Abstract
The flashlamp-pumped, solid-state, pulsed, mid-infrared, holmium:YAG laser (λ = 2120 nm) has been the clinical gold standard laser for lithotripsy for over the past two decades. However, while the holmium laser is the dominant laser technology in ureteroscopy because it efficiently ablates all urinary stone types, this mature laser technology has several fundamental limitations. Alternative, mid-IR laser technologies, including a thulium fiber laser (λ = 1908 and 1940 nm), a thulium:YAG laser (λ = 2010 nm), and an erbium:YAG laser (λ = 2940 nm) have also been explored for lithotripsy. The capabilities and limitations of these mid-IR lasers are reviewed in the context of the quest for an ideal laser lithotripsy system capable of providing both rapid and safe ablation of urinary stones.
1. Introduction
Approximately ten percent of the United States population will suffer from kidney stone disease in their lifetime [1]. There are no cures for kidney stone disease. Dietary and medical approaches are used for both prevention and treatment. While extracorporeal shockwave lithotripsy (ESWL) has long been used for noninvasive treatment of kidney stones, recent studies have shown that minimally invasive approaches such as ureteroscopy, utilizing laser lithotripsy, provide higher stone-free success rates than ESWL [2].
Laser lithotripsy is the most common laser procedure performed in urology, with approximately 300,000 cases performed on an annual basis [2]. During laser lithotripsy, for small to medium sized urinary stones (< 1.5 cm), either a rigid or flexible ureteroscope is placed through the urinary tract for illumination and imaging, while an optical fiber is inserted through the single working channel of the ureteroscope, to the location of the stone in the bladder, ureter, or kidney. The laser is then activated to fragment the stone into smaller pieces.
For over twenty years, the flashlamp-pumped, solid-state, long-pulse, Holmium:YAG infrared laser has been the laser of choice for lithotripsy [3]. Holmium laser energy is delivered through a small, flexible, low-hydroxyl, silica, multimode, optical fiber, and the stone is fragmented into multiple smaller pieces until either a stone basket can be used to extract each of the remaining stone fragments or the stones are sufficiently small (< 2 mm) to pass unimpeded through the patient’s urinary tract to be spontaneously voided.
Although laser lithotripsy is currently well established in the clinic, each of the three major components, including laser, optical fiber, and ureteroscope technologies, have continued to evolve over the past few decades. Several articles have recently been published providing comprehensive reviews of laser lithotripsy from primarily a clinical perspective [4–6]. The specific purpose of this article is to provide a review of the latest advances in laser lithotripsy from both a scientific and technical point-of-view, focusing on the mid-IR laser sources used for lithotripsy, including the standard Holmium laser, as well as experimental testing of other mid-IR lasers, including Erbium:YAG, Thulium:YAG, and Thulium fiber lasers as potential alternatives.
2. Theory of infrared hard tissue ablation
2.1 Photothermal ablation mechanism
During infrared laser lithotripsy, direct absorption of IR radiation by the urinary stone leads to non-radiative decay, heat generation, and a subsequent rise in temperature that is responsible for chemical decomposition, melting, and ablation. This process typically occurs at long laser pulse durations (> 1 µs), and low irradiances (<< 100 MW/cm2) [7]. To date, long-pulse laser lithotripsy has been considered to be primarily dependent on a photothermal ablation mechanism [8]. The increase in temperature due to laser irradiation is a function of the material’s absorption coefficient, specific heat, and radiant exposure, given by Eq. (1) [7]:
| (1) |
where µa is the absorption coefficient (cm−1), ΔT is change in temperature (°C), is material density (kg/cm3), cp is specific heat (J/kg-C) of material at constant pressure, H0 is radiant exposure (J/cm2), and z is depth in tissue (cm) [7]. The temperature rise chemically decomposes and melts stones depending on their specific thermal properties. However, the absorption coefficients of different dry stone compositions have been reported to be similar at IR wavelengths [9], which is inconsistent with the wide range of ablation thresholds and ablation rates observed for different laser wavelengths and stone compositions, leading to the hypothesis that other mechanisms may contribute to stone ablation as well. For example, ablation thresholds at the Holmium:YAG wavelength of 2120 nm have been measured for all the stone compositions listed in Table 1 [10], and for COM and UA stones at Thulium fiber laser wavelength of 1908 nm [11,12]. The ablation threshold is 2-3 times higher for COM than for UA stones, which are the two most common stone compositions encountered clinically [13].
Table 1. Thermal and mechanical properties of urinary stone compositions [14,15].
| Stone Composition | Breakdown Temperature (°C) | Hounsfield Unit Density (HU/mm) |
|---|---|---|
| Magnesium Ammonium Phosphate: | 100 | 53 ± 38 |
| Calcium Oxalate Monohydrate: | 206 | 105 ± 43 |
| Cystine: | 264 | 45 ± 4 |
| Uric Acid: | 360 | 50 ± 24 |
A list of thermal breakdown temperatures and Hounsfield unit densities for different urinary stone compositions, compiled from multiple sources in the literature, is also shown in Table 1 [14,15]. Thermal breakdown temperature is defined as chemical decomposition of a material due to heat. The Hounsfield unit describes the radiodensity of a material. Differences in kidney stone breakdown temperatures are most likely due to differences in thermal properties and density. However, it should also be noted that kidney stones are seldom of a single pure composition, so significant variability in the chemical composition and corresponding physical properties of stones encountered in the clinic is common.
2.2 Micro-explosion ablation mechanism
Kidney stones are typically immersed in a fluid environment, composed of both urine as well as saline from constant irrigation through the ureteroscope working channel during laser lithotripsy. Water serves as the dominant optical absorber of IR laser energy. Although laser lithotripsy is primarily a photothermal ablation mechanism, as summarized above, there are also secondary mechanical ablation effects, due to water absorption [16,17]. Laser irradiation causes water trapped in pores and pockets inside the hard calculi to vaporize, creating high pressure in a localized region [7]. Furthermore, even in the absence of vaporization, large differences in the thermal expansion coefficient between the stone material (10-70 x 10−6 / °C) and water (207 x 10−6 / °C) inside these pores may lead to a rapid and significant rise in pressure inside the stone [18]. The abrupt pressure change from the vapor produces a mechanical stress wave within the stone, which may be sufficient for ablation. However, even if the stress wave is not alone sufficient for ablation, it facilitates removal of weakened stone material from the irradiation site [8]. Recent studies utilizing a high power Thulium fiber laser (TFL) have reported an increase in ablation volume due to an increase in water content, as well as a change in ablation as a function of pore size [19]. Unfortunately, the physical dimensions of these pores in the stone are highly variable, based in part on stone composition and purity.
Previous studies in other medical fields have also reported on how the primary chromophore determines the laser ablation mechanism for other hard biological materials (e.g. dental enamel) by comparing different wavelengths with high water absorption versus high mineral absorption [20]. In these studies, direct absorption of carbon dioxide laser radiation in enamel resulted in the ablated surface appearing smooth and melted, due to thermal damage. However, high water absorption produced at the Erbium:YAG laser wavelength produced significant mechanical damage at the surface, resembling fragmentation, based on a different mechanism.
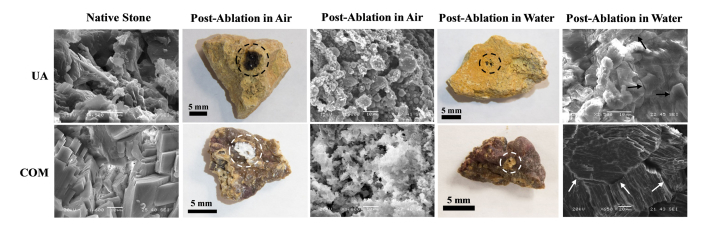
Similarly, for urinary stones, the effects of direct thermal ablation (e.g. with melting and decomposition) as well as micro-explosions (e.g. due to water present in pores) have also been observed in scanning electron microscopy (SEM) images, showing that both long pulse, infrared laser ablation processes depend on multiple factors, including laser energy, stone composition, and surrounding environment (Fig. 1) [21].
Fig. 1.
Photographs and scanning electron micrographs (SEM) of uric acid (UA) and calcium oxalate monohydrate (COM) stones before and after laser ablation with Thulium fiber laser in air and water mediums. Direct absorption of IR laser energy by stone in air results in a change from native state to more amorphous state with fusion of stone material, while absorption of IR energy by water contained in pores along stone surface may result in thermal expansion and production of cracks (shown by arrows), contributing to ablation.
For example, SEM images of COM and UA stones show that they have different physical properties, which is critical in determining the stone ablation mechanism. The Hounsfield unit density (HU/mm) for COM stones is larger than for UA, so COM stones are more dense (Table 1) [15]. UA stones have a higher melting temperature, but a lower Hounsfield unit and would thus be more likely to ablate through micro-explosions before thermal breakdown occurs. This was observed when comparing SEM images of each stone irradiated in water (Fig. 1). The UA material appeared similar to its natural state, but with significant fracturing, while COM was primarily melted with some fracturing present. Therefore, both mechanical and thermal properties of kidney stones greatly influence the primary ablation mechanism.
Another influential factor in ablation is the surrounding medium (air vs. water). During IR laser irradiation in water, the water acts as the primary absorber. This decreases the radiant heat, and also promotes the ablation process through micro-explosions. Both COM and UA stones irradiated in water exhibited more fracturing than when they were irradiated in air. However, there is still evidence of primary absorption by the material itself, indicated by melting of the natural material when examining the COM stone irradiated in water.
Furthermore, the specific technique of controlling the optical fiber during the lithotripsy procedure may also influence the primary ablation mechanism. For example, there are at least two general ways of manipulating the handheld fiber during laser irradiation, “drilling” and “scanning”. The drilling technique consists of advancing the distal fiber optic tip further into the stone during formation of the ablation crater. This approach results in rapid water evaporation, leaving no path for excess heat to diffuse, thus resulting in charring of the stone. This char formation and resultant blackening of the stone surface in turn artificially increases the absorption coefficient of the material, causing the primary ablation mechanism to be predominantly thermal in nature. On the contrary, the scanning method involves manually moving fiber optic tip laterally across stone surface during ablation. This approach allows better heat diffusion by water, and promotes micro-explosion mechanism.
Laser irradiation of the stone in air only enables ablation through direct absorption of the material, thus creating significant heat and charring of both UA and COM stones. SEM images show melting of the material with no evidence of fracturing which would be indicative of micro-explosions (Fig. 1).
3. Holmium:YAG laser lithotripsy (λ = 2120 nm)
3.1 Laser wavelength
The Holmium:YAG laser is currently the standard clinical laser for lithotripsy due in part to its ability to fragment a wide range of stone compositions (e.g. calcium oxalate, uric acid, cysteine,..etc.) (Table 1). The flashlamp-pumped configuration also makes the initial capital cost of the low-power Holmium laser affordable. The Holmium laser has several desirable technical characteristics for urology. First, the Holmium laser wavelength of 2120 nm is strongly absorbed by water. There is water contained within the pores and pockets along the stone surface due to the presence of urine in the urinary tract as well as constant saline irrigation through the working channel of the ureteroscope during the laser lithotripsy procedure, as previously mentioned above. Furthermore, this water absorbs IR laser energy, causing micro-explosions during thermal expansion and vaporization of the water. This mechanical phenomenon plays a significant role in the ablation mechanism in addition to direct IR laser absorption and thermal decomposition of stone material [8,21]. Water absorption at Holmium wavelength translates into an optical penetration depth (δ) of about 400 µm (Table 2) [22]. This property enables Holmium laser to be used for multiple urology applications requiring soft tissue ablation and/or coagulation (e.g. BPH) as well as stones.
Table 2. Low / high water absorption coefficients and optical penetration depths in water at mid-IR wavelengths [22–26].
| Laser | Wavelength λ (nm) |
Absorption Coefficient µa (cm−1) |
Optical Penetration Depth δ (μm) |
|---|---|---|---|
| Thulium fiber laser: | 1908 | 88 / 150 | 114 / 67 |
| Thulium fiber laser: | 1940 | 120 / 135 | 83 / 75 |
| Thulium:YAG: | 2010 | 62 / 60 | 161 / 167 |
| Holmium:YAG: | 2120 | 24 / 24 | 417 / 417 |
| Erbium:YAG: | 2940 | 12,000 / 1,000 | 1 / 10 |
The Holmium laser wavelength can also be transmitted through standard, multimode, low-hydroxyl (OH-), silica fibers, which are robust with desirable thermal, mechanical, and chemical properties. They allow transmission of high laser power for stone ablation, short bend diameter for use inside the working channel of flexible ureteroscopes, sterilization for medical use and re-use, resistance to corrosion in the urinary tract, and biocompatibility.
3.2 Operation mode
The flashlamp pumping scheme for the Holmium:YAG laser results in an inexpensive laser architecture, which makes the laser attractive for surgery. However, while the initial capital cost of a low power Holmium laser is modest, the need for a high voltage power supply, internal water cooling system, replacement of flashlamps, and use of bulk optical components may result in significant maintenance costs over the lifetime of the system.
While the Holmium laser is relatively mature technology, incremental improvements have been made as the laser has evolved in urology. On the one hand, smaller, lower power (e.g. 20 W), more compact Holmium laser modules dedicated specifically for laser lithotripsy have been developed for integration with other ureteroscope components (e.g. monitors, illumination and imaging systems) to conserve space in the operating room.
On the other hand, there has been an incremental evolution in larger (and more expensive) Holmium lasers with progressively higher output power (30 to 120 W) for prostate enucleation during treatment of BPH. The newer, higher power Holmium lasers package multiple laser rods into a single system, enabling operation at higher pulse rates (in contrast to older, lower power, single head, Holmium laser lithotripters). Operation at higher pulse rates has also enabled treatment of kidney stones in “dusting” mode with low pulse energy (0.2 - 0.4 J) and high pulse rate (50 - 80 Hz), as an alternative to conventional “fragmentation” mode with high pulse energy (0.6 - 1.0 J) and low pulse rate (5 - 10 Hz) [27].
While the number of laser lithotripsy modes used for ablation of urinary stones has proliferated in recent years, in general, there are at least three major techniques that are used, including “dusting” [28–33] (low pulse energy, high pulse rate) to particles typically defined as less than 1 mm in size, “fragmentation” [31,32] (high pulse energy and low pulse rate) with subsequent basketing of larger stone fragments (> 2 mm), and “popcorning” [34–38] where the fiber is held fixed in place and high pulse energy is used to create turbulent flow and break down multiple small stones trapped within a calyx in the kidney, when basketing of multiple stones is not judged to be feasible or efficient. Combinations of these operation modes (e.g. “pop-dusting”) also represent additional clinical options [5]. These major modes are briefly summarized in Table 3.
Table 3. Major Holmium:YAG laser lithotripsy operation modes [5].
| Mode | Pulse Energy (mJ) |
Pulse Rate (Hz) |
Comments |
|---|---|---|---|
| Dusting: | 200 - 500 | 50 – 80 | Low pulse energy; very high pulse rate; long pulse duration; smaller particle size; no basket used; longer treatment times |
| Fragmentation: | 500 - 1000 | 5 – 20 | High pulse energy, low pulse rate; short pulse duration; faster ablation rates; larger particle sizes; basket used |
| Popcorn: duration; |
~1500 | 20 – 40 | Very high pulse energy; high pulse rate; long pulse small fiber fixed in place; calyces of kidney; no basket used |
The recent clinical availability of higher power (100-120 W) Holmium lasers for lithotripsy has also raised additional safety concerns about potential collateral thermal damage to soft tissues (e.g. bladder, ureter or kidney wall) within the urinary tract due to direct deposition and absorption of high-power, IR energy in the surrounding saline environment. However, multiple laboratory studies utilizing feedback from thermocouples and/or thermal cameras have measured such temperatures. These studies have reported that high temperatures capable of thermally coagulating and irreversibly damaging soft urinary tissues typically only occurs in extreme circumstances, for example, where high laser power is used with low or no saline irrigation (e.g. obstructed ureter) [39–44].
3.3 Pulse shaping
Recent clinical advances in Holmium laser lithotripsy have also taken the form of manipulating the laser temporal pulse profile to reduce stone “retropulsion”, defined as movement of the stone due to the laser pulse. Stone retropulsion is typically undesirable as it results in the urologist having to follow the stone as a moving target within the urinary tract. In such cases, for example, an easily accessible stone in the ureter may be inadvertently pushed up into a less accessible calyx in the kidney, resulting in prolonged procedure time.
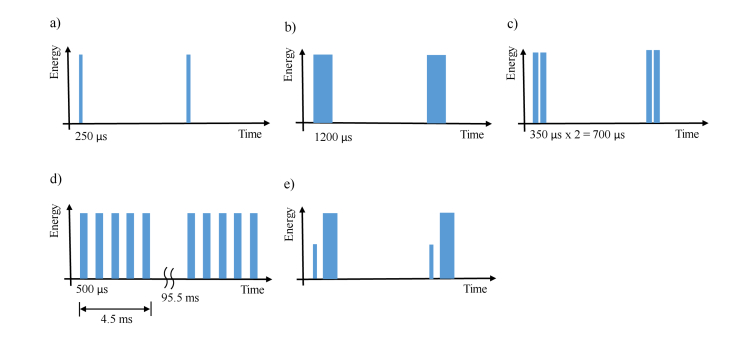
Several temporal beam shaping approaches have been utilized, including modifying the laser pulse from its standard, short pulse length of 250-350 µs, up to 700 µs by delivering two pulses together (“dual pulse mode”), stretching the laser pulse up to about 1200 µs, or delivering trains of laser pulses in bursts [45–54]. Use of longer pulse durations, lower pulse energies, and smaller fibers result in the desired effect of reduced stone retropulsion.
Alternatively, delivery of a short, low energy pulse to create a vapor bubble before delivery of a longer, higher energy pulse, has been used to reduce stone retropulsion and increase stone ablation rates. This mode is referred to as “Moses Tech” in clinical literature because the laser-induced vapor bubble created during the initial pulse effectively “parts the water”, allowing the subsequent pulse to be more delivered to the stone for enhanced ablation. This concept is not new or novel [55], however, it has recently been provided as an option on high power Holmium clinical laser systems [56]. Recent clinical studies suggest that reduced operation times do not compensate for the high cost of the laser and software [57]. The different pulse shaping approaches are summarized in Fig. 2. It should be noted that while the concept of pulse shaping for lithotripsy is relatively new, it has been previously been reported for other medical and industrial laser ablation applications [58–60].
Fig. 2.
Multiple approaches to pulsed laser lithotripsy have been utilized, including (a) short pulse (250-350 µs) for fragmentation, (b) long pulse (up to 1200 µs) to reduce stone retropulsion, (c) double-pulse delivery (2 x 350 µs), (d) delivery of pulse trains for a factor of 2 times increase in ablation rates, and (e) “Moses Tech” involving delivery of a low energy, short duration pulse to create a vapor bubble immediately followed by a higher energy, longer duration pulse for more efficient stone ablation, with reduced stone retropulsion as well.
Despite widespread adoption of Holmium laser technology for lithotripsy, several fundamental limitations of this technology remain. The advantages and disadvantages of potential alternative mid-IR lasers will be discussed below and when appropriate, compared with current Holmium laser technology, to determine if any of these new laser technologies are promising as next generation laser lithotripters.
4. Erbium:YAG laser lithotripsy (λ = 2940 nm)
Several experimental laboratory studies have been conducted with the flashlamp-pumped, solid-state, Erbium:YAG laser for lithotripsy [61–68]. The Erbium:YAG laser wavelength of 2940 nm matches a larger water absorption peak in tissue than does the Holmium laser wavelength of 2120 nm, translating into much higher absorption of the laser energy (Table 1). Both higher stone absorption and higher water absorption at this longer wavelength translates, in part, into more efficient laser ablation of kidney stones [61,64,68].
The major limitation of the Erbium laser is the lack of a suitable fiber delivery system. Standard, low-OH, silica fibers for Holmium laser lithotripsy, cannot be used at the longer, Erbium laser wavelength, because silica is not transparent beyond about 2700 nm, due to strong absorption by the OH- component in silica. Although multiple specialty mid-IR optical fibers are available (e.g. hollow silica waveguides, and sapphire, germanium oxide, fluoride, and chalcogenide fibers) for transmission of Erbium laser wavelengths for lithotripsy [62,68], all of these fibers have inferior properties compared to silica fibers (including much higher cost, poor biocompatibility, less flexibility, lower melting temperatures, and/or greater degradation in fluid environment of the urinary tract).
The flashlamp-pumped, solid-state, Erbium:YAG laser also suffers from the same inherent limitations as the Holmium:YAG laser, including low pulse rates (typically ≤ 30 Hz per laser cavity, due to thermal effects in the laser rod) and a multimodal beam profile (preventing use with smaller fibers < 200-µm-core diameter). The Erbium:YSSG laser (λ = 2790 nm) has not yet been tested for the specific application of lithotripsy, however, it is assumed that this laser would have similar limitations to that of the Erbium:YAG laser, for example, the need for a specialty, mid-IR optical fiber delivery system.
5. Thulium:YAG laser lithotripsy (λ = 2010 nm)
The Thulium:YAG laser operating at a wavelength of 2010 nm has also been used extensively in urology, primarily for soft tissue applications in treatment of benign prostatic hyperplasia (BPH). Multiple commercial diode-pumped, Thulium:YAG laser sources exist for BPH, with output powers up to 200 W. However, only a few laboratory studies have been reported for lithotripsy applications. These experimental studies involve use of either a flashlamp-pumped, short-pulse, Q-switched laser or a continuous-wave commercial laser modulated to operate in long-pulse mode [69,70]. Further studies with Thulium:YAG lasers for lithotripsy need to be performed before its utility can be properly evaluated.
6. Thulium fiber laser lithotripsy (λ = 1908 and 1940 nm)
6.1 Background
All of the previous laser systems discussed (e.g. Holmium:YAG, Thulium:YAG, Erbium:YAG, and Erbium:YSGG) are solid-state lasers. One of the latest laser technologies to be developed are fiber lasers. Rather than a bulk solid-state crystal acting as the gain medium, a chemically doped silica optical fiber is typically used. The light originates within the core of a small optical fiber, is pumped by another laser source (e.g. a diode or fiber laser), and then the light emitted from the fiber laser can be coupled into a separate, conventional, disposable, low-OH, silica, surgical fiber. The primary advantage of a fiber laser is delivery of high power output from a small fiber core, resulting in high intensity or brightness. The most common IR fiber lasers include Ytterbium (λ = 1075 nm), Erbium (λ = 1550 nm), and Thulium (λ = 1940 nm) doped silica fibers. The longer IR fiber laser wavelengths are of interest for laser ablation applications in surgery such as lithotripsy, since they target water absorption peaks in tissue.
Initial laboratory studies involving mid-IR fiber lasers were limited to low powers (a few watts), emitting either in continuous-wave (CW) or short pulse (nanosecond) modes at wavelengths near 1940 and 2940 nm water absorption peaks for tissue ablation and coagulation [71–76]. Both the low power output and CW or Q-switched modes are sub-optimal for most laser ablation applications in surgery because CW operation may result in excessive collateral thermal damage to surrounding tissue due to thermal conduction, while Q-switched operation its corresponding high peak powers may produce plasma, resulting in low tissue removal rates. For lithotripsy, the majority of studies and clinical cases are performed using long-pulse Holmium lasers (τp = 250 - 1200 µs). Also, as mentioned above, the 2940 nm wavelength requires specialty mid-IR fibers making it impractical for clinical use (due in part to high cost, poor biocompatibility, and limited flexibility).
More recently, short-pulse, Q-switched Thulium fiber lasers (TFL) have been re-explored for laser lithotripsy [77,78]. Potential advantages of the low pulse energy, high pulse rate, Q-switched operation mode for the TFL is the ability to do dusting with production of ultra-small stone particles on the scale of less than 0.1 mm, a similar advantage to that of femtosecond laser lithotripsy studies in the laboratory [79]. However, it is questionable whether stone ablation rates are sufficiently high and operation times sufficiently short to justify the use of this technology for lithotripsy. Further studies may be warranted.
Significant progress has also been achieved in power scaling from Thulium fiber lasers (TFL), which operate at a water absorption peak (λ = 1940 nm) in tissue. One major advantage of this wavelength is that TFL energy can be delivered through standard silica fibers, similar to those currently used with Holmium:YAG (λ = 2120 nm) and Thulium:YAG (λ = 2010 nm) lasers in urology. The first experimental use of high power TFLs up to 110 W in urology reported ablation of soft tissues and urinary stones [80–82]. The following sections will focus on the technical details of the TFL, since this is the most recent and perhaps the most promising new laser technology for lithotripsy, and may offer several potential advantages compared with the standard Holmium laser.
6.2 Laser wavelength
The Thulium fiber laser operates with two primary emission wavelengths of 1908 and 1940 nm, which more closely match a water absorption peak than that of the Holmium laser wavelength at 2100 nm [22–24]. As previously mentioned, absorption of IR energy by water is believed to play a major role in stone ablation, in addition to direct absorption of laser energy by the stone material, since IR absorption by dry stones are similar for different stone compositions [8,9,21]. The water absorption coefficient is µa = 120 cm−1 for Thulium fiber laser, µa = 62 cm−1 for Thulium:YAG laser, and µa = 24 cm−1 for Holmium:YAG lasers (Table 2). These values result in absorption of TFL energy that is two times higher than Thulium:YAG and 4-5 times higher than Holmium:YAG lasers. This higher water absorption directly translates into lower tissue ablation thresholds [83]. For example, ablation thresholds for the most common stone compositions, calcium oxalate monohydrate (COM) and uric acid (UA), have each been reported to be about 4 times lower for TFL (COM: 20.8 J/cm2 and UA: 6.5 J/cm2) than for Holmium:YAG laser (COM: 82.6 J/cm2 and UA: 25.9 J/cm2) [11,12]. These differences should enable either use of a lower TFL pulse energy yielding equivalent stone ablation rates, or use of an equivalent pulse energy with higher stone ablation rates.
The ability of TFL to ablate stones with lower energies than Holmium laser results in smaller laser-induced vapor bubble dimensions (1 mm vs. 5 mm) [84], which translates into an improved safety profile, since the effective working distance between the fiber optic tip and tissue directly correlates with this bubble diameter. For example, TFL-induced damage to Nitinol stone extraction baskets, frequently used during ureteroscopic laser lithotripsy procedures, has been reported at working distances up to 1.0 mm from fiber tip [85], while Holmium laser induced damage has been observed at working distances up to 5 mm [86–90].
6.3 Spatial beam profile
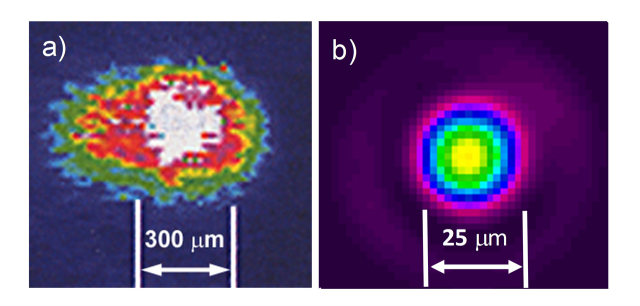
The primary advantage of fiber lasers is their high intensity or high brightness emission, due to the light originating within the small (e.g. 18-25 µm) core of the thulium-doped silica optical fiber, about 100 times smaller in cross-sectional diameter than the large (e.g. 2-3 mm-diameter) solid-state, Holmium:YAG laser crystals. This TFL property provides a near single mode, Gaussian spatial beam profile that is more uniform and symmetrical than the multimodal spatial beam profile typically produced by Holmium laser, and hence can more easily be focused to a smaller spot than Holmium multimode laser beam profile (Fig. 3).
Fig. 3.
Spatial beam profiles of (a) Holmium:YAG laser and (b) Thulium fiber laser. The multimodal Holmium laser beam prevents focusing down to small spots for coupling into fibers less than 200 µm. The TFL beam has been coupled into fibers as small as 50 µm core.
The Holmium laser’s multimode beam profile prohibits coupling of high laser power into small-core fibers (< 200 µm diameter), without risking overfilling of the input fiber core and launching of laser energy directly into the fiber cladding, which may damage the proximal fiber connector. Holmium laser beams are typically limited to larger diameters (275-500 µm) [91], which are suboptimal for complex ureteroscopy procedures that may require greater flexibility and/or saline irrigation flow. Several approaches have been explored for reducing proximal fiber failure during coupling of Holmium laser energy into small-core fibers [91,92]. These approaches have included ferrule designs which absorb incident energy or direct overflowing energy away from the fiber cladding, as well as design of thicker fiber claddings which prevent direct laser heating of the metal connector.
The flashlamp-pumped Holmium laser also generates significant heat, leading to thermal lensing in the laser rod, which may alter the spatial beam profile and lead to misalignment of the beam with the proximal fiber endface, potentially resulting in catastrophic fiber failure, sometimes exhibited in the form of fusion of the fiber connector to the laser interface [93].
Holmium fibers also experience cumulative laser-induced damage with repeated use due to the multimodal beam profile [94]. TFL lithotripsy using an improved spatial beam profile has recently been reported to reduce laser-induced damage to the proximal fiber tip surface compared to the Holmium laser, potentially allowing longer term use of fibers [95].
The smaller, more symmetrical TFL beam (Fig. 3) also enables focusing of higher power into smaller lithotripsy fibers (e.g. 50, 100, and 150 µm core) than allowed with the Holmium laser (e.g. ≥ 200-µm-core) [96–98]. Use of smaller fibers during laser lithotripsy provides several important advantages during flexible ureteroscopy, including more cross-sectional area within the single ureteroscope working channel for saline irrigation (e.g. for improved visibility and safety) as well as enabling maximal deflection of the flexible ureteroscope (e.g. for easier access to the lower pole of the kidney) [97]. Smaller fibers may also spur development of incrementally smaller instruments for use in ureteroscopy (e.g. integrated fiber/basket and miniature ureteroscopes) [99,100]. Given that engineers designing newer and progressively smaller endoscopes may devote significant time and effort attempting to reduce the endoscope outer diameter by increments as small as 50 µm, use of these smaller fiber outer diameters may enable them to more easily achieve such milestones [101]. Numerous reports have also demonstrated that stone retropulsion decreases proportionally with fiber diameter [45–47,102–106], so use of smaller fibers may further contribute to improved ablation efficiency indirectly via reduced stone retropulsion, but also potentially at the expense of higher rates of distal fiber tip burnback.
6.4 Temporal beam profile
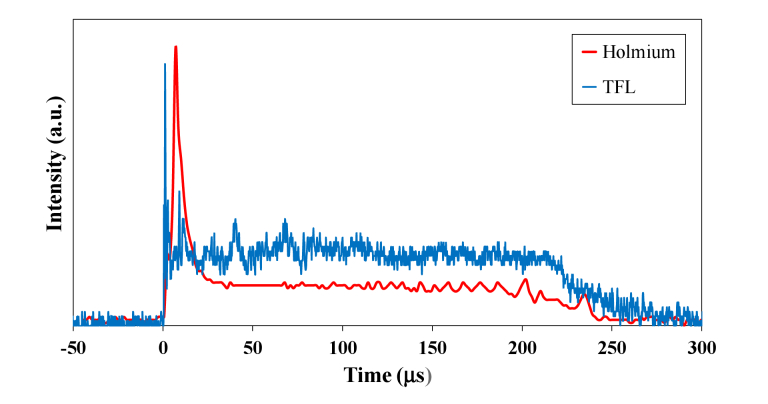
The flashlamp-pumped Holmium:YAG laser and the diode-pumped Thulium fiber laser also have different temporal beam profiles. A greater portion of the energy is contained within the initial spike in the Holmium temporal beam profile than for the more uniform TFL beam profile (Fig. 4). The duration of this first spike is important in that it directly contributes to the initial vapor bubble expansion, translating into greater pressures incident on the stone during collapse, which may in turn be partly responsible for the greater stone retropulsion commonly observed during Holmium laser lithotripsy, in comparison to TFL lithotripsy [19]. The diode-pumped architecture of the TFL also enables simple customization of the specific laser pulse duration and pulse shape, depending on the different circumstances encountered and technique desired during stone ablation (e.g. dusting, fragmentation, or popcorn modes).
Fig. 4.
Comparison of temporal beam profiles of short-pulse, flashlamp-pumped, solid-state, Holmium:YAG laser (red) and the diode-pumped, Thulium fiber laser (blue). The greater amount of energy contained in the initial spike of the Holmium temporal beam profile contributes to the initial vapor bubble expansion and collapse and consequently greater stone retropulsion observed with the Holmium laser than with the Thulium fiber laser.
6.5 Laser pulse repetition rate
The diode-pumped TFL also enables more flexibility in laser operating parameters than flashlamp-pumped, solid-state lasers. For example, the low-power Holmium laser is limited to operation at pulse rates less than about 30 Hz, due to thermal effects in the laser rod [107]. The vast majority of white light from the flashlamp pumping the laser crystal does not contribute to laser operation, but is transformed into heat, requiring bulky and expensive water cooling systems to prevent catastrophic thermal damage to the laser rod. As a result of this pumping scheme, wall-plug efficiency of Holmium lasers is less than 1-2% (with 98-99% of energy wasted as heat). While high-power Holmium lasers have recently become commercially available, and are capable of operation at pulse rates up to 80 Hz, this is due to synchronous operation of multiple laser rods integrated into a single platform (Table 4).
Table 4. Specifications of low and high power, clinical and experimental 2 µm laser lithotripsy sources.
| Laser | TFL | TFL | Thulium:YAG | Holmium:YAG | Holmium:YAG |
|---|---|---|---|---|---|
| Wavelength: | 1908 | 1940 | 2010 | 2100 | 2100 |
| Model: | TLR-100-1908 | Urolase | Revolix 200 | P20 | P120H |
| Manufacturer: | IPG Medical | IPG Medical | Lisa Laser** | Lumenis** | Lumenis** |
| Size (cm): | 50x60x80* | 55x46x29 | 42x89x95 | 52x57x32 | 47x116x105 |
| Weight (kg): | 120* | 35 | 150 | 40 | 245 |
| Cooling system: | External Water | Air | Internal Water | Internal Water | Internal Water |
| Peak power (W): | 100 | 500 | NA | NA | NA |
| Ave. power (W): | 100 | 50 | 200 | 20 | 120 |
| Pulse rate (Hz): | 1 - 1000 | 1 - 2000 | NA | 5 - 15 | 5 - 80 |
| Energy (J): | Adjustable | 0.2 - 6.0 | NA | 0.5 - 2.5 | 0.2 - 6.0 |
| Pulse width (ms): | Adjustable | 0.2 - 12 | 50 - CW | < 0.5 | Adjustable |
| Mode: | CW/modulated | Pulsed | CW/modulated | Pulsed | Pulsed |
| Fiber (µm): | ≥ 50 | ≥ 150 | NA | ≥ 200 | ≥ 200 |
Weight and dimensions for experimental 1908 nm Thulium fiber laser do not include separate recirculating chiller.
Multiple manufacturers exist for the low and high power Holmium:YAG and Thulium:YAG lasers. These specifications are representative of the standard in the field.
The diode-pumped TFL is more efficient, with a wall plug efficiency of about 12%, enabling air cooling (e.g. for a smaller form factor and reduced maintenance), and operation at pulse rates up to 2000 Hz. So far, TFL lithotripsy studies have been reported with pulse rates up to 500 Hz [108], enabling operation in “dusting” mode for lithotripsy, with low pulse energy compensated by high pulse rates, and production of smaller stone fragments.
The combination of an air cooled laser with higher wall plug efficiency additionally results in a smaller form factor. High power (e.g. 50 W), compact (e.g. tabletop) versions of the TFL have recently been manufactured and tested with higher average power output than tabletop versions of the Holmium laser (50 W vs. 20 W) [19,109–117]. Preliminary studies directly comparing this newer, TFL technology (circa 2016) with the current 120 W Holmium laser using equivalent laser parameters, demonstrated 2-4 times higher TFL stone ablation rates as well as reduced stone retropulsion. The higher wall plug efficiency may also enable TFL operation at higher powers than Holmium laser, while still utilizing a 110-volt electrical outlet. Furthermore, due to the fiber laser architecture, there is not only elimination of water cooling, but also no bulk optics (e.g. lenses and mirrors), so contamination and misalignment of optics due to handling is eliminated. Table 5 summarizes advantages and disadvantages of mid-IR lasers for lithotripsy.
Table 5. Advantages and disadvantages of mid-IR lasers for lithotripsy.
| Laser | Advantages | Disadvantages |
|---|---|---|
| TFL: | (1) 4x lower ablation threshold than Ho:YAG | (1) Experimental laser, capital cost TBD |
| (2) Use with smaller fibers (50-150 µm core) | (2) Clinical studies still lacking | |
| (3) High pulse rates (1-2000 Hz) for dusting | ||
| (4) High wall plug efficiency (~12%); air-cooled | ||
| Tm:YAG: | (1) Diode-pumped laser | (1) Limited testing, few publications on feasibility |
| (2) Continuous-wave laser, needs to be modulated | ||
| Ho:YAG: | (1) Clinically proven to fragment all stone types | (1) Does not closely match water absorption peak |
| (2) Low capital cost for low power lasers | (2) Limited to use with fibers ≥ 200-µm-core | |
| (3) Limited to low pulse rates (5-80 Hz) | ||
| (4) High maintenance costs | ||
| (5) Low wall-plug efficiency(1-2%); water cooling | ||
| Er:YAG: | (1) Higher ablation rates than Holmium | (1) No fiber optic delivery system that is low cost, biocompatible, flexible, and robust |
| (2) Limited to low pulse rates |
7. Future trends
One past trend in the field of laser lithotripsy that is likely to continue in the future has been the continual power scaling of Holmium laser lithotripters. For example, the output power from Holmium laser lithotripters has steadily increased over the past two decades, from about 20 W to the current 120 W, and the footprint and cost of such lasers has also increased along with this trend (due to limited wall plug efficiency of about 1-2%). These developments are also similar to the clinical trend observed for IR laser systems used to treat BPH in urology, as well, which have progressively increased in power from 20 W to 200 W.
It is predicted that the next generation of Holmium lasers will operate at continually higher output powers (> 120 W) and higher pulse rates (e.g. > 80 Hz), thus enabling more flexibility, especially for operation in stone dusting mode. In fact, to confirm this trend, at least one company is already marketing a 140 W Holmium laser operating at 100 Hz, the next logical incremental improvement in the technology [118]. Continued experimentation with and eventual optimization of laser pulse shapes may also further reduce stone retropulsion, in turn translating into more efficient stone ablation. However, since the fundamental architecture of flashlamp-pumped, solid-state, Holmium:YAG lasers has not changed much in recent decades, development of higher power Holmium lasers is also predicted to result in increasingly larger and more expensive systems, as observed from past trends of the sale of low power, 20-30 W, Holmium lasers $30-50k) to the sale of the newest high power, 120 W, Holmium lasers (> $200k).
Technological disruption of these trends may perhaps come in the form of a fundamentally different type of laser system, e.g. fiber lasers. Over the past decade, Thulium fiber laser output power has increased rapidly (with peak power going from 100 W to 500 W) (Table 4), while at the same time, the TFL footprint has become smaller (reduced in size from a console to tabletop version) (Fig. 5). Wall-plug efficiency has doubled from 6% to 12%, due in part to smaller diode pump laser components and newer, more efficient pump schemes, enabling high power operation with an air cooling system instead of water cooling.
Fig. 5.
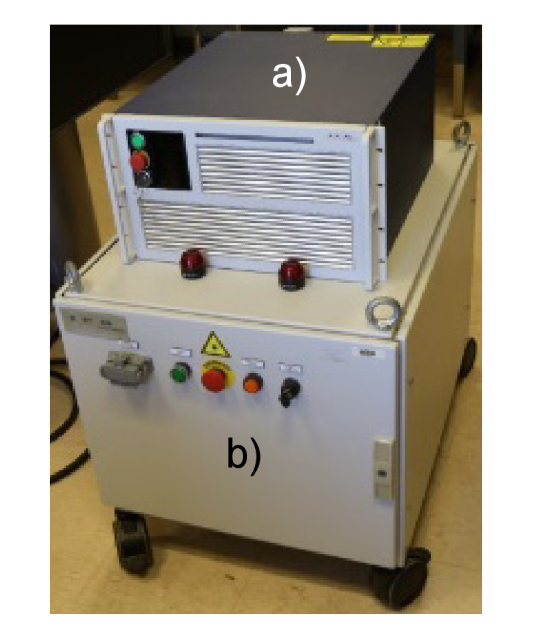
(a) Next generation (circa 2016) of compact, air-cooled, tabletop 500 W peak power, 1940 nm Thulium fiber laser sitting on top of (b) first generation, water-cooled 100 W peak power, 1908 nm Thulium fiber laser (circa 2004).
Most recently, laboratory studies and preliminary clinical studies directly comparing the TFL and high power Holmium laser using equivalent laser parameters, have demonstrated that the TFL was more efficient for lithotripsy in both dusting and fragmentation modes, providing 2-4 times faster stone ablation than the Holmium laser along with reduced stone retropulsion [108,109]. However, more extensive clinical studies are still lacking and will ultimately be required for rigorous evaluation of this new technology.
8. Conclusions
The flashlamp pumped, solid state, infrared Holmium:YAG laser is currently the clinical gold standard for lithotripsy during ureteroscopy, due in part to its cost effective treatment of all stone compositions. However, this mature technology has several fundamental technical limitations. The Holmium wavelength does not closely match a water absorption peak in tissue, its multimode spatial beam profile prevents coupling of high power into small (< 200 µm core) fibers, an inefficient pumping scheme currently limits operation to relatively low pulse rates (5-80 Hz) and the laser requires a bulky high voltage power supply and water cooling. Several mid-IR lasers have been tested as potential alternatives to the Holmium laser for lithotripsy, including Thulium:YAG, Erbium:YAG, and Thulium fiber lasers. The Er:YAG laser is limited by its inability to be used with standard, inexpensive, flexible, and biocompatible silica optical fibers. The Thulium:YAG laser has only seen limited testing and so evaluation of its performance is incomplete. The Thulium fiber laser (TFL) major emission lines at 1908 and 1940 nm closely match high and low temperature water absorption peaks, respectively, translating into 2-4 times more efficient stone ablation than for Holmium. The TFL’s improved spatial beam profile enables coupling of high power into small (e.g. 50, 100, and 150 µm core) and flexible silica fibers. Furthermore, the diode-pumped TFL architecture also enables operation at high pulse rates (up to 2000 Hz) which is attractive for stone dusting, as well as a more efficient pumping scheme, enabling packaging of a high power, compact, tabletop, air-cooled laser system. While initial clinical studies have been conducted, more extensive clinical studies with direct comparison to the Holmium laser are still lacking and will be required to validate the performance of this new technology.
Acknowledgments
The author thanks Luke Hardy for assistance in preparation of the tables and figures, as well as Gregory Altshuler and Emil Sobol for helpful discussions on ablation mechanisms.
Disclosures
Nathaniel Fried is a consultant with IPG Medical Corporation (Marlborough, MA). He does not hold any financial stake in the company.
References
- 1.Pearle M. S., Calhoun E. A., Curhan G. C., “Urologic diseases in America project: Urolithiasis,” J. Urol. 173(3), 848–857 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Scales C. D., Jr, Lai J. C., Dick A. W., Hanley J. M., van Meijgaard J., Setodji C. M., Saigal C. S., Urologic Diseases in America Project , “Comparative effectiveness of shock wave lithotripsy and ureteroscopy for treating patients with kidney stones,” JAMA Surg. 149(7), 648–653 (2014). 10.1001/jamasurg.2014.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley D., Erhard M., “Use of the holmium laser in the upper urinary tract,” Tech. Urol. 1(1), 25–30 (1995). [PubMed] [Google Scholar]
- 4.Aldoukhi A. H., Roberts W. W., Hall T. L., Ghani K. R., “Holmium laser lithotripsy in the new stone age: dust or bust?” Front Surg 29(4), 57 (2017). 10.3389/fsurg.2017.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronenberg P., Somani B., “Advances in lasers for the treatment of stones–a systematic review,” Curr. Urol. Rep. 19(6), 1– 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried N. M., Irby P. B., “Advances in laser technology and fiber optic delivery systems for use in lithotripsy,” Nat. Rev. Urol. (June), 8 (Epub) (2018) [DOI] [PubMed] [Google Scholar]
- 7.Chan K. F., Pfefer T. J., Teichman J. M. H., Welch A. J., “A perspective on laser lithotripsy: the fragmentation processes,” J. Endourol. 15(3), 257–273 (2001). 10.1089/089277901750161737 [DOI] [PubMed] [Google Scholar]
- 8.Chan K. F., Vassar G. J., Pfefer T. J., Teichman J. M., Glickman R. D., Weintraub S. T., Welch A. J., “Holmium:YAG laser lithotripsy: A dominant photothermal ablative mechanism with chemical decomposition of urinary calculi,” Lasers Surg. Med. 25(1), 22–37 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Roggan A., Bindig U., Wäsche W., Zgoda F., “Action mechanisms of laser radiation in biological tissues,” Applied Laser Medicine 3, 87 (2003) [Google Scholar]
- 10.Chan K. F., Hammer D. X., Choi B., Teichman J. M., McGuff H. S., Pratisto H., Jansen E. D., Welch A. J., “Free electron laser lithotripsy: threshold radiant exposures,” J. Endourol. 14(2), 161–167 (2000). 10.1089/end.2000.14.161 [DOI] [PubMed] [Google Scholar]
- 11.Blackmon R. L., Irby P. B., Fried N. M., “Comparison of holmium:YAG and thulium fiber laser lithotripsy: ablation thresholds, ablation rates, and retropulsion effects,” J. Biomed. Opt. 16(7), 071403 (2011). 10.1117/1.3564884 [DOI] [PubMed] [Google Scholar]
- 12.R. L. Blackmon, “Thulium fiber laser lithotripsy,” Ph.D. Thesis. University of North Carolina at Charlotte (2013). [Google Scholar]
- 13.Wilson D. M., “Clinical and laboratory approaches for evaluation of nephrolithiasis,” J. Urol. 141(3), 770–774 (1989). 10.1016/S0022-5347(17)41006-8 [DOI] [PubMed] [Google Scholar]
- 14.M. E. Mayo, “Interaction of laser radiation with urinary calculi,” Ph.D. Thesis, Department of Applied Science, Security and Resilience, United Kingdom, Cranfield University (2009). [Google Scholar]
- 15.Motley G., Dalrymple N., Keesling C., Fischer J., Harmon W., “Hounsfield unit density in the determination of urinary stone composition,” Urology 58(2), 170–173 (2001). 10.1016/S0090-4295(01)01115-3 [DOI] [PubMed] [Google Scholar]
- 16.Jansen E. D., Asshauer T., Frenz M., Motamedi M., Delacrétaz G., Welch A. J., “Effect of pulse duration on bubble formation and laser-induced pressure waves during holmium laser ablation,” Lasers Surg. Med. 18(3), 278–293 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Majaron B., Plestenjak P., Lukac M., “Thermo-mechanical laser ablation of soft biological tissue: modeling the micro-explosions,” Appl. Phys. B 69(1), 71–80 (1999). 10.1007/s003400050772 [DOI] [Google Scholar]
- 18.Schofield P. F., Knight K. S., van der Houwen J. A. M., Valsami-Jones E., “The role of hydrogen bonding in the thermal expansion and dehydration of brushite, di-calcium phosphate dihydrate,” Phys. Chem. Miner. 31(9), 606–624 (2004). 10.1007/s00269-004-0419-6 [DOI] [Google Scholar]
- 19.Zamyatina V., Enikeev D., Dymov A., Sorokin N., Minaev V., Yaroslavsky I., Kovalenko A., Vinarov A., Altshuler G., Gapontsev V., “Super pulse thulium fiber laser for lithotripsy,” Lasers Surg. Med. 48, 10 (2016). [Google Scholar]
- 20.Fried D., Zuerlein M., Featherstone J. D. B., Seka W., Duhn C., McCormack S. M., “IR laser ablation of dental enamel: mechanistic dependence on the primary absorber,” Appl. Surf. Sci. 127–129, 852–856 (1998). 10.1016/S0169-4332(97)00755-1 [DOI] [Google Scholar]
- 21.Hardy L. A., Irby P. B., Fried N. M., “Scanning electron microscopy of real and artificial kidney stones before and after Thulium fiber laser ablation in air and water,” Proc. SPIE 10468(104680G), 1–11 (2018). [Google Scholar]
- 22.Hale G. M., Querry M. R., Rusk A. N., Williams D., “Influence of temperature on the spectrum of water,” JOSA 62(9), 1103–1108 (1972). 10.1364/JOSA.62.001103 [DOI] [Google Scholar]
- 23.Hale G. M., Querry M. R., “Optical constants of water in the 200 nm to 200 mm wavelength region,” Appl. Opt. 12(3), 555–563 (1973). 10.1364/AO.12.000555 [DOI] [PubMed] [Google Scholar]
- 24.Jansen E. D., van Leeuwen T. G., Motamedi M., Borst C., Welch A. J., “Temperature dependence of the absorption coefficient of water for midinfrared laser radiation,” Lasers Surg. Med. 14(3), 258–268 (1994). 10.1002/lsm.1900140308 [DOI] [PubMed] [Google Scholar]
- 25.Lange B. I., Brendel T., Hüttmann G., “Temperature dependence of light absorption in water at holmium and thulium laser wavelengths,” Appl. Opt. 41(27), 5797–5803 (2002). 10.1364/AO.41.005797 [DOI] [PubMed] [Google Scholar]
- 26.Cummins J. P., Walsh J. T., Jr, “Erbium laser ablation: the effect of dynamic optical properties,” Appl. Phys. Lett. 62(16), 1988–1990 (1993). 10.1063/1.109512 [DOI] [Google Scholar]
- 27.Dauw C. A., Simeon L., Alruwaily A. F., Sanguedolce F., Hollingsworth J. M., Roberts W. W., Faerber G. J., Wolf J. S., Jr, Ghani K. R., “Contemporary practice patterns of flexible ureteroscopy for treating renal stones: results of a worldwide survey,” J. Endourol. 29(11), 1221–1230 (2015). 10.1089/end.2015.0260 [DOI] [PubMed] [Google Scholar]
- 28.Fahmy A., Youssif M., Rhashad H., Orabi S., Mokless I., “Extractable fragment versus dusting during ureteroscopic laser lithotripsy in children: prospective randomized study,” J. Pediatr. Urol. 12(4), 254e1 (2016). 10.1016/j.jpurol.2016.04.037 [DOI] [PubMed] [Google Scholar]
- 29.Santiago J. E., Hollander A. B., Soni S. D., Link R. E., Mayer W. A., “To dust or not to dust: a systematic review of ureteroscopic laser lithotripsy techniques,” Curr. Urol. Rep. 18(4), 32 (2017). 10.1007/s11934-017-0677-8 [DOI] [PubMed] [Google Scholar]
- 30.Li R., Ruckle D., Keheila M., Maldonado J., Lightfoot M., Alsyouf M., Yeo A., Abourbih S. R., Olgin G., Arenas J. L., Baldwin D. D., “High-frequency dusting versus conventional holmium laser lithotripsy for intrarenal and ureteral calculi,” J. Endourol. 31(3), 272–277 (2017). 10.1089/end.2016.0547 [DOI] [PubMed] [Google Scholar]
- 31.Humphreys M. R., Shah O. D., Monga M., Chang Y. H., Krambeck A. E., Sur R. L., Miller N. L., Knudsen B. E., Eisner B. H., Matlaga B. R., Chew B. H., “Dusting versus basketing during – which technique is more efficacious? A prospective multicenter trial from the EDGE research consortium,” J. Urol. 199(5), 1272–1276 (2018). 10.1016/j.juro.2017.11.126 [DOI] [PubMed] [Google Scholar]
- 32.Matlaga B. R., Chew B., Eisner B., Humphreys M., Knudsen B., Krambeck A., Lange D., Lipkin M., Miller N. L., Monga M., Pais V., Sur R. L., Shah O., “Ureteroscopic laser lithotripsy: a review of dusting vs fragmentation with extraction,” J. Endourol. 32(1), 1–6 (2018). 10.1089/end.2017.0641 [DOI] [PubMed] [Google Scholar]
- 33.Tracey J., Gagin G., Morhardt D., Hollingsworth J., Ghani K. R., “Ureteroscopic high-frequency dusting utilizing a 120-W holmium laser,” J. Endourol. Epub (2018) [DOI] [PubMed]
- 34.Chawla S. N., Chang M. F., Chang A., Lenoir J., Bagley D. H., “Effectiveness of high-frequency holmium:YAG laser stone fragmentation: the “popcorn effect”,” J. Endourol. 22(4), 645–650 (2008). 10.1089/end.2007.9843 [DOI] [PubMed] [Google Scholar]
- 35.Hecht S. L., Wolf J. S., Jr., “Techniques for holmium laser lithotripsy of intrarenal calculi,” Urology 81(2), 442–445 (2013). 10.1016/j.urology.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 36.Klaver P., de Boorder T., Rem A. I., Lock T. M. T. W., Noordmans H. J., “In vitro comparison of renal stone laser treatment using fragmentation and popcorn technique,” Lasers Surg. Med. 49(7), 698–704 (2017). 10.1002/lsm.22671 [DOI] [PubMed] [Google Scholar]
- 37.Emiliani E., Talso M., Cho S. Y., Baghdadi M., Mahmoud S., Pinheiro H., Traxer O., “Optimal settings for the noncontact Holmium:YAG stone fragmentation popcorn technique,” J. Urol. 198(3), 702–706 (2017). 10.1016/j.juro.2017.02.3371 [DOI] [PubMed] [Google Scholar]
- 38.Wollin D. A., Tom W. R., Jiang R., Simmons W. N., Preminger G. M., Lipkin M. E., “An in vitro evaluation of laser settings and location in the efficiency of the popcorn effect,” Urolithiasis epub (2018). [DOI] [PubMed]
- 39.Molina W. R., Silva I. N., Donalisio da Silva R., Gustafson D., Sehrt D., Kim F. J., “Influence of saline on temperature profile of laser lithotripsy activation,” J. Endourol. 29(2), 235–239 (2015). 10.1089/end.2014.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butticè S., Sener T. E., Proietti S., Dragos L., Tefik T., Doizi S., Traxer O., “Temperature changes inside the kidney: what happens during Holmium:Yttrium-Aluminium-Garnet laser usage?” J. Endourol. 30(5), 574–579 (2016). 10.1089/end.2015.0747 [DOI] [PubMed] [Google Scholar]
- 41.Aldoukhi A. H., Ghani K. R., Hall T. L., Roberts W. W., “Thermal response to high-power holmium laser lithotripsy,” J. Endourol. 31(12), 1308–1312 (2017). 10.1089/end.2017.0679 [DOI] [PubMed] [Google Scholar]
- 42.Wollin D. A., Carlos E. C., Tom W. R., Simmons W. N., Preminger G. M., Lipkin M. E., “Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model,” J. Endourol. 32(1), 59–63 (2018). 10.1089/end.2017.0658 [DOI] [PubMed] [Google Scholar]
- 43.Aldoukhi A. H., Hall T. L., Ghani K. R., Maxwell A. D., MacConaghy B., Roberts W. W., “Calyceal fluid temperature during high-power holmium laser lithotripsy in an in vivo porcine model,” J. Endourol. In press. [DOI] [PMC free article] [PubMed]
- 44.Hein S., Petzold R., Schoenthaler M., Wetterauer U., Miernik A., “Thermal effects of Holmium:YAG laser lithotripsy: real-time evaluation in an in vitro model,” World J. Urol. ePub (2018) [DOI] [PubMed]
- 45.Finley D. S., Petersen J., Abdelshehid C., Ahlering M., Chou D., Borin J., Eichel L., McDougall E., Clayman R. V., “Effect of holmium:YAG laser pulse width on lithotripsy retropulsion in vitro,” J. Endourol. 19(8), 1041–1044 (2005). 10.1089/end.2005.19.1041 [DOI] [PubMed] [Google Scholar]
- 46.Kang H. W., Lee H., Teichman J. M., Oh J., Kim J., Welch A. J., “Dependence of calculus retropulsion on pulse duration during Ho: YAG laser lithotripsy,” Lasers Surg. Med. 38(8), 762–772 (2006). 10.1002/lsm.20376 [DOI] [PubMed] [Google Scholar]
- 47.Kalra P., Le N. B., Bagley D., “Effect of pulse width on object movement in vitro using holmium:YAG laser,” J. Endourol. 21(2), 228–231 (2007). 10.1089/end.2005.1130 [DOI] [PubMed] [Google Scholar]
- 48.Bader M. J., Pongratz T., Khoder W., Stief C. G., Herrmann T., Nagele U., Sroka R., “Impact of pulse duration on Ho:YAG laser lithotripsy: fragmentation and dusting performance,” World J. Urol. 33(4), 471–477 (2015). 10.1007/s00345-014-1429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sroka R., Pongratz T., Scheib G., Khoder W., Stief C. G., Herrmann T., Nagele U., Bader M. J., “Impact of pulse duration on Ho:YAG laser lithotripsy: treatment aspects on the single-pulse level,” World J. Urol. 33(4), 479–485 (2015). 10.1007/s00345-015-1504-9 [DOI] [PubMed] [Google Scholar]
- 50.Wollin D. A., Ackerman A., Yang C., Chen T., Simmons W. N., Preminger G. M., Lipkin M. E., “Variable pulse duration from a new Holmium:YAG laser: the effect on stone comminution, fiber tip degradation, and retropulsion dusting model,” Urology 103, 47–51 (2017). 10.1016/j.urology.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Bell J. R., Penniston K. L., Nakada S. Y., “In vitro comparison of stone fragmentation when using various settings with modern variable pulse holmium lasers,” J. Endourol. 31(10), 1067–1072 (2017). 10.1089/end.2017.0351 [DOI] [PubMed] [Google Scholar]
- 52.Bell J. R., Penniston K. L., Nakada S. Y., “In vitro comparison of holmium lasers: evidence for shorter fragmentation time and decreased retropulsion using a modern variable-pulse laser,” Urology 107, 37–42 (2017). 10.1016/j.urology.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 53.Blackmon R. L., Irby P. B., Fried N. M., “Enhanced thulium fiber laser lithotripsy using micro-pulse train modulation,” J. Biomed. Opt. 17(2), 028002 (2012). 10.1117/1.JBO.17.2.028002 [DOI] [PubMed] [Google Scholar]
- 54.Kronenberg P., Traxer O., “MP22–13 “Burst laser lithotripsy - a novel lithotripsy mode,” J. Urol. 195(4), e258 (2016). 10.1016/j.juro.2016.02.701 [DOI] [Google Scholar]
- 55.Trost D., “Laser pulse format for penetrating an absorbing fluid,” U.S. Patent #5,321,715 1994.
- 56.Elhilali M. M., Badaan S., Ibrahim A., Andonian S., “Use of the Moses technology to improve holmium laser lithotripsy outcomes: a preclinical study,” J. Endourol. 31(6), 598–604 (2017). 10.1089/end.2017.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stern K. L., Monga M., “The Moses holmium system - time is money,” Can. J. Urol. 25(3), 9313–9316 (2018). [PubMed] [Google Scholar]
- 58.Vogel A., Schmidt P., Flucke B., “Minimization of thermomechanical side effects in IR ablation by use of multiply q-switched laser pulses,” Med. Laser Appl. 17(1), 15–20 (2002). 10.1078/1615-1615-00040 [DOI] [Google Scholar]
- 59.Joseph D. P., Allen P., Negus D., Hobart J., “A new and improved vitreoretinal erbium:YAG laser scalpel: long-term morphologic characteristics of retinal-choroidal injury,” Ophthalmic Surg. Lasers Imaging 35(4), 304–315 (2004). [PubMed] [Google Scholar]
- 60.Hendow S. T., Romero R., Shakir S. A., Guerreiro P. T., “Percussion drilling of metals using bursts of nanosecond pulses,” Opt. Express 19(11), 10221–10231 (2011). 10.1364/OE.19.010221 [DOI] [PubMed] [Google Scholar]
- 61.Teichman J. M., Chan K. F., Cecconi P. P., Corbin N. S., Kamerer A. D., Glickman R. D., Welch A. J., “Erbium: YAG versus holmium:YAG lithotripsy,” J. Urol. 165(3), 876–879 (2001). 10.1016/S0022-5347(05)66548-2 [DOI] [PubMed] [Google Scholar]
- 62.Fried N. M., “Potential applications of the erbium:YAG laser in endourology,” J. Endourol. 15(9), 889–894 (2001). 10.1089/089277901753284080 [DOI] [PubMed] [Google Scholar]
- 63.Chan K. F., Lee H., Teichman J. M., Kamerer A., McGuff H. S., Vargas G., Welch A. J., “Erbium: YAG laser lithotripsy mechanism,” J. Urol. 168(2), 436–441 (2002). 10.1016/S0022-5347(05)64653-8 [DOI] [PubMed] [Google Scholar]
- 64.Lee H., Kang H. W., Teichman J. M., Oh J., Welch A. J., “Urinary calculus fragmentation during Ho: YAG and Er:YAG lithotripsy,” Lasers Surg. Med. 38(1), 39–51 (2006). 10.1002/lsm.20258 [DOI] [PubMed] [Google Scholar]
- 65.Iwai K., Shi Y. W., Nito K., Matsuura Y., Kasai T., Miyagi M., Saito S., Arai Y., Ioritani N., Okagami Y., Nemec M., Sulc J., Jelinkova H., Zavoral M., Kohler O., Drlik P., “Erbium:YAG laser lithotripsy by use of a flexible hollow waveguide with an end-scaling cap,” Appl. Opt. 42(13), 2431–2435 (2003). 10.1364/AO.42.002431 [DOI] [PubMed] [Google Scholar]
- 66.Yang Y., Chaney C. A., Fried N. M., “Erbium:YAG laser lithotripsy using hybrid germanium/silica optical fibers,” J. Endourol. 18(9), 830–835 (2004). 10.1089/end.2004.18.830 [DOI] [PubMed] [Google Scholar]
- 67.Raif J., Vardi M., Nahlieli O., Gannot I., “An Er:YAG laser endoscopic fiber delivery system for lithotripsy of salivary stones,” Lasers Surg. Med. 38(6), 580–587 (2006). 10.1002/lsm.20344 [DOI] [PubMed] [Google Scholar]
- 68.Qiu J., Teichman J., Wang T., Elmaanaoui B., Gamez D., Milner T. E., “Comparison of fluoride and sapphire optical fibers for Er: YAG laser lithotripsy,” J. Biophotonics 3(5-6), 277–283 (2010). 10.1002/jbio.200900104 [DOI] [PubMed] [Google Scholar]
- 69.Zhang J. J., Rajabhandharaks D., Xuan J. R., Wang H., Chia R. W. J., Hasenberg T., Kang H. W., “Water content contribution in calculus phantom ablation during Q-switched Tm:YAG laser lithotripsy,” J. Biomed. Opt. 20(12), 128001 (2015). 10.1117/1.JBO.20.12.128001 [DOI] [PubMed] [Google Scholar]
- 70.Kamal W., Kallidonis P., Koukiou G., Amanatides L., Panagopoulos V., Ntasiotis P., Liatsikos E., “Stone retropulsion with Ho:YAG and Tm:YAG lasers: a clinical practice – oriented study,” J. Endourol. 30(11), 1145–1149 (2016). 10.1089/end.2016.0212 [DOI] [PubMed] [Google Scholar]
- 71.Pierce M. C., Jackson S. D., Dickinson M. R., King T. A., “Laser-tissue interaction with a high-power 2-microm fiber laser: preliminary studies with soft tissue,” Lasers Surg. Med. 25(5), 407–413 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Sumiyoshi T., Sekita H., Arai T., Sato S., Ishihara M., Kikuchi M., “High-power continuous-wave 3- and 2-µm cascade Ho3+:ZBLAN fiber laser and its medical applications,” IEEE J. Sel. Top. Quantum Electron. 5(4), 936–943 (1999). 10.1109/2944.796314 [DOI] [Google Scholar]
- 73.Pierce M. C., Jackson S. D., Dickinson M. R., King T. A., Sloan P., “Laser-tissue interaction with a continuous wave 3-mcm fibre laser: preliminary studies with soft tissue,” Lasers Surg. Med. 26(5), 491–495 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Jackson S. D., Lauto A., “Diode-pumped fiber lasers: a new clinical tool?” Lasers Surg. Med. 30(3), 184–190 (2002). 10.1002/lsm.10023 [DOI] [PubMed] [Google Scholar]
- 75.El-Sherif A. F., King T. A., “Soft and hard tissue ablation with short-pulse high peak power and continuous thulium-silica fibre lasers,” Lasers Med. Sci. 18(3), 139–147 (2003). 10.1007/s10103-003-0267-5 [DOI] [PubMed] [Google Scholar]
- 76.Pal D., Ghosh A., Sen R., Pal A., “Continuous-wave and quasi-continuous wave thulium-doped all-fiber laser: implementation on kidney stone fragmentations,” Appl. Opt. 55(23), 6151–6155 (2016). 10.1364/AO.55.006151 [DOI] [PubMed] [Google Scholar]
- 77.Keller M. D., Stafford J. A., Schmidt B. P., Wells J. D., “In vitro testing of dual-mode thulium microsurgical laser,” Proc. SPIE 8207, 820711 (2012). 10.1117/12.909516 [DOI] [Google Scholar]
- 78.Pal D., Paul A., Shekhar N. K., Chowdhury S. D., Sen R., Chatterjee K., Pal A., “COM stone dusting and soft tissue ablation with Q-switched thulium fiber laser,” IEEE J. Sel. Top. Quantum Electron. Epub (2018).
- 79.Qiu J., Teichman J. M., Wang T., Neev J., Glickman R. D., Chan K. F., Milner T. E., “Femtosecond laser lithotripsy: feasibility and ablation mechanism,” J. Biomed. Opt. 15(2), 028001 (2010). 10.1117/1.3368998 [DOI] [PubMed] [Google Scholar]
- 80.Fried N. M., “High-power laser vaporization of the canine prostate using a 110 W Thulium fiber laser at 1.91 microm,” Lasers Surg. Med. 36(1), 52–56 (2005). 10.1002/lsm.20126 [DOI] [PubMed] [Google Scholar]
- 81.Fried N. M., Murray K. E., “High-power thulium fiber laser ablation of urinary tissues at 1.94 microm,” J. Endourol. 19(1), 25–31 (2005). 10.1089/end.2005.19.25 [DOI] [PubMed] [Google Scholar]
- 82.Fried N. M., “Thulium fiber laser lithotripsy: an in vitro analysis of stone fragmentation using a modulated 110-watt Thulium fiber laser at 1.94 microm,” Lasers Surg. Med. 37(1), 53–58 (2005). 10.1002/lsm.20196 [DOI] [PubMed] [Google Scholar]
- 83.Schomacker K. T., Domankevitz Y., Flotte T. J., Deutsch T. F., “Co:MgF2 laser ablation of tissue: effect of wavelength on ablation threshold and thermal damage,” Lasers Surg. Med. 11(2), 141–151 (1991). 10.1002/lsm.1900110208 [DOI] [PubMed] [Google Scholar]
- 84.Hardy L. A., Kennedy J. D., Wilson C. R., Irby P. B., Fried N. M., “Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones,” J. Biophotonics 10(10), 1240–1249 (2017). 10.1002/jbio.201600010 [DOI] [PubMed] [Google Scholar]
- 85.Wilson C. R., Hardy L. A., Irby P. B., Fried N. M., “Collateral damage to the ureter and Nitinol stone baskets during thulium fiber laser lithotripsy,” Lasers Surg. Med. 47(5), 403–410 (2015). 10.1002/lsm.22348 [DOI] [PubMed] [Google Scholar]
- 86.Cordes J., Lange B., Jocham D., Kausch I., “Destruction of stone extraction basket during an in vitro lithotripsy--a comparison of four lithotripters,” J. Endourol. 25(8), 1359–1362 (2011). 10.1089/end.2011.0019 [DOI] [PubMed] [Google Scholar]
- 87.Cordes J., Nguyen F., Lange B., Brinkmann R., Jocham D., “Damage of stone baskets by endourologic lithotripters: a laboratory study of 5 lithotripters and 4 basket types,” Adv. Urol. 2013, 632790 (2013). 10.1155/2013/632790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bader M. J., Gratzke C., Hecht V., Schlenker B., Seitz M., Reich O., Stief C. G., Sroka R., “Impact of collateral damage to endourologic tools during laser lithotripsy--in vitro comparison of three different clinical laser systems,” J. Endourol. 25(4), 667–672 (2011). 10.1089/end.2010.0169 [DOI] [PubMed] [Google Scholar]
- 89.Freiha G. S., Glickman R. D., Teichman J. M., “Holmium:YAG laser-induced damage to guidewires: experimental study,” J. Endourol. 11(5), 331–336 (1997). 10.1089/end.1997.11.331 [DOI] [PubMed] [Google Scholar]
- 90.Honeck P., Wendt-Nordahl G., Häcker A., Alken P., Knoll T., “Risk of collateral damage to endourologic tools by holmium:YAG laser energy,” J. Endourol. 20(7), 495–497 (2006). 10.1089/end.2006.20.495 [DOI] [PubMed] [Google Scholar]
- 91.Griffin S., “Fiber optics for destroying kidney stones,” Biophoton. Int. 11, 44–47 (2004). [Google Scholar]
- 92.Nazif O. A., Teichman J. M. H., Glickman R. D., Welch A. J., “Review of laser fibers: a practical guide for urologists,” J. Endourol. 18(9), 818–829 (2004). 10.1089/end.2004.18.818 [DOI] [PubMed] [Google Scholar]
- 93.Marks A. J., Mues A. C., Knudsen B. E., Teichman J. M. H., “Holmium:YAG lithotripsy proximal fiber failures from laser and fiber mismatch,” Urology 71(6), 1049–1051 (2008). 10.1016/j.urology.2007.10.060 [DOI] [PubMed] [Google Scholar]
- 94.Knudsen B. E., Pedro R., Hinck B., Monga M., “Durability of reusable holmium:YAG laser fibers: a multicenter study,” J. Urol. 185(1), 160–163 (2011). 10.1016/j.juro.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 95.Wilson C. R., Hardy L. A., Irby P. B., Fried N. M., “Microscopic analysis of laser-induced proximal fiber tip damage during Holmium:YAG and Thulium fiber laser lithotripsy,” Opt. Eng. 55(4), 046102 (2016). 10.1117/1.OE.55.4.046102 [DOI] [Google Scholar]
- 96.Scott N. J., Cilip C. M., Fried N. M., “N. M, “Thulium fiber laser ablation of urinary stones through small-core optical fibers,” IEEE J. Sel. Top. Quantum Electron. 15(2), 435–440 (2009). 10.1109/JSTQE.2008.2012133 [DOI] [Google Scholar]
- 97.Blackmon R. L., Irby P. B., Fried N. M., “Thulium fiber laser lithotripsy using tapered fibers,” Lasers Surg. Med. 42(1), 45–50 (2010). 10.1002/lsm.20883 [DOI] [PubMed] [Google Scholar]
- 98.Blackmon R. L., Hutchens T. C., Hardy L. A., Wilson C. R., Irby P. B., Fried N. M., “Thulium fiber laser ablation of kidney stones using a 50-µm-core silica optical fiber,” Opt. Eng. 54(1), 011004 (2014). 10.1117/1.OE.54.1.011004 [DOI] [Google Scholar]
- 99.Wilson C. R., Hutchens T. C., Hardy L. A., Irby P. B., Fried N. M., “A miniaturized, 1.9-French integrated optical fiber and stone basket for use in Thulium fiber laser lithotripsy,” J. Endourol. 29(10), 1110–1114 (2015). 10.1089/end.2015.0124 [DOI] [PubMed] [Google Scholar]
- 100.Wilson C., Kennedy J. D., Irby P., Fried N., “Miniature ureteroscope distal tip designs for potential use in thulium fiber laser lithotripsy,” J. Biomed. Opt. 23(7), 1–9 (2018). 10.1117/1.JBO.23.3.030901 [DOI] [PubMed] [Google Scholar]
- 101.Leiner D., Digital Endoscope Design (SPIE Press, 2016), p. 12. [Google Scholar]
- 102.Marguet C. G., Sung J. C., Springhart W. P., L’Esperance J. O., Zhou S., Zhong P., Albala D. M., Preminger G. M., “In vitro comparison of stone retropulsion and fragmentation of the frequency doubled, double pulse nd:yag laser and the holmium:yag laser,” J. Urol. 173(5), 1797–1800 (2005). 10.1097/01.ju.0000154341.08206.69 [DOI] [PubMed] [Google Scholar]
- 103.White M. D., Moran M. E., Calvano C. J., Borhan-Manesh A., Mehlhaff B. A., “Evaluation of retropulsion caused by holmium:YAG laser with various power settings and fibers,” J. Endourol. 12(2), 183–186 (1998). 10.1089/end.1998.12.183 [DOI] [PubMed] [Google Scholar]
- 104.Lee H., Ryan R. T., Teichman J. M., Kim J., Choi B., Arakeri N. V., Welch A. J., “Stone retropulsion during holmium:YAG lithotripsy,” J. Urol. 169(3), 881–885 (2003). 10.1097/01.ju.0000046367.49923.c6 [DOI] [PubMed] [Google Scholar]
- 105.Lee H., Ryan R. T., Kim J., Choi B., Arakeri N. V., Teichman J. M. H., Welch A. J., “Dependence of calculus retropulsion dynamics on fiber size and radiant exposure during Ho:YAG lithotripsy,” J. Biomech. Eng. 126(4), 506–515 (2004). 10.1115/1.1786297 [DOI] [PubMed] [Google Scholar]
- 106.Sea J., Jonat L. M., Chew B. H., Qiu J., Wang B., Hoopman J., Milner T., Teichman J. M., “Optimal power settings for Holmium:YAG lithotripsy,” J. Urol. 187(3), 914–919 (2012). 10.1016/j.juro.2011.10.147 [DOI] [PubMed] [Google Scholar]
- 107.Struve B., Huber G., “Properties and medical applications of near-IR solid-state lasers,” J. Phys. IV 1(C7), 3–6 (1991). 10.1051/jp4:1991701 [DOI] [Google Scholar]
- 108.Hardy L. A., Wilson C. R., Irby P. B., Fried N. M., “Rapid Thulium fiber laser lithotripsy at pulse rates up to 500 Hz using a stone basket,” IEEE J. Sel. Top. Quantum Electron. 20(5), 0902604 (2014). 10.1109/JSTQE.2014.2305715 [DOI] [Google Scholar]
- 109.Glybochko P., Altshuler G., Vinarov A., Rapoport L., Enikeev M., Grigoriev N., Enikeev D., Sorokin N., Dymov A., Sukhanov R., Taratkin M., Zamyatina V., “Comparison between the possibilities of holmium and thulium laser in lithotripsy in vitro,” Eur. Urol. 16(3), 391 (2017). [Google Scholar]
- 110.Glybochko P., Altshuler G., Yaroslaksy I., Vinarov A., Enikeev D., Sorokin N., Dymov A., Sukhanov R., Vinnichenko V. V., “Comparative in vitro study of Ho:YAG and Tm fiber laser lithotripters in dusting mode of operation,” J. Urol. 197 (4S), e815 (2017). 10.1016/j.juro.2017.02.1899 [DOI] [Google Scholar]
- 111.Dymov A., Glybochko P., Alyaev Y., Vinarov A., Altshuler G., Zamyatina V., Sorokin N., Enikeev D., Lekarev V., Proskura A., Koshkarev A., “Thulium lithotripsy: from experiment to clinical practice,” J. Urol. 197(4S), 11 (2017) [Google Scholar]
- 112.Hardy L. A., Gonzalez D. A., Irby P. B., Fried N. M., “Fragmentation and dusting of large kidney stones using a compact, air-cooled, high peak power, 1940-nm, Thulium fiber laser,” Proc. SPIE 10468(104680O), 1–5 (2018). [Google Scholar]
- 113.Gross A., Becker B., Taratkin M., Enikeev D., Rapoport L., Netsch C., “Wavelength and pulse shape effects on stone fragmentation of laser lithotripters,” J. Urol. 199(4S), e293–e294 (2018). 10.1016/j.juro.2018.02.763 [DOI] [Google Scholar]
- 114.Yaroslavsky I., Vinnichenko V., McNeill T., Novoselteva A., Perchuk I., Vybornov A., Altshuler G., Gapontsev V., “Optimization of a novel Tm fiber laser lithotripter in terms of stone ablation efficiency and retropulsion reduction,” Proc. SPIE 10468(104680H), 1–9 (2018). [Google Scholar]
- 115.Martov A. G., Ergakov D. V., Guseinov M. A., Andronov A. S., Dutov S. V., Vinnichenko V. A., Kovalenko A. A., “[Initial experience in clinical application of thulium laser con7act lithotripsy for transurethral treatment of urolithiasis],” Urologiia Mar 2018(1), 112–120 (2018). 10.18565/urology.2018.1.112-120 [DOI] [PubMed] [Google Scholar]
- 116.Ergakov D., Martov A. G., Guseynov M., “The comparative clinical study of Ho:YAG and superpulse Tm fiber laser lithotripters,” J. Urol. 17(2), e1391 (2018). [Google Scholar]
- 117.Traxer O., Rapoport L., Tsarichenko D., Dymov A., Enikeev D., Sorokin N., Ali S., Akopyan G., Korolev D., Proskura A., Lekarev V., Klimov R., “First clinical study on superpulse thulium fiber laser lithotripsy,” J. Urol. 199 (4S), e321–e322 (2018). 10.1016/j.juro.2018.02.827 [DOI] [Google Scholar]
- 118.Dornier website (https://dornier.com/products/dornier-medilas-h-140/).