Abstract
Fluoroquinolones (FQs) and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are two classes of Active Pharmaceutical Ingredients (APIs), widespreadly used in human healthcare and as veterinary drugs, and that have been found throughout the water cycle in the past years. These two classes of APIs are commonly present in aqueous streams in concentrations ranging from ng.L-1 to µg.L-1. Despite such low concentrations, these contaminants tend to bioaccumulate, leading to serious environmental and health issues after chronic exposure. The low concentrations of FQs and NSAIDs in aqueous media also render their difficult identification and quantification, wich may result in an unefficient evaluation of their environmental impact and persistence. Therefore, the development of alternative pre-treatment techniques for their extraction and concentration from aqueous samples is a crucial requirement. In this work, liquid-liquid systems, namely ionic-liquid-based aqueous biphasic systems (IL-based ABS), were tested as simultaneous extraction and concentration platforms of FQs and NSAIDs. ABS composed of imidazolium-, ammonium- and phosphonium-based ILs and a citrate-based salt (C6H5K3O7) were evaluated for the single-step extraction and concentration of three FQs (ciprofloxacin, enrofloxacin and norfloxacin) and three NSAIDs (diclofenac, naproxen and ketoprofen) from aqueous samples. Outstanding one-step extraction efficiencies of APIs close to 100% were obtained. Furthermore, concentration factors of both FQs and NSAIDs were optimized by an appropriate manipulation of the phase-forming components compositions to tailor the volumes of the coexisting phases. Concentration factors of 1000-fold of both FQS and NSAIDs were obtained in a single-step, without reaching the saturation of the IL-rich phase. The concentration of APIs up to the mg.L-1 allowed their easy and straightforward identification and quantification by High-Performance Liquid Chromatography (HPLC) coupled to an UV detector, as shown either with model aqueous samples or real wastewater effluent samples.
Introduction
The presence of active pharmaceutical ingredients (APIs) in non-negligible levels in sewage treatment plants (STPs), wastewater treatment plants (WWTPs), surface water effluents, river waters and seawater has been a topic of growing concern.1–4 The increasing consumption of large number of different pharmaceuticals along time has had a significant impact in the public health and wildlife. Within APIs, antibiotics, and in particular fluoroquinolones (FQs), and non-steroidal anti-inflammatory drugs (NSAIDs) are of particular concern since they are consumed in relatively high amounts,5, 6 which results in their inherent excretion into the waste water cycle (either as metabolized or unchanged species) or by the simple direct discharge of expired or non-consumed drugs.7–9 APIs are known as mutagenic, carcinogenic and endocrine disruptors and have been detected in worldwide effluents in concentrations up to µg.L-1 and in rivers and oceans in concentrations generally up to ng.L-1.1–3
FQs (ciprofloxacin, norfloxacin and enrofloxacin) are synthetic antibiotics broadly used in the treatment of infectious diseases, such as respiratory and urinary tract infections, since they act against a wide range of aerobic gram-positive and gram-negative organisms.10 Due to their high effectiveness, FQs have been largely used by humans, food producing animals (cattle and aquaculture fish, e.g.), and companion animals. However, these pharmaceutical drugs have negative impacts towards humans and wildlife, namely in the development and reproductive functions of fish, invertebrates, plants and algae, particularly after prolonged exposure periods. Nowadays, ciprofloxacin and norfloxacin are the second-generation FQs most prescribed in the world. In Europe, for example, in 2012 ciprofloxacin accounted for 71% of the consumption of second generation quinolones in all countries.11–14 Due to this large consumption, ciprofloxacin was already found not only in effluents from WWTP/STP but also in river waters. In effluents, the levels tend to be higher ranging from 40 to 3353 ng.L-1 in Europe; from 110 to 1100 ng.L-1 in North America; and from 42 to 720 ng.L-1 in Asia and Australia.15 In WWTP influents and hospital wastewaters, the levels are, as expected, much higher (in the µg.L-1 range).4, 10 In Switzerland, e.g., ciprofloxacin levels in hospital wastewater ranged between 3 and 87 µg.L-1.16 In river waters, the levels are generally in the low ng.L-1 range. Nevertheless, exceptionally high levels have also been reported: concentrations of ciprofloxacin as high as 2745 ng.L-1 were found in one location of a Polish river.17
NSAIDs (diclofenac, naproxen and ketoprofen) are a class of pain killers used in human and veterinary medicine, being one of the primary classes of pharmaceutical compounds prescribed in human medical care, with many compounds sold without prescription.2 Due to their widespread use, NSAIDs are continuously discharged in the aquatic environment where they are pseudo-persistent. They have the potential to bioaccumulate and they can be reactive to non-target organisms. They are known to be toxic towards a wide variety of organisms including invertebrates and fish.2 Diclofenac was already found in effluents from WWTP/STP in levels ranging from 460 to 3300 ng.L-1 in Europe; from < 0.5 to 177 ng.L-1 in North America; and from 8.8 to 127 ng.L-1 in Asia and Australia.15 It was also detected in freshwater rivers in levels varying between 2 and 41 ng.L-1 in Europe, 11-82 ng.L-1 in North America and 1.1 and 6.8 ng.L-1 in Asia and Australia.15
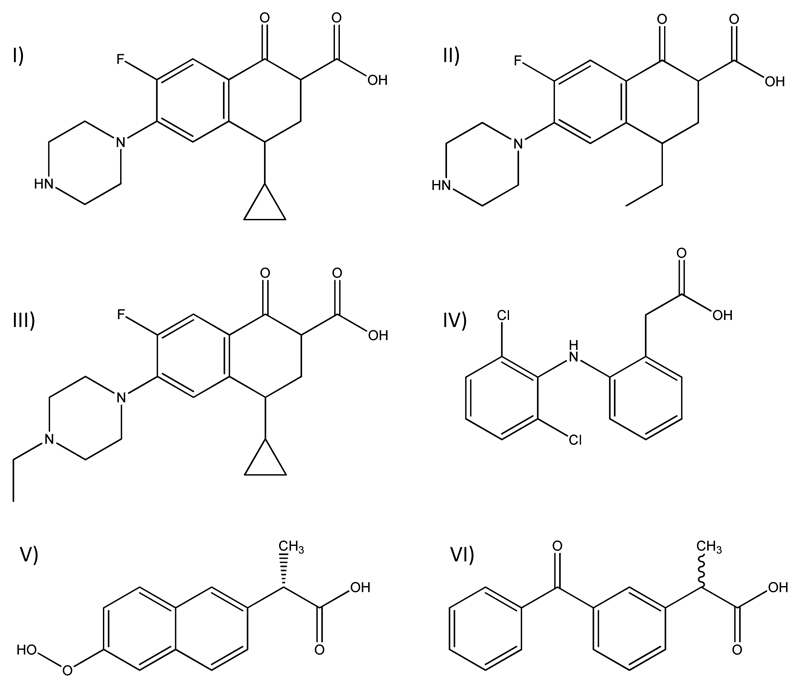
Since diclofenac, naproxen and ciprofloxacin are the most frequently found APIs in water cycles, the Global Water Research Coalition (GWRC) pointed them out as high priority pharmaceutical drugs.5, 18, 19 Furthermore, diclofenac is considered as priority hazardous substance in the European Union.20 In Fig. 1, the chemical structures of the FQs and NSAIDs studied in this work are depicted.
Fig. 1.
Chemical structures of fluoroquinolones: I) ciprofloxacin, II) enrofloxacin, and III) norfloxacin; and non-steroidal anti-inflammatory drugs: IV) diclofenac, V) naproxen, and VI) ketoprofen.
Although STPs and WWTPs use advanced technologies for the removal and elimination of pollutants/contaminants, the complete elimination of APIs is extremely difficult and thus these contaminants were detected even in drinking water.1 According to Deblonde et al.21, the removal efficiency of ciprofloxacin, norfloxacin, diclofenac, naproxen and ketoprofen in WWTPs is 62.3, 54.3, 34.6, 81.6 and 31.1 %, respectively. Therefore, the entry of these contaminants into the environment is a continuous process which will result in significant environmental and human hazards in the near future. For an adequate monitoring of their concentration, environmental risks, persistence and occurrence, there is the need to develop improved analytical methods for their detection and quantification. The accurate identification and quantification of APIs often requires pre-treatment strategies of aqueous samples, both to increase their concentrations up to values that can be quantified by analytical equipment or to remove major interferences. The commonly used technique for the pre-treatment of aqueous samples is solid-phase extraction (SPE). For instance, Vieno et al.22 applied SPE as an isolation and concentration procedure, aiming at an improved detection of three FQS, in which concentration factors of 2000, 1000, 500 and 200 for ground water, surface water, STP effluent and STP influent samples, respectively, were obtained. Prat et al.23 used SPE to concentrate 10 quinolones (concentration factor up to a 250-fold) followed by reversed-phase high-performance liquid chromatography coupled to a fluorescence detector analysis. Lopes et al.24 developed a modified SPE method for identification of emerging contaminants from large sample volumes, where the concentration of bisphenol-A, acetaminophen, salicylic acid and diclofenac was determined by high-pressure liquid chromatography combined with time-of-flight mass spectrometry (HPLC-MS-TOF). Although SPE is the most commonly used technique for the pre-treatment and concentration of aqueous samples containing APIs, it requires an additional desorption step of the analyte, usually carried out with hazardous volatile organic solvents. In this context, it is of high relevance to develop alternative and more efficient pre-treatment techniques for aqueous samples containing APIs envisaging their accurate monitoring in the aquatic environment.
In this work, ionic-liquid-based aqueous biphasic systems (IL-based ABS) will be evaluated as an alternative pre-treatment strategy for two major families of APIS, FQs and NSAIDS. ABS are liquid-liquid systems formed by at least two (ideally non-volatile) compounds dissolved in a water-rich medium. In general, two polymers, a salt and a polymer or two salts above given concentrations lead to the creation of two-phase aqueous systems.25, 26 In 2003, Rogers and co-workers27 demonstrated that the addition of a “kosmotropic” salt to aqueous solutions of ILs results in two-phase separation. Since then, IL-based ABS, formed by the combination of ILs with a large number of salts, amino acids, carbohydrates or polymers, have been the focus of intensive research regarding their use in extraction, separation and purification approaches.28, 29 This boom in research derives from the ILs exceptional properties, namely a negligible vapor pressure, non-flammability, high thermal and chemical stabilities, and the ability to tailor the phases’ polarities and affinities by an adequate choice of the chemical structures of the ILs ions.30–33 While most studies reported in the literature are devoted to the use of IL-based ABS for purification purposes,29 their use in the extraction and concentration of target compounds has also been investigated. Passos et al.,34 envisaging an adequate monitoring of endocrine disruptors in human fluids, demonstrated the complete extraction of bisphenol A from human urine and its concentration up to a 100-fold using IL-based ABS. Later, Dinis et al.35 studied the simultaneous extraction and concentration of ethinylestradiol with IL-based ABS, achieving a concentration factor up to 1000-fold in a single-step. In conclusion, IL-based ABS are promising candidates to pre-treatment strategies of aqueous samples allowing for a better monitoring of APIs in aqueous environmental samples. Therefore, in this work, ABS composed of a wide range of ILs and a citrate-based biodegradable salt (potassium citrate, C6H5K3O7) were investigated for the extraction and concentration of FQS, namely ciprofloxacin, norfloxacin and enrofloxacin, and of NSAIDs, namely diclofenac, naproxen and ketoprofen. An initial screening of the ability of these systems to extract FQS and NSAIDs was carried out, followed by the use of the most promising systems for the simultaneous extraction and concentration the two classes of APIs. Model systems, using distilled water and also real effluent samples from a WWTP were used to evaluate the matrix effect on the extraction performance of the proposed technology.
Experimental Section
Materials
Three FQs, namely ciprofloxacin hydrochloride (CAS# 86393-32-0), enrofloxacin (CAS# 93107-08-5), and norfloxacin (CAS# 70458-96-7), and three NSAIDs, namely diclofenac sodium salt (CAS# 15307-79-6), naproxen (CAS# 22204-53-1) and ketoprofen (CAS# 22071-15-4), were used in this work. Ciprofloxacin hydrochloride, norfloxacin, diclofenac sodium salt, naproxen and ketoprofen were acquired from Sigma-Aldrich, whereas enrofloxacin was purchased from BioChemika. The chemical structures of the studied FQs and NSAIDs are depicted in Fig. 1.
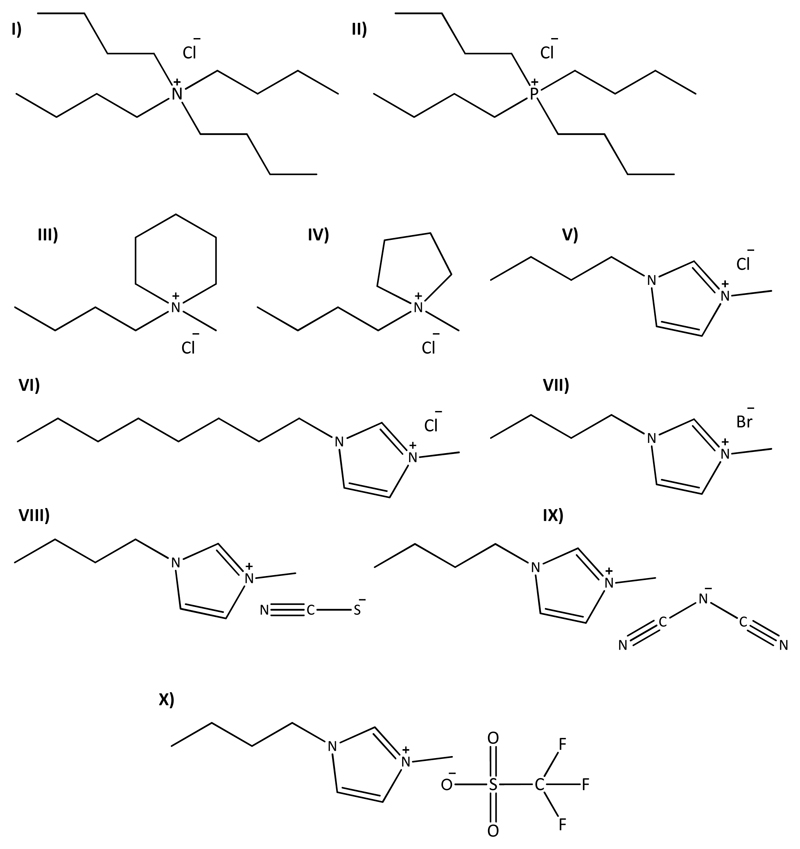
The ILs investigated to form ABS were tetrabutylphosphonium chloride, [P4444]Cl, (97 wt %); tetrabutylammonium chloride, [N4444]Cl, (≥ 97 wt %); 1-butyl-1-methylpiperidinium chloride, [C4C1pip]Cl, (≥ 99 wt %); 1-butyl-1-methylpyrrolidinium chloride, [C4C1pyr]Cl, (≥ 99 wt %); 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl, (99 wt %); 1-methyl-3-octylimidazolium chloride, [C8C1im]Cl, (99 wt %); 1-butyl-3-methylimidazolium bromide, [C4C1im]Br, (99 wt %); 1-butyl-3-methylimidazolium thiocyanate, [C4C1im][SCN], (98 wt %); 1-butyl-3-methylimidazolium dicyanamide, [C4C1im][N(CN)2], (98 wt %); 1-butyl-3-methylimidazolium trifluoromethanesulfonate, [C4C1im][CF3SO3], (99 wt %). Phosphonium-based ILs were gently supplied by Cytec Industries Inc., while the imidazolium-, piperidinium-, and pyrrolidinium-based fluids were purchased from Iolitec. Tetrabutylammonium chloride was purchased from Sigma-Aldrich. To decrease the volatile impurities and water contents, individual samples of ILs were purified at room temperature under constant stirring and vacuum for a minimum of 24 h. In particular for [P4444]Cl, the temperature was raised up to 373 K and this sample was kept under vacuum for a minimum of 72 h, due to its higher amounts of water. The purity of each IL was confirmed by 1H and 13C NMR and found to be in accordance with the purities given by the suppliers. The chemical structures of the studied ILs are depicted in Fig. 2.
Fig. 2.
Chemical structures of the ionic liquids used: I) [N4444]Cl, II) [P4444]Cl, III) [C4C1pip]Cl, IV) [C4C1pyr]Cl, V) [C4C1im]Cl,VI) [C8C1im]Cl, VII) [C4C1im]Br, VIII) [C4C1im][SCN], IX) [C4C1im][N(CN)2], and X) [C4C1im][CF3SO3].
The potassium citrate tribasic monohydrate salt, C6H5K3O7.H2O (≥ 99 wt %) used in the ABS was acquired from Sigma–Aldrich. The water used in the extractions experiments was double distilled, passed across a reverse osmosis systems and further treated with a Milli-Q plus 185 water purification equipment. Effluent samples from a waste water treatment plant serving a population of about 20 000 inhabitants located in central Portugal were used to test the validity of the developed concentration technique. Buffers solutions of pH of 4.00 and 7.00, acquired from Panreac, were used for the calibration of the pH meter.
Screening of IL-based ABS for the complete extraction of APIs
The liquid-liquid ternary phase diagrams corresponding to the ABS used for the extraction and concentration purposes carried out in this work were previously reported by Passos et al.36 Each tie-line (TL), which gives the mixture compositions to be used in the extraction experiments, was determined in this work by an established gravimetric method proposed by Merchuk et al.37 Further details are provided in the ESI†.
ABS composed of IL + C6H5K3O7 + water for the extraction of FQs and NSAIDs, corresponding to ternary mixtures in the biphasic region, were prepared gravimetrically using a Sartorius CPA225D Analytical Balance, within ± 2×10-5 g. Glass ampoules (15 cm3) were used for the ABS preparation, by adding appropriate amounts of IL, inorganic salt and water solutions containing each of the FQs and NSAIDs. The concentration of ciprofloxacin, norfloxacin, enrofloxacin, diclofenac, naproxen and ketoprofen used in the initial aqueous solutions was 5x10-2 g.L-1, while a ternary mixture composed of 40 wt% of IL, 19 wt% of C6H5K3O7 and 41 wt% of aqueous solution of APIs was used for extraction/concentration purposes. These mixtures were vigorously stirred and left to equilibrate for 24 h at (25 ± 1) ºC, to allow the equilibrium and complete separation of both phases. Subsequently, both the IL and salt-rich phases were carefully separated and weighted. The amount of each FQ and NSAID in each phase was quantified through UV-spectroscopy, using a Shimadzu UV-1800, Pharma-Spec UV-Vis Spectrophotometer, at a wavelength of 276, 275, 275, 276, 230 and 256 nm for ciprofloxacin, norfloxacin, and enrofloxacin, diclofenac, naproxen and ketoprofen, respectively, using calibration curves previously established. Ternary mixtures at the same weight fraction composition were prepared, using pure water instead of the aqueous solutions containing the FQs or NSAIDs, for blank control purposes. The pH values (± 0.02) of the IL-rich phase were measured at (25 ± 1) ºC, using a Mettler Toledo S47 SevenMulti™ dual meter pH/conductivity equipment.
The extraction efficiencies of FQs (%EEFQs) and NSAIDs (%EENSAIDs) are defined as the ratio between the total mass of each FQ or NSAID present in the IL-rich phase to that in the total mixture (both phases). Three replicates were prepared for each extraction assay, allowing for the determination of the average extraction efficiency and respective standard deviation.
Concentration of APIs using IL-based ABS
After the ILs screening for the extraction of both FQs and NSAIDs, the system composed of [N4444]Cl + C6H5K3O7 + water with a tie-line length (TLL) of 88 was selected to develop APIs´s concentration platforms. The concentration factors of FQs and NSAIDs were determined using ternary systems with different initial compositions along the same TL. In the same TL, the composition of both phases in equilibrium remains constant, only the ratio of the volume or mass of the two phases changes. This step was initially carried out using the same initial concentration (5x10-2 g.L-1) of ciprofloxacin and diclofenac in the aqueous solutions used in the screening step. Furthermore, and in order to simulate representative concentrations of APIs in aqueous environments, aqueous solutions of ciprofloxacin and diclofenac, at concentrations of circa 7×10-6 and 5×10-6 g.L-1, respectively, were used.
ABS with a total weight of 50 g were prepared. After equilibration and careful separation of the phases, the amount of ciprofloxacin and diclofenac in each phase was quantified through UV-spectroscopy, using a Shimadzu UV-1800, Pharma-Spec UV-Vis Spectrophotometer, at a wavelength of 276 nm. For the studies of real water samples, high-performance liquid chromatography (HPLC) was used to quantify ciprofloxacin and diclofenac, using a HPLC-UV from Shimadzu, Prominence Modular HPLC, using calibration curves previously determined. The chromatographic separation was achieved using a Reprosil C-18 analytical column, with porous spherical silica of 5 µm and pore diameter of 100 Å, from GmbH. The column size was of 250 × 4.6 mm. The operation temperature of the column was set at 25 ºC. The mobile phase used was a mixture of methanol (A), and water adjusted to pH 2.5 with concentrated formic acid (B). The volume ratio of solvent A to solvent B was 70:30. The elution was performed at the flow-rate of 0.8 mL.min-1 and the injection volume was 10 µL. The wavelength of the UV detector was set at 278 and 275 nm for the quantification of ciprofloxacin and diclofenac, respectively.
The concentration factor was determined as the ratio between the concentrations of each API in the IL-rich phase and in the initial aqueous solution/sample. The concentration factors of FQs and NSAIDs were determined with model systems (distilled water) and real wastewater samples from a municipal waste water treatment plant. Since in the real sample, no detectable levels of the target APIs were found, the sample was spiked with 0.002 g.L-1 of ciprofloxacin and diclofenac.
An important parameter when developing IL-based ABS as concentration techniques, is the solubility of ciprofloxacin and diclofenac in the IL-rich phase. Thus, the solubility of both APIs at 25 ± 1 ºC in the [N4444]Cl-rich phase, namely in an aqueous solution composed of 62.4 wt% [N4444]Cl + 2.1 wt% C6H5K3O7 + 35.5 wt% H2O, was determined. A total weight of 1 g of the IL-rich phase was used, in which individual amounts of FQs and NSAIDs were continuously added - (0.002 – 0.005) g - and kept under controlled stirring and temperature (25 ± 1 ºC). The solubility was determined by the visual detection of the cloud point, i.e. the appearance of solid that does not dissolve in 24 h. Three replicates were prepared for each solubility assay, allowing the determination of the average solubility value and respective standard deviation.
Results and Discussion
Screening of IL-based ABS for the complete extraction of APIs
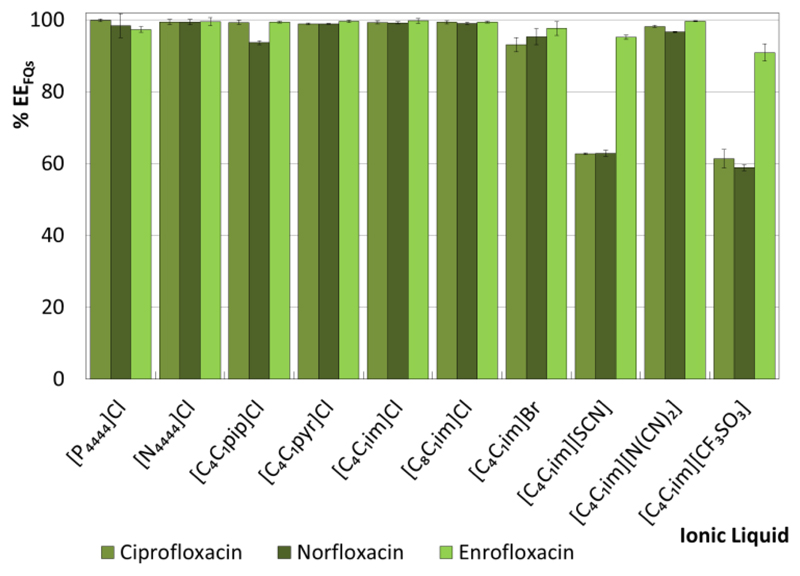
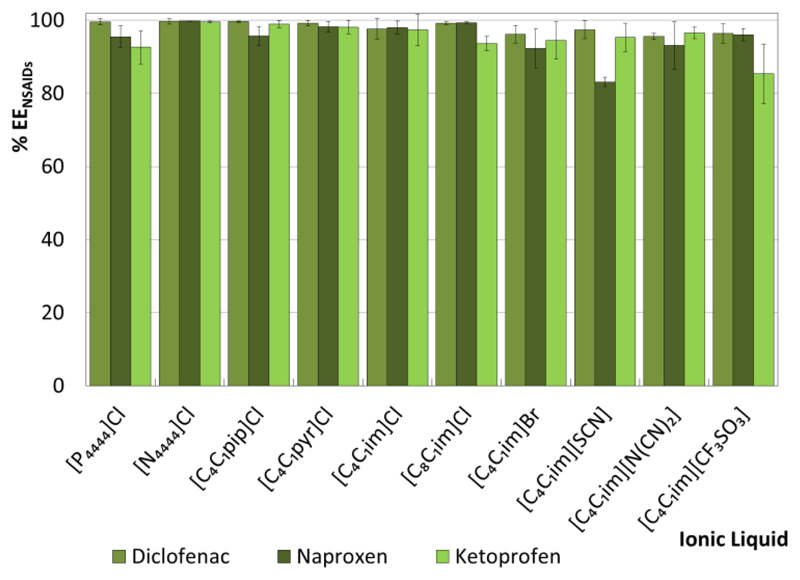
A fixed ternary mixture composition (IL ≈ 40 wt %, salt ≈ 19 wt %) was initially used to evaluate the ability of ILs of different chemical structures to extract FQs and NSAIDs from aqueous media. Although the liquid-liquid ternary phase diagrams used in the current work were previously reported by Passos et al.,36 the compositions of the two phases in equilibrium (TLs) for each extraction experiment were determined in this work and are reported in the ESI†. Figs. 3 and 4 depict the single-step extraction efficiencies of the investigated ABS at 25 ºC for FQs (%EEFQs) and NSAIDs (%EENSAIDs) (cf. the ESI† with detailed data). In all systems investigated, the studied FQs and NSAIDs preferentially partition to the IL-rich phase (top phase of the studied systems, with the exception of the system formed by [C4C1im][CF3SO3] in which an inversion on the phases densities occurs).
Fig. 3.
Extraction efficiencies of ABS composed of IL + C6H5K3O7 + H2O at 25 ºC for FQs (%EEFQs).
Fig. 4.
Extraction efficiencies of ABS composed of IL + C6H5K3O7 + H2O at 25 ºC for NSAIDs (%EENSAIDs).
The %EEFQs and %EENSAIDs of the studied ABS for the IL-rich phase range between 59% and 100%, and between 83% and 100%, respectively. Overall, the extraction efficiencies of IL-based ABS for FQs and NSAIDs follow the rank: [N4444]Cl ≈ [C4C1pip]Cl ≈ [C4C1pyr]Cl ≈ [C4C1im]Cl ≈ [C8C1im]Cl ≈ [P4444]Cl > [C4C1im]Br > [C4C1im][N(CN)2] > [C4C1im][SCN] ≈ [C4C1im][CF3SO3]. No significant differences are found between the extraction efficiencies of the investigated IL-based ABS for the different FQs and NSAIDs when using ILs with the chloride (Cl-) anion (combined with the [P4444]+, [N4444]+, [C4C1pip]+, [C4C1pyr]+ and [C4C1im]+ cations), suggesting that the IL anion plays a dominant role in the systems extraction performance. In the same line, an increase in the IL cation alkyl side chain length (from [C4C1im]Cl to [C8C1im]Cl) has no significant impact on the %EEFQs and %EENSAIDs.
When analyzing ILs with the same cation core ([C4C1im]+) combined with different anions, namely Cl-, Br-, [SCN]-, [N(CN)2]-, and [CF3SO3]-, more significant differences in the extraction efficiencies can be found. Among these, ABS containing ILs bearing the chloride-anion or anions of higher hydrogen-bond basicity38 are more efficient extraction platforms for both NSAIDs and FQs.
The pH values of the IL-rich phase range between 7 and 10, as a result of the alkaline character of the C6H5K3O7 aqueous solutions. According to the FQs and NSAIDs pKa values, all compounds are mainly present in their zwiterionic and negatively charged forms. The detailed pH data and speciation curves of each API are shown in the ESI†. In previous works,39, 40 IL-based ABS composed of IL + Al2(SO4)3 + H2O were proposed as removal techniques of APIs from aqueous streams. Since these systems were highly acidic, both FQs and NSAIDs were mainly present in their protonated form. Very high %EEFQs and %EENSAIDs, up to 98%39 and 100%,40 were obtained in a single-step extraction, respectively. Taking into account this information and the maximum extraction efficiencies found in this work, it can be concluded that electrostatic interactions between the charged APIs and IL or salt ions do not play a major role in the solutes partitioning/extraction.
Considering the extraction efficiencies of the studied APIs by the different IL-based ABS, it can be concluded that [N4444]Cl is one of the most promising candidates for extracting FQs and NSAIDs from aqueous media. Although rarely explored as phase-forming component of ABS,36, 41quaternary ammonium-based ILs present a higher aptitude to form ABS, due to their higher hydrophobicity, afforded by the four alkyl side chains. As a result, there is also a small loss of this type of ILs to the salt-rich phase (or cross-contamination). For the investigated mixtures, the amount of [N4444]Cl in the salt-rich phase is circa 2 wt % (TL data shown in the ESI†). In addition, [N4444]Cl. also has a low cost42 and low toxicity.43 Due to these advantages, the ABS constituted by [N4444]Cl and C6H5K3O7 was used in the concentration studies.
Concentration of APIs using IL-based ABS
A primary requisite to use ABS as concentration platforms is the existence of long tie-lines. The TLL is an indicator of the differences in the compositions between the two phases and it is usually used to correlate the partitioning trend of solutes between both phases.29 A long TLL not only decreases the cross-contamination of each phase by the component enriched in the opposite layer, but also affords higher concentration factors.29 The manipulation of the mixture compositions along the same TL enables the tailoring of the volumes of the coexisting phases, without changing their composition. As it was mentioned before, the ABS formed by [N4444]Cl + C6H5K3O7 exhibits a large biphasic region, leading to long TLs and thus the possibility of obtaining high concentration factors. For the study of the concentration factors, ciprofloxacin and diclofenac were chosen as representatives of the FQs and NSAIDs classes, mainly because these two APIs are classified as high priority pharmaceuticals.5
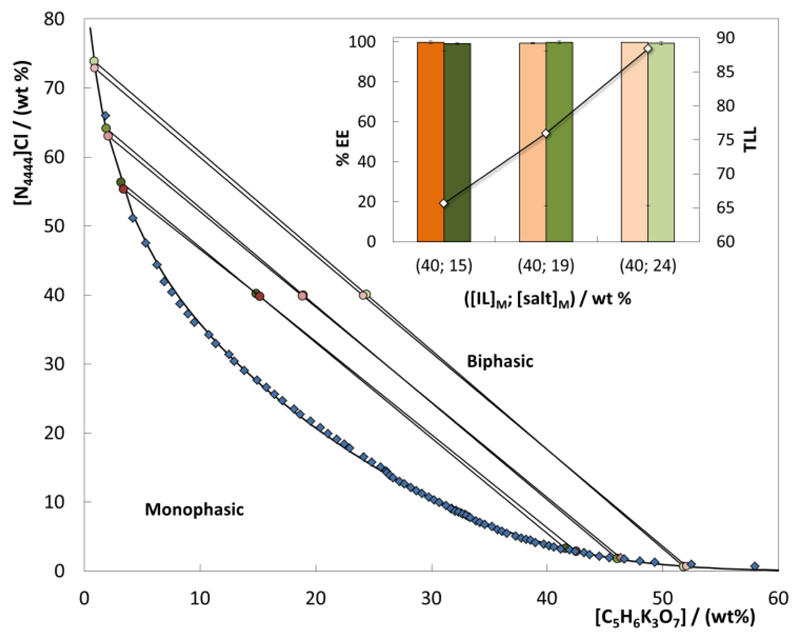
Fig. 5 presents the extraction efficiencies of the [N4444]Cl-based system as a function of the TLL for ciprofloxacin and diclofenac. Detailed data are provided in the ESI†. In general, the extraction efficiencies of the studied ABS for FQs and NSAIDs are maintained at 100% in a single-step in the different TLs evaluated, meaning that no saturation of APIs in the IL-rich phases occurs. Therefore, the longest TLL (circa 88) was further studied for concentration purposes, since it fulfills three criteria: (i) it allows a high concentration factor to be obtained; (ii) the total extraction of FQs and NSAIDs is achieved in a single-step; and (iii) there is a low amount of IL in the salt-rich phase or cross-contamination (circa 1 wt%).
Fig. 5.
Extraction efficiencies (%EE) of the [N4444]Cl-based ABS for ciprofloxacin (orange) and diclofenac (green) at (25 ± 1 ºC) using different TL: binodal curve data (◆)36; TL data (●); and TLL values (◇).
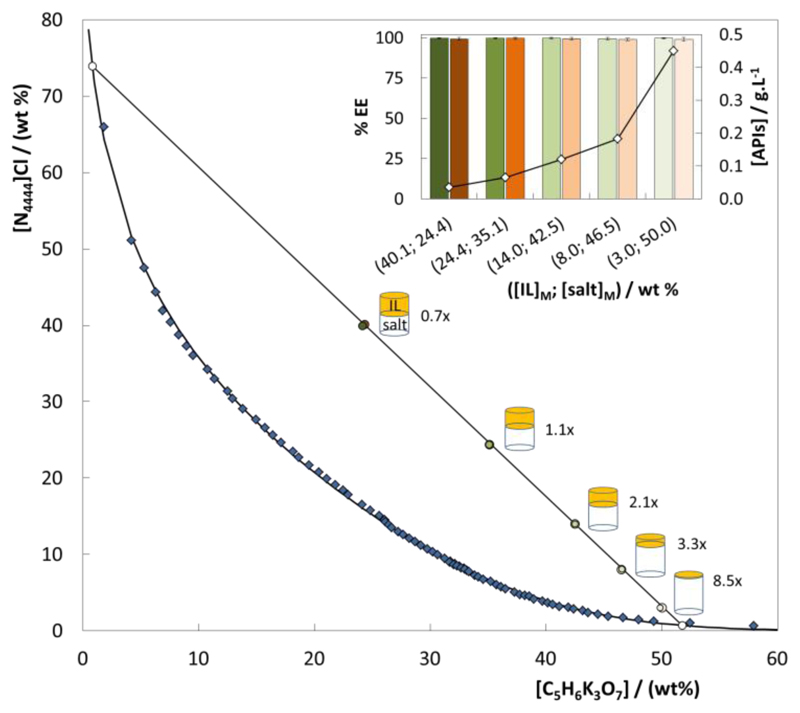
Fig. 6 shows the composition of the initial mixtures (cf. the ESI† with detailed data) along the TL with a TLL circa 88 for the [N4444]Cl + C6H5K3O7 + H2O ABS,36 (cf. the ESI† with detailed data). The extraction efficiencies values and concentration factors afforded by these mixtures for ciprofloxacin and diclofenac are also shown. As discussed before, ciprofloxacin and diclofenac are enriched in the IL-rich phase, due to their preferential migration to this phase. Concentration factors ranging from 0.6 to 9.0-fold for ciprofloxacin and from 0.7 to 8.0-fold for diclofenac were attained, showing that it is possible to concentrate FQs and NSAIDs in the IL-rich phase up to a 9-fold without losing the extraction efficiency performance or saturating the IL-rich phase. These preliminary results carried out with model systems allow to conclude about the possibility to use IL-based systems to concentrate FQs and NSAIDs, and thus overcome their difficult detection and quantification resulting from their low concentrations in aquatic real samples. It should be highlighted that in these experiments, initial concentrations of ciprofloxacin and diclofenac of circa 5x10-2 g.L-1 were used and thus the final concentrations of both APIs in the two phases in equilibrium were here determined by UV-spectroscopy. Therefore, the maximum obtained concentration factors of 9.0-fold and 8.0-fold represent concentrations of ciprofloxacin and diclofenac in the IL-rich phase of 0.45 and 0.40 g.L-1, respectively.
Fig. 6.
Extraction efficiencies (%EE) of the [N4444]Cl-based ABS for ciprofloxacin (orange) and diclofenac (green) for different initial compositions of ABS phase forming components, along the same TL at 25 ºC: (◆), binodal curve data36; (○), TL data, (●), initial composition ([IL]M; [salt]M); and (◇), final concentration of ciprofloxacin and diclofenac in the IL-rich phase ([APIs]/g.L-1).
In order to explore higher concentration factors, still along the same TL, lower initial concentrations of IL and APIs had to be used in the ABS formation and concomitantly the quantification of APIs in the IL-rich phase was carried out by HPLC-UV. The initial mixture compositions of ciprofloxacin and diclofenac used here are presented in ESI† (Table S.I.5). Since the determined limit of detection (LOD) of ciprofloxacin and diclofenac in the HPLC is of 0.5 mg.L-1 and 0.2 mg.L-1, respectively, it is possible to quantify samples contaminated with APIs in the order of µg.L-1. HPLC chromatograms of aqueous solutions of ciprofloxacin and diclofenac, with concentrations ranging between 1×10-4 and 5×10-2 g.L-1 are presented in the ESI†. Aqueous solutions of ciprofloxacin and diclofenac with a concentration circa 7×10-6 and 5×10-6 g.L-1, respectively, representative of the APIs levels that may be found in aqueous environments, were used in the study of higher concentration factors. According to Fig. 6, the complete extraction of both APIs and experimental concentration factors of 1000-fold were achieved in a single-step (cf. the ESI†, 1085 and 1164 for ciprofloxacin and diclofenac, respectively). Furthermore, and since the retention time of ciprofloxacin and diclofenac are different (2.9 min and 13.2 min, respectively), aqueous solutions containing both ciprofloxacin and diclofenac were further concentrated, and again concentration factors of 1000-fold were obtained (cf. the ESI†, 1010 and 998 for ciprofloxacin and diclofenac, respectively). This fact allows the conclusion that IL-based ABS are powerful tools to simultaneously extract and concentrate different classes of APIs.
In order to confirm that the saturation the two studied APIs in the IL-rich phase was not reached, their solubility was determined. Solubility values of 2.0 ± 0.2 g.L-1 and 5.4 ± 0.2 g.L-1 in the IL-rich phase were obtained at 25 ± 1 ºC for ciprofloxacin and diclofenac, respectively. These values are well above the equipment LOD and final concentrations determined for the highest concentration factors obtained.
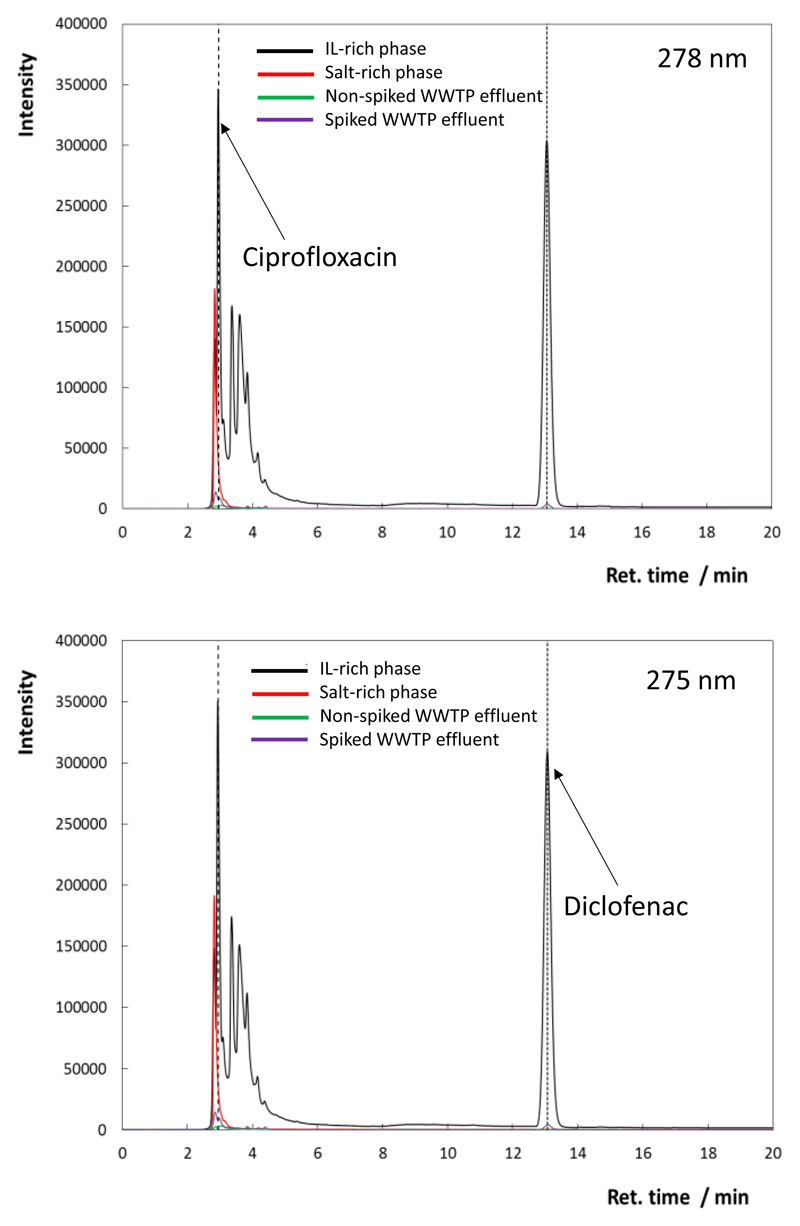
In order to further evaluate the feasibility of using the [N4444]Cl-based ABS as extraction and concentration platforms for ciprofloxacin and diclofenac, real effluent samples from a wastewater treatment plant spiked with ciprofloxacin and diclofenac were used. Although a preliminary concentration step was carried out with non-spiked samples, diclofenac and ciprofloxacin were not identified, meaning that, they are present in concentrations below µg.L-1. Such results are not a surprise, since many surveys conducted in Europe disclosed concentrations in the order of ng.L-1. Also, our effluent samples come from a WWTP that serves a population of only 20 000 inhabitants. Fig. 7 shows the HPLC-UV chromatograms at 278 nm and 275 nm, in which the peaks corresponding to ciprofloxacin and diclofenac are clearly identified and can be quantified, after reaching concentration factors of 1000-fold in a single-step (experimental concentration factors of 1006 and 1014 for ciprofloxacin and diclofenac, respectively). The chromatograms of the non-spiked and spiked WWTP effluent samples with no ABS pre-treatment are also shown. In summary, analysis of the chromatograms shows that there is no interference of the ABS phase-forming components and any other compounds present in the real sample and thus that it is possible to individually quantify ciprofloxacin and diclofenac.
Fig. 7.
HPLC-UV chromatograms corresponding to the identification/quantification of ciprofloxacin and diclofenac simultaneously extracted from WWTP effluent and 1000-fold concentrated. Peaks corresponding to ciprofloxacin and diclofenac are identified at both wavelengths. The remaining peaks correspond to the phase-forming components of the ABS and other compounds present in the real sample.
The highest detected concentration values of ciprofloxacin and diclofenac in WWTP effluents in Europe was 3.3 µg.L-1 and 3.3 µg.L-1.15 Therefore, the here proposed technique allows to identify and quantify APIs, with concentration in the µg.L-1 range, in real effluents by HPLC-UV, with LOD of 0.5 mg.L-1 and 0.2 mg.L-1 for ciprofloxacin and diclofenac, respectively, This is due to the high concentration factors achieved, ca. 1000. Although some studies have been found in the literature23,24 regarding the pre-treatment of water samples for the identification and quantification of FQs and NSAIDs, most of them reached lower enrichment factors and use of a SPE approach, which requires an additional desorption step, usually carried out with hazardous volatile organic solvents, for the target contaminants analysis. The use of IL-based ABS overcome some of these drawbacks, namely the need of an additional desorption step. From the obtained results it is expected that the proposed extraction/concentration procedure using IL-based ABS for the monitoring of APIs could be also applied to influents of a WWTP and in environmental routine analysis of other contaminants present in aqueous samples, namely in river water and seawater were APIs have already been detected.1, 2, 44
Conclusions
We propose the use of IL-based ABS as effective extraction/concentration techniques for FQs and NSAIDs in order to improve the monitoring of aqueous environmental samples, while overcoming some limitations of SPE pre-treatment techniques. ABS composed of C6H5K3O7 and imidazolium-, phosphonium- and ammonium-based ILs were firstly screened to identify the most promising systems able to completely extract the two classes of APIs. Due to the high extraction efficiencies, ability to allow high concentration factors, and lower environmental hazards, [N4444]Cl-based ABS were further investigated as concentration platforms for ciprofloxacin and diclofenac as model FQs and NSAIDs. By playing around with the initial mixture compositions along the same TL, it was possible to decrease the IL-rich phase volume and reach concentration factors of ciprofloxacin and diclofenac of 1000-fold in a single-step, shown for both model aqueous samples and real WWTP effluent samples. The proposed technology allows the simultaneous extraction/concentration of the two important classes of APIs up to values of mg.L-1 and their simple identification and quantification by HPLC-UV. IL-based ABS are thus potential candidates to pre-treat environmental aqueous samples aiming at improving the monitoring of APIs.
Supplementary Material
†Electronic Supplementary Information (ESI) available: weight fraction percentage (wt%); composition of the coexisting phases (tie-lines, TLs); Tie-line lengths (TLL); pH values of the IL-rich phases (pHIL); extraction efficiencies of FQs and NSAIDs (%EEFQs and (%EENSAIDs) and HPLC chromatograms. See DOI: 10.1039/x0xx00000x
Acknowledgements
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. H.F.D. Almeida acknowledges FCT for the PhD grant SFRH/BD/88369/2012. M.G. Freire acknowledges the funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 337753. I.M. Marrucho acknowledges the FCT Investigator Program (IF/363/2012). This work was carried out under the Research unit GREEN-it "Bioresources for Sustainability" (UID/Multi/04551/2013). The authors thank António Alçada from EPAL – “Grupo Águas de Portugal” for kindly providing the WWTP effluent.
References
- 1.aus der Beek T, Weber F-A, Bergmann A, Hickmann S, Ebert I, Hein A, Küster A. Environ Toxicol Chem. 2016;35:823–835. doi: 10.1002/etc.3339. [DOI] [PubMed] [Google Scholar]
- 2.Puckowski A, Mioduszewska K, Łukaszewicz P, Borecka M, Caban M, Maszkowska J, Stepnowski P. J Pharm Biomed Anal. 2016;127:232–255. doi: 10.1016/j.jpba.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 3.Gaw S, Thomas KV, Hutchinson TH. Phil Trans R Soc B. 2014;369 doi: 10.1098/rstb.2013.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timraz K, Xiong Y, Al Qarni H, Hong P-Y. Environmental Science: Water Research & Technology. 2017;3:293–303. [Google Scholar]
- 5.de Voogt P, Janex-Habibi M-L, Sacher F, Puijker L, Mons M. Water Sci Technol. 2009;59:39–46. doi: 10.2166/wst.2009.764. [DOI] [PubMed] [Google Scholar]
- 6.Andreu V, Blasco C, Picó Y. Trends Anal Chem. 2007;26:534–556. [Google Scholar]
- 7.Salgado R, Noronha JP, Oehmen A, Carvalho G, Reis MAM. Water Sci Technol. 2010;62:2862–2871. doi: 10.2166/wst.2010.985. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz de García S, Pinto Pinto G, García Encina P, Irusta Mata R. Sci Total Environ. 2013;444:451–465. doi: 10.1016/j.scitotenv.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 9.Kosma CI, Lambropoulou DA, Albanis TA. Sci Total Environ. 2014;466–467:421–438. doi: 10.1016/j.scitotenv.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 10.Janecko N, Pokludova L, Blahova J, Svobodova Z, Literak I. Environ Toxicol Chem. 2016;35:2647–2656. doi: 10.1002/etc.3552. [DOI] [PubMed] [Google Scholar]
- 11.European Food Safety Authority (EFSA) at www.efsa.europa.eu/ (Accessed on October 2016).
- 12.European Centre for Disease Prevention and Control (ECDC) at http://ecdc.europa.eu/en/Pages/home.aspx (Accessed on Abril 2017).
- 13.European Medicines Agency at http://www.ema.europa.eu/ema/ (Accessed on Abril 2017).
- 14.European Centre for Disease Prevention and Control at http://ecdc.europa.eu/en/publications/Publications/antimicrobial-consumption-europe-esac-net-2012.pdf (Accessed on Abril 2017).
- 15.Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F. N Biotechnol. 2015;32:147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann A, Alder AC, Koller T, Widmer RM. Environ Toxicol Chem. 1998;17:377–382. [Google Scholar]
- 17.Wagil M, Kumirska J, Stolte S, Puckowski A, Maszkowska J, Stepnowski P, Białk-Bielińska A. Sci Total Environ. 2014;493:1006–1013. doi: 10.1016/j.scitotenv.2014.06.082. [DOI] [PubMed] [Google Scholar]
- 18.GWRC, London, UK., 2004.
- 19.Tixier C, Singer HP, Oellers S, Müller SR. Environ Sci Technol. 2003;37:1061–1068. doi: 10.1021/es025834r. [DOI] [PubMed] [Google Scholar]
- 20.European Comission at http://ec.europa.eu/smart-regulation/impact/ia_carried_out/docs/ia_2012/com_2011_0876_en.pdf (Accessed on Abril 2017).
- 21.Deblonde T, Cossu-Leguille C, Hartemann P. Int J Hyg Environ Health. 2011;214:442–448. doi: 10.1016/j.ijheh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Vieno NM, Tuhkanen T, Kronberg L. J Chromatogr A. 2006;1134:101–111. doi: 10.1016/j.chroma.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 23.Prat MD, Benito J, Compañó R, Hernández-Arteseros JA, Granados M. J Chromatogr A. 2004;1041:27–33. doi: 10.1016/j.chroma.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Lopes VSA, Riente RR, da Silva AA, Torquilho DF, Carreira RdS, Marques MRdC. Mar Pollut Bull. 2016;110:572–577. doi: 10.1016/j.marpolbul.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Albertsson PA. 3rd ed. Wiley; New York: 1986. [Google Scholar]
- 26.Zaslavsky BY. Marcel Dekker, Inc.; New York: 1994. [Google Scholar]
- 27.Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. J Am Chem Soc. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- 28.Ventura SPM, e Silva FA, Quental MV, Mondal D, Freire MG, Coutinho JAP. Chem Rev. 2017;117:6984–7052. doi: 10.1021/acs.chemrev.6b00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freire MG, Claudio AFM, Araujo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN. Chem Soc Rev. 2012:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- 30.Welton T. Chem Rev. 1999;99:2071–2084. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- 31.Gaune-Escard M, Seddon KR. John Wiley; Hoboken, N.J.: 2010. [Google Scholar]
- 32.Rogers RD, Seddon KR. Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 33.Conceicao LJA, Bogel-Lukasik E, Bogel-Lukasik R. RSC Adv. 2012;2:1846–1855. [Google Scholar]
- 34.Passos H, Sousa ACA, Pastorinho MR, Nogueira AJA, Rebelo LPN, Coutinho JAP, Freire MG. Anal Methods. 2012;4:2664–2667. [Google Scholar]
- 35.Dinis TBV, Passos H, Lima DLD, Esteves VI, Coutinho JAP, Freire MG. Green Chem. 2015;17:2570–2579. doi: 10.1039/C5GC00077G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passos H, Ferreira AR, Cláudio AFM, Coutinho JAP, Freire MG. Biochem Eng J. 2012;67:68–76. [Google Scholar]
- 37.Merchuk JC, Andrews BA, Asenjo JA. J Chromatogr B Biomed Sci Appl. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- 38.Claudio AFM, Swift L, Hallett JP, Welton T, Coutinho JAP, Freire MG. Phys Chem Chem Phys. 2014;16:6593–6601. doi: 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- 39.Almeida HFD, Freire MG, Marrucho IM. Green Chem. 2016;18:2717–2725. doi: 10.1039/c5gc02464a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida HFD, Marrucho IM, Freire MG. ACS Sustain Chem Eng. 2017;5:2428–2436. doi: 10.1021/acssuschemeng.6b02771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bridges NJ, Gutowski KE, Rogers RD. Green Chem. 2007;9:177–183. [Google Scholar]
- 42.Passos H, Freire MG, Coutinho JAP. Green Chem. 2014;16:4786–4815. doi: 10.1039/C4GC00236A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munoz M, Domínguez CM, de Pedro ZM, Quintanilla A, Casas JA, Ventura SPM, Coutinho JAP. Sep Purif Technol. 2015;150:252–256. [Google Scholar]
- 44.Hughes SR, Kay P, Brown LE. Environ Sci Technol. 2013;47:661–677. doi: 10.1021/es3030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.