Supplemental Digital Content is available in the text.
Keywords: Perfluoroalkyl substance, Perfluorooctanoic acid, Prenatal, Birth weight, Growth, Prenatal, Children
Abstract
Background:
Prenatal perfluoroalkyl substance (PFAS) exposure has been associated with reduced birth weight and excess child adiposity, but the relationship between PFAS and early life growth is unknown.
Objective:
To determine if prenatal PFAS exposure was associated with birth weight, body composition, and growth until 2 years of age.
Methods:
In a prospective cohort of women and their children from Cincinnati, OH, we quantified perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS) in pregnant women’s serum. We used linear regression to estimate associations of PFAS with birth weight z-scores (n = 345) and linear mixed models to estimate associations with repeated weight and length/height measurements (n = 334) at 4 weeks and 1 and 2 years of age, after adjusting for sociodemographic, perinatal, nutritional, and environmental factors.
Results:
We found nonsignificant inverse associations between PFAS and infant birth weight. For example, each log2 increase in PFOA was associated with a 0.03 SD reduction in birth weight z-score (95% confidence interval [CI] = −0.17, 0.10). Compared to associations with birth weight, we observed stronger associations between PFAS and child anthropometry from 4 weeks to 2 years. For instance, each log2 increase in PFOA was associated with a 0.12 SD decrease in BMI z-score (95% CI = −0.25, 0.01). We did not observe any differences in growth rate associated with PFAS.
Conclusions:
We observed inverse associations between prenatal serum PFAS concentrations and anthropometry until 2 years of age. Prenatal serum PFAS concentrations were not associated with growth rate in the first 2 years of life.
Introduction
Perfluoroalkyl substances (PFAS) are a class of synthetic chemicals that have been used for over 60 years in many consumer products including some food packaging, stain- and water-repellent textiles, nonstick coatings, and fire-fighting foams.1 PFAS were produced in high volumes, and almost all people (98%) in the United States have detectable serum concentrations of some PFAS.2 Several PFAS, including perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS), are persistent in the environment, have biological half-lives of several years, can cross the placenta, are suspected endocrine-disrupting chemicals, and may adversely affect human health.1,3,4
PFAS can activate the peroxisome proliferator-activated receptors (PPARs) or may disrupt other aspects of endocrine function, potentially influencing a host of processes during fetal development associated with adiposity, including cell differentiation, development of adipose tissue, and carbohydrate or lipid metabolism.5–7 While many epidemiological and animal studies have consistently shown that prenatal serum PFOA concentrations are associated with reduced fetal growth,8,9 some have not.10–12 A single study found that both PFOA and PFOS were associated with altered growth in the first 2 years of life.13
Because growth-restricted newborns are more likely to experience rapid weight gain during the first 2 years of life, they may be at higher risk of cardiometabolic disease later in life.14–17 Thus, infants with higher prenatal PFAS exposure may be at increased risk for cardiometabolic diseases as rapid infant growth may be both an early marker of the potential effects of PFAS exposure and a risk factor for future cardiometabolic disorders and obesity. This hypothesis is consistent with some animal and human studies suggesting that PFAS exposure is associated with greater adiposity and increased risk of being overweight or obese.7,13,18–20
While numerous studies have examined prenatal PFAS exposure and size at birth or adiposity later in childhood, fewer studies have examined prenatal PFAS exposure and growth in the first years of life, and results have been discrepant.13,21,22 The objective of this study was to determine if maternal serum PFAS concentrations during pregnancy were associated with fetal growth and growth from 4 weeks to 2 years of age in a prospective cohort study of 345 mother–child pairs.
Methods
Study participants
We analyzed data collected from an ongoing prospective pregnancy and birth cohort, the Health Outcomes and Measures of the Environment (HOME) study. Eligibility requirements included that women were ≥18 years of age, recruited at 16 ± 3 weeks of gestation, living in a Cincinnati, OH, area in a home built before 1978, without a history of HIV infection, and not taking medications for seizure or thyroid disorders.23 We restricted the present analysis to women with singleton pregnancies. Three hundred eighty-nine eligible women were recruited from seven prenatal care clinics affiliated with three Cincinnati, OH, hospitals between 2003 and 2006.
All women provided informed consent for themselves and their children, and the institutional review boards at Cincinnati Children’s Hospital Medical Center (CCHMC) and affiliated delivery hospitals approved this study. The Centers for Disease Control and Prevention (CDC) Institutional Review Board (IRB) deferred to CCHMC as the IRB of record.
PFAS exposure assessment
Maternal blood samples were collected during pregnancy, at approximately 16 or 26 weeks gestation, or within 48 hours of delivery. Serum concentrations of PFOA, PFOS, PFNA, and PHFxS were quantified at the Centers for Disease Control and Prevention. PFAS levels were quantified in a 100-µl serum aliquot diluted with formic acid and fortified with isotopically labeled PFAS internal standards using online solid-phase extraction with high-performance liquid chromatography-isotope dilution tandem mass spectrometry.24 Each analytic batch included reagent blanks and low- and high-concentration quality control (QC) materials that had coefficients of variation of ~6%. The limits of detection were 0.082 (PFNA), 0.1 (PFHxS, PFOA), and 0.2 ng/ml (PFOS); the four PFAS were detected in all samples. Because of possible changes in plasma volume during pregnancy, we measured PFAS concentrations in the 16-week serum samples if available (86%), the 26-week sample if the 16-week sample was not available (9%), and if neither of those was available, then the samples from delivery were used (5%). PFOA, PFOS, PFNA, and PHFxS concentrations were analyzed as log2-transformed variables (i.e., change in outcome per doubling of PFAS) or terciles.
Birth weight and infant/child anthropometry
We abstracted neonatal weight (grams) and gestational age (weeks) from hospital records. Trained research assistants who were blinded to women’s PFAS concentrations took standardized measurements of weight and length/height (centimeters) when children were approximately 4 weeks, 1 year, and 2 years old. For growth analyses, we used the 4-week anthropometry measures instead of those taken at birth to maintain consistency with the subsequent anthropometry measures at 1 and 2 years of age as birth measurements were abstracted from medical records and subsequent measurements were collected using research quality measurements.
For analyses of fetal growth, we calculated birth weight z-scores, a measure of birth weight, standardized for gestational age using U.S. reference data.25 For analysis of infant/child growth, we calculated weight-for-age, length-for-age, weight-for-length, and body mass index (BMI) z-scores using World Health Organization (WHO) reference data among children with at least one anthropometry measurement between 4 weeks and 2 years of age. We chose to use WHO reference data because it allowed us to calculate BMI z-scores for children <2 years of age. In addition, we created a binary variable for rapid weight gain, which was defined as an increase in weight z-score >0.67 SDs anytime between 4 weeks and 2 years of age.17,26
Our analyses examining fetal growth and infant/child anthropometry included 345 and 334 singleton infants, respectively, who had complete data on maternal serum PFAS concentrations, anthropometry, and covariates. Our analysis examining average change in anthropometry (i.e., growth) and rapid growth included 299 singleton infants with at least two anthropometry measures between 4 weeks and 2 years of age, as well as complete exposure and covariate data.
Covariates
We used a directed acyclic graph (DAG) to select maternal sociodemographic, perinatal, nutritional, and environmental factors as covariates in our analysis (Supplemental Figure 1; http://links.lww.com/EE/A5).27,28 We assessed maternal sociodemographic factors including maternal age, race, household income, marital status, and medical insurance status using standardized interviews administered by trained research assistants during pregnancy. Maternal nutritional factors, including food security, prenatal vitamin use, and frequency of fruit/vegetable and fish consumption during pregnancy were assessed using standardized interviews. We measured serum concentrations of cotinine, a sensitive and specific biomarker of active and secondhand tobacco smoke exposure, using previously described methods.29,30 Perinatal factors, including parity and maternal BMI at ~16 weeks gestation, were abstracted from medical records, and depressive symptoms at 20 weeks gestation were assessed using the Beck Depression Inventory-II.31
All models were adjusted for maternal age at delivery (continuous), race (categorical: non-Hispanic white, non-Hispanic black, other), marital status (categorical), insurance status (dichotomous: insured, uninsured), income (continuous), education (categorical: less than high school, high school or some college, bachelors+), parity (categorical: 0, 1, 2+), serum cotinine (continuous), depressive symptoms (categorical: Beck’s Depression Index (BDI) <14, BDI 14–19, BDI >19), mid-pregnancy BMI (continuous), food security (categorical: sometimes/often not enough, enough but not always what is wanted, enough), fruit/vegetable consumption during pregnancy (categorical: less than once per week, weekly, daily), fish consumption during pregnancy (categorical: less than once per month, monthly, weekly+), and prenatal vitamin use (dichotomous: less than weekly, weekly+).
Statistical analyses
We began by describing the mean birth weight z-score as well as the median serum PFOA/PFOS concentrations according to covariates. We then examined univariate characteristics of maternal serum PFAS concentrations and calculated the correlation coefficient between the four individual PFAS. We also evaluated the presence of nonlinear relationships between PFAS concentrations and anthropometry z-scores using restricted cubic splines, but there was no indication of nonlinearity (nonlinearity P values were > 0.05) so spline terms were not included in our final models.
We performed four different analyses. First, we used linear regression to estimate the difference in birth weight z-score with increasing maternal serum PFAS concentrations. Next, we used linear mixed models to estimate the association between maternal serum PFAS concentrations and repeated weight-for-age, length-for-age, weight-for-length, and BMI z-scores. We began with a fully adjusted model that included both between- and within-subject variability, including random effects for infant/child age, uncorrelated errors, and time-specific variances. Our final model did not retain terms for the random intercept or slope of age based on model fit (Akaike information criteria). Third, among children with two or more anthropometry measures from 4 weeks to 2 years of age, we estimated the average change in anthropometry (i.e., growth) during this time period in each PFAS concentration tercile by including a product interaction term between age and PFAS tercile in our model. This allowed us to determine if the rate of growth in the first 2 years of life differed by PFAS concentration tercile. Finally, we examined rapid weight gain as a dichotomous outcome using modified Poisson regression with a log-link and robust standard errors to estimate the relative risk of rapid growth (i.e., >0.67 SD change in weight) according to tercile of maternal serum PFAS concentrations.
As previous studies reported sex-specific associations of PFAS with birth weight and child adiposity, we examined whether infant sex modified the association between PFAS concentrations and birth weight z-score or growth by including product interaction terms between maternal serum PFAS concentrations and child sex.18,22
Sensitivity analyses
We performed the following sensitivity analyses. First, we created models that included all four PFAS concentrations in the same model. Next, because of potential differences in birth weight and growth patterns, we excluded infants who were born preterm from analyses (n = 30 for birth weight z-score analyses, n = 29 for growth analyses). Third, for analyses of repeated anthropometry measures from 4 weeks to 2 years of age, we conducted separate analyses adjusting for birth weight and breastfeeding, although we acknowledge that these factors may be causal intermediates as prenatal PFAS concentrations have been associated with both fetal growth and breastfeeding.8,32 We also conducted analyses using CDC weight-for age, length-for-age, and weight-for length z-scores in the growth analyses as opposed to WHO z-scores. Finally, we conducted analyses using only the 86% of women with serum PFAS concentrations measured at 16 weeks gestation.
Results
Mothers in our study were predominantly white (62%), married (66%), and had household income >$40,000 per year (60%) (Table 1). Characteristics of women included in the analyses did not meaningfully differ from characteristics of the full sample of women in the study.23
Table 1.
Birth weight z-score and maternal serum PFOA and PFOS (ng/ml) concentrations according to covariates among HOME study participants.
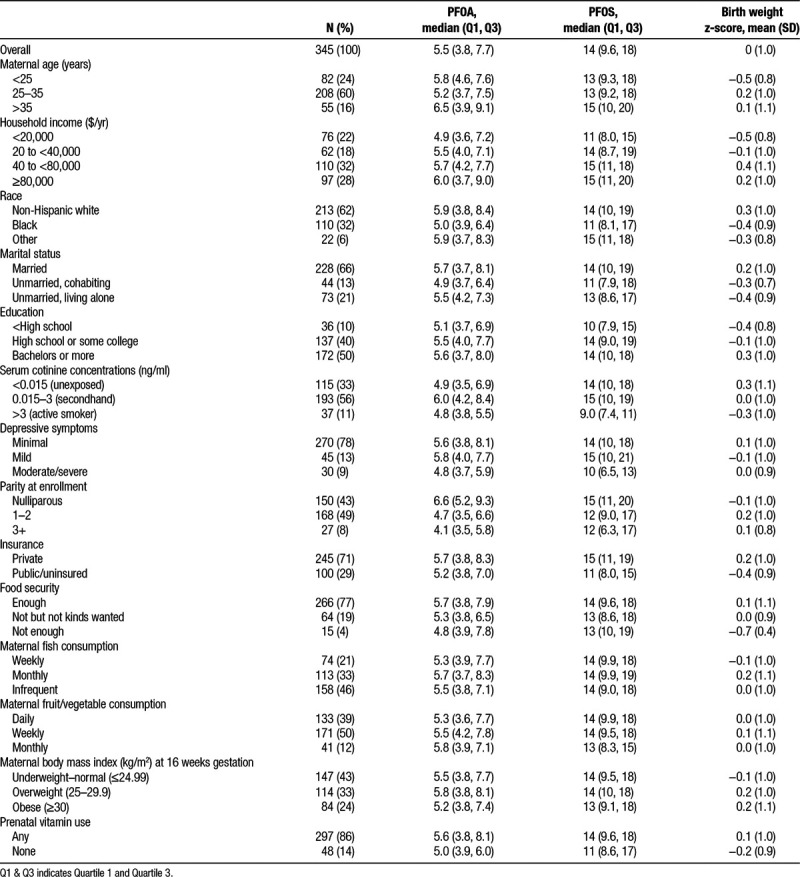
Median serum concentrations of PFOA, PFOS, PFNA, and PFHxS were 5.5, 14, 0.9, and 1.5 ng/ml, respectively. Serum concentrations of the four PFAS were moderately correlated with each other; Pearson correlation coefficients ranged from 0.32 (PFNA and PFHxS) to 0.60 (PFOA and PFOS).
When we examined the dose–response relation between PFAS and birth weight or growth, nonlinearity P values were >0.05 except for the association between PFHxS and rapid growth (P = 0.04). However, the 95% confidence interval of the spline function was imprecise at the tails of PFHxS concentrations and a linear estimate of this relationship fell within the 95% confidence interval of the spline function. Thus, spline terms were not included in our models.
In covariate-adjusted models, we did not observe significant associations between prenatal serum PFAS and birth weight z-scores; however, we did observe weak inverse associations. For example, each doubling (log2 increase) of serum PFOA was associated with a 0.03 SD reduction in birth weight z-score (95% CI = −0.17, 0.10). Further, compared with infants born to women in the first PFOA concentration tercile, infants born to women in the third PFOA tercile had lower birth weight z-scores (−0.15; 95% CI = −0.40, 0.10; P value for trend = 0.14) (Table 2). However, we did not observe a monotonic dose–response relationship between PFOA and birth weight z-scores. We observed weak inverse associations between the other PFAS and birth weight z-scores and did not consistently observe monotonic dose–response relations (Table 2).
Table 2.
Adjusted difference in birth weight z-score and repeated measures of weight-for-age, length-for-age, weight-for-height, and BMI z-score from age 4 weeks to 2 years by terciles (T1-T3) of PFAS and per doubling (log2) increase in maternal serum PFAS concentrations.a
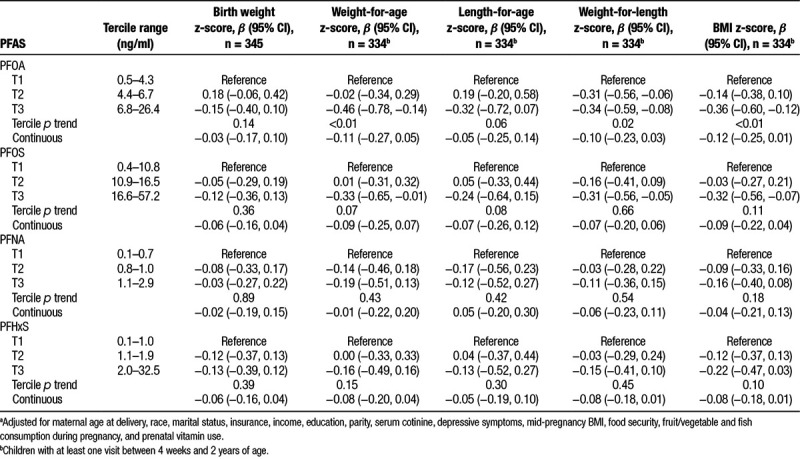
After covariate adjustment, maternal serum PFAS concentrations were inversely associated with repeated weight-for-age, height-for-age, weight-for-height, and BMI z-scores from 4 weeks to 2 years of age. For example, compared with children born to mothers in the first tercile of PFOA concentrations, those born to mothers in the second and third terciles had BMI z-scores that were on average 0.14 (95% CI = −0.38, 0.10) and 0.36 (95% CI = −0.60, −0.12) SDs lower, respectively, (P value for trend < 0.01) (Table 2). Furthermore, each doubling of maternal serum PFOA concentration was associated with a 0.12 SD decrease in BMI z-score (95% CI = −0.25, 0.01) (Table 2). Results were similar for PFOS, PFNA, and PFHxS, but weaker than the associations we observed for PFOA.
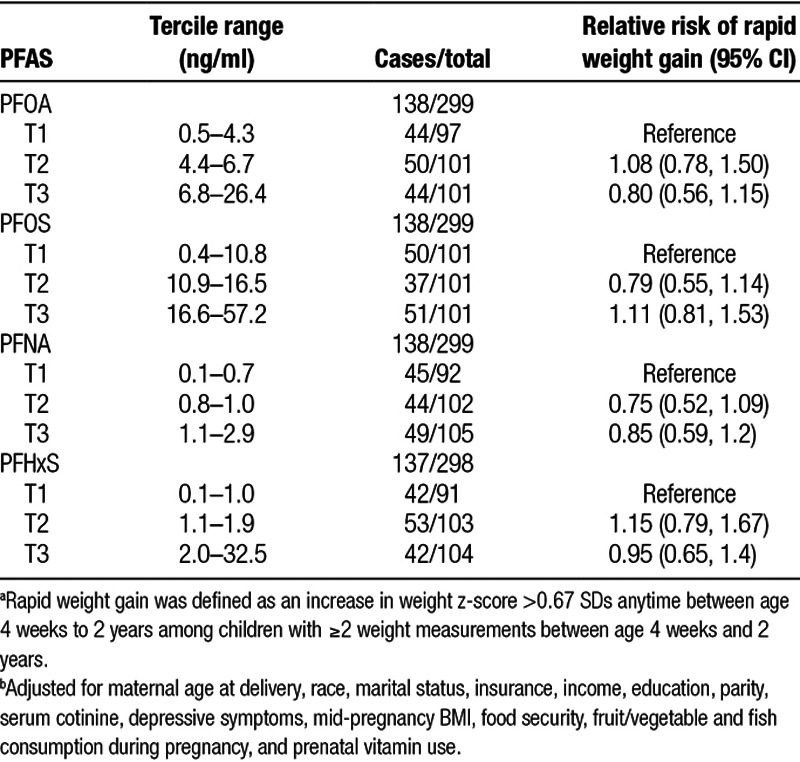
Infant and child growth rates from 4 weeks to 2 years of age did not differ across terciles of PFAS concentration, suggesting that the rate of change in child anthropometry from 4 weeks to 2 years of age was not modified by maternal PFAS concentration (Supplemental Table 1; http://links.lww.com/EE/A5). Further, we found no association between terciles of maternal PFAS concentration and risk of rapid weight gain between 4 weeks and 2 years of age (Table 3). We did not find evidence that associations of maternal serum PFAS concentrations with birth weight z-scores or repeated anthropometry measures were modified by child sex (all PFAS × sex product interaction P values ≥ 0.65).
Table 3.
Relative risk of rapid weight gain between age 4 weeks and 2 years by tercile (T1-T3) of PFAS serum concentrations (n = 299).a,b
Sensitivity analyses
When we adjusted for all four PFAS in the same model, the associations between each individual PFAS and birth weight z-score were attenuated toward the null and became more imprecise (Supplemental Table 2; http://links.lww.com/EE/A5). However, in our BMI z-score analyses, when adjusting for all four serum PFAS concentrations, associations of PFOS, PFNA, and PFHxS with BMI z-score were attenuated to the null while the inverse association between PFOA and BMI z-score remained, but became less precise (Supplemental Table 3; http://links.lww.com/EE/A5). Our results were slightly attenuated when we excluded preterm infants from analyses (Supplemental Tables 2 and 3; http://links.lww.com/EE/A5) and when we adjusted for birth weight in models of maternal serum PFAS concentrations and average BMI z-score (Supplemental Table 3; http://links.lww.com/EE/A5). However, our results were not changed when we adjusted for duration of breastfeeding (Supplemental Table 3; http://links.lww.com/EE/A5) or when we used CDC z-scores as opposed to WHO z-scores. When we restricted our analyses to include only women who provided blood samples at 16 weeks gestation, our results were largely unchanged; however, associations between prenatal PFOA concentrations and BMI z-score were stronger and statistically significant (β: −0.15; 95% CI = −0.29, −0.02 per doubling of PFOA) (Supplemental Tables 2 and 3; http://links.lww.com/EE/A5).
Discussion
Median concentrations of PFOS, PFNA, and PFHxS among women in our study were similar to those of pregnant women in 2003–2004 and 2005–2006 National Health and Nutrition Examination Survey (NHANES) cycles; median PFOA concentrations were more than two times higher among women in our study (5.5 vs. 2.3 ng/ml) compared with NHANES.33
In this prospective cohort, we found suggestive evidence that maternal serum PFAS concentrations, particularly PFOA, were inversely associated with longitudinal measures of infant/child anthropometry from 4 weeks to 2 years of age. In contrast, we observed weak and nonsignificant associations between all four PFAS and birth weight z-scores. Finally, we did not find evidence that maternal serum PFAS concentrations were associated with rapid infant growth between 4 weeks and 2 years of age. These results suggest that infants born to mothers with higher PFAS concentrations tend to be smaller at birth and stay smaller through 2 years of age, while maintaining similar growth trajectories to children born to mothers with lower serum PFAS concentrations. Notably, after adjusting for all four PFAS in the same model, associations of BMI z-scores from 4 weeks to 2 years of age with PFOS, PFNA, or PFHxS were attenuated; however, associations with PFOA remained.
While the inverse association that we observed between maternal PFAS concentrations and infant birth weight z-score was not statistically significant, it is consistent with the magnitude of effect observed in prior studies.8 A meta-analysis conducted by Johnson et al8 estimated a 19-g reduction in birth weight with each 1 ng/ml increase in maternal serum PFOA concentrations. When we conducted a comparable analysis, we found an 8 g decrease (95% CI = −24, 9) in birth weight for each 1 ng/ml increase in maternal serum PFOA concentrations.
While there are numerous studies of prenatal PFAS exposure and size at birth, few have examined growth in the first years of life. Maisonet et al13 examined associations between prenatal serum PFAS concentrations and growth from birth to age 20 months in 447 girls. They reported higher weight at 20 months of age and more rapid weight gain in the first 20 months of life in infants born to women with higher serum PFOS concentrations compared to women with lower PFOS concentrations.13 Consistent with our findings, Andersen et al22 reported inverse associations between prenatal PFOA and PFOS exposure and infant anthropometry at 5 and 12 months of age in a sample of 1,010 mothers and infants from the Danish National Birth. Alkhalawi et al21 examined associations between prenatal exposure to PFOA, PFOS and PFHxS and anthropometry at 1, 4, 6, and 12 months of age in 156 mother–infant pairs. While they reported a significant decrease in ponderal index at birth with increasing PFOA, PFOS, and PFHxS, associations with subsequent weight and length were null.21 In a much larger study, Manzano-Salgado et al34 examined associations between maternal serum PFAS concentrations and infant growth until 6 months of age. They observed positive associations between prenatal PFOA concentrations and weight gain in boys, but not girls.34
In our previous work using the HOME study, we reported that prenatal serum PFOA concentrations were associated with more rapid gains in adiposity from 2–8 years of age and excess adiposity at 8 years of age.20 Thus, in this cohort, it appears that prenatal serum PFOA concentrations are not associated with growth in the first 2 years of life, but may affect growth later in childhood. Future studies should consider examining if PFAS exposure is associated with growth trajectories from delivery to adolescence to better characterize how prenatal PFAS exposures may affect both absolute adiposity and relative changes in adiposity over time.
After adjusting for other PFAS, we did not observe associations between PFOS and infant/child anthropometry, while Maisonet et al13 only found associations between PFOS and growth. A possible reason for the discrepant results is the different PFAS concentration ranges. Median PFOA concentrations were approximately 50% higher in HOME study women than they were in women in the study by Maisonet et al13 (5.5 vs. 3.7 ng/ml), and median PFOS concentrations were approximately 25% lower in the HOME study women (14 vs. 20 ng/ml). Further, animal studies have shown that the effects of prenatal exposure to PFOA, but not PFOS, depend on PPAR-α activation. Thus, it is possible that distinct PFAS, acting through different mechanisms at different concentrations, could have different health effects,35,36 but it is unclear how these effects would differ across different populations.
Our study has several strengths, including a prospective study design, which allowed us to examine longitudinal anthropometry measures between birth and 2 years of age, a critical time period for the development of childhood obesity.14,15,17 Moreover, our rich covariate information allowed us to adjust for a variety of potential confounders, including sociodemographic, nutritional, environmental, and perinatal factors. However, our measures of diet were crude, and it is possible that there is residual confounding from diet as maternal diet may be associated with PFAS exposure and diet quality may be associated with fetal growth. There is also the potential for residual confounding from maternal glomerular filtration rate (GFR).37,38 GFR is the rate at which the kidneys filter blood and it increases as pregnancy progresses, particularly in the first half of pregnancy. GFR is positively associated with both fetal growth and PFAS excretion.37,39 Thus, GFR, which is inversely associated with serum PFAS concentrations and positively associated with birth weight, could be responsible for observed associations between PFAS and birth weight.38,40 However, three studies suggest that the associations between prenatal PFAS exposure and birth weight are not fully explained by confounding from GFR.12,34,38 Finally, while our study had a modest sample size, women in our had above average PFOA concentrations compared with the general population, which provides a unique opportunity to assess potential health effects of higher PFOA concentrations that are still relevant to the general population.
In this cohort of pregnant women and their children, maternal serum PFOA concentrations during pregnancy were inversely associated with repeated measures of weight-for-height and BMI from 4 weeks to 2 years of age. However, we found no associations of prenatal PFAS concentrations with rapid growth in the first 2 years of life. Our weak inverse association between prenatal PFAS concentrations and fetal growth was consistent with the prior literature. Additional studies are needed to verify these findings, and long-term studies that examine growth trajectories from birth through childhood and adolescence would help elucidate the associations between prenatal PFAS exposure and growth.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
This work was supported by National Institute of Environmental Health Science (NIEHS) grants R01 ES025214, R01 ES020349, and P01 ES011261.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgments
We thank Stephen McGarvey and David Savitz and for their helpful comments on this work. We also thank Kayoko Kato and Tao Jia for PFAS measurements.
Footnotes
Published online 24 April 2018
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007; 99366–394. [DOI] [PubMed] [Google Scholar]
- 2.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 2007; 1151596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexane sulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 2007; 1151298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 2017; 13161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casals-Casas C, Feige JN, Desvergne B. Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int J Obes (Lond) 2008; 32suppl 6S53–S61. [DOI] [PubMed] [Google Scholar]
- 6.Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol Sci 2003; 76247–249. [DOI] [PubMed] [Google Scholar]
- 7.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 2009; 30497–105. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PI, Sutton P, Atchley DS, et al. The Navigation Guide—evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 2014; 1221028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koustas E, Lam J, Sutton P, et al. The navigation guide—evidence-based medicine meets environmental health: systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect 2014; 1221015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int 2017; 108278–284. [DOI] [PubMed] [Google Scholar]
- 11.Bach CC, Bech BH, Nohr EA, et al. Perfluoroalkyl acids in maternal serum and indices of fetal growth: the Aarhus Birth Cohort. Environ Health Perspect 2016; 124848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early pregnancy perfluoroalkyl substance plasma concentrations and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect 2012; 1201432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 2000; 320967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006; 95904–908. [DOI] [PubMed] [Google Scholar]
- 16.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 2007; 165849–857. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev 2005; 6143–154. [DOI] [PubMed] [Google Scholar]
- 18.Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 2012; 120668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Z, Li G, Li Y, et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol 2013; 28532–542. [DOI] [PubMed] [Google Scholar]
- 20.Braun JM, Chen A, Romano ME, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity (Silver Spring) 2016; 24231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhalawi E, Kasper-Sonnenberg M, Wilhelm M, Völkel W, Wittsiepe J. Perfluoroalkyl acids (PFAAs) and anthropometric measures in the first year of life: results from the Duisburg Birth Cohort. J Toxicol Environ Health A 2016; 791041–1049. [DOI] [PubMed] [Google Scholar]
- 22.Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am J Epidemiol 2010; 1721230–1237. [DOI] [PubMed] [Google Scholar]
- 23.Braun JM, Kalloo G, Chen A, et al. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 2017; 4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 2011; 12182133–2137. [DOI] [PubMed] [Google Scholar]
- 25.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003; 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valvi D, Casas M, Romaguera D, et al. Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect 2015; 1231022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 1037–48. [PubMed] [Google Scholar]
- 28.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011; 22 [DOI] [PubMed] [Google Scholar]
- 29.Braun JM, Daniels JL, Poole C, et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health 2010; 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997; 432281–2291. [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation;; 1996. [Google Scholar]
- 32.Romano ME, Xu Y, Calafat AM, et al. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res 2016; 149239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17-39 years: data from National Health and Nutrition Examination Survey 2003-2008. J Toxicol Environ Health A 2013; 76409–421. [DOI] [PubMed] [Google Scholar]
- 34.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA Birth Cohort Study. Environ Health Perspect 2017; 125097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol 2009; 27246–257. [DOI] [PubMed] [Google Scholar]
- 36.Abbott BD, Wolf CJ, Schmid JE, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci 2007; 98571–581. [DOI] [PubMed] [Google Scholar]
- 37.Morken NH, Travlos GS, Wilson RE, Eggesbø M, Longnecker MP. Maternal glomerular filtration rate in pregnancy and fetal size. PLoS One 2014; 9e101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verner MA, Loccisano AE, Morken NH, et al. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect 2015; 1231317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson HM. Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw 1973; 801067–1074. [DOI] [PubMed] [Google Scholar]
- 40.Watkins DJ, Josson J, Elston B, et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 2013; 121625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]


