provided in the ESI†.
Fig. 4.
Selective separation of HMF from fructose that did not react through the addition of KOH and formation of ABS.
In all investigated systems, HMF preferentially migrates to the IL-rich phase while fructose is enriched in the opposite layer, allowing thus the separation of the two compounds. Remarkable extraction efficiencies of HMF to the IL-rich phase ranging between 92 and 96%, and of fructose to the salt-rich phase ranging between 45 and 59%, were obtained in a single-step. This migration for opposite phases and selective separation results from the higher affinity of HMF to more hydrophobic (IL-rich) phases in contrast to the higher affinity of fructose to more hydrophilic (salt-rich) phases, as reflected by their octanol-water partition coefficients (Kow) (log(Kow) of HMF = -0.10; log(Kow) of fructose = -2.76).24 Although the almost complete extraction of HMF to the IL-rich phase was achieved in a single-step in all studied systems, the extraction of fructose to the opposite phase seems to be more dependent on the nature of the IL employed; the selective separation of HMF and fructose increases in the following order: [C4mim]Cl < [C4mpy]Cl < [C4mpip]Cl. This trend follows the IL hydrophobic nature as described by their ternary phase diagrams at a fixed pH shown in the ESI†.
In what concerns the extraction performance and selectivity of the systems to HMF (ranging between 14 and 48; detailed data shown in the ESI†), our results are quite promising when compared to those reported in the literature.42, 43 Partition coefficients of HMF ranging between 0.5 and 2.0 have been reported, with biphasic systems composed of water and butanol, methyl-iso-butylketone (MIBK), toluene, dichloromethane or other mixtures of organic volatile solvents.42 More recently, Blumenthal et al.43 suggested biphasic systems formed by water and o-propylphenol or o-isopropylphenol for the separation of HMF from the aqueous reaction medium, in which an increase up to five times in the partition coefficients was reported when compared to the previously applied solvents.42 The partition coefficients obtained in the current work range between 9 and 17 (cf. the ESI† with detailed data). Our results on the separation of HMF are thus better than the most promising ones reported hitherto in the literature. Moreover, with IL-based ABS, the use of volatile and often toxic organic solvents is avoided; instead, aqueous-rich media are used. Additionally, an integrated methodology is here proposed for the separation of HMF by the creation of two-phase systems only through a pH increase, representing thus a step forward on the development of more sustainable production-separation processes.
In summary, the results here reported demonstrate that pH-driven reversible ABS composed of ILs can be easily prepared by playing around with the speciation of at least one of the phase-forming components. Furthermore, these systems show potential to become remarkable integrated platforms, in which reaction and separation steps can be carried out sequentially by taking advantage of their switchable behaviour, as demonstrated here with the HMF production and separation from fructose as the reaction precursor. In addition to the example shown herein, reversible IL-based ABS can be certainly tailored to fit the requirements of other separation processes by a proper selection of the medium pH and composition.
Supplementary Material
† Electronic Supplementary Information (ESI) available: Materials and experimental procedure, binodal weight fraction data, the phases diagrams for the different ILs in study at fixed pH, and the speciation curves of citric acid. See DOI: 10.1039/C7GC00157F
Acknowledgments
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials (Ref. FCT UID /CTM /50011/2013) and QOPNA research unit (FCT UID/QUI/00062/2013), financed by national funds through the FCT/MEC and when applicable co-financed by FEDER under the PT2020 Partnership Agreement. A. M. Ferreira acknowledges FCT for the PhD grant SFRH/BD/92200/2013. This research was undertaken, in part, thanks to funding from the Canada Excellence Research Chairs Program. The research leading to reported results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 337753.
Footnotes
ILs used: 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl; 1-butyl-2,3-dimethylimidazolium chloride, [C4C1C1im]Cl; 1-butyl-1-methylpiperidinium chloride, [C4C1pip]Cl; 1-butyl-1-methylpyridinium chloride, [C4C1py]Cl; 1-butyl-3-methylimidazolium bromide, [C4C1im]Br; tetrabutylphosphonium chloride, [P4444]Cl.
References
- 1.Fifield FW, Kealey D. Principles and Practice of Analytical Chemistry. 5th edn. 2000. [Google Scholar]
- 2.Rydberg J, Cox M, Musikas C, Choppin GR. Principles and practices of solvent extraction. Second Edition, Revised and Expanded. 2nd edn. 2004. [Google Scholar]
- 3.Rogers RD, Seddon KR. Science (New York, N.Y.) 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 4.Earle MJ, Esperanca JMSS, Gilea MA, Canongia Lopes JN, Rebelo LPN, Magee JW, Seddon KR, Widegren JA. Nature. 2006;439:831–834. doi: 10.1038/nature04451. [DOI] [PubMed] [Google Scholar]
- 5.Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. J Am Chem Soc. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- 6.Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- 7.Freire MG, Louros CLS, Rebelo LPN, Coutinho JAP. Green Chem. 2011;13:1536–1545. [Google Scholar]
- 8.Freire MG, Neves CMSS, Marrucho IM, Canongia Lopes JN, Rebelo LPN, Coutinho JAP. Green Chem. 2010;12:1715–1718. [Google Scholar]
- 9.Kohno Y, Ohno H. Chem Commun. 2012;48:7119–7130. doi: 10.1039/c2cc31638b. [DOI] [PubMed] [Google Scholar]
- 10.Jessop PG, Mercer SM, Heldebrant DJ. Energy Environ Sci. 2012;5:7240–7253. [Google Scholar]
- 11.Luo J, Xin T, Wang Y. New J Chem. 2013;37:269–273. [Google Scholar]
- 12.Saita S, Kohno Y, Nakamura N, Ohno H. Chem Commun. 2013;49:8988–8990. doi: 10.1039/c3cc45302b. [DOI] [PubMed] [Google Scholar]
- 13.Kohno Y, Arai H, Ohno H. Chem Commun. 2011;47:4772–4774. doi: 10.1039/c1cc10613a. [DOI] [PubMed] [Google Scholar]
- 14.Xiong D, Wang H, Li Z, Wang J. ChemSusChem. 2012;5:2255–2261. doi: 10.1002/cssc.201200307. [DOI] [PubMed] [Google Scholar]
- 15.Xiong DZ, Cui GK, Wang JJ, Wang HY, Li ZY, Yao KS, Zhang SJ. Angew Chem Int Ed. 2015;54:7265–7269. doi: 10.1002/anie.201500695. [DOI] [PubMed] [Google Scholar]
- 16.Freire MG, Neves CMSS, Shimizu K, Bernardes CES, Marrucho IM, Coutinho JAP, Lopes JNC, Rebelo LPN. J Phys Chem B. 2010;114:15925–15934. doi: 10.1021/jp1093788. [DOI] [PubMed] [Google Scholar]
- 17.Nockemann P, Thijs B, Pittois S, Thoen J, Glorieux C, Van Hecke K, Van Meervelt L, Kirchner B, Binnemans K. J Phys Chem B. 2006;110:20978–20992. doi: 10.1021/jp0642995. [DOI] [PubMed] [Google Scholar]
- 18.Saita S, Kohno Y, Ohno H. Chem Commun. 2013;49:93–95. doi: 10.1039/c2cc37006a. [DOI] [PubMed] [Google Scholar]
- 19.Lachwa J, Szydlowski J, Makowska A, Seddon KR, Esperanca JMSS, Guedes HJR, Rebelo LPN. Green Chem. 2006;8:262–267. [Google Scholar]
- 20.Fukumoto K, Ohno H. Angew Chem Int Ed. 2007;46:1852–1855. doi: 10.1002/anie.200604402. [DOI] [PubMed] [Google Scholar]
- 21.Kohno Y, Saita S, Murata K, Nakamura N, Ohno H. Polym Chem. 2011;2:862–867. [Google Scholar]
- 22.Xie Z-L, Taubert A. ChemPhysChem. 2011;12:364–368. doi: 10.1002/cphc.201000808. [DOI] [PubMed] [Google Scholar]
- 23.Passos H, Luis A, Coutinho JAP, Freire MG. Sci Rep. 2016;6:7. doi: 10.1038/srep20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemspider. The free chemical database. [accessed October 2016]; http://www.chemspider.com.
- 25.Zafarani-Moattar MT, Hamzehzadeh S. Fluid Phase Equilib. 2011;304:110–120. [Google Scholar]
- 26.Li S, He C, Liu H, Li K, Liu F. J Chromatogr B. 2005;826:58–62. doi: 10.1016/j.jchromb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Kurnia KA, Freire MG, Coutinho JAP. J Phys Chem B. 2014;118:297–308. doi: 10.1021/jp411933a. [DOI] [PubMed] [Google Scholar]
- 28.Hong-Shum JSL. In: Food Additives Data Book. B. S. Ltd, editor. Blackwell Science Ltd, Wiley-Blackwell; 2003. pp. i–xix. [DOI] [Google Scholar]
- 29.Cláudio AFM, Ferreira AM, Shahriari S, Freire MG, Coutinho JAP. J Phys Chem B. 2011;115:11145–11153. doi: 10.1021/jp204865a. [DOI] [PubMed] [Google Scholar]
- 30.Cláudio AFM, Swift L, Hallett JP, Welton T, Coutinho JAP, Freire MG. Phys Chem Chem Phys. 2014;16:6593–6601. doi: 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- 31.Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM. Green Chem. 2011;13:754–793. [Google Scholar]
- 32.Song J, Zhang B, Shi J, Fan H, Ma J, Yang Y, Han B. RSC Adv. 2013;3:20085–20090. [Google Scholar]
- 33.van Putten R-J, van der Waal JC, de Jong E, Rasrendra CB, Heeres HJ, de Vries JG. Chem Rev. 2013;113:1499–1597. doi: 10.1021/cr300182k. [DOI] [PubMed] [Google Scholar]
- 34.Konduri SKM, Thoota SK, Javvadi C, Muddasani PR, Adibhatla KSBR, Nannapaneni VC. WO/2015/155784. 2015
- 35.Roman-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA. Nature. 2007;447:982–985. doi: 10.1038/nature05923. [DOI] [PubMed] [Google Scholar]
- 36.Sousa AF, Vilela C, Fonseca AC, Matos M, Freire CSR, Gruter G-JM, Coelho JFJ, Silvestre AJD. Polym Chem. 2015;6:5961–5983. [Google Scholar]
- 37.Gupta P, Singh SK, Pathak A, Kundu B. Tetrahedron. 2002;58:10469–10474. [Google Scholar]
- 38.Mascal M, Dutta S. Green Chem. 2011;13:3101–3102. [Google Scholar]
- 39.Salak Asghari F, Yoshida H. Ind Eng Chem Res. 2006;45:2163–2173. [Google Scholar]
- 40.Teong SP, Yi G, Zhang Y. Green Chem. 2014;16:2015–2026. [Google Scholar]
- 41.Sievers C, Musin I, Marzialetti T, Olarte MB, Agrawal PK, Jones CW. ChemSusChem. 2009;2:665–671. doi: 10.1002/cssc.200900092. [DOI] [PubMed] [Google Scholar]
- 42.Fan C, Guan H, Zhang H, Wang J, Wang S, Wang X. Biomass Bioenergy. 2011;35:2659–2665. [Google Scholar]
- 43.Blumenthal LC, Jens CM, Ulbrich J, Schwering F, Langrehr V, Turek T, Kunz U, Leonhard K, Palkovits R. ACS Sustain Chem Eng. 2016;4:228–235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.