Abstract
Background
Glandular odontogenic cyst (GOC) demonstrates a significant predilection toward localized biologic aggressiveness and recurrence. GOC shares certain histopathologic features with intraosseous mucoepidermoid carcinoma (IMEC). The current investigation evaluates a group of recurrent, biologically aggressive GOCs to determine if any cases demonstrated unique histologic features or Mastermind – like2 (MAML2) rearrangements common to IMEC.
Methods
Microscopic slides from eleven previously diagnosed GOGs were stained with hematoxylin and eosin and assessed by two study participants for ten classic histopathologic features required to establish a diagnosis of GOC. Cases were evaluated utilizing break-apart fluorescent in situ hybridization (FISH) analysis for the presence of MAML2 gene rearrangements. Clinical and demographic data on all patients were recorded.
Results
The mean age for patients included in the study was 55.27 years with a range of 36 to 72 years. The most common presenting symptom was a jaw expansion and all cysts presented initially as a unilocular or multilocular radiolucency. CYSTS displayed a minimum of 6 of 10 histologic parameters necessary for a diagnosis of GOC. One case demonstrated MAML2 rearrangements by FISH. That case also showed marked ciliation of cyst lining epithelial cells and extensive mucous secreting goblet cell proliferation.
Conclusion
Findings in the current study are in concert with previous investigations, and although this study finds ONLY LIMITED molecular evidence to support the premise that recurrent biologically aggressive GOCs are a precursor to IMEC, detection of MAML2 rearrangements in one case suggests that such a theoretic transition, while rare, is possible.
Keywords: Glandular odontogenic cyst, intraosseous mucoepidermoid carcinoma, MAML2 gene rearrangements, FISH
INTRODUCTION
The glandular odontogenic cyst (GOC) is a well-recognized yet uncommon developmental cyst of the gnathic bones (1). First characterized as sialo-odontogenic cyst in 1987, GOC has a propensity for recurrences and locally aggressive behavior (2).
Primary (central) intraosseous mucoepidermoid carcinoma (IMEC) is an infrequent malignant salivary gland neoplasm of the jaws with a predilection for the mandible (3). Accounting for 2–4% of all reported cases of mucoepidermoid carcinoma (MEC), the origin of IMEC remains a matter of conjecture. One theory suggests that IMEC arises from ectopic salivary glands in the jaw bone, while a second developmental theory suggests that the tumor stems from the pluripotent nature of odontogenic epithelium (4). There has been debate among investigators as to whether or not the GOC might represent a transitional precursor to IMEC.
Most MECs, including intraosseous tumors harbor mastermind-like 2 (MAML2) gene rearrangements, a family of genetic alterations characterized primarily by translocation t(11;19)(q21;p13), resulting in CRTC1-MAML2 fusion (5). CRTC1 is also known as mucoepidermoid carcinoma translocated 1 (MECT1) (6). The frequency of MAML2 gene rearrangements in IMECs is variable with studies reporting a frequency ranging between 75–85%. (1, 6–8).
Many of the histologic features of GOC closely mimic those of low grade IMEC, making the diagnosis of, and distinction between the two entities, quite challenging. Most investigations utilizing fluorescent in situ hybridization (FISH) analysis have demonstrated that GOC lacks MAML2 gene rearrangements. These studies have lessened enthusiasm for assigning GOC a potential role as an IMEC precursor, concluding that the high sensitivity and specificity of MAML2 rearrangement seen in MEC should theoretically allow one to clearly distinguish IMEC from GOC (1).
Although such a premise may have validity, it fails to address a significant issue that has not been pursued vigorously in previous investigations. Most GOCs evaluated for MAML2 rearrangements have represented a random assessment of a small number of surgical pathology samples derived from various pathology department archives. No studies to our knowledge have investigated MAML2 rearrangements in a subset of lesions categorized exclusively as biologically aggressive, recurrent GOCs. Therefore, the present investigation was undertaken.
METHODS
Case Selection
The surgical pathology archives of the University of Colorado School of Dental Medicine Oral Pathology Laboratory, Western States Regional Oral and Maxillofacial Pathology Laboratory, and Oral Pathology Laboratory, Inc., Flushing, New York were reviewed for cysts diagnosed and coded as either glandular odontogenic cyst or sialo-odontogenic cyst between the years 1989 to 2015. To be included in this study, all cases had to have demonstrated a minimum of one recurrence after definitive treatment. No cases were accepted in which the recurrence appeared less than 18 months after the original cyst’s presentation and diagnosis. A total of eleven recurrent cases were identified.
This investigation was approved by the Colorado Multiple Institutional Review Board, Protocol #00–1094.
All cases assessed had to display at least six of ten previously established histopathologic parameters required for the diagnosis of GOC as described by Fowler et al. (9). Formalin-fixed, paraffin-embedded tissue blocks of all the eleven cases were available. Hematoxylin and eosin stained slides were prepared and reviewed for each case by two study participants who recorded the presence or absence of the microscopic parameters characteristic of GOC.
Special and immunohistochemical stains were available for review on six cases including cytokeratin 19 (CK19), mucicarmine (MUC), and periodic acid schiff (PAS) stains.
Detection of MAML2 Gene Break by FISH
Formalin-fixed, paraffin-embedded sections of eleven specimens were assessed. Sections were subjected to a dual-color FISH assay using the probe ZytoLight SPEC MAML2 Dual Color Break Apart (ZYTOVISION catalog number Z-2014–200). The probe to the 5’ gene sequence is labeled in green and the probe to the 3’ gene sequence is labeled in red. FISH assays were performed as described by Flacco et al., (10).
Analysis was performed on an epifluorescence microscope using single interference filter sets for red (tailored also to detect orange fluorescence) and green. For each interference filter, monochromatic images were acquired and merged using CytoVision (Leica Microsystems). Analyses were performed in a minimum of 50 nuclei harboring at least one copy of each signal. A specimen was considered positive for MAML2 rearrangement when a minimum of 20% of analyzed cells displayed split 3’MAML2 and 5’MAML2 signals or single signals.
RESULTS
Patient Demographics are Clinical Symptoms
The mean age at diagnosis for the patients included in this study was 55.27 years with a range of 36–72 years (n=11). Six patients were female and five were male. Nine cases occurred in the mandible; one case presented in the maxilla and in one case the site of the lesion was not identified. The most common symptom documented at the time of initial cyst presentation and at the time of recurrence was “expansion of the jaw or swelling.”
Radiographic Findings
All eleven cases assessed presented initially as a radiolucency of the jaw. Four of eleven cases were described initially as multilocular lesions. The other seven cases were described as a jaw radiolucency without any additional descriptors. One lesion was described as being in a periapical location and one lesion was described as having a classic “traumatic bone cyst” appearance. Of the four lesions that were described as being multilocular at their initial presentation, two were described subsequently as being a multilocular radiolucency at the time of recurrence. One lesion recurred for a second time and was described as multilocular at the time of that second recurrence.
Treatment Modalities and Follow Up
Although follow up information was available for only seven of the cases evaluated, all of the GOCs included in this study were known to be recurrent lesions. THE AVERAGE LENGTH OF FOLLOW UP WAS 6.1 YEARS. THE AVERAGE PERIOD OF TIME BETWEEN THE INITIAL TREATMENT OF ANY CYST AND FIRST RECURRENCE WAS 3.6 YEARS WITH A RANGE OF 1.5 TO 5.5 YEARS.
All cysts were treated initially in a conservative manner that included either local excision, enucleation, cystectomy, curettage, or peripheral ostectomy. Information regarding the management of recurrences was available on six of the eleven cases. Four cases were retreated at a second surgery by aggressive curettage and peripheral ostectomy. Two cases were retreated with peripheral ostectomy. The one case that demonstrated a second recurrence was retreated by peripheral ostectomy.
Microscopic Findings
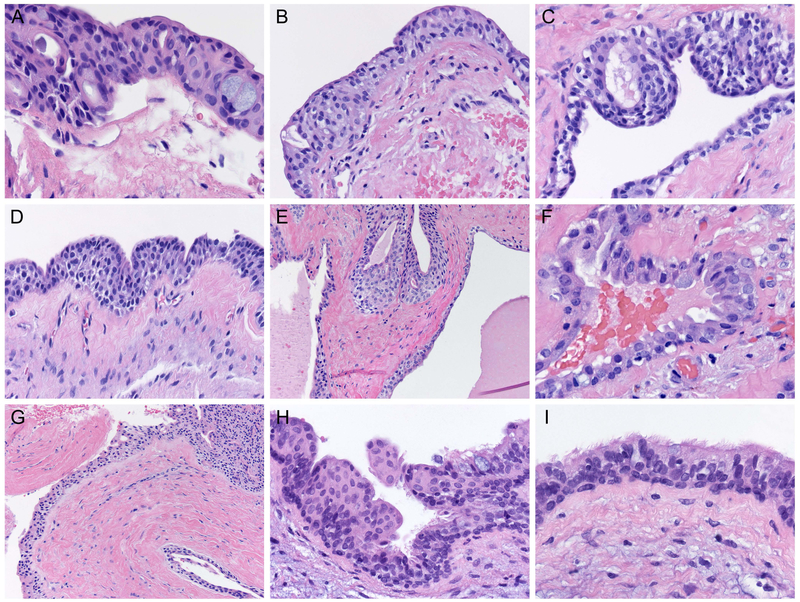
Ten specific and well delineated microscopic parameters were employed to characterize and establish a diagnosis of GOC. These microscopic features were adapted from those described by Fowler et al. (9). For each case studied, the presence or absence of these ten features were recorded independently by two study participants. A minimum of six of ten histopathologic parameters were required for a lesion to be diagnosed as GOC. Those microscopic parameters are delineated below:
Intraepithelial microcysts or duct-like spaces (figure 1A).
Vacuolated cells or cells with clear cytoplasm within the basal epithelial and parabasal layers of the epithelial cyst lining (figure 1B).
Epithelial papillary projections into the cyst lumen (figure 1C).
Nodular epithelial spherules or plaque-like thickenings of lining epithelial cells (figure 1D).
Multiple cystic compartments (figure 1E).
Eosinophilic cuboidal cells, known as “hobnail cells” along the surface of the epithelial cyst lining (figure 1F).
Apocrine snouting or rounding of hobnail cells (figure 1F).
Variations in the thickness of cyst lining epithelium (figure 1G).
Solitary mucous cells or mucous secreting goblet cells within the cyst lining epithelium (figure 1H).
Ciliation of cyst lining epithelial cells (figure 1I).
Figure 1: Histopathologic features characterizing GOC.
A. The epithelial lining of a GOC demonstrating microcysts and duct-like spaces (H&E stain, orig. mag x200).
B. Vacuolated (clear) cells within the basal and parabasal layers of the epithelial cyst lining (H&E stain, orig. mag x400).
C. Papillary projections (tufting) of the epithelial lining into the cyst lumen (H&E stain orig. mag x400).
D. Nodular epithelial spherules, which represent plaque-like thickenings of the epithelial cyst lining (H&E stain orig. mag x400).
E. Multiple cystic compartments (H&E stain, orig. mag x200).
F. Presence of cuboidal and eosinophilic hobnail cells with occasional basilar clear cells in the cyst lining. Apocrine rounding (snouting) is a common feature of these hobnail cells (H&E stain orig. mag x400).
G. Variable thickness of the epithelial lining that ranges from a few cell layers to greater than 30-cell layers in thickness (H&E stain orig. mag x200).
H. Presence of mucous cells within the epithelial cyst lining. Occasionally these cells may line the duct-like structures in the cyst lining (H&E stain orig. mag x400).
I. GOC epithelial lining characterized by clear cells, eosinophilic hobnail cells, and cells with cilia (H&E stain, orig. mag x600).
FISH Analysis Findings
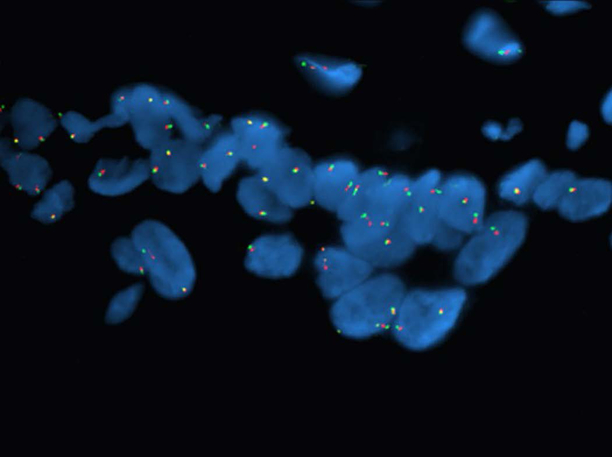
Successful FISH analysis was performed on all eleven recurrent GOCs. Molecular studies were negative for MAML2 rearrangements in ten of our recurrent cases with the majority of nuclei showing intact MAML2 copies (figure 2). One case showed positive MAML2 rearrangements demonstrating split 3’ MAML2 and 5’ MAML2 signals (figure 3A). This case involved a 46 year-old female with a multilocular lesion of the left mandible. The cyst recurred 18 months after initial treatment. At the time of recurrence, the treating oral and maxillofacial surgeon described the lesion as a recurrent multilocular radiolucency of the left mandible (figure 3B). There were no definitive clinical findings that would seem to favor or disfavor transition of the recurrent GOC to IMEC. The surgical specimen of the initial lesion was unavailable for histologic and molecular analyses.
Figure 2:
Representative photomicrograph of a case of recurrent GOC demonstrating negative pattern for MAML2 rearrangement. Note the intact and fused 5’ and 3’ MAML2 signals in the majority of the cell nuclei (5’ green signal, 3’ red signal).
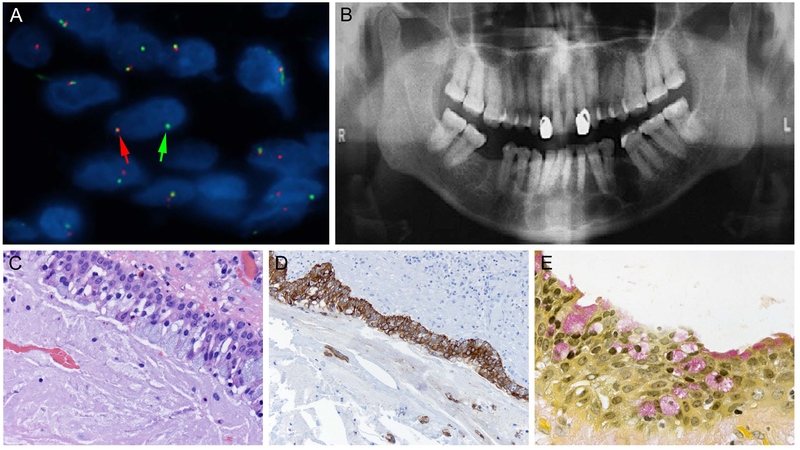
Figure 3: MAML2 positive, recurrent GOC.
A. Hybridization with MAML2 break-apart probe reveals evidence of fusion transcript demonstrated by split signals (arrows) or individual signals on greater than 20% of the cell nuclei (5’ green signal, 3’ red signal).
B. Panoramic radiograph of MAML2 positive case demonstrating a well-defined multilocular radiolucency on the left mandible extending through the midline, with some tooth displacement and no evidence of root resorption.
C. Photomicrograph showing the arrangement of mucous secreting goblet cells along the luminal aspect of the cyst lining epithelium in MAML2 positive GOC (H&E stain, orig. mag x400)
D. Immunohistochemical staining with CK19 highlights the epithelial cyst lining of MAML2 positive GOC (orig. mag x200).
E. MUC stains demonstrate arrangement of mucous secreting goblet cells within the epithelium and along the luminal aspect of the cyst lining (orig. mag x400).
DISCUSSION
GOC is an extensively studied developmental odontogenic cyst that has been well described clinically, radiographically and histopathologically since its initial recognition. Two significant investigations, those of Fowler et al. (9) and Kaplan et al. 11), have delineated the histologic criteria required for a diagnosis of GOC. Kaplan et al. have suggested that at least four major and two minor histologic features must be identified by the pathologist in order to definitively arrive at a diagnosis of GOC. Fowler et al. however, maintain that it is not a group of major or minor histologic criteria that must be met in order to establish a diagnosis of GOC, but rather a combination of ten characteristic histologic features that best aid in establishing that diagnosis. We accepted a cyst as a GOC if six or more of the histologic parameters described by Fowler et al. were identified microscopically.
In a univariant analysis of the 46 cases of GOCs examined by Fowler et al., these investigators found that 43 of the cases studied displayed 7 of the aforementioned diagnostic histologic parameters (9). These investigators further point out that recurrent GOCs sometimes fail to exhibit many of these classic histologic features when compared to the initial biopsy specimens. This finding IS supported by the current study, where three of our recurrent cases displayed only six classic GOC histologic features. All eleven cases in this study met that six parameter requirement.
Although most of the existing scientific literature supports the concept that IMECs and GOCs are separate and non-transitional entities, Fowler et al. has demonstrated that IMEC and GOC clearly share a histologic spectrum (9). These investigators also report finding MEC-like islands in 3 of the 46 cases of GOC they studied; islands similar to those seen in IMECs. Interestingly, these investigators also report that in 2 of those 3 cases, the MEC-like islands that were identified invaded the bone. However, they conclude that the presence of these MEC-like islands likely has no clinical significance. The investigation did not include molecular analysis for MAML2 gene rearrangements in the GOCs that were studied.
FISH analysis revealed that ten of the eleven recurrent GOCs lacked MAML2 gene rearrangements. This finding IS in agreement with the study of Bishop et al., which revealed that GOCs lack MAML2 gene rearrangements (1). THE MOST SIGNIFICANT FINDING IN THE CURRENT STUDY HOWEVER, WAS THE DETECTION OF MAML2 FUSION TRANSCRIPTS IN ONE OF OUR RECURRENT CASES. THIS IS TO THE BEST OF OUR KNOWLEDGE, THE FIRST CASE OF GOC REPORTED IN THE ENGLISH LANGUAGE LITERATURE TO DEMONSTRATE POSITIVE MAML2 GENE REARRANGEMENTS.
The MAML2 positive GOC in the study demonstrated seven of the ten classic GOC histopathologic parameters described by Fowler et al. We did not identify any MEC-like islands of the type described by Fowler et. al (9) in any of the cysts examined in this study including the recurrent GOC with MAML2 gene rearrangements. Although scattered mucous secreting goblet cells were present in the cyst lining of six of the recurrent cases studied, only one case, the MAML2 positive GOC, demonstrated prominent goblet cell proliferation that was almost entirely along the luminal aspect of the cyst lining epithelium (figure 3C). Mucicarmine stains were positive and immunohistochemical studies, as expected, revealed strong CK19 reactivity in epithelial cells lining that GOC (figures 3D and3E). Whether or not this unique distribution of goblet cells is of histologic and clinical significance and whether it is in any way predictive of IMEC transitional behavior can only be speculated.
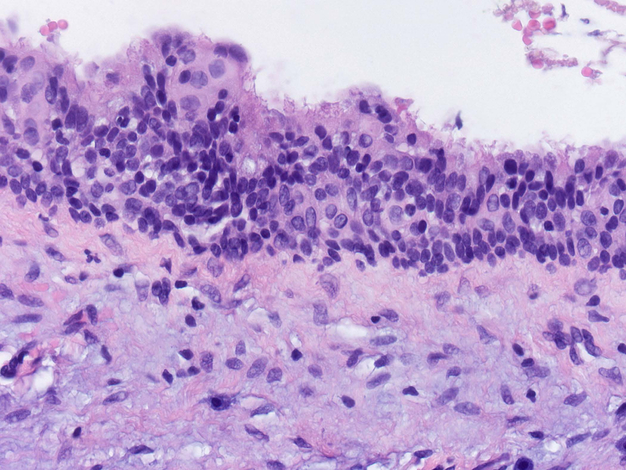
One additional unique finding was the presence of prominent ciliated cells along the epithelial cyst lining of the MAML2 positive GOC. This ciliation was far more prominent than in other recurrent GOCs included in this study (figure 4).
Figure 4:
GOC lining epithelium exhibits marked ciliation in comparison to other cases in the study (H & E stain, orig. mag x400).
IT CAN ONLY BE SPECULATED WHETHER MARKED CILIATION AND MUCOUS SECRETING GOBLET CELL PROLIFERATION ALONG THE LUMINAL ASPECT OF THE CYST LINING EPITHELIUM HAS ANY CORRELATION WITH MOLECULAR EVIDENCE OF MAML2 GENE REARRANGEMENT. However, this finding warrants further investigation in order to determine if such histologic features are of predictive value in assessing recurrent GOCs for potential risk of transition to IMEC, and to possibly redefine those GOC histopathologic parameters that can be of value in determining the future biologic behavior and potential neoplastic transformational risk of a GOC.
Our study DOES NOT provide DEFINITIVE molecular evidence to support a theoretic transition of biologically aggressive, recurrent GOCs to IMEC. However, we believe the finding of MAML2 gene rearrangements in one biologically aggressive recurrent GOC, HERETOFORE NEVER REPORTED, IS NONETHELESS A SIGNIFICANT enough finding to warrant further investigation of a larger series of similar behaving GOCs.
The FISH findings in the current study also raise the question of the reliability of MAML2 gene rearrangement as a diagnostic tool in the differentiation of IMEC from GOC. It should be remembered that the absence of MAML2 rearrangements does not necessarily exclude a diagnosis of IMEC. Current studies have demonstrated that only 75–85% of low grade MECs carry such rearrangements (1, 6–8). It is interesting as well, that Luk et al. found that MAML2 gene rearrangements were not present in 4 of 41 cases of primary salivary gland MECs they studied (6). It is also important to note that non-salivary primary MECs, such as thyroid sclerosing MEC with eosinophilia, lack MAML2 gene rearrangements (12), and that sclerosing mucoepidermoid carcinoma with eosinophilia has also been reported in salivary glands (13). To our knowledge, such salivary gland tumors have not been studied for MAML2 gene rearrangements. Furthermore, it should be noted that MAML2 rearrangements have been identified in other odontogenic cysts besides GOC, including dentigerous and radicular cysts (14). These findings demonstrate the limitations of molecular analysis for MAML2 rearrangements in such cystic lesions, despite its potential diagnostic value.
Cytokeratin (CK) expression in IMEC and GOC has been reported to be quite different. Pires et al., (15) demonstrated CK19 expression in 100% of the GOCs they studied while only 50% of the six IMECs they studied showed CK19 expression. The one GOC that demonstrated MAML2 positivity in the current study was also strongly CK19 positive, further supporting a GOC diagnosis and classification despite the molecular findings.
CONCLUSION
The distinction between GOC and IMEC can be a difficult diagnostic challenge for the pathologist, especially when dealing with recurrent, biologically aggressive GOCs. NO APPARENT CLINICAL OR RADIOGRAPHIC PARAMETERS ALLOW ONE TO EASILY SEPARATE THE TWO ENTITIES. Although of great diagnostic value, FISH analysis for MAML2 gene rearrangements also has its limitations. Therefore, the unique microscopic parameters that are considered highly predictive of recurrent GOCs should be routinely relied upon by the pathologist when rendering a GOC diagnosis. The presence of at least six histopathologic parameters as described Fowler et al. is mandatory for a diagnosis of GOC, especially in recurrent GOCs, where histologic parameters are often lost. The presence of less than five of these parameters tends to favor the diagnosis of a cyst other than a GOC. Absence of classic GOC histopathologic features or a reduction in their numbers below five, in the presence of MAML2 fusion transcripts tends to favor a diagnosis of IMEC.
ACKNOWLEDGEMENTS
This work was partially supported by University of Colorado Cancer Center (Molecular Pathology Shared Resources) Grant NCI-CCSG P30CA046934.
Footnotes
CONFLICE OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- 1.Bishop JA, Yonescu R, Batista D, Warnock GR, Westra WH. Glandular odontogenic cysts lack MAML2 rearrangements: a finding to discredit the putative nature of GOC as a precursor to central mucoepidermoid carcinoma. Head and Neck Pathol. 2014;8:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascitti M, Santarelli A, Sabatucci A, et al. Glandular odontogenic cyst: review of literature and report of new case with cytokeratin-19 expression. The Open Dent J. 2014;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan KC, Pharoah M, Lee L, Weinreb I, Perez-Ordonez B. Intraosseous mucoepidermoid carcinoma: a review of the diagnostic imaging features of four jaw cases. Dentomaxillofacial Radiol. 2013;doi:4:20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atarbashi Moghadam S, Atarbashi Moghadam F. Intraosseous mucoepidermoid carcinoma: report of two cases. J Dent Shiraz Univ Med Sci. 2014;15:86–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Brandwein-Gensier M, Bell D, Inagaki H, Katabi N, Levio I, Seethala R, Triantafyllou A. Mucoepidermoid carcinoma In: WHO classification of head and neck tumours, 4th ed 2017, International Agency for Research on Cancer: Lyon, 163–164. [Google Scholar]
- 6.Luk PP, Wykes J, Selinger CI, et al. Diagnostic and prognostic utility of mastermind-like 2 (MAML2) gene rearrangement detection by fluorescence in situ hybridization (FISH) in mucoepidermoid carcinoma of salivary glands. Oral Surg, Oral Med, Oral Pathol, Oral Radiol. 2016;121:530–541. [DOI] [PubMed] [Google Scholar]
- 7.Barrett AW, Abdullakutty A, Norris PM, et al. Molecular diagnostics in the differential diagnosis of glandular odontogenic cysts and mucoepidermoid carcinoma- case reports. Oral Surg. 2016;9:193–200. [Google Scholar]
- 8.Seethala RR, Dacic S, Cieply K, Kelly L, Nikiforova MN. A reappraisal of the METC1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–1121. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CB, Brannon RB, Kessler HP, Castle JT, Kahn MA. Glandular odontogenic cyst: analysis of 46 cases with special emphasis on microscopic criteria for diagnosis. Head and Neck Pathol. 2011;5:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flacco A, Ludovini V, Bianconi F, et al. MYC and human telomerase gene (TERC) copy number gain in early non-small cell lung cancer. Am J Clin Oncol. 2015;38:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan I, Anavi Y, Hirshberg A. Glandular odontogenic cyst: a challenge in diagnosis and treatment. Oral Dis. 2008;14:575–581. [DOI] [PubMed] [Google Scholar]
- 12.Shah AA, La Fortune K, Miller C, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol. 2017; 30:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urano M, Masato A, m Yosimune H, Makoto M, Yoshikazu M. Sclerosing mucoepidermoid carcinoma with eosinophilia of salivary glands. Pathology – Research and Practice. 2002;198:305–310. [DOI] [PubMed] [Google Scholar]
- 14.Argyris PP, Wehrs RN, Garcia JJ, Koutlas IG. Florescence in-situ hybridization identifies mastermind-like 2 (MAML2) rearrangement in odontogenic cysts with mucous prosoplasia: a pilot study. Histopathol. 2015;66:791–797. [DOI] [PubMed] [Google Scholar]
- 15.Pires FR, Chen SY, da Cruz Perez DE, de Almeida OP, Kowalski LP. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol. 2014;40: 545–551. [DOI] [PubMed] [Google Scholar]