Abstract
Background:
Although individuals with, or at risk for, psychotic disorders often show difficulties with performance monitoring and feedback processing, findings from studies using event-related potentials (ERPs) to index these processes are not consistent. This meta-analytic review focused on studies of two different indexes of performance monitoring, the early error-related negativity (ERN; n = 25) and the later error positivity (Pe; n = 17), and one index of feedback processing, the feedback negativity (FN; n = 6).
Methods:
We evaluated whether individuals (1) with psychotic disorders, or (2) at heightened risk for these disorders differ from healthy controls in available studies of the ERN, Pe, and FN.
Results:
There was a significant, large ERN reduction in those with psychosis (g = − .96) compared to controls, and a significant, moderate ERN reduction in those at-risk (g = −.48). In contrast, there were uniformly non-significant, small between-group differences for Pe and FN (gs ≤ ∣.16∣).
Conclusions:
The results reveal a differential pattern of impairment in psychosis. Early performance monitoring (ERN) impairments are substantial among those with psychotic disorders in general and may be a useful vulnerability indicator for these disorders. However, later performance monitoring (Pe) and basic feedback processing (FN) appear to be relatively spared in psychosis.
Keywords: ERN, Pe, FN, error-related negativity, error positivity, feedback-related negativity
1. Introduction
Impairments in daily life functioning, including diminished engagement in productive and pleasurable activities, are hallmarks of psychotic disorders (Barch and Dowd, 2010; Blanchard et al., 2011). These impairments are directly linked to cognitive deficits, which have been extensively documented in psychosis and psychosis-risk. The ability to accurately monitor one’s performance, and integrate internal (e.g., self-generated comparisons of whether performed actions match their intended outcomes) and external performance feedback (e.g., externally-generated information indicating favorable vs. unfavorable outcomes), are critical aspects of cognition, reward processing, and learning as they guide adaptive decision-making and productive behavior (Falkenstein et al., 1990; Gehring et al., 1993; Holroyd and Coles, 2002). A number of investigators have used event-related potentials (ERPs) to assess whether distinct aspects of performance monitoring and feedback processing are impacted in those with schizophrenia, with psychotic disorders more broadly, or at heightened risk for developing one of these disorders. Most investigations have focused on two ERP components that measure performance monitoring, the error-related negativity (ERN) and error positivity (Pe), and one that measures feedback processing, the feedback negativity (FN). Study findings have varied considerably across these three components, making it hard to draw conclusions. To date, there has not been an integrative quantitative review of this literature.
The purpose of this meta-analytic review is to determine whether individuals with psychotic disorders (including schizophrenia, schizoaffective disorder, delusional disorder, schizophreniform disorder, schizophrenia spectrum disorders otherwise specified, and mood disorders with psychotic features) or at heightened risk for these psychotic disorders (either genetic risk, clinical high-risk, or psychometric high-risk samples) differ from healthy controls on these three ERP components. Additionally, we will evaluate potential moderators of these components, where applicable. This information can shed light on how performance monitoring and feedback processing is impacted in psychosis-related psychopathology.
1.1. ERPs associated with performance monitoring: ERN and Pe
The ERN (also known as the Ne) is a response-locked ERP that has been associated with performance monitoring of actions and detecting errors (Falkenstein et al., 1990; Gehring et al., 1993; Simons, 2010). It is generally assessed with choice reaction time tasks, such as the flanker or go/no go paradigms. The onset of the ERN occurs shortly before or at the moment of an erroneous response and peaks approximately 100 ms later at midline frontocentral scalp locations (Gehring et al., 2012). Initial evidence from source localization, functional magnetic resonance imaging, and single unit recording studies suggests that the ERN may be generated within the dorsal region of the anterior cingulate cortex (ACC) (e.g., Debener et al., 2005; Holroyd and Coles, 2002), a structure centrally involved in performance monitoring and error detection (Taylor et al., 2007).
In psychosis research, the ERN has received the most attention of the three ERP components considered in this review. Across 22 separate studies of individuals with schizophrenia, the vast majority have reported reduced ERN compared to healthy controls. The overall magnitude of the reduction is unclear and the potential impact of methodological differences across studies (e.g., sample characteristics, type of paradigm) has not been evaluated. A smaller number of studies have examined the ERN in individuals with more broadly defined psychotic disorders (n = 4) or at-risk groups (n = 7). Although ERN reductions are also typically reported in these samples, the overall magnitude of the reductions has not been evaluated.
The ERN is typically followed by the Pe component. The Pe peaks in the centroparietal region between 200 and 400 ms after an erroneous response. Despite some debate (Gehring et al., 2012; e.g., Van Veen and Carter, 2002), the Pe is typically thought to index error awareness or the ability to detect errors (Endrass et al., 2007; Nieuwenhuis et al., 2001), and it has been reported that the Pe may be generated by the rostral ACC (Endrass et al., 2007).
Compared to the ERN, fewer studies have examined the Pe in those with schizophrenia (n = 13), those with broadly defined psychotic disorders (n = 3), or at-risk groups (n = 7). In contrast to the consistent reports of reduced ERN, studies of the Pe have been decidedly mixed, finding either relatively small reductions or no differences between these groups and healthy controls. It is unclear whether differences in methodologies or clinical characteristics may account for inconsistencies across studies.
1.2. ERP associated with external feedback processing: FN
The FN is typically assessed using simple gambling or feedback-based learning paradigms (Simons, 2010) and, in contrast to the ERN and Pe, is elicited by externally provided feedback about positive versus negative outcomes. The feedback stimulus-locked FN peaks between 250 and 300 ms after feedback onset and is maximal over the frontocentral region. In addition, it is relatively more negative-going after unfavorable versus favorable feedback (e.g., a monetary loss compared to a monetary gain). The FN has historically been viewed as tracking the occurrence of unfavorable outcomes (negative reward prediction errors). Some, however, have argued that the FN tracks the occurrence of favorable outcomes (positive reward prediction error), resulting in a reward-related positivity (i.e., “Reward Positivity”) that is absent or suppressed following an unfavorable outcome (for a review, see Proudfit, 2015). This is supported by tentative evidence that the FN originates from the striatum (e.g., Carlson et al., 2011; Foti et al., 2011). Others propose the FN reflects an unsigned salience/surprise signal or that multiple processes (e.g., positive reward prediction error and unsigned salience signal) may contribute to the FN (Hauser et al., 2014; Cavanaugh & Frank, 2014; Sambrook & Goslin, 2016). For the sake of consistency with previous research in this area, the current review will refer to this component as the "FN".
Compared to the ERN and Pe, relatively few studies investigated the FN in those with schizophrenia (n = 4), broadly defined psychosis (n = 1) or at risk for psychotic disorders (n = 1). Almost all reported intact FN in schizophrenia across these groups. However, the sample sizes were relatively small, and it is unclear whether reliable differences between these groups and healthy controls are detectable.
1.3. The current study
Overall, findings from the ERP literature regarding performance monitoring and feedback processing in psychotic disorders and at-risk populations are mixed. To clarify these findings, we employed meta-analysis, a powerful statistical technique that can identify trends across relatively small studies. For the ERN and Pe, the goals of the review were to: (1) determine whether individuals with psychotic disorders or at-risk groups show reliable impairments compared to non-psychiatric controls and to quantify the corresponding effect sizes, and (2) evaluate potential methodological (type of paradigm, ERP quantification methods) and patient characteristic (diagnosis, patient status, phase of illness) moderators of these components. Given the smaller database for the FN, we focused on determining whether individuals with psychosis show a reliable impairment compared to healthy controls and quantifying the effect size.
2. Materials and method
2.1. Eligibility Criteria for Meta-Analysis
The current meta-analysis followed PRISMA guidelines (Moher et al., 2009) for transparent and replicable methods and findings. Please see the Supplementary Table 1 for the PRISMA checklist.
Inclusion criteria for the current analyses were as follows: 1) the study included a sample of either 1A) all patients meeting DSM-III-R (APA, 1987) or DSM-IV (APA, 2000) criteria for schizophrenia or schizoaffective disorder, 1B) patients with any DSM disorder also reporting psychotic symptoms (e.g., schizophrenia, major depressive disorder with psychotic features) or 1C) individuals "at risk" for schizophrenia-spectrum disorders identified by either a structured clinical interview (clinical risk), a family history of a 1st degree family member with schizophrenia/schizoaffective disorder (genetic risk), or standardized questionnaire measures (psychometrically-defined risk); 2) a nonpsychiatric control sample (i.e., sample with no history of psychopathology determined by study-specific methods/criteria); 3) the study task required overt participant responses and is generally recognized as a reliable elicitor of ERN, Pe, or FN ERPs; 4) amplitude of the ERN, Pe, or FN ERP waveform was reported for patients/at risk and control subjects; 5) statistics were reported that allowed for calculation of effect size (standardized mean difference or Hedges’ g) of ERN/Pe/FN ERP waveform amplitude; and 6) study findings were reported in an English language, peer-reviewed journal article. Studies were excluded if they did not meet inclusion criteria. There were no other exclusion criteria. The literature search began on February 2, 2017, and ended on March 21, 2017.1
There were inconsistencies in the literature regarding nomenclature, measured time windows, specific electrodes included, and quantification of the waveforms. However, of the included studies, the ERN was characterized as a negative-going waveform, recorded at the frontocentral region (e.g., Fz, FCz, Cz; American Encephalographic Society, 1994), and occurring between 0 and 100 msec after an incorrect response. Similarly, the Pe was categorized as a positive-going waveform, recorded at the midline (e.g., Pz; American Encephalographic Society, 1994), and occurring approximately 200-500 msec after an incorrect response. Finally, the FN was categorized as a negative-going waveform, recorded at the frontocentral region, and occurring between 250-350 msec after a response performance feedback was given.
2.2. Information Sources, Search Terms, and Study Selection
We identified relevant studies using PubMed and PsycINFO online databases using the following search terms: (schizophrenia OR schizoaffective OR psychotic* OR psychosis) AND (EEG OR ERP) AND (ERN OR Pe OR FN or FRN or error* or feedback*). For all of the relevant papers that were identified by the computer searches, we examined the references to see if other relevant articles would be identified. Additionally, we conducted a cited reference search of each included article. For every article identified in the computer search, the authors on the current article reviewed the title and abstract to ensure that it was appropriate to be included in the meta-analysis. Multiple studies from the same research group were flagged for further review to ensure that the samples were nonoverlapping. When the study results were ambiguous or insufficient for meta-analysis (e.g., information required to calculate effect size was not reported), the corresponding author of that particular study was contacted for further information.
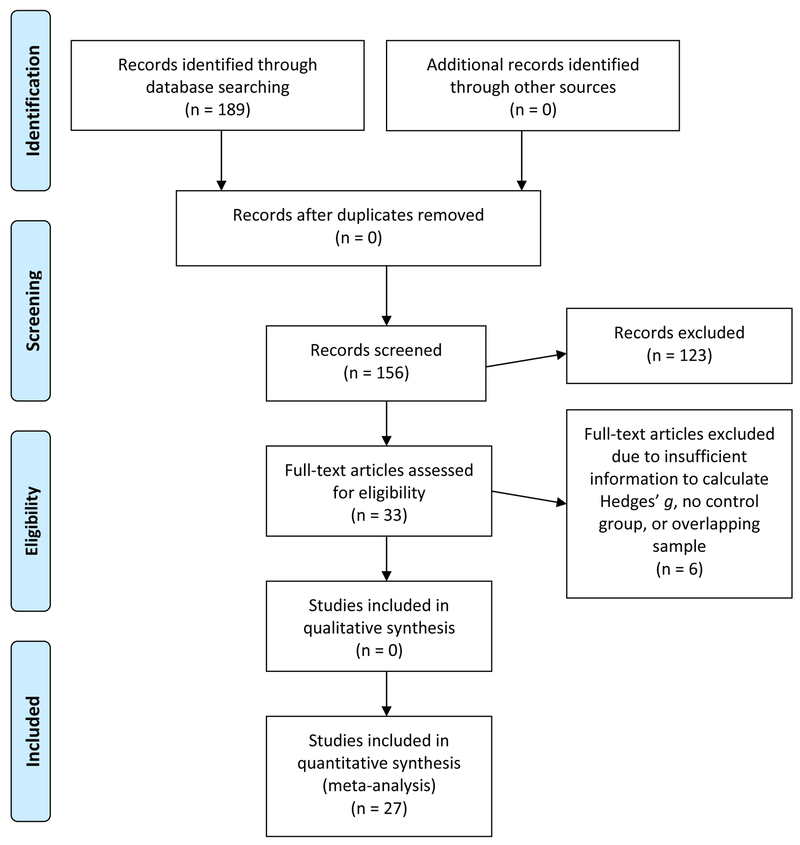
A PRISMA diagram outlining the systematic review and selection of studies is presented in Figure 1 (Moher et al., 2009). Combined searches of PubMed and PsycINFO yielded 189 unique records. After screening the titles and abstracts, 33 records were identified as possibly fulfilling inclusion criteria and were subject to further review. Six articles were subsequently excluded for the following reasons: 3 studies did not include sufficient information to calculate Hedges’ g and requests for information from the first and corresponding authors were unsuccessful (Alain et al., 2002; Charles et al., 2017; Kopp and Rist, 1999); 2 studies did not include a nonpsychiatric control group (Foti et al., 2016; Schneider et al., 2013); and 1 study had an overlapping sample that was already included (Foti et al., 2013). Thus, 27 full-text articles were included in the current analyses: 5 reporting ERN only, 2 reporting FN only, 16 reporting both ERN and Pe, 2 reporting ERN and FN, and 2 reporting all three components. No articles reported the Pe only or both Pe and FN only. Subsequent searches (i.e., reference lists and cited reference searches of included articles) yielded no additional records. Two coders (E. Martin and M. Moore) recorded relevant data from each article and confirmed the accuracy through consensus meetings of the information coded. For access to the review protocol, including coding book, please contact the corresponding author (E. Martin).
Figure 1.
PRISMA Flow Diagram.
2.3. Moderators Examined
For ERN and Pe, each identified article was coded for several potential moderators related to study methodology and sample characteristics. From the information available, we examined moderators when the effect size distributions were heterogeneous. One moderator was type of activation task employed (i.e., non-verbal vs. verbal task). A "non-verbal" task was one that involved simple visual stimuli and a speeded motor response to stimuli (Go/No Go, Flanker) whereas a "verbal" task (Stroop, picture-word matching task) was one that involved word reading followed by a response. Another moderator examined was method of ERP component calculation (difference score between error and correct trials vs. baseline comparison). A third moderator was diagnosis of psychosis patient samples (schizophrenia/schizoaffective disorder only vs. broadly-defined psychotic disorders). A fourth moderator examined was clinical status of the patient samples (inpatients vs. outpatients). Finally, we examined whether phase of illness of the patient samples (early vs. chronic phase) moderated any effects. Patients were characterized as being in the early phase of illness if their onset of psychosis was 5 years or less before testing, and were characterized as being in the chronic phase if the onset was greater than 5 years prior to testing.
2.4. Data Analysis Plan
The primary variables of interest were the amplitudes of the ERN, Pe, and FN ERP waveforms for the patient and at-risk samples compared to healthy control subjects. For the ERN and Pe, we used or calculated delta scores for ERN (error – correct trials) and Pe (error - correct trials) whenever possible. This was not possible for 10 ERN and 8 Pe studies, and we instead used the response to error trials for ERN and Pe. For all FN studies, a delta score (non-reward – reward) trials was calculated. For each study, Hedges’ g and SE, 95% confidence interval (CI), inverse variance weight, and weighted effect size (ES) of ERN, Pe, and/or FN waveform amplitude were calculated. For studies with multiple psychosis groups, i.e., a schizophrenia/schizoaffective group and a broadly-defined psychosis group (Foti et al., 2012; Minzenberg et al., 2014), the overall difference between cases and controls in ERP response was used for calculation of Hedges’ g in the primary analysis. Similarly, for studies with multiple conditions (e.g., Morris et al., 2006), the overall difference between groups in ERP response across conditions was used for calculation of Hedges’ g.
Across studies, the overall weighted mean effect size and SE and 95% CI were calculated for the ERN, Pe, and FN waveforms, and z tests were performed to determine if they were significantly different from zero. Estimates were derived from fixed effect models. Random effects models were not employed due to an increased risk for Type I error when the number of studies is small (Guolo and Varin, 2017). Homogeneity of the effect size distributions was assessed using the Q and I2 statistic. For analyses with fifteen or more studies, potential publication bias was assessed using funnel plots, which graph the effect size against the total sample size of each study, and Egger’s regression test of funnel plot asymmetry. For the funnel plot, a symmetrical, inverted cone-shaped distribution of effect sizes centered about the overall weighted mean effect size suggests absence of publication bias. Impact of potential moderators was assessed using the partitioned Q statistic.
3. Results
3.1. Characteristics of included studies and samples
Information about the study paradigms, including type of activation task and ERP component(s) examined, are presented in Table 1. The total sample size of the studies (N = 27) ranged from 18 to 267 (median = 36). For studies that examined the ERN or Pe, 17 used a nonverbal task (e.g., flanker task) and 9 used a verbal behavioral task (e.g., Stroop task). Regarding method of ERP component calculation, 10 reported peak amplitude and 17 reported mean amplitude; of these 27 studies, 17 reported delta amplitudes (i.e., error-correct amplitudes).
Table 1:
Study characteristics
| Study and Country of Origin | Sample size (Cases: Controls) | Activation Task | ERP assessed |
|---|---|---|---|
|
Araki et al. (2013)
USA |
18:18 | Stroop task | Mean amplitude ΔERN |
|
Bates et al. (2002) Canada |
21:21 | Go/No-Go | Peak amplitude ΔERN |
|
Bates et al. (2004) Canada |
9:9 | Go/No-Go | Peak amplitude ΔERN, ΔPe |
|
Bedwell et al. (2016) USA |
31:13 | Pavlovian monetary reward | Mean amplitude ΔFN |
|
Chan et al. (2015a) USA |
22:20 | Flanker task | Mean amplitude ERN, Pe |
|
Chan et al. (2015b) USA |
14:12 | Flanker task | Mean amplitude ERN, Pe |
|
de la Asuncion et al. (2015) Belgium |
22:21 | Simon Task | Peak amplitude ERN |
|
Ford et al. (2009) USA |
11:10 | Go/No-Go | Peak amplitude ERN |
|
Foti et al. (2012) USA |
104:33 | Flanker task | Mean amplitude ΔERN, ΔPe |
| Horan et al. (2012) | 16:14 | Flanker task | Mean amplitudeΔERN, ΔPe, ΔFN |
| USA | Monetary gambling task | ||
|
Houthoofd et al. (2013) Belgium |
12:12 | Flanker task | Mean amplitude ΔERN |
|
Kansal et al. (2014) Canada |
18:18 | Stroop task | Mean amplitude ΔERN, ΔPe |
| Karcher et al. (2016) USA |
42:20 | Reversal learning task | Mean amplitude ΔFN |
| Kim et al. (2006) Korea |
15:15 | Stroop task | Mean amplitude ΔERN, ΔPe |
|
Kim et al. (2015) Korea |
17:20 | Simon Task | Peak amplitude ΔERN, ΔPe |
|
Laurens et al. (2010) UK |
22:26 | Go/No-Go | Peak amplitude ΔERN, ΔPe |
| Llerena et al. (2016) | 82:48 | Flanker task | Mean amplitude ΔERN, ΔPe, ΔFN |
| USA | Time estimation task | ||
|
Mathalon et al. (2002) USA |
18:18 | Picture-word matching task | Mean amplitude ERN, Pe |
|
Minzenberg et al. (2014) USA |
73:54 | Stroop task | Mean amplitude ΔERN, ΔPe |
|
Morris et al. (2006) USA |
16:11 | Flanker task | Mean amplitude ERN, Pe |
|
Morris et al. (2008) USA |
26:27 | Probabilistic learning task | Mean amplitude ΔERN, ΔFN |
| Morris et al. (2011) | 20:15 | Flanker task | Peak amplitude ΔERN, ΔFN |
| USA | Passive gambling task | ||
|
Perez et al. (2012) USA |
132:135 | Picture-word matching task | Mean amplitude ERN, Pe |
|
Rabella et al. (2016) Spain |
9:12 | Flanker task | Peak amplitude ERN, Pe |
| Reinhart et al. (2015) USA |
17:18 | Feedback learning task | Mean amplitude ΔERN |
|
Simmonite et al. (2012) UK |
29:35 | Go/No-Go | Peak amplitude ERN, Pe |
|
Zou et al. (2014) China |
15:15 | Go/No-Go | Peak amplitude ΔERN, ΔPe |
Table 2 presents descriptive data for studies of patient and at-risk samples. For the psychosis studies, the grand mean age was 37.6 years (SD = 9.3), and the grand mean for level of education was 13.6 years (SD = 0.8). For the at-risk samples, the grand mean age was 19.57 years (SD = 4.66), and the grand mean for level of education was 14.41 years (SD = 0.41).
Table 2.
Sample characteristics, a) psychosis studies (k=23) and b) at-risk studies (k=8).
| Study | Mean age years (s.d.) |
Gender Female: Male |
Mean education years (s.d.) |
Mean duration of illness years (s.d.) |
Mean symptom ratings (s.d.) |
Clinical status and diagnostic group |
|||
|---|---|---|---|---|---|---|---|---|---|
| Case | Ctrl | Case | Ctrl | Case | Ctrl | ||||
| a. Psychosis studies | |||||||||
| Araki et al. (2013) | 44.0 (10.3) |
36.9 (12.0) |
0:18 | 0:18 | 13.3 (1.4) |
15.3 (1.3) |
NR | PANSS Total= 80.4 (23.6) |
NR Schizophrenia |
| Bedwell et al. (2016) | NR | NR | NR | NR | NR | NR | NR | PANSS Positive= 12.3 (5.5) PANSS Negative=12.6 (5.6) |
Outpatients Psychotic d/o |
| Bates et al. (2002) | 35.0 (8.7) |
34.0 (8.1) |
4:17 | 4:17 | NR | NR | NR | NR | Inpatients Schizophrenia |
| Bates et al. (2004) | 35.8 (7.2) |
32.8 (6.6) |
0:9 | 0:9 | NR | NR | NR | NR | Inpatients Schizophrenia |
| Chan et al. (2015b) | 36.9 (7.8) |
37.2 (8.8) |
9:05 | 8:4 | 14.5 (3.2) |
14.5 (1.9) |
NR | PANSS Positive= 16.1 (6.0) PANSS Negative= 15.9 (6.6) PANSS General= 28.3 (7.8) |
Outpatients Psychotic d/o |
| de La Asuncionet al. (2015) | 33.0 (9.7) |
28.1 (8.5) |
4:18 | 2:19 | NR | NR | 9.0 (8.0) |
SAPS Total=12.8 (11.6) SANS Total=33.3 (18.8) |
NR Schizophrenia |
| Ford et al. (2009) | 37.9 (12.7) |
37.7 (10.3) |
3:8 | 3:7 | 12.8 (3.4) |
18.9 (2.5) |
NR | BPRS Total=39.0 (9.6) |
Inpatients and outpatients Schizophrenia |
| Foti et al. (2012) | 43.7 (9.2) |
43.8 (12.8) |
25:53 | 11:22 | NR | NR | NR | SAPS Psychotic= 2.5 (5.5) SAPS Disorganized= 2.2 (3.7) SANS Total= 12.5 (10.9) |
Outpatients Psychotic d/o |
| Horan et al. (2012) | 46.9 (7.6) |
43.5 (9.3) |
9:26 | 8:25 | 13.1 (1.5) |
14.6 (1.6) |
23.5 (8.6) |
BPRS Positive=2.1 (0.8) BPRS Negative=1.7 (0.9) BPRS Total=41.6 (10.5) |
Outpatients Schizophrenia |
| Houthoofd et al. (2013) | 31.1 (11.3) |
28.0 (8.8) |
1:11 | 4:7 | NR | NR | NR | PANSS Positive= 22.2 (7.3) PANSS Negative= 21.9 (8.0) PANSS General= 40.4 (9.6) |
Inpatients Schizophrenia |
| Kansal et al. (2014) | 43.2 (7.8) |
41.1 (9.8) |
7:11 | 10:8 | NR | NR | NR | SAPS Total= 4.2 (4.3) SANS Total= 8.9 (4.0) |
Outpatients Schizophrenia |
| Kim et al. (2006) | 27.9 (5.4) |
26.1 (4.3) |
6:9 | 6:9 | 15.1 (3.0) |
16.0 (1.3) |
5.5 (4.4) |
PANSS Positive= 16.2 (7.4) PANSS Negative= 16.0 (7.1) PANSS General= 29.5 (9.8) |
NR Schizophrenia |
| Llerena et al. (2016) | 59.5 (10.7) |
47.5 (8.8) |
23:70 | 23:40 | 13.1 (1.8) |
14.6 (1.9) |
NR | PANSS Positive= 18.0 (7.2) PANSS Negative= 14.8 (6.3) |
Outpatients Schizophrenia |
| Mathalon et al. (2002) | 40.0 (8.0) |
43.0 (10.0) |
1:17 | 1:17 | 14.2 (2.0) |
16.9 (2.0) |
NR | BPRS Total= 42.6 (7.8) |
Inpatients and outpatients Schizophrenia |
| Minzenberg etal. (2014) | 21.1 (3.4) |
20.1 (2.4) |
24:75 | 26:28 | 12.6 (2.1) |
13.6 (2.0) |
<1 | SAPS Total= 8.6 (11.5) SANS Total= 10.7 (9.0) |
Outpatients Psychotic d/o (early phase) |
| Morris et al. (2006) | 31.4 (7.4) |
31.6 (6.1) |
5:11 | 4:7 | 14.1 (1.6) |
15.2 (1.2) |
7.38 (6.5) |
SANS Total= 6.4 (3.8) BPRS Total= 27.9 (5.4) |
Outpatients Schizophrenia |
| Morris et al. (2008) | 45.0 (6.3) |
43.4 (11.3) |
8:18 | 11:16 | 12.9 (2.9) |
15.2 (3.0) |
NR | SANS Total= 33.1 (17.6) BPRS Total= 37.0 (10.5) |
Outpatients Schizophrenia |
| Morris et al. (2011) | 47.1 (6.7) |
46.7 (11.0) |
6:26 | 5:18 | 14.5 (2.3) |
13.0 (1.7) |
NR | SANS Total=32.2 (16.2) BPRS Total= 36.1 (11.3) |
Outpatients Schizophrenia |
| Perez et al. (2012) | 29.6 (11.6) |
27.9 (9.8) |
20:64 | 42:68 | NR | NR | NR | PANSS Total= 66.4 (16.4) |
Outpatients Schizophrenia (early phase and chronic phase) |
| Reinhart et al. (2015) | 43.1 (7.8) |
38.2 (10.8) |
8:11 | 8:10 | 12.6 (1.98) |
NR | 22.6(7.86) | SAPS Total=16.8 (15.4) SANS Total= 31.7 (16.9) |
Outpatients Schizophrenia |
| Simmonite et al. (2012) | 19.5 (1.7) |
17.9 (2.2) |
10:19 20:15 NR | NR | <5 | NR | NR Schizophrenia (early phase) |
||
| b. At-risk studies | |||||||||
| Chan et al. (2015a) | 19.2 (1.7) |
19.9 (2.2) |
11:11 | 11:9 | NR | NR | n/a | SPQ-BR Total= 73.7 (16.1) |
College students, not seeking treatment, psychometric schizotypy |
| Karcher et al. (2016) | PE= 18.5 (0.6) SocAn h= 18.7 (0.8) |
18.4 (0.8) |
15:17 | 10:11 | NR | NR | n/a | NR | College students, not seeking treatment, psychometric schizotypy |
| Kim et al. (2015) | 21.0 (1.5) |
21.4 (2.0) |
9:8 | 10:10 | 14.82 (.81) |
14.55 (.95) |
n/a | SPQ Total= 41.7(5.5) |
College students, not seeking treatment, psychometric schizotypy |
| Laurens et al. (2010) | 11.2 (0.9) |
11.3 (0.8) |
8:14 | 14:12 | NR | NR | n/a | NR | School-age children, psychometrically defined putative antecedents of Sz |
| Perez et al. (2012) | 18.9 (4.1) |
20.0 (4.3) |
19:29 | 41:47 | NR | NR | n/a | SOPS Total= 32.3 (13.7) |
Clinical high-risk |
| Rabella et al. (2016) | 30.4 (5.8) |
28.2 (6.8) |
7:2 | 5:7 | NR | NR | n/a | SPQ Total = 44.7(6.8) O-LIFE Total= 61.7(9.4) |
not seeking treatment, Schizotypal personality disorder |
| Simmonite et al. (2012) | 17.9 (2.2) |
17.9 (2.2) |
21:15 | 20:15 | NR | NR | n/a | NR | not seeking treatment, Unaffected siblings of Sz probands |
| Zou et al. (2014) | 20.3 (1.8) |
20.8 (1.5) |
8:7 | 9:6 | 14.00 (1.34) |
14.87 (1.73) |
n/a | SPQ Total= 42.0 (6.6) |
College sample, not seeking treatment, psychometric schizotypy |
Note: BPRS, Brief Psychiatric Symptom Scale; Ctrl, healthy control group; NR, not reported; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences; PANSS, Positive and Negative Syndrome Scale; PE, psychotic experiences group; Psychotic d/o; broadly-defined psychotic disorders; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; SocAnh, social anhedonia group; SOPS, Scale of Prodromal Symptoms; SPQ, Schizotypal Personality Questionnaire; SPQ-BR, SPQ-Brief version; Schizophrenia, schizophrenia/schizoaffective/schizophreniform disorder.
Across all studies, eight reported medication dosing information and 22 reported symptom ratings. Exclusion criteria for patients included history of neurological conditions (14 studies) or head injury (12 studies), current substance abuse (18 studies), intellectual disability (5 studies), or previous electroconvulsive therapy (2 studies). Regarding inclusion criteria for nonpsychiatric control samples, 18 studies screened participants for DSM-IV Axis I psychotic conditions using a structured or semi-structured clinical interview, 5 screened for DSM-IV Axis II conditions, 19 excluded for recent history of substance abuse, and 13 studies excluded participants with a history of psychotic disorder in a first-degree relative. Seventeen studies excluded control participants with a history of neurological disorder, and 12 studies excluded controls with a history of head injury.
3.2. ERN results
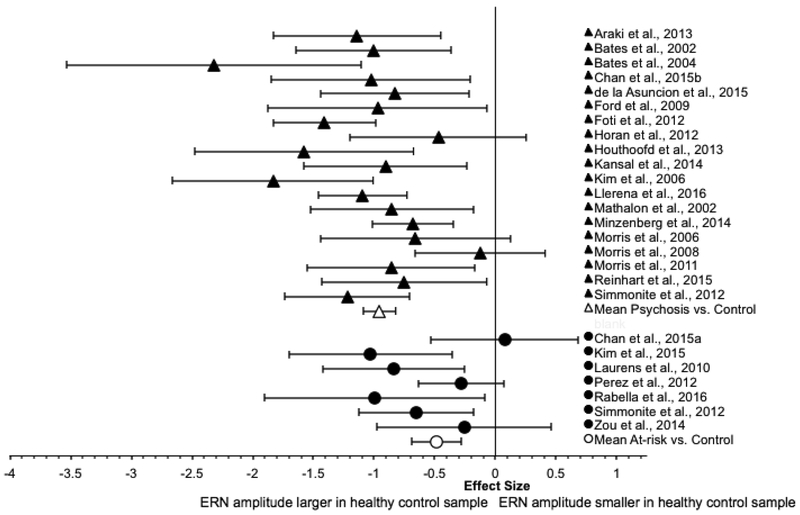
The ERN results for the two groups are summarized in Table 3, and corresponding forest plots of the effect size and 95% CI for each study is presented in Figure 2.
Table 3.
ERN effect sizes.
| Study Name | n Case: n Ctrl | g (SE) | 95% CI | Weighted ES | |
|---|---|---|---|---|---|
| Psychosis vs. Ctrl | Araki et al., 2013 | 18: 18 | −1.14 (0.35) | −1.83, −0.45 | −9.23 |
| Bates et al., 2002 | 21: 21 | −1.01 (0.33) | −1.65, −0.36 | −9.37 | |
| Bates et al., 2004 | 9: 9 | −2.32 (0.62) | −3.54, −1.10 | −6.02 | |
| Chan et al., 2015b | 14: 12 | −1.02 (0.42) | −1.84, −0.20 | −5.85 | |
| de la Asuncion et al., 2015 | 22: 21 | −0.83 (0.31) | −1.44, −0.21 | −8.44 | |
| Ford et al., 2009 | 11: 10 | −0.97 (0.46) | −1.87, −0.07 | −4.60 | |
| Foti et al., 2012 | 78: 33 | −1.41 (0.22) | −1.83, −0.98 | −30.01 | |
| Horan et al., 2012 | 16: 14 | −0.47 (0.37) | −1.20, 0.25 | −3.45 | |
| Houthoofd et al., 2013 | 12: 12 | −1.58 (0.46) | −2.48, −0.67 | −7.45 | |
| Kansal et al., 2014 | 18: 18 | −0.91 (0.34) | −1.58, −0.23 | −7.63 | |
| Kim et al., 2006 | 15: 15 | −1.83 (0.42) | −2.66, −1.01 | −10.34 | |
| Llerena et al., 2016 | 82: 48 | −1.09 (0.19) | −1.46, −0.73 | −31.51 | |
| Mathalon et al., 2002 | 18: 18 | −0.85 (0.34) | −1.53, −0.18 | −7.23 | |
| Minzenberg et al., 2014 | 99: 54 | −0.68 (0.17) | −1.01, −0.35 | −23.34 | |
| Morris et al., 2006 | 16: 11 | −0.66 (0.40) | −1.44, 0.12 | −4.12 | |
| Morris et al., 2008 | 26: 27 | −0.13 (0.27) | −0.66, 0.41 | −1.69 | |
| Morris et al., 2011 | 20: 15 | −0.86 (0.35) | −1.55, −0.17 | −6.92 | |
| Reinhart et al., 2015 | 17: 18 | −0.75 (0.35) | −1.43, −0.07 | −6.26 | |
| Simmonite et al., 2012 | 29: 35 | −1.22 (0.26) | −1.74, −0.71 | −17.69 | |
| Total | 541:409 | −0.96(0.07) | −1.09, −0.82 | ||
| At-risk vs. Ctrl | Chan et al., 2015a | 22: 20 | 0.08 (0.31) | −0.53, 0.68 | 0.80 |
| Kim et al., 2015 | 17: 20 | −1.03 (0.34) | −1.70, −0.35 | −8.67 | |
| Laurens et al., 2010 | 22: 26 | −0.84 (0.30) | −1.42, −0.25 | −9.49 | |
| Perez et al., 2012 | 48: 88 | −0.28 (0.18) | −0.63, 0.07 | −8.61 | |
| Rabella et al., 2016 | 9: 12 | −1.00 (0.46) | −1.91, −0.09 | −4.62 | |
| Simmonite et al., 2012 | 36: 35 | −0.65 (0.24) | −1.12, −0.18 | −11.26 | |
| Zou et al., 2014 | 15: 15 | −0.26 (0.37) | −0.98, 0.46 | −1.92 | |
| Total | 169: 216 | −0.48 (0.11) | −0.69, −.28 |
Note: CI, confidence interval; Ctrl, healthy control subject sample; ES, effect size; SE, standard error.
Figure 2.
Forest plot of ERN effect sizes.
3.2.1. Psychosis group
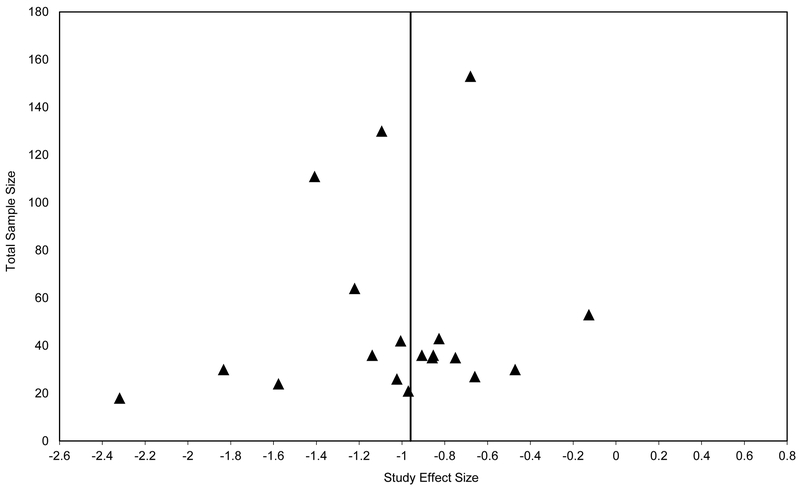
For the psychosis group, the weighted mean effect size of the 20 studies was large in magnitude [ES=−0.84]. However, the distribution of effect sizes was significantly heterogeneous [Qtotal(19)=48.73, p<0.001; I2=63.06%]. Subsequent analysis indicated that a single study (Perez et al., 2012) made an unusually large contribution to the heterogeneity statistic Q due to its relatively small effect size (accounting for 34.72% of Qtotal). The data were re-analyzed with the outlying study removed. The weighted mean effect size of the remaining 19 studies was large in magnitude [ES=−0.96, SE=0.07, 95% CI: −1.09, −0.82], with the ERN amplitude of the psychosis group being smaller than that of the control group. The effect size was significantly different from zero [z=−13.87, p<0.001]. The distribution of effect sizes remained significantly heterogeneous [Qtotal(18)=31.81, p=0.02; I2=49.70%]. The funnel plot was roughly symmetrical, giving little indication of publication bias (see Figure 3). That is, there was more variability of effect sizes for studies with smaller sample sizes, and the effect sizes of the larger studies more closely approximate the overall weighted mean effect size. Egger’s regression test of funnel plot asymmetry was not statistically significant [intercept=−0.95, SE=0.96, p=0.34, 95% CI: −2.97, 1.07].
Figure 3.
Funnel plot of ERN effect sizes, Psychosis vs. Control studies
We conducted follow-up analyses to examine the impact of possible moderators on study effect size. Regarding task characteristics, the type of activation task significantly contributed to the heterogeneity of effect sizes. Twelve studies used non-verbal tasks (e.g., Flanker task, Go/No-Go) and seven studies used verbal tasks (e.g., Stroop). The weighted mean effect size from studies that employed non-verbal tasks was larger than the weighted mean effect size from studies that used verbal tasks [Qbetween(1)=5.82, p=0.02; ESnon-verbal=−1.10, SE=0.09; ESverbal=−0.76, SE=0.11] However, the method of ERN calculation (difference waves [13 studies] vs. ERPs to error trials [6 studies]) did not significantly contribute to heterogeneity of effect sizes [Qbetween(1)=0.001, p=0.97; ESERN=−0.96, SE=0.14; ESΔERN=−0.96, SE=0.08].
Regarding sample characteristics, diagnosis of the patient participant sample, i.e., schizophrenia/schizoaffective disorder [16 studies] vs. broadly-defined psychotic disorders [3 studies], did not account for a significant proportion of the heterogeneity of effect sizes [Qbetween(1)=0.01, p=0.92; ESschizophrenia=−0.95, SE=0.08; ESpsychotic=−0.96, SE=0.13]. Clinical status (10 outpatient studies, 5 inpatient or mixed studies, 4 not reported) did not significantly contribute to heterogeneity of effect sizes [Qbetween(1)=2.37, p=0.12; ESoutpatient=−0.85, SE=0.08; ESinpatient/mixed sample=−1.16, SE=0.18], nor did phase of illness (2 recent-onset studies, 17 chronic or mixed sample studies) [Qbetween(1)=0.86, p=0.35; ESrecent-onset=−0.84, SE=0.14; ESchronic=−0.99, SE=0.08]. Moreover, mean age of the patient sample was not significantly correlated with study effect size [r=0.23, p=0.34].
3.2.2. At-risk group
For the at-risk group, the weighted mean effect size of the seven studies was medium in magnitude [ES=−0.48, SE=0.11, 95% CI: −0.69, −0.28], with the ERN amplitude of the high-risk group being smaller than that of the control group. The weighted mean effect size significantly differed from zero [z=−4.60, p<0.001], and the distribution of the effect sizes was not significantly heterogeneous [Qtotal(6)=10.58, p=0.10; I2=43.3%].
3.3. Pe results
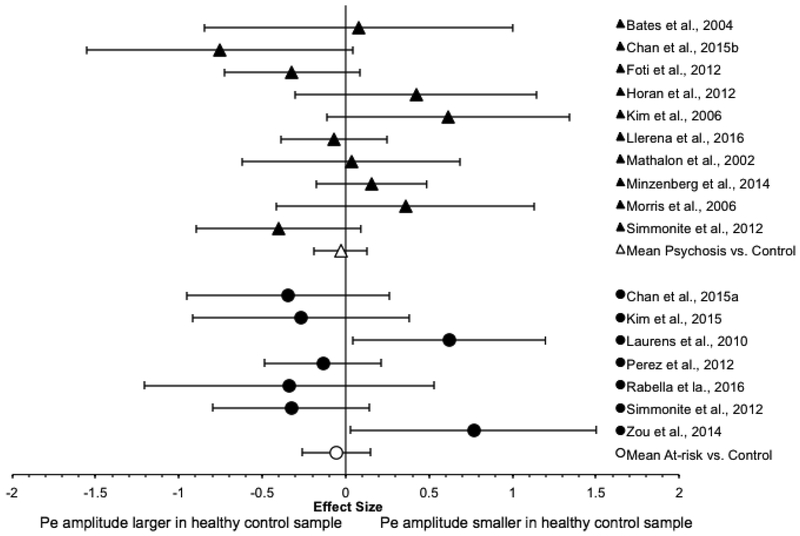
The Pe results are summarized in Table 4, and a forest plot of the effect size and 95% CI of each study is presented in Figure 4.
Table 4.
Pe effect sizes
| Study Name | n Case: n Ctrl | g (SE) | 95% CI | Weighted ES | |
|---|---|---|---|---|---|
| Psychosis vs. Ctrl† | Bates et al., 2004 | 9: 9 | 0.08 (0.47) | −0.85, 1.00 | 0.37 |
| Chan et al., 2015b | 14: 12 | −0.75 (0.41) | −1.55, 0.04 | −4.55 | |
| Foti et al., 2012 | 78: 33 | −0.32 (0.21) | −0.73, 0.09 | −7.43 | |
| Horan et al., 2012 | 16: 14 | 0.42 (0.37) | −0.30, 1.14 | 3.10 | |
| Kim et al., 2006 | 15: 15 | 0.62 (0.37) | −0.11, 1.34 | 4.46 | |
| Llerena et al., 2016 | 93: 63 | −0.07 (0.47) | −0.39, 0.32 | −2.67 | |
| Mathalon et al., 2002 | 18: 18 | 0.03 (0.33) | −0.62, 0.69 | 0.30 | |
| Minzenberg et al., 2014 | 99: 54 | 0.15 (0.17) | −0.18, 0.49 | 5.36 | |
| Morris et al., 2006 | 16: 11 | 0.36 (0.39) | −0.41, 1.13 | 2.31 | |
| Simmonite et al., 2012 | 29: 35 | −0.40 (0.25) | −0.89, 0.09 | −6.37 | |
| Total | 387: 264 | −0.03 (0.08) | −0.19, 0.13 | ||
| At-risk vs. Ctrl | Chan et al., 2015a | 22: 20 | −0.34 (0.31) | −0.95, 0.26 | −3.57 |
| Kim et al., 2015 | 17: 20 | −0.27 (0.33) | −0.92, 0.38 | −2.45 | |
| Laurens et al., 2010 | 22: 26 | 0.62 (0.29) | 0.05, 1.20 | 7.20 | |
| Perez et al., 2012 | 48: 88 | −0.14 (0.18) | −0.49, 0.22 | −4.24 | |
| Rabella et al., 2016 | 9: 12 | −0.34 (0.44) | −1.21, 0.53 | −1.72 | |
| Simmonite et al., 2012 | 36: 35 | −0.33 (0.24) | −0.79, 0.14 | −5.76 | |
| Zou et al., 2014 | 15: 15 | 0.77 (0.37) | 0.03, 1.50 | 5.45 | |
| Total | 169: 216 | −0.06 (0.10) | −0.26, 0.15 |
Note: CI, confidence interval; Ctrl, healthy control subject sample; ES, effect size; SE, standard error.
Figure 4.
Forest plot of Pe effect sizes
3.3.1. Psychosis group
For the psychosis group, the weighted mean effect size of the 12 studies was small in magnitude [ES=−0.24], with the Pe amplitude of the psychosis group being smaller than that of the control group. However, the distribution of effect sizes was significantly heterogeneous [Qtotal(11)=39.63, p<0.001; I2=74.8%]. Subsequent analysis indicated that a single study (Perez et al., 2012) made an unusually large contribution to the heterogeneity statistic Qtotal due to its relatively large effect size (accounting for 43.2% of Qtotal). The data were then re-analyzed with the outlying study removed. The weighted mean effect size of the remaining 11 studies was small in magnitude [ES=−0.09, SE=0.08, 95% CI: −0.24, 0.07], with the Pe amplitude of the psychosis group being slightly smaller than that of the control group. The effect size was not significantly different from zero [z=−1.10, p=0.27]. The distribution of effect sizes was significantly heterogeneous [Qtotal(10)=22.50, p=0.01; I2=55.6%].
Follow-up analyses were conducted to investigate potential sources of heterogeneity of the effect sizes. Regarding task characteristics, the method of Pe calculation (difference waves [7 studies] vs. ERPs to error trials [4 studies]) did not account for a statistically significant proportion of the heterogeneity of the effect sizes [Qbetween(1)=0.90, p=0,34; ESpe=−0.22, SE=0.16, 95% CI: −0.54, 0.10; ESΔPe=−0.05, SE=0.09, 95%CI: −0.22, 0.13]. Similarly, the type of activation task (7 non-verbal, 4 verbal) did not significantly contribute [Qbetween(1)= 113, p=0.29; ESnon-verbal=−0.15, SE=0.10; ESverbal=0.02, SE=0.13].
Regarding patient characteristics, diagnosis of the patient participant sample, i.e., schizophrenia/schizoaffective disorder [8 studies] vs. broadly-defined psychotic disorders [3 studies], did not account for a significant proportion of the heterogeneity of effect sizes [Qbetween(1)=0.03, p=0.86; ESschizophrenia=−0.08, SE=0.10; ESpsychotic=−0.10, SE=0.12]. Similarly, the clinical status of the patient sample (7 outpatient studies, 2 inpatient studies) did not account for a significant proportion of the heterogeneity [Qbetween(1)=0.28, p=0.59; ESoutpatient=−0.10, SE=0.09; ESinpatient/mixed sample=0.05, SE=0.27]. Likewise, phase of illness of the patient sample (2 recent-onset studies, 9 chronic phase studies) did not significantly contribute [Qbetween(1)=0.33, p=0.56; ESrecent-onset=−0.02, SE=0.14; ESchronic=−0.11, SE=0.10], and mean age of the patient sample was not significantly correlated with study effect size [r=−0.15, p=0.66].
Given that we were unable to identify the source(s) of heterogeneity, we re-analyzed the data to identify a subset of homogeneous studies. One study (Kansal et al., 2014) made a large contribution to heterogeneity (accounting for 37.12% of Qtotal). Removing this study from the analysis yielded a homogeneous subset of 10 studies with a weighted mean effect size that was small in magnitude [ES=−0.03, SE=0.08, 95%CI: −0.19, 0.13, Qtotal(9)= 14.15, p=0.12, I2=36.4%], with the Pe amplitude of the psychosis group being slightly smaller than that of the control group. The effect size was not significantly different from zero [z=−0.42, p=0.68].
3.3.2. At-risk group
For the at-risk group, the weighted mean effect size of the seven studies was very small in magnitude [ES=−0.06, SE=0.10, 95% CI: −0.26, 0.15], with the Pe amplitude of the high-risk group being smaller than that of the control group. The weighted mean effect size did not significantly differ from zero [z=−0.53, p=0.60]. The distribution of the effect sizes was moderately heterogeneous [Qtotal(6)= 13.29, p=0.04; I2=54.9%].
Follow-up analyses indicated that method of Pe calculation (difference waves [3 studies] vs. ERPs to error trials [4 studies]) accounted for a statistically significant proportion of the heterogeneity of the effect sizes [Qbetween(1)=7.10, p=0.008], with the two calculation methods yielding weighted small to moderate mean effect sizes in opposing directions [ESPe=−0.24, SE=0.12, 95% CI: −0.48, 0.01, z=−1.91, p=0.06; ESΔPe=0.37, SE=0.19, 95%CI:−0.01, 0.74, z=1.93, p=0.05]. Note, however, that the confidence intervals both overlap with zero. The type of activation task (5 non-verbal studies, 2 verbal studies) did not significantly contribute to heterogeneity of effect sizes [Qbetween(1)=0.49, p=0.48; ESnon-verbal=−0.12, SE=0.14; ESverbal=0.03, SE=0.16].2
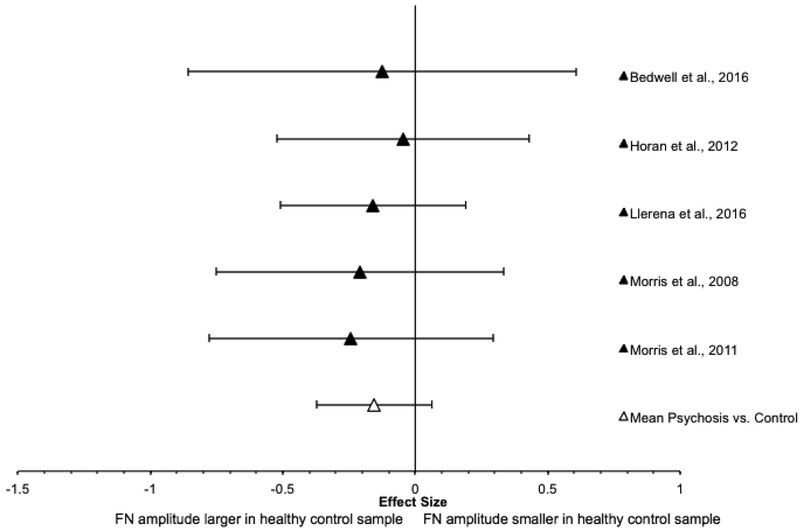
3.4. FN results
3.4.1. Psychosis group
For the psychosis group, FN results are presented in Table 5, and a forest plot of the effect size and 95% CI of each study is presented in Figure 3. The weighted mean effect size of the five studies was small in magnitude [ES=−0.15, SE=0.11, 95% CI: −0.37, 0.06], with smaller FN amplitude in the psychosis group compared to the control group. The weighted mean effect size did not significantly differ from zero [z=−1.39, p=0.08], and the distribution of effect sizes was homogeneous [Qtotal(4)=0.32, p=0.99; I2=0%].
Table 5.
FN effect sizes
| Study Name | n Case: n Ctrl | g (SE) | 95% CI | Weighted ES | |
|---|---|---|---|---|---|
| Psychosis vs. Ctrl | Bedwell et al., 2016 | 16: 13 | −0.12 (0.37) | −0.86, 0.61 | −0.89 |
| Horan et al., 2012 | 35: 33 | −0.05 (0.24) | −0.52, 0.43 | −0.78 | |
| Llerena et al., 2016 | 74: 55 | −0.16 (0.18) | −0.51, 0.19 | −5.00 | |
| Morris et al., 2008 | 26: 26 | −0.21 (0.28) | −0.75, 0.33 | −2.76 | |
| Morris et al., 2011 | 32: 23 | −0.24 (0.27) | −0.78, 0.29 | −3.23 | |
| Total | 183:150 | −0.16 (0.12) | −0.38, 0.07 |
Note: CI, confidence interval; Ctrl, healthy control subject sample; ES, effect size; SE, standard error.
3.4.2. At-risk group
FN has been examined in only one at-risk group study (Karcher et al., 2016). Karcher and colleagues examined FN in separate non-clinical samples with elevated psychotic experiences (PE group) or social anhedonia (SocAnh group), and these two groups were compared to healthy controls. The effect size was moderate in magnitude for the PE group [g=−0.49, SE=0.32, 95% CI: −1.11, 0.13], with the FN amplitude of the PE group being smaller than that of the control group. For the SocAnh group, the effect was very small in size [g=0.05, SE=0.31, 95% CI: −0.54, 0.65], with the FN amplitude being slightly larger in the SocAnh group than the control group. The confidence intervals for both comparisons overlap with zero.
3.5. Association between behavioral performance accuracy and Pe amplitude
Last, we examined the relationship between performance accuracy on the activation task and ERP amplitude. Only six studies reported correlations between performance accuracy and ERN amplitude, four studies with Pe amplitude, and one with FN amplitude. The results were mixed, with some studies reporting a significant positive association between performance accuracy and ERP amplitude in at-risk or psychosis samples (ERN: Bates et al., 2002, Kim et al., 2015, Perez et al., 2012; Pe: Chan et al., 2015b; FN: Morris et al., 2008) and others reporting no significant association (ERN: Chan et al., 2015b; Minzenberg et al., 2014; Morris et al., 2008; Pe: Kim et al., 2015; Minzenberg et al., 2014; Perez et al., 2012). Next, we examined whether effect size for performance accuracy on the activation task was associated with effect size for the ERP components across studies. Based on data provided in the papers, we were able to calculate the effect size for performance accuracy for 23 studies of ERN and 16 studies of Pe. Given that only one study measuring FN also reported performance accuracy data, we could not conduct the same analysis for that component. Across studies, there were no statistically significant correlations between performance accuracy effect size and ERN or Pe effect size (ps ≥ 0.14).
4. Discussion
The results of this meta-analysis indicate that not all aspects of performance monitoring and feedback processing are disrupted in psychosis. A large number of studies indicate that early performance monitoring (ERN) impairments are substantial among those with psychotic disorders in general and are also detectable among those at risk for developing these disorders. In contrast, the smaller literatures on later performance monitoring (Pe) and feedback processing (FN) indicate that these processes appear relatively spared. These findings shed light on the nature of how performance monitoring and feedback processing is impacted in psychosis-related disorders and provide guidance for further research.
4.1. ERN: Substantial impairment in psychotic disorders and disorder risk in general
The results provide clear and consistent evidence of a large ERN reduction in those with psychosis, accompanied by a moderate ERN reduction in those at risk for these disorders. For the psychosis studies, there was no strong evidence for presence of publication bias, though there were too few studies to evaluate this in the at-risk group. There was significant heterogeneity in effect sizes for the psychosis group and this was partly accounted for by the type of activation task. The magnitude of impairment was significantly larger for non-verbal tasks, such as the flanker task (which shows the strongest psychometric properties of all tasks used to elicit the ERN (Weinberg et al., 2015)) than verbal tasks. Notably, a study using a picture-matching task (Perez et al., 2012) yielded unusual findings for both the ERN and Pe, indicating that the task may not be optimized to index the typical ERN/Pe response. Heterogeneity of effect size estimates was not impacted by factors such as method of ERN calculation, clinical diagnosis or status, or phase of illness. Overall, this robust pattern of ERN reduction across those with and at-risk for psychosis suggests that the ERN may be a useful biomarker or endophenotype (Gottesman and Gould, 2003) for psychosis.
One factor that makes the ERN an attractive biomarker is that a fair amount is known about the neural generators of this component. Given the considerable evidence that the ERN is generated by the dorsal ACC (e.g., Debener et al., 2005; Holroyd and Coles, 2002), our results suggest dysfunction of this region is associated with both psychosis and psychosis-risk. This is indeed consistent with substantial evidence of structural and functional ACC abnormalities in those with (Nelson et al., 2015; Salgado-Pineda et al., 2014) and at risk for (e.g., Fornito et al., 2008; Park et al., 2013) psychotic disorders. Furthermore, studies employing simultaneous fMRI and ERP recording have linked error processing abnormalities to ACC dysfunction in those with and at risk for psychosis (Ford et al., 2009). It should be noted, however, that error processing involves coordinated activity in a network of regions and the ERN deficits seen in psychosis may reflect dysfunction within, or in connectivity among, these regions (e.g., anterior PFC, anterior insula, inferior parietal lobe, thalamus, cerebellum; Becerril and Barch, 2013; Ford et al., 2009; Ramyead et al., 2017), rather than dysfunction specific to the ACC.
Several factors support the viability of the ERN as an endophenotype for psychosis. Endophenotypes are defined as biological or psychological phenomena that are associated with genetic contributions to a disorder (e.g., Glahn et al., 2014; Miller and Rockstroh, 2016; Ritsner and Gottesman, 2011). Aside from its consistent association with psychosis, there is evidence that the ERN meets several additional criteria for an endophenotype (Gottesman and Gould, 2003). For example, the ERN is a heritable trait in the general population with heritability estimates ranging from .30-.50 (Anohkin et al., 2008). Along these lines, initial evidence indicates that unaffected relatives of probands with psychotic disorders show significant ERN reductions compared to healthy controls (Simmonite et al., 2012). Further, among those with psychotic disorders, the ERN reduction appears to be largely state-independent and longitudinally stable: the majority of relevant studies reported no significant correlations between ERN and positive, disorganized, or negative symptoms (Chan et al., 2015b, Horan et al., 2012; Kim et al., 2006; Llerena et al., 2016; Minzenberg et al., 2014; but see Foti et al., 2012, Reinhart et al., 2015; Mathalon et al., 2002), and good longitudinal stability has been found from one month to four years (Foti et al., 2016; Llerena et al., 2016). These converging lines of evidence suggest that performance monitoring abnormalities indexed by the ERN are an endophenotype associated with genetic predisposition for psychosis, perhaps aiding researchers to identify genes associated with the illness.
At this point, however, the ERN reduction does not appear to be uniquely associated with psychosis. Although this reduction does differ from individuals with obsessive-compulsive disorder, depression, and generalized anxiety disorder who show elevated ERN, reduced ERN is also seen in ADHD, substance use disorders, and other externalizing disorders (for a review, see Weinberg et al., 2015). It is possible that more fine-grained paradigms could identify certain characteristics of abnormal ERN that are more specifically associated with psychosis. Initial work hints as the possibility that the ERN reduction in psychosis may be less responsive to reward manipulations than other disorders. For example, baseline ERN reductions have been found to significantly improve during a performance-based reward incentive condition in young people with ADHD (i.e., providing monetary rewards for good performance increase the amplitude of the ERN; Groom et al., 2013), whereas similar incentives did not ameliorate the ERN reduction in individuals with schizophrenia (Morris et al., 2006). Further research to more precisely define the scope of ERN reductions in psychotic compared to other types of disorders will be an important next step.
4.2. Pe and FN: Largely spared in psychosis
In contrast to the robust early ERN impairments, later performance monitoring indexed by Pe appears largely normal across the two groups. This result is tempered to some extent by the significant heterogeneity of effect sizes for the Pe for the psychosis and at-risk groups. We were unable to identify significant subgroups or moderators of the heterogeneity for the schizophrenia group. For the at-risk group, only method of Pe calculation accounted for a statistically significant proportion of the heterogeneity, but this is not a robust finding since the confidence intervals associated with both calculation methods overlapped with zero. Our evaluation of potential moderators was necessarily constrained by the information that was reported with sufficient frequency across the studies. Overall, the amplitude of the Pe appears to be very similar in psychosis and psychosis-risk groups compared to healthy individuals. Given the limited number of studies available, further investigation is warranted to determine whether a relatively small Pe impairment is associated with a certain patient subgroup or methodological characteristic.
Feedback processing indexed by the FN also appears largely spared in psychosis. For the FN, the distribution of effect sizes was homogeneous, indicating that methodological and clinical factors do not appear to have an important impact on the findings. These results must be interpreted cautiously since they are based on a small number studies and our power to detect a small effect was limited. However, confidence in the FN results is bolstered by consistent findings of broadly intact hedonic responses in schizophrenia across a range of complementary methods. For example, individuals with schizophrenia subjectively report normal levels of “in the moment” pleasure and show normal psychophysiological responses when they are exposed to pleasant stimuli (Cohen and Minor, 2010). They also show responses biases towards more rewarding stimuli that are similar to healthy controls on behavioral paradigms (Barch and Sheffield, 2017; Heerey et al., 2008). Furthermore, fMRI studies indicate that striatal responses to monetary reward in both medicated and unmedicated individuals with schizophrenia are similar to healthy individuals (Barch and Dowd, 2010; Gilleen et al., 2015; Nielsen et al., 2012; Schlagenhauf et al., 2009; Simon et al., 2010; Waltz et al., 2010). Thus, several lines of evidence support the current review's finding of normal feedback processing in schizophrenia. As noted above, the precise process(es) that the FN indexes remains an active area of research.
At this point, it is not possible to draw conclusions about the consistency or magnitude of FN disturbances with regard to the at-risk group since only one relevant study was available. Given the evidence pointing toward normal FN in psychosis, one would likely expect similarly normal findings in the at-risk group. However, this is a research gap that remains to be filled.
4.3. Integration: what does the pattern of findings mean for performance monitoring in psychosis?
Despite a substantial ERN deficit, the largely intact Pe indicates relatively preserved error awareness or error detection capacity in those with psychosis and psychosis risk. This pattern converges with experimental findings in healthy samples demonstrating that ERN and Pe are functionally dissociable (e.g., Hughes and Yeung, 2011; Overbeek et al., 2005). The intact Pe also illustrates a means by which individuals with psychosis area able to adjust their performance to task demands. Although the efficiency of their trial and error learning is likely compromised by an early performance monitoring impairment, their error awareness/detection abilities may at least partly compensate for this limitation, enabling them to make strategic adjustments to improve task performance.
The finding of a relatively intact FN indicates typical external feedback responsivity in psychosis. This area of preserved functioning reflects a relative rarity in psychosis research and an important strength that could, for example, be exploited in novel psychosocial rehabilitation approaches. However, translating normal “in-the-moment” external feedback processing into complex adaptive behavior, such as seeking out potentially rewarding events, depends on involves several other processes that may be impaired in schizophrenia. For example, individuals with schizophrenia have deficits associated with learning, anticipating, or decisionmaking to guide goal-directed behavior (Barch and Dowd, 2010, Morris et al., 2011), which may in turn influence responding to and integrating reward feedback.
Taken together, performance monitoring and feedback processing play essential roles in action selection and goal-directed behavior. Thus, abnormalities in performance monitoring, coupled with intact feedback processing, may reflect impairments in the ability to attribute values linked to different choices (e.g., one response option is associated with a small, immediate reward whereas another option is associated with a larger, future reward; Gold et al., 2008), leading to difficulties in predicting associations between responses and outcomes (Morris et al., 2011). This reduced ability to predict outcomes may impede patients' ability to guide future action selection.
As mentioned above, decreased ERN is not diagnostically specific to psychosis spectrum disorders. It is possible, however, that the pattern across the three ERP components examined in this review has greater specificity. A recent review reported a similar pattern of decreased early performance monitoring (ERN) accompanied by relatively intact Pe and FN in people with autism spectrum disorders (Hupen et al., 2016). These comparable patterns are intriguing to consider in the context of the longstanding interest in the potential overlapping vulnerability to psychosis and autism spectrum disorders (Stone and Iguchi, 2011).
4.4. Limitations and conclusions
Although the database for the ERN meta-analyses was fairly large for psychosis, an important limitation of this review is the smaller number of studies that examined the ERN in at-risk groups, and the generally smaller number of Pe and FN studies. This is particularly true for the FN results, which should be viewed as preliminary. In addition, given the limited number of studies available, we were unable to directly compare effect sizes across components. This review was also limited by the large number of studies that did not report correlations between clinical symptoms or medication dosage and ERP components, which made it impossible to test for moderation by these factors. Finally, because we did not seek out unpublished data for this review, the findings could be biased by studies that found significant, publishable results. Importantly though, the funnel plot for ERN does not suggest the file drawer problem is a major concern. The more limited number of Pe and FN studies precluded assessment of potential publication bias.
Despite these limitations, the current meta-analytic review is the first to quantitatively highlight the similar early performance monitoring abnormalities in psychosis/psychosis-risk and the relatively normal feedback processing ability in psychosis. It also identifies gaps in the literature (e.g., research on FN in psychosis and psychosis-risk) and avenues for future research (e.g., the use of the ERN as an endophenotype for psychosis risk, identification of moderators of Pe). Further investigation of these ERP components will refine our understanding of performance monitoring and feedback processing in psychosis.
Supplementary Material
Figure 5.
Forest plot of FN effect sizes.
Highlights.
There were moderate to large ERN reductions in psychosis and psychosis-risk.
There were non-significant, small differences from controls for Pe and FN.
Early responding monitoring is impaired in psychosis/psychosis-risk.
Later responding monitoring appears relatively intact across groups.
External feedback processing also appears to be relatively intact.
Acknowledgments
Funding Sources: A. McCleery is supported by a career development award from NIH (5K23MH108829).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We used the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS; Kim et al., 2013). Two raters (M. Moore and A. McCleery) completed independent ratings of each study with good inter-rater agreement (91% agreement, Cohen’s kappa=0.80). Scoring discrepancies were resolved by consensus ratings. For the majority of studies, risk of potential bias was low. A summary of the RoBANS data can be found in Supplementary Table 2.
Delta scores (i.e., ERP response to error trials minus response to correct trials) were calculated from available data for six ERN studies and four Pe studies. Weighted mean effect sizes for ERN and Pe in both the psychosis and high-risk groups, calculated using the originally reported metric for these studies (i.e., ERN or Pe to error trials only), were similar to those reported above calculated using delta scores.
References
- Alain C, McNeely HE, He Y, Christensen BK, West R, 2002. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb. Cortex 12, 840–846. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society, 1994. Guideline thirteen: guidelines for standard electrode position nomenclature. American Electroencephalographic Society. J. Clin. Neurophysiol 11, 111–113. [PubMed] [Google Scholar]
- American Psychiatric Association, 1987. Diagnostic and statistical manual of mental disorders, 3rd edition, revised. American Psychiatric Association, Washington, DC. [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and statistical manual of mental disorders, 4th ed text revision. American Psychiatric Association, Washington, DC. [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC, 2008. Heritability of frontal brain function related to action monitoring. Psychophysiology 45, 524–534. [DOI] [PubMed] [Google Scholar]
- Araki T, Niznikiewicz M, Kawashima T, Nestor PG, Shenton ME, McCarley RW, 2013. Disruption of function-structure coupling in brain regions sub-serving self monitoring in schizophrenia. Schizophr. Res. 146, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC, 2010. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull. 36, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Sheffield JM, 2017. Cognitive control in schizophrenia, in: Egner T (Ed.), The Wiley Handbook of Cognitive Control. John Wiley & Sons, pp. 556–580. [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF, 2002. Error-related negativity and correct response negativity in schizophrenia. Clin. Neurophysiol. 113, 1454–1463. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET, 2004. State dependent changes in error monitoring in schizophrenia. J. Psychiatr. Res. 38, 347–356. [DOI] [PubMed] [Google Scholar]
- Becerril KE, Barch DM, 2013. Conflict and error processing in an extended cingulo-opercular and cerebellar network in schizophrenia. Neuroimage Clin 3, 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell JS, Potts GF, Gooding DC, Trachik BJ, Chan CC, Spencer CC, 2016. Transdiagnostic Psychiatric Symptoms and Event-Related Potentials following Rewarding and Aversive Outcomes. PLoS One 11, e0157084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R, 2011. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr. Bull. 37, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G, 2011. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage 57, 1608–1616. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Spencer CC, West C, Viegas C, Bedwell JS, 2015a. Metacognitive processes in psychometrically defined schizotypy. Psychiatry Res. 230, 279–286. [DOI] [PubMed] [Google Scholar]
- Chan CC, Trachik BJ, Bedwell JS, 2015b. An event-related potential investigation of error monitoring in adults with a history of psychosis. Clin. Neurophysiol. 126, 1717–1726. [DOI] [PubMed] [Google Scholar]
- Charles L, Gaillard R, Amado I, Krebs MO, Bendjemaa N, Dehaene S, 2017. Conscious and unconscious performance monitoring: Evidence from patients with schizophrenia. Neuroimage 144, 153–163. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS, 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 36, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Asuncion J, Docx L, Morrens M, Sabbe B, de Bruijn ER, 2015. Neurophysiological evidence for diminished monitoring of own, but intact monitoring of other's errors in schizophrenia. Psychiatry Res. 230, 220–226. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK, 2005. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci 25, 11730–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N, 2007. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur. J. Neurosci. 26, 1714–1720. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L, 1990. Effects of errors in choice reaction tasks on the ERP under focused and divided attention, in: C.H.M B, A.W.K G, A K (Eds.), Psychological Brain Research. Tilburg University Press, Tilburg, Netherlands, pp. 192–195. [Google Scholar]
- Ford JM, Jorgensen KW, Roach BJ, Mathalon DH, 2009. Error detection failures in schizophrenia: ERPs and FMRI. Int. J. Psychophysiol 73, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, Velakoulis D, McGorry PD, Pantelis C, Yucel M, 2008. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry 64, 758–765. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet E, Hajcak G, 2012. Beyond the broken error-related negativity: functional and diagnostic correlates of error processing in psychosis. Biol. Psychiatry 71, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Hajcak G, 2013. Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J. Abnorm. Psychol. 122, 520–531. [DOI] [PubMed] [Google Scholar]
- Foti D, Perlman G, Hajcak G, Mohanty A, Jackson F, Kotov R, 2016. Impaired error processing in late-phase psychosis: Four-year stability and relationships with negative symptoms. Schizophr. Res. 176, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G, 2011. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum. Brain Mapp 32, 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E, 1993. A neural system for error detection and compensation. Psychol. Sci. 4, 385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J, 2012. The error-related negativity (ERN/Ne), in: Luck SJ, Kappenman ES (Eds.), Oxford Handbook of Event-Related Potential Components. Oxford University Press, New York, pp. 231–291. [Google Scholar]
- Gilleen J, Shergill SS, Kapur S, 2015. Impaired subjective well-being in schizophrenia is associated with reduced anterior cingulate activity during reward processing. Psychol. Med. 45, 589–600. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Knowles EE, McKay DR, Sprooten E, Raventos H, Blangero J, Gottesman II, Almasy L, 2014. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am. J. Med. Genet. B Neuropsychiatr. Genet 165B, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA, 2008. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr. Bull. 34, 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD, 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Liddle EB, Scerif G, Liddle PF, Batty MJ, Liotti M, Hollis CP, 2013. Motivational incentives and methylphenidate enhance electrophysiological correlates of error monitoring in children with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 54, 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guolo A, Varin C, 2017. Random-effects meta-analysis: the number of studies matters. Stat. Methods Med. Res. 26, 1500–1518. [DOI] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Stampfli P, Drechsler R, Brandeis D, Walitza S, Brem S, 2014. The feedback-related negativity (FRN) revisited: New insights into the localization, meaning, and network organization. Neuroimage 84, 159–168. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM, 2008. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol. Psychiatry 64, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 109, 679–709. [DOI] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, Green MF, 2012. Impaired neural response to internal but not external feedback in schizophrenia. Psychol. Med. 42, 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd S, Morrens M, Sabbe B, Schrijvers D, Vandendriessche F, Hulstijn W, de Bruijn ER, 2013. Trait and state aspects of internal and external performance monitoring in schizophrenia. Int. J. Psychophysiol. 87, 42–51. [DOI] [PubMed] [Google Scholar]
- Hughes G, Yeung N, 2011. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia 49, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupen P, Groen Y, Gaastra GF, Tucha L, Tucha O, 2016. Performance monitoring in autism spectrum disorders: A systematic literature review of event-related potential studies. Int. J. Psychophysiol. 102, 33–46. [DOI] [PubMed] [Google Scholar]
- Kansal V, Patriciu I, Kiang M, 2014. Illness insight and neurophysiological error-processing deficits in schizophrenia. Schizophr. Res. 156, 122–127. [DOI] [PubMed] [Google Scholar]
- Karcher NR, Bartholow BD, Martin EA, Kerns JG, 2017. Associations between Electrophysiological Evidence of Reward and Punishment-Based Learning and Psychotic Experiences and Social Anhedonia in At-Risk Groups. Neuropsychopharmacology 42, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jang KM, Kim MS, 2015. Deficits in error-monitoring by college students with schizotypal traits: an event-related potential study. PLoS One 10, e0122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, Jang BH, Son HJ, 2013. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 66, 408–414. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, 1999. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J. Abnorm. Psychol. 108, 337–346. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM, Taylor EA, 2010. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol. Psychiatry 67, 238–245. [DOI] [PubMed] [Google Scholar]
- Llerena K, Wynn JK, Hajcak G, Green MF, Horan WP, 2016. Patterns and reliability of EEG during error monitoring for internal versus external feedback in schizophrenia. Int. J. Psychophysiol. 105, 39–46. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM 2002. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J. Abnorm. Psychol 111, 22–41. [PubMed] [Google Scholar]
- Miller GA, Rockstroh BS, 2016. Progress and prospects for endophenotypes for schizophrenia in the time of genomics, epigenetics, oscillatory brain dynamics, and the Research Domain Criteria, in: Nickl-Jockschat TAT (Ed.), The Neurobiology of Schizophrenia. Elsevier, The Netherlands, pp. 17–38. [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS, 2014. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Res. 221, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012. [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB, 2008. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr. Res. 99, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Holroyd CB, Mann-Wrobel MC, Gold JM, 2011. Dissociation of response and feedback negativity in schizophrenia: electrophysiological and computational evidence for a deficit in the representation of value. Front. Hum. Neurosci. 5, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH, 2006. Electrophysiological analysis of error monitoring in schizophrenia. J. Abnorm. Psychol. 115, 239–250. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Bjorkquist OA, Olsen EK, Herbener ES, 2015. Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: An fMRI investigation. Psychiatry Res. 234, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, Glenthoj B, 2012. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol. Psychiatry 71, 898–905. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A, 2001. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38, 752–760. [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR, 2005. Dissociable Components of Error Processing. On the Functional Significance of the Pe Vis-a-vis the ERN/Ne. Journal of Psychophysiology. [Google Scholar]
- Park HY, Hwang JY, Jung WH, Shin NY, Shim G, Jang JH, Kwon JS, 2013. Altered asymmetry of the anterior cingulate cortex in subjects at genetic high risk for psychosis. Schizophr. Res. 150, 512–518. [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, Loewy RL, Vinogradov S, Mathalon DH, 2012. Error monitoring dysfunction across the illness course of schizophrenia. J. Abnorm. Psychol. 121, 372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, 2015. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology 52, 449–459. [DOI] [PubMed] [Google Scholar]
- Rabella M, Grasa E, Corripio I, Romero S, Mananas MA, Antonijoan RM, Munte TF, Perez V, Riba J, 2016. Neurophysiological evidence of impaired self-monitoring in schizotypal personality disorder and its reversal by dopaminergic antagonism. Neuroimage Clin 11, 770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramyead A, Kometer M, Studerus E, Andreou C, Ward LM, Riecher-Rossler A, 2017. Abnormal brain connectivity during error-monitoring in the psychosis high-risk state. Schizophr. Res in press. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Gottesman II, 2011. The Schizophrenia construct after 100 years of challenges, in: Ritsner MS (Ed.), Handbook of Schizophrenia Spectrum Disorders. Springer; The Netherlands, pp. 1–44. [Google Scholar]
- Salgado-Pineda P, Landin-Romero R, Fakra E, Delaveau P, Amann BL, Blin O, 2014. Structural abnormalities in schizophrenia: further evidence on the key role of the anterior cingulate cortex. Neuropsychobiology 69, 52–58. [DOI] [PubMed] [Google Scholar]
- Sambrook TD, Goslin J, 2016. Principal components analysis of reward prediction errors in a reinforcement learning task. Neuroimage 124 (Pt A), 276–286. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Juckel G, Gallinat J, Heinz A, 2009. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol. Psychiatry 65, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Simmonite M, Bates AT, Groom MJ, Jackson GM, Hollis C, Liddle PF, 2012. Error processing-associated event-related potentials in schizophrenia and unaffected siblings. Int. J. Psychophysiol. 84, 74–79. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S, 2010. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophr. Res. 118, 154–161. [DOI] [PubMed] [Google Scholar]
- Simons RF, 2010. The way of our errors: theme and variations. Psychophysiology 47, 1–14. [DOI] [PubMed] [Google Scholar]
- Stone WS, Iguchi L, 2011. Do Apparent Overlaps between Schizophrenia and Autistic Spectrum Disorders Reflect Superficial Similarities or Etiological Commonalities? N. Am. J. Med. Sci. (Boston) 4, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ, 2007. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist 13, 160–172. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS, 2002. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 14, 593–602. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, Gold JM, Stein EA, 2010. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology 35, 2427–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LQ, Wang K, Qu C, Lui SS, Shum DH, Cheung EF, Chan RC, 2014. Verbal self-monitoring in individuals with schizotypal personality traits: an exploratory ERP study. Asian J. Psychiatr. 11, 53–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.