Abstract
Objective:
To identify the prevalence of monoclonal gammopathy of undetermined significance (MGUS) in patients with transthyretin (ATTR) amyloidosis.
Patients and Methods:
We performed a retrospective analysis of patients with biopsy-proven ATTRwt and genopositive V122I ATTRm amyloidosis evaluated at the Amyloidosis Center at Boston University and Boston Medical Center between January 1, 2003 and December 31, 2016.
Results:
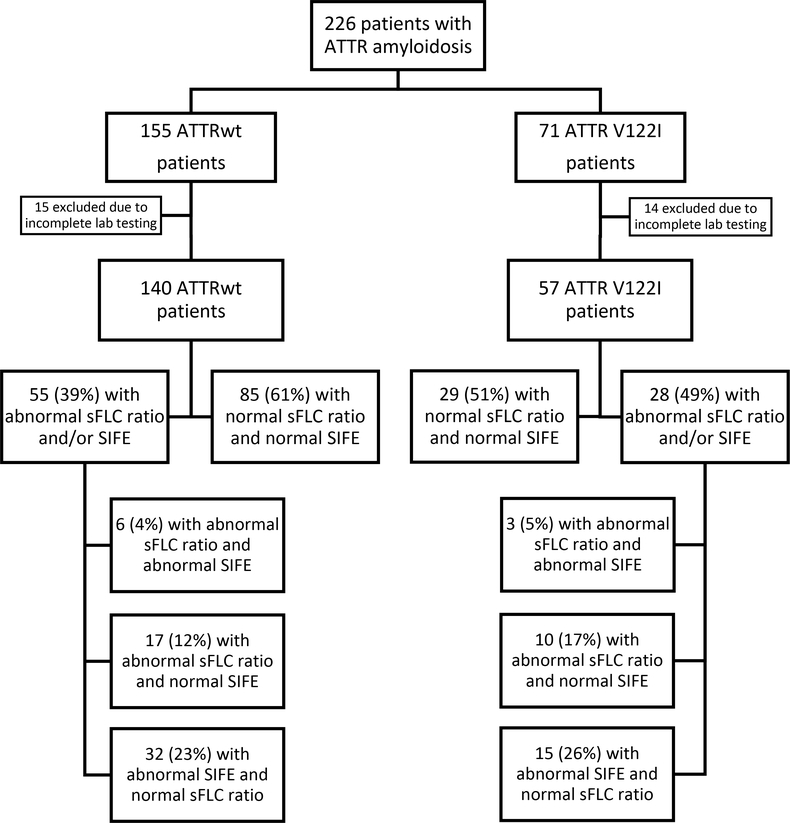
There were a total of 226 patients with ATTRwt and V122I ATTRm amyloidosis evaluated during the specified time frame with 155 and 71 patients in each cohort, respectively. Those with complete medical records, 140 patients with ATTRwt and 57 V1221 ATTRm subjects, were included in the analyses. Fifty-five patients (39%) in the ATTRwt cohort and 28 patients (49%) in the V122I ATTRm cohort had an MGUS, as indicated by an abnormality in the serum free light chain ratio and/or serum immunofixation electrophoresis.
Conclusion:
These data confirm the high prevalence of coexistent MGUS with ATTR amyloidosis in this patient population, with an MGUS rate that is higher than the general population. These findings also highlight the importance of a thorough diagnostic evaluation in patients with amyloidosis to determine the precursor protein, as the clinical course and treatment of AL and ATTR amyloidosis are distinct.
Keywords: transthyretin amyloidosis, amyloid cardiomyopathy, MGUS, V122I ATTRm, ATTRwt
Introduction
Amyloidosis is a disease characterized by tissue deposition of insoluble fibrils created by the aggregation of misfolded proteins [1]. This aberrant protein deposition can affect nearly all major organ systems, producing varied clinical presentations which reflect the organs affected by this infiltrative process [1]. Cardiac involvement is common and associated with an increased risk of morbidity and mortality related to the development of congestive heart failure, arrhythmias, and conduction defects [2,3]. Of more than 30 proteins implicated in the development of amyloidosis, at least 9 have been associated with amyloid cardiomyopathy [4,5]. The specific type of cardiac amyloidosis is defined by the underlying misfolded protein; with light-chain (AL) and transthyretin (ATTR), being the most common in the United States and other developed nations [5,6].
Transthyretin is a protein produced by the liver that functions as a transporter for thyroxine (T4) and retinol [7]. Misfolding and fibril deposition of transthyretin may be caused by hereditary or genetic mutations (ATTRm) or by an acquired “wild-type” form of the disease (ATTRwt), previously referred to as senile systemic amyloidosis [6]. A common inherited cause of ATTRm cardiac amyloidosis is a valine-to-isoleucine substitution at position 122 of the TTR gene (referred to as ATTR V122I) [8,9]. The V122I mutation almost exclusively affects patients of African descent and appears in 3–4% of black Americans, with an age-dependent, variable penetrance [8,10]. A recent analysis of Afro-Caribbean patients >60 years of age with congestive heart failure demonstrated a prevalence of at least 12% for the V122I allele in that population [8].
Traditionally, the diagnosis of cardiac amyloidosis has required the demonstration of amyloid deposits by Congo red staining, thioflavin T or Alcian blue [5]. Recently, non-invasive imaging modalities such as cardiac magnetic resonance imaging, nuclear scintigraphy with bone-avid compounds, and echocardiography with strain quantification are being employed to establish the diagnosis of cardiac amyloidosis without tissue confirmation [6,9,11]. When biopsy specimens are obtained, techniques such as immunohistochemistry, mass spectrometry, and immunogold electron microscopy are used to identify the major protein constituent of the amyloid fibrils [3]. Concomitant genetic testing of TTR coding regions using DNA extracted from peripheral blood identifies any mutations associated with ATTR amyloidosis to distinguish between wild-type or mutant forms of the disease [11].
The diagnosis of AL amyloidosis requires evidence of organ dysfunction related to a monoclonal paraprotein, which can be detected by the serum free light chain (SFLC) assay, serum or urine immunofixation electrophoresis (SIFE/UIFE), serum or urine protein electrophoresis (SPEP/UPEP), or bone marrow biopsy [12]. A monoclonal paraprotein is also present in patients with a monoclonal gammopathy of undetermined significance (MGUS), which is a premalignant disorder characterized by the presence of a low-level monoclonal protein in the absence of the sequelae of a monoclonal plasma cell disorder [13,14]. MGUS can be distinguished from AL amyloidosis by careful physical exam, history, and laboratory testing to evaluate organ function. If characteristic organ dysfunction is discovered, the diagnosis of AL amyloidosis can be confirmed by the demonstration of monoclonal light chain as the precursor protein in the amyloid fibrils on tissue biopsy.
Diagnostic accuracy is required to differentiate between ATTRwt, ATTR V122I, and AL amyloidosis which all have a similar age of onset (approximately 60 to 65 years) [3,15,16]. The presence of an MGUS, which also has increasing incidence with age [15], may confound the diagnosis of amyloidosis because the finding of a monoclonal protein in these patients could be an indication of AL amyloidosis or ATTR amyloidosis with a concomitant MGUS.
Given the increasing incidence of both ATTR amyloidosis and monoclonal gammopathies in the elderly population, it is not uncommon to discover a monoclonal protein in elderly patients with ATTR amyloidosis. Previous retrospective analyses of patients with ATTRwt amyloidosis noted a 10–18% prevalence of MGUS, with an additional 16% of patients with abnormal serum free light chains [3,17]. These reviews were not limited to patients with tissue biopsy and confirmed amyloidosis typing. We sought to determine the prevalence of MGUS in patients with tissue biopsy-proven ATTRwt or genopositive ATTR V122I amyloidosis in the largest single center cohort of V122I ATTRm and ATTRwt patients reported to date, and restricted our analysis to include only patients with biopsy proven, confirmatory tissue typed ATTRwt or genopositive ATTR V122I amyloidosis
Patients and methods
We performed a retrospective review of all 226 subjects with biopsy-proven ATTRwt or genopositive ATTR V122I amyloidosis evaluated at the Amyloidosis Center of Boston University School of Medicine and Boston Medical Center between January 1, 2003 and December 31, 2016. The study was approved by the Institutional Review Board. All patients consented as per Declaration of Helsinki. Only subjects with biochemical or immunochemical proof of TTR in amyloid deposits, and negative testing for an amyloidogenic TTR mutant by genotyping or mass spectral analyses of the serum protein, were included in the ATTRwt subgroup. All patients in the ATTR V122I subgroup had serum isoelectric focusing showing the presence of a circulating mutant and DNA sequencing demonstrating a V122I mutation (Table 1). Confirmation of ATTR amyloidosis was obtained by mass spectrometry, immunohistochemistry, immunogold electron microscopy, immunofluorescence, immunoblot, or a combination of these methods. Pertinent clinical data, including cardiac biomarkers, cardiac imaging, and testing for evidence of a monoclonal protein were collected. The presence of a monoclonal gammopathy was defined as an abnormal serum free light chain ratio and/or detection of a monoclonal protein on serum immunofixation electrophoresis, both of which are routinely checked during a complete evaluation at our center. Serum free light chains were measured using the Freelite assay, with 0.26 to 1.65 being the normal range for the free light chain ratio. Indicators of renal function were also collected given the known effect of renal function on the concentration of serum free light chains [18].
TABLE 1:
Patient demographic and clinical characteristics.
| ATTRwt N=140 | ATTR V122I N=57 | |
|---|---|---|
|
Clinical Median age at initial visit, y Male, N (%) Female, N (%) Deceased, N (%) Death due to amyloid, N (% of all deaths) Median overall survival, y |
75.5 (51–87) 136 (97) 4 (3) 71 (51) 39 (55) 4.3 |
71 (50–90) 44 (77) 13 (23) 28 (49) 8 (29) 3.1 |
|
Diagnostic testing, N (%) Amyloidogenic TTR mutant identified Confirmation of TTR amyloid Mass Spectrometry Immunohistochemistry (IHC) Immunogold electron microscopy (EM) Immunofluorescence Immunoblot IHC + additional typing (EM or MS) |
0 (0) 140 (100) 45 (32) 64 (46) 18 (13) 3 (2) 5 (4) 5 (4) |
57 (100) 26 (45) 10 (38) 10 (38) 6 (23) 0 0 0 |
|
Disease Markers Cardiac MRI performed, N (%) Late gadolinium enhancement, N (%) 99mTechnetium-pyrophosphate scintigraphy, N (%) Mean heart-lung contralateral ratio, (range) Mean left ventricular ejection fraction, % (range) Median interventricular septal diameter, mm (range) New York Heart Association (NYHA) Stage reported, N (%) NYHA Stage >1, N (%) Troponin I reported, N (%) Mean troponin I, ng/mL (range) B-type Natriuretic Peptide (BNP) reported, N (%) Mean BNP, pg/ml (range) Estimated glomerular filtration rate < 60 ml/min, N (%) Estimated glomerular filtration rate < 30 ml/min, N (%) |
44 (31) 39 (89) 30 (21) 1.96 (1.03–3.5) 49 (45–70) 16 (8–25) 134 (96) 115 (86) 116 (83) 0.19 (<0.006–2.15) 136 (97) 436 (12–1756) 79 (56) 8(6) |
15 (26) 11 (73) 16 (28) 1.73 (1.13–2.4) 44.6 (20–70) 16 (11–23) 54 (95) 43 (80) 47 (82) 0.22 (<0.006–1.90) 56 (98) 804.1 (5–5411) 21(37) 4 (7) |
Results
Subjects with both SIFE and SFLC measured at the initial diagnostic evaluation were included in the final analysis. This included 140 of 155 subjects (90%) in the ATTRwt subgroup and 57 of 71 (80%) in the V1221 subgroup. The ATTRwt group was comprised of 136 males (97%) with a median age of 75.5 years (range, 51–87). The median age in the ATTR V122I group was 71 years (range, 50–90) and included 44 males (77%). In the ATTRwt cohort, 116 patients (83%) were diagnosed by cardiac biopsy and the remaining subjects had biopsy proof from other organ sites. Within the V122I group, 44 patients had biopsies obtained and 26 of these (59%) were obtained from cardiac tissue. The baseline clinical and diagnostic characteristics of both subgroups are presented in Table 1.
Of the ATTRwt subjects, 55 patients (39%) had an abnormality in the laboratory evaluation for a monoclonal protein conferring an MGUS (Figure 1). Of these patients, 6 (11%) had both an abnormal FLC ratio and a corresponding monoclonal protein seen on SIFE; 17 (31%) had an abnormal FLC ratio with a negative SIFE; and 32 (58%) had a monoclonal protein detected on SIFE with a FLC ratio that was within normal limits. Of the 55 patients with evidence of a monoclonal protein, 28 (51%) had bone marrow biopsies available for review; 8 (29%) of which were abnormal. Two biopsies confirmed the monoclonal protein seen on SIFE, three confirmed the kappa monoclonal light chain noted in the corresponding FLC ratio, two showed a monoclonal lambda predominance in patients with SIFE demonstrating a kappa monoclonal protein, and the remaining abnormal biopsy revealed the presence of a B-cell lymphoma.
FIGURE 1.
Flow diagram
Of the ATTR V122I subjects, 28 (49%) had abnormalities in one or both of the laboratory tests of interest conferring the diagnosis of MGUS (Figure 1). Within this group, 3 (11%) subjects had an abnormal FLC ratio with a monoclonal protein on SIFE; 10 (36%) had an abnormal FLC ratio with a negative SIFE; and 15 (54%) had a detectable monoclonal protein on SIFE with a normal FLC ratio. Fifteen of these patients (54%) had bone marrow biopsies available for review and 3 (20%) of these were abnormal. All three of these bone marrow biopsies yielded results that correlated directly with the monoclonal protein detected on the corresponding SIFE. There was no significant difference between the incidence of MGUS in the ATTRwt group compared to the V122I ATTRm group (P=0.268).
Of the individuals in the ATTRwt group, 79 patients (56%) had an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73m2 and 21 patients (37%) in the ATTR V122I subgroup had renal dysfunction by the same criteria. Of the seventeen ATTRwt subjects with abnormal SFLC ratio and negative SIFE, nine met this criteria for renal dysfunction; of which two patients had confirmation of the monoclonal protein on bone marrow biopsy, one had an abnormal ratio with elevated lambda free light chains (not expected in renal dysfunction), and six individuals had no other evidence of monoclonality. In the ATTR V122I group, of the ten patients with abnormal FLC ratios and negative SIFE, four had renal dysfunction. Within this cohort, the patients had no other evidence of monoclonality.
Discussion
This study was comprised entirely of patients with tissue-biopsy proven ATTRwt amyloidosis with confirmatory tissue typing and genopositive V122I mutation testing to ensure accurate diagnosis and exclusion of AL amyloidosis. We found that 55 ATTRwt patients (39%) had an MGUS; 38 of 140 patients (27%) had a monoclonal gammopathy based on an abnormal SIFE and an additional 17 of 140 patients (12%) with light chain monoclonal gammopathy based on an abnormal SFLC ratio. A slightly higher rate of monoclonal gammopathy was seen in the ATTR V122I patients with 28 patients (49%) diagnosed with a MGUS; 18 of 57 patients (32%) with diagnosis based on SIFE and 10 of 57 (18%) with light chain monoclonal gammopathy based on an abnormal serum FLC ratio. These rates of MGUS are higher than the 10–18% previously reported in the literature for patients with ATTR amyloidosis and also higher than the reported prevalence of 5.3% in patients greater than 70 years of age [19]. The reason for the increased rate of MGUS in the ATTR population is unknown and is an area that requires additional exploration. These findings could potentially be related to differences in the demographics of the current population compared with prior studies or perhaps a more direct relationship between destabilization or mutation of the transthyretin protein and monoclonal plasma cells.
Although each patient in this study had a tissue biopsy and amyloid typing in the wild-type cohort and confirmed genetic mutation in the V122I cohort, it is not currently the standard of care to obtain a biopsy and genotyping in all patients with suspected ATTR amyloidosis. Noninvasive diagnostic techniques, such as nuclear imaging, have been increasingly utilized to diagnosis ATTR cardiac amyloidosis. These imaging techniques have high sensitivity and specificity for cardiac involvement associated with TTR amyloid fibril deposits, but have been associated with false positive results in patients with AL cardiac amyloidosis [20]. Therefore, despite the development of accurate noninvasive testing, tissue biopsy remains vital in any suspected ATTR amyloidosis case in which there is clinical uncertainty surrounding the diagnosis, including those cases with the discovery of a monoclonal protein during initial evaluation.
The evaluation of a monoclonal protein discovered in a patient with amyloidosis should include serum free light chains, serum immunofixation electrophoresis, serum protein electrophoresis, urine immunofixation electrophoresis, and urine protein electrophoresis as recommended by the International Myeloma Working Group [14]. A bone marrow biopsy should be considered in patients with intermediate or high risk MGUS [21]. Of these screening tools, the serum free light chain assay has the highest sensitivity, followed by the SIFE and subsequently the SPEP when screening for a monoclonal protein associated with AL amyloidosis [22]. Due to the high sensitivity of these tests, they are commonly used as a screening test prior to a more invasive bone marrow biopsy. As expected, the higher sensitivity of the serum free light chains for detection of a monoclonal protein decreases the specificity of the assay and therefore has a false positive rate of approximately 3.8% [23,24]. Even in the absence of a positive SIFE and SPEP, an abnormal SFLC ratio can be an indication of a light chain MGUS and continued monitoring is recommended as light chain MGUS accounts for approximately 4% of all MGUS cases and has a rate of progression to multiple myeloma of 0.3% per 100-person years [25]. In all patients with suspected amyloidosis we would recommend checking serum free light chains and serum immunofixation electrophoresis, two tests with high sensitivity for a monoclonal gammopathy, to evaluate for the possibility of AL amyloidosis. If a monoclonal gammopathy is discovered during this evaluation this should prompt confirmatory tissue typing. If the monoclonal protein is determined to be related to an MGUS it is important to follow these patients for progression to multiple myeloma, AL amyloidosis or other lymphoplasmacytic disorders.
In the case of an abnormal SFLC ratio, without other evidence of monoclonal gammopathy, the patient’s renal function should be evaluated. Serum free light chains are typically cleared via the kidneys, with κ light chains more readily cleared by the renal system due to the increased tendency of λ light chains to dimerize [26]. This physiologic production and clearance pattern produces a κ/λ ratio which typically ranges from 0.26 to 1.65 [18]. Renal impairment alters the normal rate of light chain clearance, as the light chains are cleared by the reticuloendothelial system, disproportionately increasing in the serum concentration of κ light chains relative to that of λ light chains. In fact, research has shown that a κ/λ ratio of 2 or greater may be normal in individuals with underlying renal disease [18]. In our population there were six patients in the ATTRwt and four patients in the ATTR V122I who had renal dysfunction and an elevated SFLC ratio without other evidence of monoclonality. The SFLC abnormality in these patients could potentially be attributed to renal dysfunction, although these patients did not have a bone marrow biopsy or 24 hour urine studies at our institution to complete the work-up for a monoclonal gammopathy, therefore some of these patients may have a confirmed monoclonal gammopathy with additional testing.
We found a higher rate of MGUS in the ATTR V122I population than the ATTRwt patients (49% v. 39%), although this difference was not statistically significant (P=0.268). While this finding was not significant, it is important to recognize the higher prevalence of MGUS in patients with African ancestry [27], a group comprising the majority of ATTR V122I patients. The younger median age of 71 years in the ATTR V122I group versus 75.5 years in the ATTRwt cohort and the small sample size is the likely explanation for the lack of statistical difference in the rates of MGUS in these two populations.
There are some limitations to this study, including mainly the retrospective nature of the study, which limits the analysis to data that is available in the previous medical record at our institution. In addition, not all patients who were evaluated at our center completed a full evaluation. This limited the analysis to 90% of patients in the ATTRwt group and 80% of those in the ATTR V122I group, therefore introducing a potential selection bias. Despite these limitations the coexistence of MGUS and ATTR amyloidosis is still evident.
In conclusion, the prevalence of both ATTRwt and ATTR V122I amyloidosis increases with age, as does the rate of monoclonal gammopathies. The data from this study confirm the high prevalence of MGUS in the context of ATTR amyloidosis and highlight the importance of a thorough evaluation of all patients with suspected TTR amyloidosis. Given the disparities in the clinical course and treatment of ATTR and AL amyloidosis, it is critical to properly identify the precursor protein in these patients. As the diagnostic approach in ATTR cardiac amyloidosis has shifted significantly toward non-invasive imaging, it remains essential to perform a complete evaluation for a plasma cell disorder, including as a minimum serum free light chains and a serum immunofixation electrophoresis, in all patients with suspected amyloidosis. Biopsy of the affected organ should be obtained to confirm the diagnosis when a monoclonal protein is identified. Additional research is needed to explore the relationship between ATTR amyloidosis and monoclonal gammopathy with the goal of discovering the underlying pathophysiology that explains the higher rate of monoclonal gammopathy in the ATTR amyloidosis population compared with the general population.
Acknowledgements
This work was supported by the Amyloid Research Fund of the Amyloidosis Center at Boston University School of Medicine, The National Institutes of Health 2R56AG031804–06A1 (LHC), R01AG031804 (LHC), and the Boston University School of Medicine Young Family Amyloid Research Fund.
Abbreviations:
- ATTR
transthyretin amyloid protein
- ATTRm
hereditary or mutant transthyretin amyloid protein
- ATTRwt
wild-type transthyretin amyloid protein
- V122I
valine-to-isoleucine substitution at position 122 of the TTR gene
- AL
light chain amyloid protein
- MGUS
monoclonal gammopathy of undetermined significance
- SFLC
serum free light chain
- SIFE
serum immunofixation electrophoresis
- SPEP
serum protein electrophoresis
Footnotes
Disclosure of interest
There are no relevant conflicts of interest to disclose.
References
- [1].Karafiatova L, Pika T. Amyloid cardiomyopathy. Biomed Pap. 2017;161:117–127. [DOI] [PubMed] [Google Scholar]
- [2].Tuzovic M, Yang EH, Baas AS, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19:46. [DOI] [PubMed] [Google Scholar]
- [3].Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23:209–213. [DOI] [PubMed] [Google Scholar]
- [5].Guan J, Mishra S, Falk RH, et al. Current perspectives on cardiac amyloidosis. Am J Physiol - Hear Circ Physiol. 2012;302:H544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meier-Ewert HK, Sanchorawala V, Berk JL, et al. Cardiac amyloidosis: evolving approach to diagnosis and management. Curr Treat Options Cardiovasc Med. 2011;13:528. [DOI] [PubMed] [Google Scholar]
- [7].Chen R, Chen CP, Preston JE. Effects of transthyretin on thyroxine and β-amyloid remo[val from cerebrospinal fluid in mice. Clin Exp Pharmacol Physiol. 2016;43:844–850. [DOI] [PubMed] [Google Scholar]
- [8].Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Givens RC, Russo C, Green P, et al. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health. 2013;9:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin Variant in elderly black Americans. N Engl J Med. 2014;372:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brunjes DL, Castano A, Clemons A, et al. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2017;22:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanchorawala V Light-Chain (AL) Amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331–1341. [DOI] [PubMed] [Google Scholar]
- [13].Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548. [DOI] [PubMed] [Google Scholar]
- [15].Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desport E, Bridoux F, Sirac C, et al. AL Amyloidosis. Orphanet J Rare Dis. 2012;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Geller HI, Singh A, Mirto T, et al. Prevalence of monoclonal gammopathy of unknown significance in ATTR-wild type patients. XVth Int Symp Amyloidosis. 2016;62 (Abstr.) [Google Scholar]
- [18].Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kyle RA, Therneau TM, Rajkumar S V, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354. [DOI] [PubMed] [Google Scholar]
- [20].Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- [21].Kyle RA, Durie BGM, Rajkumar S V, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Katzmann JA. Screening panels for monoclonal gammopathies: Time to change. Clin Biochem Rev. 2009;30:105–111. [PMC free article] [PubMed] [Google Scholar]
- [23].Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- [24].Katzmann JA. Serum free light chain specificity and sensitivity: A reality check. Clin Chem. 2006;52:1638–1639. [DOI] [PubMed] [Google Scholar]
- [25].Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2017;375:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abadie JM, Van Hoeven KH, Wells JM. Are renal reference intervals required when screening for plasma cell disorders with serum free light chains and serum protein electrophoresis? Am J Clin Pathol. 2009;131:166–171. [DOI] [PubMed] [Google Scholar]
- [27].Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]