Abstract
To date, there has been limited evidence to reveal the effect of terahertz radiation on sperm. In this study, semen samples were collected from males who had just finished a prepregnancy computer-assisted semen analysis (CASA). The motility, intracellular concentration of free Ca2+ and DNA integrity of sperm with or without terahertz (0.1 to 3 THz) irradiation at 60 µW/cm2 were assessed. We found that terahertz irradiation for more than 5 minutes significantly increased the progressive motility percentage of sperm, and the DNA integrity was not changed. We also found that the effect of terahertz irradiation on spermatozoa was weakened by reducing the concentration of extracellular calcium ions or by blocking calcium channels.
1. Introduction
Electromagnetic waves (EMWs) affect living cells. The effects of electromagnetic irradiation on organisms can be divided into ionizing irradiation and nonionizing irradiation effects. The harmful effects of ionizing irradiation on biological systems are obvious, while the effect of nonionizing irradiation on organisms is one of the hotspots of current research [1]. The sperm cell is very sensitive and fragile, especially in vitro, where these cells are more sensitive to a variety of stimulators. Studies have found that nonionizing irradiation has significant effects on sperm in vitro. Infrared and some regions of visible light have a positive effect on sperm. Visible light can induce nitric oxide (NO) formation in bovine endothelial and sperm cells [2] and can increase the intracellular calcium concentration in mouse and bovine sperm [3] [4]; these changes account for the enhanced fertilization rate in these conditions. In sperm cells, NO has a major effect on the mobility of the spermatozoa [5], as well as an essential role in the acrosome reaction [6]. Another study found that the concentration of intracellular free calcium ions is higher in hyperactivated sperm than in nonhyperactivated sperm [7]. Radiofrequency irradiation has a negative effect on sperm. Electromagnetic fields are recognized as hazards that affect testicular function by generating reactive oxygen species (ROS) and reducing the bioavailability of androgen to maturing spermatozoa [8]. Microwave irradiation usually has negative effects on sperm. It increased the mitochondrial ROS generation in human spermatozoa, decreased the motility and vitality of these cells, stimulated DNA base adduct formation and ultimately led to DNA fragmentation [9]. However, there is a “forgotten” EMW, the terahertz wave, which was hard to stably generate until the past few decades. Terahertz waves are EMWs with frequencies from 0.1 to 10 THz, which is between the microwave and far-infrared frequencies. Unlike the other EMWs, which have been studied for centuries, people knew very little about terahertz waves until a stable irradiation generator and detector were produced in the late 1980s. Terahertz waves have been well studied in the industrial field, and the associated production processes and safety inspections are well developed. At present, the research on terahertz radiation in the field of biomedicine is limited and reported studies have already tried to use terahertz waves to facilitate the diagnosis of diseases and the detection of biological substances [10]. However, very few studies explored the effect of terahertz irradiation on biological cells or tissues, and no studies reported the effect of terahertz irradiation on sperm.
In this study, we found that terahertz irradiation increased the mobility of sperm in vitro, and we examined the potential mechanism of the effect of terahertz irradiation on sperm, revealing that Ca2+ influx activities might mediate this effect.
2. Materials and methods
2.1. Collection of semen samples
From January 2017 to December 2017, 2 ml of semen that was obtained by masturbation and that remained after patients’ routine computer-assisted semen analysis (CASA) in our hospital was retained after their informed consent. This study was carried out in accordance with the ethical standards of the Helsinki Declaration. According to the fifth edition of the WHO laboratory manual for the examination and processing of human semen, patients with severe asthenospermia (1%≤progressive motility (PR)<10%) were excluded because these samples are usually considered to have significant organic contaminants. In other words, this study included normal males and patients with mild (20%≤PR<32%) or moderate (10%≤PR<20%) asthenospermia.
2.2. Semen preparation
The semen was placed in 37°C for 1 hour. If the semen was liquefied, the next step of the experiment was carried out. A total of 100 semen specimens were obtained for this experiment, including 20 from normal patients, 60 from mild asthenospermia patients and 20 from moderate asthenospermia patients. Each semen specimen was divided into experimental group and control group for self-control.
2.3. CASA
A standard count four chamber 20 mm-depth slide was prewarmed at 37°C. Five-microliter semen samples were placed on the slide and analyzed by CASA with software (IVOS CASA system, Hamilton-Thorne Biosciences). For each sample, up to 10 30-s sequences were acquired. According to human sperm motility parameters, at least 100 cells in each sample were analyzed.
2.4. Terahertz irradiation system
A terahertz irradiation generation and inspection equipment (Mini-Z, Zomega Terahertz Corporation, Troy, NY 12180) is set up for terahertz irradiation and detection (0.1-3 THz). The average power emitted by our terahertz generator was measured by a Bolometer (CA-CFW-S,IRLabs Ins. US) and the measured power was about 4.2 μW. Such THz wave was focused on the sample through a convex lens and generated a 3mm diameter spot which can cover most of the droplet of the semen specimens, corresponding to an average intensity about 60 μW/cm2. The semen counting plate was fixed by a combined metal holder. All experiments were performed in a warm room, and the environmental temperature and relative humidity were steadily controlled at 37°C and 60%, respectively.
2.5. Assessment of DNA integrity
The DNA fragmentation index (DFI) was assessed by the sperm chromatin dispersion test using a Halosperm kit (Halotech DNA, S.L, Spain). The method was performed according to the manufacturer’s directions. Sperm was embedded in agarose and exposed a lysis solution, followed by alcohol dehydration and staining. Then slides were examined to detect presence of halos. Sperm with large /medium sized halos were considered to have intact DNA. Sperm cells with small, degraded or absent halos were considered to have fragmented DNA. For each sample, 500 spermatozoa were used.
2.6. Determination of the intracellular calcium concentration in sperm cells
We assessed the intracellular concentration of free Ca2+ by the fluorescent calcium indicator, Fluo-4/AM. Washed sperm cells (1 Ч 107 cells/ml) were incubated with 1 mM Fluo-4/AM for 1 hour. To remove the extracellular Fluo-4/AM, the loaded cells were washed three times. The cells were resuspended in a transparent medium containing calcium and then placed in a black 96-well plate. Immediately after irradiation, the fluorescence was immediately measured by a Tecan Spectro fluorometer microplate reader (excitation wavelength of 488 nm and emission wavelength of 516 nm).
2.7. Statistical analysis
All statistical analyses were performed with IBM SPSS, v.21.0 (IBM, Armonk, NY) and GraphPad Prism 7 (La Jolla, CA). Continuous variables were presented as the mean (standard error of mean) and were compared by Student’s t test. All statistical tests were two sided, and p<0.05 was considered significant.
3. Results
3.1. Terahertz irradiation increased the progressive motility percentage of sperm, with an effect lasting more than 60 minutes
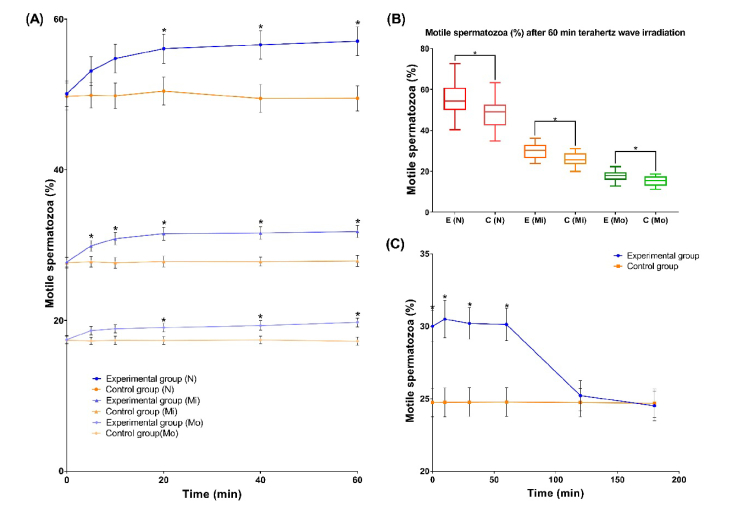
A total of 60 samples of semen from 20 normal males, 20 mild asthenospermia patients and 20 moderate asthenospermia patients were taken. After liquefaction, 5 µl of semen was placed in two sperm counting plates, plate A and plate B. Plate A was the experimental group, and plate B was control group. Before and at 5, 10, 20, 40, and 60 minutes after the start of irradiation, under an Olympus phase-contrast microscope (model CX31), CASA was performed for both groups. For semen samples from the mild asthenospermia patients, the PR percentage was higher in the experimental group with terahertz irradiation than in the control group without terahertz irradiation, the difference between the experimental group and the control group was statistically significant from the fifth minute. However, for normal and moderate asthenospermia patients, the differences were statistically significant from the 20th minute. Therefore, sperm from mild asthenospermia patients was considered the most sensitive to the terahertz irradiation, and subsequent experiments were carried out by using samples from such patients (Fig. 1(A).). At the end of 60-minute irradiation, the differences between the experimental group and the control group reached maximum (Fig. 1(B).). The detail data of progressive motility percentage of sperm during irradiation is provided in Appendix A, Table 1.
Fig. 1.
The effect of terahertz irradiation on sperm motility. (A) The experimental groups received terahertz irradiation, while the control groups did not. The PR percentage of sperm was measured from the beginning to 60 minutes by CASA. The results are presented as median with the standard error of the mean. “N” stands for results from normal patients, “Mi” stands for results from mild asthenospermia patients, and “Mo” stands for results from moderate asthenospermia patients. (B) The experimental groups received terahertz irradiation for 60 minutes, while the control groups did not. The PR percentage of sperm was measured at the end of irradiation. The results are presented as median with quartile and extremum. “E” stands for experimental group, “C” stands for control group, “N” stands for results from normal patients, “Mi” stands for results from mild asthenospermia patients, and “Mo” stands for results from moderate asthenospermia patients. Samples were taken from 20 normal patients, 20 mild asthenospermia patients and 20 moderate asthenospermia patients. Each sample was divided into experimental and control group. (C) The experimental groups received terahertz irradiation for 60 minutes, while the control groups did not. After irradiation, the PR percentage of sperm was measured by CASA from the beginning to 180 minutes. Samples were taken from 10 mild asthenospermia patients and each sample was divided into experimental and control group. *p<0.05
Next, a total of 10 samples of semen from mild asthenospermia patients were taken. The experimental group received additional terahertz irradiation for 60 minutes. Then, the two groups were placed in the original environment. After 0, 10, 30, 60, 120 and 180 minutes, the sperm activity of two groups was determined by the aforementioned methods. After 60 minutes of terahertz irradiation, the improvement in sperm quality in the experimental group compared to that in the control group was statistically significant for more than 60 minutes after the irradiation stopped (Fig. 1(C).). The detail data of progressive motility percentage of sperm after irradiation is provided in Appendix B, Table 2.
3.2. Terahertz irradiation did not affect the DNA integrity
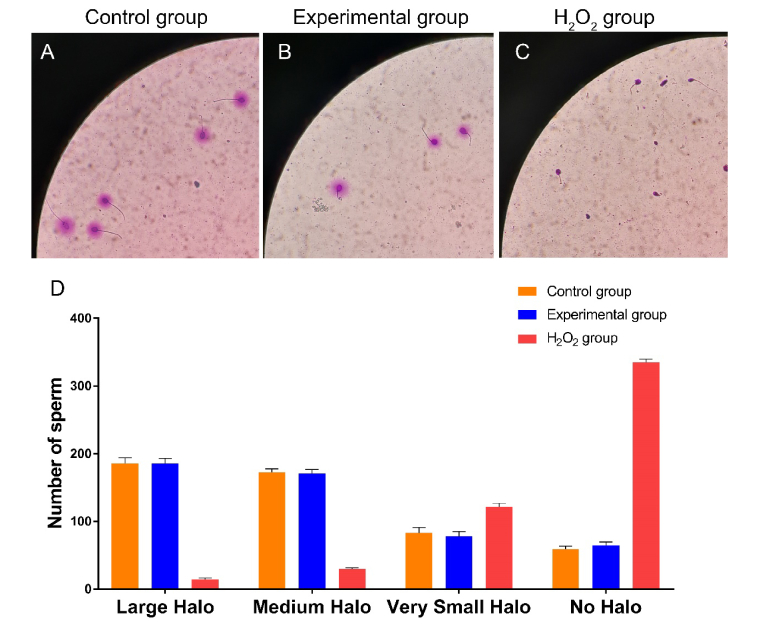
A total of 10 samples of the semen from mild asthenospermia patients were taken and each was divided into 3 groups, the experimental group, the control group and the H2O2 group (for positive control). H2O2, a kind of peroxide, was chosen for positive control to induce sperm nucleus fragmentation. The experimental group was irradiated for 60 minutes. The H2O2 group were incubated with 100 mM H2O2 for 10 min. After irradiation, the mean DFI of the experimental group was 28.62 ± 2.05%, and the mean DFI of the control group was 28.34 ± 1.98%. There was no significant difference in DFI between the experimental group and the control group (Fig. 2).
Fig. 2.
Effect of terahertz irradiation on the DNA integrity of sperm. The experimental group received terahertz irradiation for 60 minutes, while the control group did not. The H2O2 group were incubated with 100 mM H2O2 for 10 min for positive control. After irradiation, the DFI of these samples was assessed by the sperm chromatin dispersion test using a Halosperm kit (Halotech DNA, S.L, Spain). For each sample, 500 spermatozoa were accounted. (A) Representative vision of control group in sperm chromatin dispersion test. (B) Representative vision of experimental group in sperm chromatin dispersion test. (C) Representative vision of H2O2 group in sperm chromatin dispersion test. (D) This graph represents average number of sperm with different Halo size in different group. Samples were taken from 10 mild asthenospermia patients and each sample was divided into experimental, control group and H2O2 group.
3.3. Terahertz irradiation can increase the intracellular calcium concentration in sperm
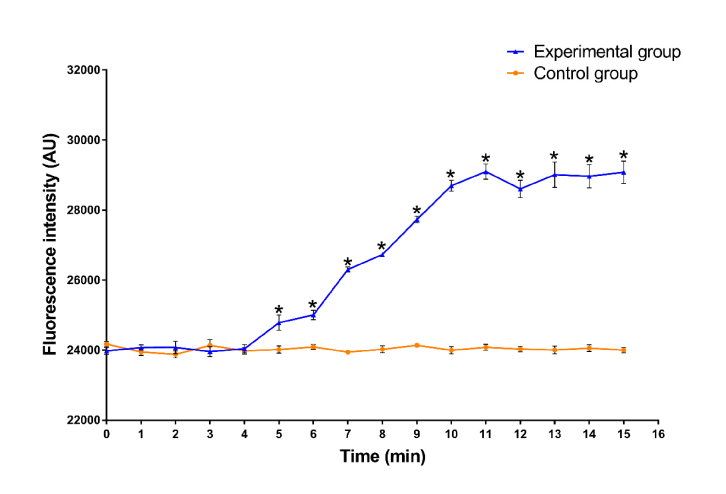
Another 10 samples of semen from mild asthenospermia patients were taken and each was divided into the experimental and control groups. In the experimental group, after 5 minutes of terahertz irradiation, the calcium concentration in the sperm was significantly higher than that in the control group, which did not receive irradiation (see Fig. 3). The detail data of intracellular calcium concentration during irradiation is provided in Appendix C, Table 3.
Fig. 3.
Effect of terahertz irradiation on the intracellular calcium concentration in sperm. Sperm cells were incubated with Fluo-4/AM as described and then irradiated for the specified duration. Fluorescence was measured immediately after the irradiation. Samples were taken from 10 mild asthenospermia patients and each sample was divided into experimental, control group. *p<0.05
3.4. Effects of terahertz irradiation on sperm motility are affected by calcium channels and the extracellular calcium concentration
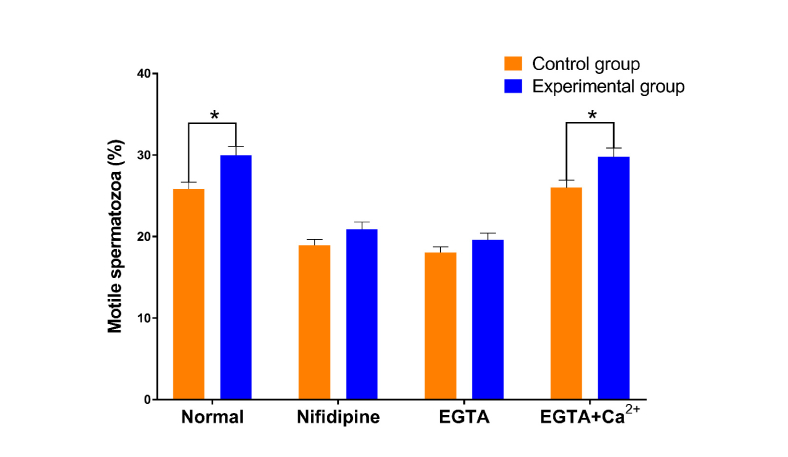
Another 10 samples of semen from mild asthenospermia patients were taken and each was divided into the experimental and control groups. Washed sperm cells (100 µl) from these samples were incubated with 1 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 30 mM nifedipine or EGTA with Ca2+. Ten minutes later, the experimental group was irradiated for 60 minutes. Sperm motility was measured using CASA after irradiation. When calcium channels were inhibited and when the extracellular calcium concentration decreased, the motility of sperm decreased, but the sperm activity in the experimental group receiving terahertz irradiation was still higher than that in the control group without irradiation, although the difference was not significant. However, for the sperm incubated with EGTA with Ca2+, irradiation still caused significant enhancement of motility (see Fig. 4). The detail data of sperm motility is provided in Appendix D, Table 4.
Fig. 4.
Effect of terahertz irradiation on sperm motility after blocking calcium channels or changing the extracellular calcium concentration. Washed sperm cells (100 µl) were incubated with phosphate-buffered saline with 1 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), 30 mM nifedipine, EGTA with Ca2+ or nothing. Ten minutes later, the experimental group was irradiated for 60 minutes. Sperm motility was measured using CASA after the irradiation. Samples were taken from 10 mild asthenospermia patients and each sample was divided into all groups. *p<0.05
4. Discussion
Terahertz waves, also known as T-rays, are a form of EMW. The frequency range of terahertz waves is 0.1 THz to 10 THz, (1 THz = 1012 Hz), the wavelength is 1 Ч 10−3 m to 1.5 Ч 10−5 m, and the corresponding photon energy is 2 Ч 10−22 J to 11.3 Ч 10−20 J. The photon energy of a terahertz wave is low, and the photon energy at the 1 THz frequency is approximately 4 mV, which is 107-108 times weaker than the photon energy of an X-ray. Therefore, terahertz waves do not cause ionizing effects on biological tissue, and terahertz waves are safe for both biological samples and operators. Recently, terahertz pulsed imaging (TPI) technology has been used to assist in the diagnosis of basal cell carcinoma [11], breast cancer [12], liver cancer [13], cervical cancer [14], etc. In addition to being used for the diagnosis of tumors, researchers have also applied TPI to the detection of bacteria, successfully identifying the type of gram staining of 5 different bacteria in the experiment [15]. In addition, some scholars suggest that the use of terahertz waves can both qualitatively and quantitatively detect Escherichia coli [16]. Other studies have confirmed that the concentration of prostate cancer-specific antigen and a variety of biological materials can be quantitatively detected in real time by a label-free THz biosensor [17].
However, no previous studies have reported the effect of terahertz irradiation on sperm. Thus, our study is the first to discuss the effect of terahertz irradiation on human sperm. In our study, sperm from mild asthenospermia patients was the most sensitive to terahertz irradiation. Only 5-minute irradiation can cause significant enhancement of motility, and the effect can last for more than 60 minutes. In addition, the irradiation did not affect the DNA integrity, with the proportion of forward spermatozoa increasing and DFI not increasing. Some studies also reported the effect of other rays on sperm motility. Shahar’s study demonstrated that exposure to visible light at 40 mW/cm2 for 3 minutes increased the hyperactivated motility of capacitated sperm for at least 3 hours, but no effect was seen on total motility [18]. A 30-minute infrared laser pulse of 50 mW/cm(2) at 905 nm can also increase sperm motility, especially in asthenospermic samples [2]. In contrast, 6-hour daily radio frequency radiation from a cell phone for 18 weeks can significantly decrease sperm motility of rats [19].
Terahertz waves, as a kind of EMW, produce a thermal effect on the irradiated object, which may increase the enzyme activity when the radiation acts on the cell. However, in this study, the environmental temperature was strictly controlled, and the emission power of our terahertz generator was only 60 µW/cm2. Therefore, the thermal effect caused by terahertz irradiation in this experiment was negligible. We guess that terahertz irradiation may stimulate some cellular processes by a photochemical reaction. Our study showed that the terahertz irradiation-enhanced sperm motility was achieved by increasing the calcium concentration in sperm cells by affecting calcium channels. The effects of the terahertz irradiation were weakened when we reduced the concentration of extracellular calcium ions by adding EGTA and when we blocked calcium channels by using nifedipine. However, the effect of terahertz irradiation did not completely disappear upon inhibition of calcium channels. Compared with no irradiation, terahertz irradiation can still increase sperm activity, suggesting that terahertz irradiation may act through other mechanisms. Similar to the study on visible light, the effect of light irradiation on sperm motility was mediated by a Ca2+ influx. Moreover, this study also revealed other mechanisms such as ROS production and activation of protein kinase A and sarcoma protein kinase [18]. Another study concluded that visible light illumination can induce NO formation in sperm cells [2]. In addition, a study assessed the effect of radiofrequency irradiation on sperm cell surface adhesion proteins and found that the irradiation can upregulate the mRNA levels of cadherin-1 and interstitial cell adhesion molecule 1 [19]. Overall, differing from conventional molecular signaling pathways, irradiation does not affect only a specific downstream molecule or pathway. Irradiation causes changes in sperm motility through various mechanisms.
Though some preliminary results have been obtained in our study, in regard to the research on the mechanism underlying the effects of terahertz irradiation, we only observed briefly that terahertz irradiation may act by increasing the intracellular calcium concentration of sperm cells. Determining its effects on the whole calcium signaling pathway and the possibility of other effects, such as changing the activity of mitochondria, the production of ROS and the activation of other relevant proteins, needs further experimental research.
5. Conclusion
Terahertz irradiation can increase sperm activity, which may be achieved by increasing the concentration of calcium ions in the sperm cell. This result suggests that terahertz irradiation may be applied in assisted reproductive technology in the future and provide a new method and possibility for improving sperm activity in vitro, but further research on the mechanism and effect will be needed. We believe this study will lay a theoretical foundation for the future application of terahertz irradiation in assisted reproductive technology.
Acknowledgments
We thank all the patients who provided us with their sperm.
Appendix A: Sperm motility during terahertz irradiation
Table 1. The effect of terahertz irradiation on sperm motility during terahertz irradiation.
| Motile spermatozoa (%) | 0 min | 5 min | 10 min | 20 min | 40 min | 60 min | |
|---|---|---|---|---|---|---|---|
| Normal (N* = 20) |
Experimental (n# = 20) | 48.16 ± 1.73 | 51.17 ± 1.88 | 52.83 ± 1.91 | 54.13 ± 1.94 | 54.66 ± 1.86 | 55.12 ± 1.91 |
| Control (n# = 20) | 47.80 ± 1.79 | 47.93 ± 1.68 | 47.88 ± 1.69 | 48.50 ± 1.86 | 47.53 ± 1.86 | 47.54 ± 1.68 | |
| p | 0.89 | 0.21 | 0.06 | 0.04 | 0.01 | 0.01 | |
| Mild asthenospermia (N* = 20) |
Experimental (n# = 20) | 25.82 ± 0.68 | 27.96 ± 0.73 | 28.91 ± 0.82 | 29.57 ± 0.85 | 29.66 ± 0.82 | 29.86 ± 0.82 |
| Control (n# = 20) | 25.73 ± 0.69 | 25.84 ± 0.70 | 25.70 ± 0.72 | 25.87 ± 0.70 | 25.86 ± 0.60 | 25.95 ± 0.72 | |
| p | 0.92 | 0.04 | 0.01 | 0.00 | 0.00 | 0.00 | |
| Moderate asthenospermia (N* = 20) |
Experimental (n# = 20) | 15.51 ± 0.51 | 16.7 ± 0.59 | 16.94 ± 0.54 | 17.10 ± 0.58 | 17.39 ± 0.63 | 17.80 ± 0.58 |
| Control (n# = 20) | 15.44 ± 0.53 | 15.35 ± 0.50 | 15.43 ± 0.57 | 15.40 ± 0.53 | 15.47 ± 0.50 | 15.30 ± 0.54 | |
| p | 0.92 | 0.09 | 0.06 | 0.04 | 0.02 | 0.00 | |
| *Samples were taken from 20 patients and #each sample was divided into experimental and control group. | |||||||
Appendix B: Sperm motility after terahertz irradiation
Table 2. The effect of terahertz irradiation on sperm motility after terahertz irradiation.
| Motile spermatozoa (%) | 0 min | 10 min | 30 min | 60 min | 120 min | 180 min | |
|---|---|---|---|---|---|---|---|
| Mild asthenospermia (N* = 10) |
Experimental (n# = 10) | 30.01 ± 1.08 | 30.50 ± 1.28 | 30.20 ± 1.09 | 30.13 ± 1.11 | 25.22 ± 1.03 | 24.53 ± 1.03 |
| Control (n# = 10) | 24.74 ± 1.00 | 24.75 ± 0.99 | 24.77 ± 0.99 | 24.77 ± 0.99 | 24.74 ± 0.98 | 24.70 ± 0.97 | |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 | 0.90 | |
| *Samples were taken from 10 patients and #each sample was divided into experimental and control group. | |||||||
Appendix C: Intracellular calcium concentration in sperm during terahertz irradiation
Table 3. Effect of terahertz irradiation on the intracellular calcium concentration in sperm.
| Fluorescence intensity (AU) | 0 min | 1 min | 2 min | 3 min | 4 min | 5 min | 6 min | 7 min | 8 min | 9 min | 10 min | 11 min | 12 min | 13 min | 14 min | 15 min |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental (n# = 10) | 23976.6 ± 99.5 | 24074.5 ± 78.8 | 24077.0 ± 179.8 | 23957.9 ± 141.5 | 24387.0 ± 118.1 | 24785.7 ± 220.0 | 25003.6 ± 134.2 | 26303.1 ± 73.0 | 26729.5 ± 51.3 | 27728.6 ± 97.6 | 28694.2 ± 153.7 | 29098.5 ± 215.9 | 28605.7 ± 248.1 | 29011.2 ± 365.7 | 28963.5 ± 332.8 | 29080.7 ± 321.9 |
| Control (n# = 10) |
24171.7 ± 76.6 | 23948.3 ± 95.5 | 23879.5 ± 97.9 | 24113.5 ± 166.0 | 23982.5 ± 96.1 | 24015.0 ± 103.0 | 24092.9 ± 68.8 | 23843.8 ± 53.6 | 24023.1 ± 98.9 | 24137.1 ± 60.5 | 23997.7 ± 101.2 | 24081.8 ± 84.4 | 24030.5 ± 82.1 | 24005.4 ± 110.9 | 24057.7 ± 96.3 | 24000.5 ± 80.2 |
| p | 0.14 | 0.32 | 0.35 | 0.43 | 0.72 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| #Samples were taken from 10 mild asthenospermia patients and each sample was divided into experimental and control group. | ||||||||||||||||
Appendix D: Sperm motility after blocking calcium channels or changing the extracellular calcium concentration
Table 4. Effect of terahertz irradiation on sperm motility after blocking calcium channels or changing the extracellular calcium concentration.
| Motile spermatozoa (%) | Normal | Nifidipine | EGTA | EGTA + Ca2+ | |
|---|---|---|---|---|---|
| Mild asthenospermia (N* = 10) |
Experimental (n# = 10) | 24.85 ± 0.85 | 18.96 ± 0.72 | 18.05 ± 0.71 | 26.01 ± 0.92 |
| Control (n# = 10) | 29.98 ± 1.08 | 20.90 ± 0.90 | 19.62 ± 0.83 | 29.78 ± 1.09 | |
| p | 0.01 | 0.17 | 0.11 | 0.02 | |
| *Samples were taken from 10 patients and #each sample was divided into experimental and control group. | |||||
Funding
Natural Science Foundation of Hubei Province (2013cfb095); Innovation foundation of Huazhong University of Science and Technology (2015YGYL017).
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References and links
- 1.Romanenko S., Begley R., Harvey A. R., Hool L., Wallace V. P., “The interaction between electromagnetic fields at megahertz, gigahertz and terahertz frequencies with cells, tissues and organisms: risks and potential,” J. R. Soc. Interface 14(137), 20170585 (2017). 10.1098/rsif.2017.0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankri R., Friedman H., Savion N., Kotev-Emeth S., Breitbart H., Lubart R., “Visible light induces nitric oxide (NO) formation in sperm and endothelial cells,” Lasers Surg. Med. 42(4), 348–352 (2010). 10.1002/lsm.20849 [DOI] [PubMed] [Google Scholar]
- 3.Cohen N., Lubart R., Rubinstein S., Breitbart H., “Light irradiation of mouse spermatozoa: stimulation of in vitro fertilization and calcium signals,” Photochem. Photobiol. 68(3), 407–413 (1998). 10.1111/j.1751-1097.1998.tb09700.x [DOI] [PubMed] [Google Scholar]
- 4.Lubart R., Friedmann H., Levinshal T., Lavie R., Breitbart H., “Effect of light on calcium transport in bull sperm cells,” J. Photochem. Photobiol. B 15(4), 337–341 (1992). 10.1016/1011-1344(92)85139-L [DOI] [PubMed] [Google Scholar]
- 5.Lewis S. E., Donnelly E. T., Sterling E. S., Kennedy M. S., Thompson W., Chakravarthy U., “Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial to sperm motility,” Mol. Hum. Reprod. 2(11), 873–878 (1996). 10.1093/molehr/2.11.873 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez P. C., O’Flaherty C. M., Beconi M. T., Beorlegui N. B., “Nitric oxide-induced capacitation of cryopreserved bull spermatozoa and assessment of participating regulatory pathways,” Anim. Reprod. Sci. 85(3-4), 231–242 (2005). 10.1016/j.anireprosci.2004.05.018 [DOI] [PubMed] [Google Scholar]
- 7.Suarez S. S., Varosi S. M., Dai X., “Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle,” Proc. Natl. Acad. Sci. U.S.A. 90(10), 4660–4664 (1993). 10.1073/pnas.90.10.4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S., Kesari K. K., Behari J., “The therapeutic effect of a pulsed electromagnetic field on the reproductive patterns of male Wistar rats exposed to a 2.45-GHz microwave field,” Clinics (Sao Paulo) 66(7), 1237–1245 (2011). 10.1590/S1807-59322011000700020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Iuliis G. N., Newey R. J., King B. V., Aitken R. J., “Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro,” PLoS One 4(7), e6446 (2009). 10.1371/journal.pone.0006446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmink And Grundt G. J., “Current State of Research on Biological Effects of Terahertz Radiation,” J Infrared Millim. THz Waves. 32(10), 1074–1122 (2011). [Google Scholar]
- 11.Joseph C. S., Yaroslavsky A. N., Neel V. A., Goyette T. M., Giles R. H., “Continuous wave terahertz transmission imaging of nonmelanoma skin cancers,” Lasers Surg. Med. 43(6), 457–462 (2011). 10.1002/lsm.21078 [DOI] [PubMed] [Google Scholar]
- 12.Ashworth P. C., Pickwell-MacPherson E., Provenzano E., Pinder S. E., Purushotham A. D., Pepper M., Wallace V. P., “Terahertz pulsed spectroscopy of freshly excised human breast cancer,” Opt. Express 17(15), 12444–12454 (2009). 10.1364/OE.17.012444 [DOI] [PubMed] [Google Scholar]
- 13.Nishizawa J., Sasaki T., Suto K., Yamada T., Tanabe T., Tanno T., Sawai T., Miura Y.,“THz imaging of nucleobases and cancerous tissue using a GaP THz-wave generator,” Opt. Commun. 2004(1–6), 469–474 (2005). [Google Scholar]
- 14.Jung E.-A., Lim M.-H., Moon K.-W., Do Y.-W., Lee S.-S., Han H.-W., Choi H.-J., Cho K.-S., Kim K.-R., “Terahertz pulse imaging of micro-metastatic lymph nodes in early-stage cervical cancer patients,” J. Opt. Soc. Korea 15(2), 155–160 (2011). 10.3807/JOSK.2011.15.2.155 [DOI] [Google Scholar]
- 15.Berrier A., Schaafsma M. C., Nonglaton G., Bergquist J., Rivas J. G., “Selective detection of bacterial layers with terahertz plasmonic antennas,” Biomed. Opt. Express 3(11), 2937–2949 (2012). 10.1364/BOE.3.002937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazhorova A., Markov A., Ng A., Chinnappan R., Skorobogata O., Zourob M., Skorobogatiy M., “Label-free bacteria detection using evanescent mode of a suspended core terahertz fiber,” Opt. Express 20(5), 5344–5355 (2012). 10.1364/OE.20.005344 [DOI] [PubMed] [Google Scholar]
- 17.Lee H. J., Lee Choi J. H., “Asymmetric split-ring resonator-based biosensor for detection of label-free stress biomarkers,” Appl. Phys. Lett. 103(5), 86 (2013). [Google Scholar]
- 18.Shahar S., Wiser A., Ickowicz D., Lubart R., Shulman A., Breitbart H., “Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility,” Hum. Reprod. 26(9), 2274–2282 (2011). 10.1093/humrep/der232 [DOI] [PubMed] [Google Scholar]
- 19.Yan J. G., Agresti M., Bruce T., Yan Y. H., Granlund A., Matloub H. S., “Effects of cellular phone emissions on sperm motility in rats,” Fertil. Steril. 88(4), 957–964 (2007). 10.1016/j.fertnstert.2006.12.022 [DOI] [PubMed] [Google Scholar]