Abstract
Many human activities increase concentrations of major geochemical ions (Na+1, K+1, Ca+2, Mg+2, Cl−1, SO4−2, and HCO3−1/CO3−2) in fresh water systems, and can thereby adversely affect aquatic life. Such effects involve several toxicants, multiple toxicity mechanisms, various ion interactions, and widely varying ion compositions across different waterbodies. Previous studies of individual salt toxicities have defined some useful relationships, but adding single salts to waters results in atypical compositions and does not fully address mixture toxicity. To better understand mechanisms and interactions for major ion toxicity, 29 binary mixture experiments, each consisting of 7–8 toxicity tests, were conducted on the acute toxicity of major ion salts and mannitol to Ceriodaphnia dubia. These tests demonstrated multiple mechanisms of toxicity, including 1) non-specific ion toxicity, correlated with osmolarity and to which all ions contribute, and 2) cation-dependent toxicities for K, Mg, and Ca best related to their chemical activities. These mechanisms primarily operate independently, except for additive toxicity of Mg- and Ca-dependent toxicities. These mixture studies confirmed ameliorative effects of Ca on Na and Mg salt toxicities and of Na on K salt toxicity, and further indicated lesser ameliorative effects of Ca on K salt toxicity and Mg on Na salt toxicity. These results provide a stronger basis for assessing risks from the complex mixtures of ions found in surface waters.
Keywords: Aquatic toxicology, Toxicity mechanisms, Concentration addition, Independent action, Chemical activity
INTRODUCTION
As discussed in Mount et al. [1] and references therein, anthropogenic increases of major geochemical ions (Na+1, K+1, Ca+2, Mg+2, Cl−1, SO4−2, and HCO3−1/CO3−2) pose significant and widespread risks to aquatic communities, and adequately characterizing those risks requires understanding how the toxicity of such ion mixtures varies with their composition. In [1], we examined effects of dilution water chemistry on the acute toxicity of individual major ion salts to Ceriodaphnia dubia. Although a wide range of dilution water compositions were evaluated, significant effects found in these experiments were limited to a reduction in the toxicity of Na and Mg salts with increasing Ca in the dilution water and a reduction in the toxicity of K salts with increasing Na.
Although our background waters studies were not explicitly intended to evaluate mechanisms of toxicity, the large differences in the toxicities of different salts indicated that major ion toxicity involves multiple mechanisms of action. For K and Mg salts, toxicity appeared to be primarily or solely attributable to the specific cation, whereas for Na salts both the cation and the anions apparently contributed to toxicity. For CaCl2, comparisons to other salts were confounded by the ameliorative effect of Ca, but the data again suggested toxicity to be primarily or solely attributable to the cation. For Na salts, an exposure metric of osmolarity provided good coherence among different salts and dilution waters after accounting for Ca effects. The implication that the mechanism for Na salt toxicity to C. dubia was linked to osmotic stress was reinforced by close agreement of median lethal concentrations (LC50s) for Na salts and mannitol when expressed as osmolarity. To better compare toxicities across all major ion salts, we also found it important to express toxicity on the basis of molarity rather than mass per volume, and as chemical activity rather than concentration.
A limitation of previous work by us and others is that it focused on waters enriched with single salts. Even with evaluations spanning different dilution waters, increasing concentrations of only a single cation and a single anion is not representative of most field settings in which multiple ions co-vary, and also provides limited ability to infer toxic interactions among the various ions. Such interactions can be better established by experiments with binary mixtures of salts, but little has been done previously in this regard. Mount et al. [2] evaluated 1:1 mixtures of different salts, which indicated additivity of mixtures sharing the same cation, but nonadditivity across different cations. In evaluating the effects of Cl on Na2SO4 toxicity, Soucek and coworkers [3] provided some evidence for additivity of NaCl and Na2SO4. The present study builds on these efforts with more comprehensive mixture toxicity tests.
Mechanistic insights that can be obtained from binary mixture tests are illustrated by the isobolograms in Figure 1. We use LC50s as the toxicity metric here, but the concepts illustrated in Figure 1 also apply to other types and/or magnitudes of effect. The lines (isoboles) represent concentration pairs of chemicals A and B (CA, CB) that together cause 50% mortality, the intercepts being the LC50s for each chemical in the absence of the other chemical (LC50Aonly, LC50Bonly), which in this example are 1.0 for chemical A and 2.0 for chemical B. Each isobole shape represents a different type of joint action. Terminology used here is based on classification schemes described by Plackett and Hewlett [4] and by Anderson and Weber [5], as reviewed and further developed by Könemann [6]. Figure 1 is organized according to the 4 categories of Plackett and Hewlett [4], who classified joint action by whether the “toxic action” of the 2 chemicals are “similar” or “dissimilar” and whether “additional interactions” are present or absent.
Figure 1.
Theoretical isobolograms (solid lines) according to the classification of Plackett and Hewlett (1952) of similar or dissimilar toxicity with additional interactions present or absent. The additional interaction in panels B and D is an ameliorative effect of chemical B on chemical A depicted by the dashed line. The dotted line in panel B denotes extrapolating the linear concentration-addition portion at high chemical B concentrations to estimate the LC50 of chemical A at high chemical B.
For similar toxic action, the chemicals A and B interact such that their toxic units (TUs) add to 1.0 along the isobole, a TU being a chemical’s concentration divided by its effect concentration. With no additional interactions between the chemicals (Figure 1A), a straight isobole will connect the axes, representing the equation CA/LC50Aonly+CB/LC50Bonly = TUA+TUB = 1.0. Because this involves adding normalized concentrations, it is typically referred to as “concentration addition” [5,6]. Figure 1A further qualifies this straight isobole as “simple concentration addition” to indicate that concentration additivity can also be part of more complex interactions.
Figure 1B illustrates similar action with an interaction present, the example interaction here being an ameliorative effect of chemical B on the toxicity of chemical A, such as the effect of Ca on the toxicities of Na and Mg [1]. The dashed curve on this figure represents a hypothetical increase in LC50Aonly as CB increases (i.e., the toxicity of chemical A decreases at higher concentrations of chemical B). This ameliorative effect is not directly observable except at low CB because chemical B is also contributing additively to toxicity. Because the action of the 2 chemicals is similar, the TUs will still add to 1.0, causing the isobole (solid curve) to veer from the dashed curve at relatively low CB. How far this isobole swings out and up from the simple concentration addition line will depend on the shape and degree of the underlying ameliorative effect. Because the ameliorative effect of chemical B is only directly observable at low CB, definitive conclusions about additivity therefore will be difficult. However, if the ameliorative effect on LC50Aonly approaches a limit at CB<LC50Bonly (i.e., the vertical asymptote of the dashed curve in Figure 1B), the isobole becomes approximately linear at low CA and high CB, such a linear portion being indicative of concentration addition. Furthermore, extrapolating this linear portion of the isobole to the CA (dotted line on Figure 1B) axis can provide an estimate of the asymptote for LC50Aonly on the dashed curve.
In contrast, for dissimilar action with additional interactions absent (Figure 1C), an individual organism reacts only to chemical A or chemical B, depending on which exerts the greater toxicity in a particular mixture. Therefore, the isobole stays at LC50Aonly when CB<LC50Bonly and vice versa, with the curvature when both chemicals are near 1 TU being due to some individual organisms reacting to chemical A and others reacting to chemical B. The shape and degree of this curvature depends on the steepness of the mortality versus concentration curves for each chemical and the degree to which the sensitivities of the individual organisms to one chemical are correlated to their sensitivities to the other chemical [6]. In the simplest case, for a binary endpoint such as mortality and when tolerance distributions are independent between the chemicals, the isobole reflects combining probabilities of mortality (P) according to the relationship PMixture=1-(1-PA)(1-PB), where PA and PB are the probabilities of survival at CA and CB for exposures to the individual salts and PMixture is the resultant probability of survival for the mixture. Because this involves combining independent responses to the chemicals, it has been referred to as “independent action” [4], but also has been referred to as “no addition” [6] and “response addition” [5].
Figure 1D illustrates dissimilar action with the same ameliorative effect of chemical B on the toxicity of chemical A as shown in Figure 1B. In this case, the independent action of the chemicals causes the isobole (solid curve) to follow the dashed curve for the ameliorative effect of CB on LC50Aonly until TUB is nearly 1, at which point the isobole curves off to approach LC50Bonly. The shape of the ameliorative effect will thus be more evident than when the chemicals are additive, and independent action in this case will be easier to establish.
Deviations from the simple isoboles in Figures 1A and 1C were termed by Könemann [6] as “supra-addition” when below the concentration-addition isobole (synergistic interactions), “partial-addition” when between the 2 isoboles, and “antagonism” when beyond the independent action isobole. While these terms have utility, they are problematic for the types of interactions shown in Figures 1B and 1D. For example, in Figure 1B, the ameliorative effect of CB on LC50Aonly can certainly be considered antagonism of chemical B to the toxicity of chemical A, and the isobole’s swing to the right is in the region thus classified by Könemann [6] as antagonism, but the bulk of the isobole would be in the region classified as partial-addition, even though it actually represents complete concentration addition, as modified by the ameliorative effect. The point here is that formulating and interpreting isoboles requires some caution.
In addition, it should be noted that the isobole shapes in Figure 1 presume that the concentrations of both chemicals are the appropriate exposure metrics for their toxicities and interaction. However, for major ion toxicity, each salt itself is a mixture of 2 ions, possibly reflecting different toxicological actions; also, the salts in a binary mixture can share an ion, thereby partially or wholly representing the same toxicological action. Therefore, to adequately demonstrate even simple concentration addition and independent action, the isobologram axes should be exposure metrics representing the toxicological action(s), rather than simple salt concentrations.
In the present study, we conducted a wide variety of acute lethality tests with C. dubia exposed to binary mixtures of major geochemical ion salts and mannitol. Isobolograms were used to interpret these experiments with regard to different toxicological mechanisms and other interactions among major ions. Better defining these interactions will improve the foundation for assessing the toxicity of ion mixtures expected in natural waters.
MATERIALS AND METHODS
Study design
Twenty-nine binary toxicity experiments were conducted, consisting of 209 individual toxicity tests. These included 18 of the possible 28 combinations of Na, Mg, and Ca salts (Table 1) (excluding CaCO3, for which solubility issues precluded any meaningful tests). For salt pairs sharing the same cation but having different anions, all possible combinations (7) were tested. For Na/Ca salt combinations, 3 of the 6 possible combinations were tested, these being selected based on Ca solubility considerations. For Mg/Ca salt combinations, 3 of the 6 combinations were also tested, again based on Ca solubility considerations. For Na/Mg salt combinations, 5 of the possible 9 combinations were tested, including all 3 combinations with a shared anion and 2 with mixed anions; testing more combinations was impractical and considered unnecessary, as well as entailing more Ca precipitation issues.
Table 1.
Combinations of the major geochemical salts and mannitol tested in binary toxicity experiments. Letters denote groups of experiments – “A” for salts with same cation, “B” for Na/Mg salts, “C” for Ca/Na salts, “D” for Ca/Mg salts, “E” for KCl with other chloride salts, and “F” for mannitol with chloride salts. “Ca” denotes combination also tested at elevated Ca. Shaded blocks denote untested chemical pairs.
| NaCl | Na2SO4 | NaHCO3 | MgCl2 | MgSO4 | MgCO3 | CaCl2 | CaSO4 | |
|---|---|---|---|---|---|---|---|---|
| Na2SO4 | A,Ca | |||||||
| NaHCO3 | A | A | ||||||
| MgCl2 | B,Ca | |||||||
| MgSO4 | B | B,Ca | A,Ca | |||||
| MgCO3 | B | B | A | A | ||||
| CaCl2 | C | C | D | D | ||||
| CaSO4 | C | D | A | |||||
| KCl | E | E | E | |||||
| Mannitol | F | F | F |
All these combinations were tested in amended Lake Superior water (ALSW) [1] and 4 of them were also conducted in ALSW with additional Ca (Table 1) to test whether joint toxicity relationships were consistent across a factor known to affect the toxicity of Mg and Na salts. The Na2SO4/MgSO4 combination was tested twice to test replicability of the observed joint toxicity pattern. Because K should rarely be a major factor in ion toxicity, testing was limited to mixtures of KCl with NaCl, MgCl2, and CaCl2 (Table 1) to provide a basic evaluation of the independence of K toxicity from these other cations. The final 3 experiments (Table 1) addressed mixtures of mannitol with NaCl, CaCl2, and MgCl2 to test the hypothesis [1] that osmolarity is a primary determinant for Na salt toxicity and to test the independence of Ca and Mg toxicities from that of mannitol.
Twenty-six of these binary toxicity experiments followed a design in which 7–8 separate toxicity tests were simultaneously conducted, with each test being assigned a concentration ratio for the 2 salts that remained fixed across all concentrations within the test. These ratios were based on the relative toxicities determined by Mount et al. [1], with the ratio R=TUB:TUA set to 1:0, 5:1, 2:1, 1:1, 1:2, 1:5, and 0:1 for most experiments; these ratios produce evenly-spaced points along a simple concentration addition line and also provide good coverage of a simple independent action isobole. In some experiments, an eighth ratio (1:10) was also used when low concentrations of the B salt (e.g., Ca salts) were known or suspected to have ameliorative effects on the other salt. The remaining 3 experiments involved CaSO4, whose limited solubility required using a different design. Instead of both salt concentrations varying in fixed proportions, these experiments consisted of 7 toxicity tests, each at a fixed CaSO4 concentration ranging from 0 to about 90% of saturation, with only the concentrations of the other salt (CaCl2, NaCl, or MgCl2) varying within the tests.
Test organisms, chemicals, and dilution waters
The dilution water for most tests was ALSW, which was developed by Mount et al. [1] to address concerns about ion ratios in synthetic dilution waters being atypical of natural waters. ALSW is prepared by amending sand filtered and UV-treated Lake Superior water (LSW, obtained from an intake located offshore from our laboratory at 46.840º N, 92.004º W) with major ion salts to provide ion ratios equal to median values for U.S. water, while maintaining its alkalinity and other natural constituents. For the 4 tests at elevated Ca, CaCl2 was added to ALSW to raise the Ca concentration to 2.5 mM. This is 7-fold higher than in ALSW, so as to encompass most of the ameliorative effect of increasing Ca above that in ALSW [1], but represents only 0.15 TU for CaCl2, so that there would be little or no toxicity from Ca.
Test organisms were <24 h old Ceriodaphnia dubia obtained from in-house cultures and cultured in ALSW. General culture procedures followed those described by [7]. The major ion salts listed in Table 1 and mannitol were obtained from Sigma-Aldrich Chemical Company or Fisher Scientific. All chemicals were ACS reagent grade or better, with the exception of MgCO3 which was specified as USP quality. The certificate of analysis for this salt was used to determine the nominal concentration of MgCO3 and also that of Ca added incidentally with the salt.
Toxicity test procedures
Static 48-h toxicity tests were conducted in 30-ml plastic cups (Berry Plastics Corporation) filled with 10 ml test solution and held in polystyrene boards floated in a temperature-controlled water bath with a glass sheet covering all test cups. Each cup received food in the form of 100 μl of a 50:50 mixture of YCT (a mixture of yeast, cereal leaves, and trout chow [7]) and algae (Pseudokirchneriella subcapitata at 3.5×107 cells/ml). Food was added based on previous work indicating that it had minimal effect on toxicity of major ions [2] and to avoid possible stress. Test temperature was 25 ± 1° C with fluorescent lighting on a 16:8 h (light:dark) photoperiod. Five C. dubia neonates were added to each of 2 duplicate cups for each treatment, and survival was determined after 24 and 48 h of exposure; death was defined as no visible movement after gentle prodding and at least 10 s of observation. Temperature, pH, dissolved oxygen, and conductivity were monitored as described by [1].
For each of the several toxicity tests within a binary experiment, test solutions were prepared by dissolving both test salts, in the desired ratio, in the dilution water to achieve a high test mixture concentration with an estimated 2 to 4 total TUs, these total TUs depending on the expected type of joint toxicity relationship. For tests with MgCO3, dilution of the salt was CO2-assisted as described in [1]. The high test mixture was then diluted with the dilution water to produce 9 test concentrations ranging from 16% to 100% strength with a 0.8 dilution factor. For tests with CaSO4, a near-saturated solution (≈90%) was first produced by adding an excess of CaSO4·2H2O to ALSW, aerating for 3 hours, allowing the solution to settle, and decanting the overlying water, resulting in a CaSO4 solution of about 15 mM. This solution was mixed with ALSW to produce 7 dilution waters with a range of CaSO4 concentrations. For each of these dilution waters, the other test salt (NaCl, MgCl2, or CaCl2) was added to create a high test solution, which was then mixed with the dilution water to produce the desired exposure concentration series.
Exposure monitoring
For analysis of the major ions, analytical samples were collected at the start of exposure from the dilution water(s) and from the highest concentration of each exposure series. In addition, analytical samples were collected from each exposure series at 24 h in the lowest concentration with 100% mortality, and at 48 h from the concentration nearest the LC50. Every dilution water was analyzed for all 4 cations plus Cl and SO4. The other samples from the exposure series were analyzed only for the test salt cations because cation analysis was more time-efficient than anion analysis. For tests involving NaHCO3 and MgCO3, which caused oversaturation of CaCO3, Ca concentrations were also measured.
For cation analysis in early experiments, samples were filtered (0.45 μm nylon syringe filter, Grainger) only for tests involving NaHCO3 and MgCO3, and unfiltered otherwise because comparisons in [1] showed no difference between total and dissolved cations; however, in later experiments all samples were filtered to provide more consistency. Samples for cation analysis were acidified by adding 0.2% (v/v) concentrated HNO3 and held at room temperature; for tests with NaHCO3 and MgCO3, this acid addition was increased by an amount calculated to neutralize the extra alkalinity. Samples for Cl and SO4 analysis were filtered (0.45 μm) into a polypropylene centrifuge tube and refrigerated. Quantifications were by flame atomic absorption spectrophotometry and ion chromatography for cations and anions, respectively, using methods and equipment described in [1].
Across all test solutions (i.e., samples other than controls), the ratio of measured to nominal concentrations had a mean (standard deviation, 10th-90th percentile) of 101% (5%, 96–108%) for K, 100% (4%, 96–105%) for Na, 99% (4%, 95–104%) for Ca, and 99% (6%, 95–105%) for Mg. Across 1001 comparisons, only 1.9% of the measurements deviated by more than 10% from nominal, with a maximum deviation of 15%. Conductivity measurements on all treatments confirmed that the intended gradient of exposure existed among samples not sampled for cation analysis. Based on these results, and consistent with [1], nominal concentrations of the added salts were used for calculating LC50 values and for characterizing solution chemistry, except for tests involving CaSO4, for which nominal values did not exist and concentrations were based on measurements of the 100% solutions. Ca measurements were also used where CaCO3 precipitation was indicated in tests involving NaHCO3; in those cases, the measured Ca at 24 and 48 hours were used to estimate a Ca concentration to associate with the mixture LC50 per the procedures described in [1].
Data analysis
Median lethal concentrations and their confidence limits were estimated as described in [1], except that these were calculated as a percentage of the highest mixture exposure for the test, and then converted to concentrations of the added salts. These concentrations were then added to the dilution water chemistry and the resultant concentrations for all major ions were subject to chemical speciation/ion activity and osmolarity calculations as also described in [1]. Confidence limits for LC50s expressed as individual salt concentration, chemical activities, or osmolarities were assigned the same relative values as those for the percent test mixture, and do not reflect any additional uncertainty associated with the speciation calculations.
Isobole plots were examined based both on total added salt concentrations and on the exposure metrics suggested by Mount et al. [1] (i.e., osmolarity for Na salts and mannitol, and cation activities for the other salts). For isobolograms of salts with a shared cation, the cation activity at the LC50 was partitioned between the salts in proportion to the contribution of each salt to the total cation concentration. Osmolarities for tests with 2 Na salts were similarly partitioned based on the contribution of each salt to the nominal (ideal) osmolarity.
Where appropriate, isoboles were drawn to assess the adherence of the interactions to simple concentration addition or simple independent action. For salt combinations that shared a cation and for the NaCl/mannitol mixture, a straight isobole line (simple concentration addition) was drawn connecting the LC50s for the pure salts. For combinations of Mg salts with Na salts, KCl with MgCl2 or CaCl2, and MgCl2 with mannitol, a simple independent action isobole was based on assigning the values for the ends of the isobole to the average of 2–3 LC50s nearest each axis. The curvature at the corner of this isobole was estimated using the relationships of PA to CA and PB to CB for the individual chemicals to determine CA, CB pairs expected to produce PMixture=0.5 according to the equation PMixture=1-(1-PA)(1-PB) (discussed in the Introduction in regard to Figure 1C). For combinations of Ca salts with Na or Mg salts or mannitol and of KCl with NaCl, isobole lines were not drawn due to the greater complexity of the relationships.
These isoboles are not intended to provide definitive quantitative models for these binary relationships because there typically are additional considerations (e.g., variation in Ca across tests with Na and Mg salts) that could cause some deviations from these simple relationships. Rather, they are intended only to support qualitative, visual evaluation of the adherence of the data to the basic interactions of additivity or independence, based on overlap of LC50 confidence limits with the isoboles. More quantitative analysis of all these interactions will be the subject of a subsequent paper on modeling toxicity using the data from both the present study and [1].
RESULTS AND DISCUSSION
A spreadsheet in the Supplemental Data (Table S1) provides, for each of the 209 tests: the LC50 (with confidence limits) as total added concentrations of each salt, and estimated total component concentrations, ion activities, pH, and osmolarity at the LC50. Dissolved oxygen was always above 7.5 mg/L, and measured temperatures were within 25 ± 1° C. All reported tests had conductivity measurements that varied across treatments in a way consistent with the intended exposure concentrations. Survival in the control treatments averaged 99% and was ≥90% in all but 3 tests, and for those tests there were a sufficient number of low treatments with high survival to define a background survival ≥92%. One test was discarded due to 30% control mortality and even higher mortalities at low mixture concentrations that were greatly inconsistent with other tests. The following material will present these results first as isobolograms for each experiment based on the added concentrations of each salt, and then as isobolograms or alternative formats based on other exposure metrics we previously suggested [1].
Binary mixtures of KCl with MgCl2, CaCl2, and NaCl
Mount et al. [1] concluded that K salt toxicity involved a mechanism different from that for salts of the other cations, based on the greater toxicity of K salts, its dependence on Na, and physiological arguments. The binary experiments in the present study of KCl with MgCl2, CaCl2, and NaCl more definitively established the existence of a K-dependent mechanism (Figure 2). Even though K concentrations in surface waters or effluents rarely will be high enough to be the primary cause of toxicity, this set of experiments is presented first as a useful example of how isobolograms can inform inferences about mechanisms, salt interactions, and exposure metrics.
Figure 2.
Isobolograms for 48-h LC50s from binary mixture experiments of KCl with MgCl2, CaCl2, and NaCl. Upper panels (A-C) provide LC50s on the basis of added salt concentration, while lower panels (D-F) use exposure metrics of osmolarity and K, Mg, and Ca activities. Small x’s denote LC50s for KCl at different NaCl concentrations from Mount et al. [1]. Dashed lines denote approximate isoboles for simple independent action.
The results from the KCl×MgCl2 binary experiment are plotted based on added salt concentration in Figure 2A and on calculated K and Mg activities as exposure metrics in Figure 2D. Both plots adhere closely to the pattern expected for simple independent toxicity (Figure 1C). Not only do these isobolograms demonstrate different mechanisms for KCl and MgCl2, but the mechanism for KCl is clearly dominated by K, as indicated by K remaining constant in the vertical part of the isobolograms despite Cl increasing by about 6-fold as MgCl2 increases (Supplemental Data Table S1).
Figures 2A and 2D also demonstrate the benefit of addressing chemical activity rather than total concentration. In Figure 2A, for the horizontal portion of the isobole, there is a trend of increasing MgCl2 at the LC50 as KCl increases, and the 1:1 mixture also lies outside the isobole. One reason for such trends would be decreased activity coefficients and increased complexation of Mg by Cl as KCl increases; consistent with this, there is improved fit when the isobologram is based on Mg and K activities (Figure 2D). Although the differences between Figure 2A and 2D are small, they illustrate the role that chemical speciation and activity can play in addressing salt interactions, and which can be more substantial for other ion interactions [1].
The results of the KCl×CaCl2 experiment also show a pattern of simple independent toxicity both as added salt concentration (Figure 2B) and as cation activities (Figure 2E), with the use of activities again improving fit. In the vertical part of the isobologram, there is a small trend of decreasing toxicity (increased K at the LC50) as Ca increases. This is more pronounced for the added salt plot, but is still present in the cation activity plot. This might indicate some amelioration of K toxicity by Ca, although far less than for Na and Mg salts (see Mount et al. [1] and later sections on Binary mixtures of Mg and Ca salts and Binary mixtures of Na and Ca salts).
In contrast to the simple patterns for KCl×MgCl2 and KCl×CaCl2, the results of the KCl×NaCl experiment show a complicated pattern when expressed as added salt concentration (Figure 2C). At lower NaCl concentrations, K concentrations at the LC50s were expected to vary because Mount et al. [1] demonstrated that Na had an ameliorative effect on K toxicity; the data from [1] for KCl LC50s at different NaCl additions are included in Figure 2C to better illustrate this aspect of the isobole. However, at higher NaCl concentrations, there is also complexity in that the added NaCl concentrations at the LC50s decrease as KCl concentrations increase. This would appear to be contrary to the notion that KCl acts by a mechanism independent of NaCl.
However, this apparent contradiction can be explained when the isobologram is reformulated based on the exposure metrics of osmolarity and K activity rather than added salt concentrations (Figure 2F). As reported previously [1], for acute toxicity to C. dubia, Na salts appear to act by a mechanism involving a non-specific, aggregate effect of all ions which correlates well with osmolarity. Because both KCl and NaCl would contribute to osmolarity, less added NaCl is needed to reach the toxic osmolarity as KCl concentrations increase. The reformulated isobologram in Figure 2F accounts for KCl contributing both to this non-specific toxicity and to K-specific toxicity, and shows the pattern in Figure 1D of independent action with an ameliorative effect – a virtually flat upper portion of constant osmolarity that intersects the rising function for the ameliorative effect of Na on K toxicity. Our previous work inferred the non-specific mechanism based only on the toxicity of individual sodium salts; the results of this NaCl×KCl binary experiment show that an ion other than Na+1, Cl−1, SO4−2 and HCO3−1 also can contribute, in addition to any more specific action it might have.
Figure 2F also further establishes important limitations regarding the contribution of K to major ion toxicity in field exposures. As previously noted in [1], the high K toxicity to C. dubia in typical laboratory test waters depends on Na concentrations much lower than K and much lower than would occur in realistic exposures. Figure 2F further demonstrates that any K-specific toxicity depends on Na concentrations being near or below the K concentrations, which would also be very unusual.
Binary mixtures of Na and Mg salts
Binary experiments with several combinations of Na salts with Mg salts also suggested independent action by 2 different mechanisms (Figure 3), but interpretation of these data depends on how exposure is expressed. When LC50s are expressed based on added salt (Figures 3A-C), there are some consistent deviations from the simple independent action isoboles fitted to these data. First, for all 7 data sets, the LC50 for 1 or 2 mixtures most dominated by Mg have an added Mg salt concentration greater than the LC50 of the pure Mg salt (i.e., mixture points on Figure 3A-C higher than the point for the pure Mg salt). Second, for 6 of the data sets, the LC50 for 1 or 2 mixtures most dominated by Na have an added Na salt concentration greater than the LC50 of the pure Na salt (i.e., mixture points lying to the right of the point for the pure Na salt). Third, except for this rightward deviation of mixture points at low Mg salt additions, the NaCl concentrations for the mixture LC50s consistently decline with increasing Mg salt concentration (i.e., mixture points cutting diagonally across the isobole rather than following it).
Figure 3.
Isobolograms for 48-h LC50s from binary mixture experiments of Na and Mg salts. Upper panels (A-C) provide LC50s on the basis of added salt concentration, while lower panels (D-F) use exposure metrics of osmolarity and Mg activity. Dashed lines denote approximate isoboles for simple independent action. Primary colors denote different anions (yellow=Cl, red=SO4, blue=HCO3/O3), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
Basing the isobolograms on the exposure metrics of osmolarity and magnesium activity (Figures 3D-F) reduces some of these deviations. Magnesium activities for mixture LC50s do not lie above that for the pure Mg salt; this is attributable to the fact that added Na salt reduces activity coefficients and increases Mg complexation, thereby requiring higher total Mg concentrations to reach the same Mg activity. Using osmolarity rather than Na salt concentration eliminates or reduces the diagonal “cut” across the isobole; this is because this exposure metric accounts for both the Mg salt and the Na salt contributing to osmolarity and thus to the nonspecific ion toxicity. This adds further to the evidence from Figure 2F that cations other than Na contribute to non-specific ion toxicity, and that interpreting major ion toxicity requires basing it on more appropriate exposure metrics than added salt concentrations.
However, reformulating the isobolograms based on osmolarity and activities does not explain the rightward deviations of points in the vertical portion of the isobole; in fact, these deviations are actually greater when based on osmolarity, and all 7 sets now show them (Figures 3D-F). This might reflect a weak ameliorative effect of Mg on Na salt toxicity analogous to the Ca effect, although the magnitude of the effect is less than that caused by Ca (see [1] and the later section Binary mixtures of Na and Ca salts). More generally, it should be noted that the single salt tests in these binary experiments represent rather extreme ion compositions, in which several ions are at unusually low concentrations relative to those of the salt being tested. That such ion imbalances might contribute to observed ion toxicities has been suggested by other investigators [8]. Nevertheless, our overall conclusion is that these binary tests with Na salts versus Mg salts demonstrate 2 independent mechanisms of action – one a Mg-specific toxicity and the other a non-specific ion toxicity to which all the ions contribute – that are well described by the exposure metrics of Mg activity and osmolarity.
The NaHCO3×MgCO3 experiment was not included in Figure 3, but rather is separately provided in Figure 4, because Ca complexation and precipitation created a nearly 5-fold variation in Ca activity, resulting in a markedly different isobole. On an added salt basis, Figure 4A actually shows a stronger pattern of independent action than the Na×Mg salt combinations in Figure 3; however, when the isobologram is based on osmolarity and Mg activity, the isobole in Figure 4B deviates substantially from the simple independent toxicity shape, unlike Figures 3D-F, with a substantial decline in the Mg activity associated with the LC50 as osmolarity increases. This trend is correlated with declining Ca activities, which are due to increasing complexation by HCO3−1/CO3−2 and by CaCO3 precipitation. Further interpretation of these data requires modeling the Ca effect and will be provided in our subsequent modeling paper.
Figure 4.
Isobolograms for 48-h LC50s from binary mixture experiment of NaHCO3 and MgCO3. Upper panel (A) provides LC50s on the basis of added salt concentration, while lower panel (B) uses exposure metrics of osmolarity and Mg activity. Numbers adjacent to points on panel B denote Ca activity in mM.
Binary mixtures of Ca salts
Although Mount et al. [1] found that CaSO4 was not acutely toxic to C. dubia at saturation, its interaction with CaCl2 in the present study was still informative (Figure 5). Expressed as concentration of added salt (Figure 5A), CaCl2 concentrations at the mixture LC50 decreased linearly as CaSO4 concentration increased, indicating simple concentration addition. If the linear isobole based on these data is extrapolated to the y-axis (dashed line), it suggests that the LC50 for CaSO4 would be about 37 mM (more than 2-fold that of CaCl2) if the solubility constraint did not preclude testing at this concentration.
Figure 5.
Results of binary mixture experiment with CaCl2 and CaSO4. Upper panel (A) is isobologram for LC50s on the basis of added salt concentration and dashed line is isobole for simple concentration addition. Lower panel (B) shows 48-h LC50s on the basis of cation activity as a function of the amount of CaSO4 in the mixture and also includes results for CaCl2 and Ca gluconate toxicity in different dilution waters from [1]. Primary colors denote different anions (yellow=Cl, red=SO4), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
Although the results in Figure 5A indicate additive toxicity for CaCl2 and CaSO4, they still leave open the question of which ions are responsible for the toxicity, because plotting various combinations of ion concentrations or activities (e.g., Cl vs SO4; Ca activity contributed by CaCl2 versus that contributed by CaSO4) also produce linear relationships (not shown). We speculated in our previous work [1] that Ca was the primary or sole source of toxicity for CaCl2, based on it occurring at lower osmolarity than for Na salts, and this is further supported by the present study. For the tests in Figure 5, the osmolarities at the LC50 range from 44–48 mOsm/L, compared to >80 mOsm/L for Na salts tested at higher Ca (Supplemental Data Table S1). In addition, although KCl apparently contributes to osmolarity-related toxicity in its interactions with NaCl (Figure 2C), its toxicity is independent of CaCl2 (Figure 2B), further indicating a Caspecific mechanism.
Even stronger evidence for Ca being the primary source of toxicity is that its activity at the LC50 is essentially constant across the different mixtures (Figure 5B) and for the LC50s determined for Ca-gluconate and for CaCl2 in various dilution waters from [1], which are also included on Figure 5B. A requirement for any exposure metric representing the proximate stress causing toxicity is that it be constant across different tests or be a consistent function of a factor known to affect toxicity. While this standard is met by Ca, for it to be met jointly by Cl and SO4 would require these anions to (a) have specific toxicities in addition to and greater than that manifested in the Na salt toxicity tests, (b) share the same specific action or otherwise act additively despite markedly different chemical properties, and (c) coincidentally result in constant Ca activities across widely variant anion ratios.
Binary mixtures of Na salts
Binary toxicity experiments with all combinations of the 3 Na salts were conducted in ALSW, as well as an experiment with NaCl×Na2SO4 at elevated Ca (Figure 6). In all cases, isobolograms based on added salt concentrations (Figures 6A-C) are close to the linear relationship indicative of simple concentration addition. Binary plots using Na activity and osmolarity are similarly linear (not shown), but neither Na activity nor osmolarity show a constant value across all experiments, as was the case in Figure 5B for Ca activity. This lack of a constant value is expected because of the ameliorative effect of Ca [1] on Na salt toxicity and the fact that Ca concentration and activity vary by more than 10-fold (Supplemental Data Table S1) across the tests included in Figure 6.
Figure 6.
Results of binary mixture experiments with different pairs of Na salts. Upper panels (A-C) are isobolograms for LC50s on the basis of added salt concentration and dashed lines are isoboles for simple concentration addition. Lower panels (D-F) show 48-h LC50s as a function of calcium on the basis of different exposure metrics. In panel F, the small squares are data from [1] for tests with single Na salts in different dilution waters. Primary colors denote different anions (yellow=Cl, red=SO4, blue=HCO3/CO3), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
To better evaluate the underlying interactions, the data in Figures 6A-C are replotted with alternative metrics in Figures 6D-F. Figure 6D simply plots the sum of the added salt concentrations versus Ca concentration. Although the effect of Ca is evident across tests with low and high Ca, there is considerable variation in the LC50 at a given Ca concentration that is correlated with the anion ratios, reaffirming that added salt concentration is not a good exposure metric to address toxicity variation across these mixtures.
Figure 6E replots the data as Na activity vs Ca activity. This also produces a poor relationship, leaving considerable vertical spread in the data, as well as a trend within both NaCl×Na2SO4 experiments of LC50s decreasing with increasing Ca activity, contrary to the LC50s increasing between these experiments. This supports our earlier findings [1] that using Na activity as the basis for the LC50 did not account well for variations across the salts.
Figure 6F aggregates Na and anion effects through osmolarity, which greatly reduces the vertical spread within the NaCl×Na2SO4 experiments, and provides a consistent relationship across all experiments as a function of Ca activity. There still is significant vertical spread for some of the Na2SO4×NaHCO3 LC50s, which might reflect inaccuracies in estimating Ca concentrations or chemical activities, or effects of pH and CO3/HCO3 beyond their influence on calculated activity. The single Na salt LC50s from our earlier work [1] are also plotted on Figure 6F (small squares) and overlay the present data quite well. That the vertical data spread is little greater than the confidence limits on the LC50s argues for the utility of osmolarity as an effective exposure metric, although this does not prove that osmotic stress is the sole and proximate cause of toxicity.
Binary mixtures of Mg salts
Binary toxicity experiments with all combinations of the 3 Mg salts were conducted in ALSW, as well an experiment with MgCl2×MgSO4 at elevated Ca (Figure 7). In all cases, isobolograms based on added salt concentrations are close to the linear relationship indicative of simple concentration addition (Figures 7A-C). Binary plots using Mg activity are similarly linear (not shown), but do not show constant Mg activity values, unlike the constant Ca activity in Figure 5B. As was the case for the Na salt mixtures in Figure 6, this lack of a constant Mg activity is expected because of the ameliorative effect of Ca [1] on Mg salt toxicity and the fact that Ca concentrations and activities vary substantially (Supplemental Data Tables S1) across the tests included in Figure 7.
Figure 7.
Results of binary mixture experiments with different pairs of Mg salts. Upper panels (A-C) are isobolograms for LC50s on the basis of added salt concentration and dashed lines are isoboles for simple concentration addition. Lower panels (D-E) show 48-h LC50s as a function of calcium on the basis of different exposure metrics. In panel E, the small squares are data from [1] for tests with single Mg salts in different dilution waters. Primary colors denote different anions (yellow=Cl, red=SO4, blue=HCO3/CO3), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
As was done for the Na salt mixtures in Figure 6, the aggregated data from all the Mg salt mixture tests are replotted versus Ca in Figures 7D and 7E. Figure 7D shows LC50s based on the total added concentrations of all salts versus Ca concentration. The ameliorative effect of Ca is evident in comparing the MgCl2×MgSO4 experiment at elevated Ca to the other mixture experiments; however, all the experiments show substantial and systematic LC50 trends across their mixture ratios, with higher LC50s for SO4-dominated mixtures and lower LC50s for CO3-dominated mixtures, indicating that total concentration is not a good exposure metric. When plotted as Mg activity vs Ca activity (Figure 7E), the vertical data spreads for the MgCl2×MgSO4 experiments are reduced, because the greater complexation of Mg by SO4 than by Cl causes the Mg activity to be proportionately lower in the MgSO4-dominated tests. However, the data collectively still show slightly higher LC50s for MgSO4, and lower LC50s for MgCO3, than for MgCl2; these residual trends may relate to uncertainties in characterizing the chemistry of these tests, or to some other factor affecting toxicity (e.g., the high pH in the MgCO3-dominated tests). Nevertheless, when data from the present study are combined with data from our earlier study [1] (small squares in Figure 7E), there is a clear and consistent relationship of Mg salt toxicity to Ca with residual variability generally within 1.5-fold of the mean at any Ca activity.
Binary mixtures of Mg and Ca salts
When expressed on an added salt basis, LC50s for the experiments combining Mg and Ca salts show large variation (Figure 8A); shapes and slopes differ greatly among the 3 salt pairs and LC50s vary by as much as 3-fold at a given Ca concentration. The mixture series including SO4 falls above and to the right of the MgCl2 versus CaCl2 series, consistent with the greater complexation of Ca and Mg by SO4 than by Cl. However, when the isobologram is based on Mg and Ca activities (Figure 8B), a common relationship is indicated, with confidence limits overlapping except for a single data point for the MgSO4×CaCl2 experiment, which is still within a factor of 1.5 of the other experiments.
Figure 8.
Isobolograms for 48-h LC50s from binary mixture experiments of Mg and Ca salts. Upper panel (A) provides LC50s on the basis of added salt concentration, while lower panel (B) uses exposure metrics of Mg and Ca activities. Primary colors denote different anions (yellow=Cl, red=SO4), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
The activity-based curve in Figure 8B is similar to the theoretical plot for “similar action with an ameliorative interaction present” shown in Figure 1B. At low Ca, there is the ameliorative effect of Ca on Mg toxicity, but when Ca activity exceeds about 0.25 TU, the LC50 on the basis of Mg activity starts decreasing and there is an extended, approximately linear, region suggestive of additive toxicity between these cations. Extrapolating this linear portion to the Mg activity axis (in accord with the dotted line in Figure 1B) would suggest that the LC50 for Mg activity at high Ca is approximately 12 mM. However, as noted in the discussion of Figure 1 in the Introduction, inferences about additivity and the ameliorative effect in such a case are difficult since the ameliorative effect is not directly observable in the concentration ranges with apparent additivity.
Binary mixtures of Na and Ca salts
When expressed on an added salt basis, LC50s for the experiments combining Na and Ca salts also show large variation among the 3 salt pairs (Figure 9A). However, when the isobologram is based on osmolarity and Ca activities (Figure 9B), a common relationship again emerges, and is even more consistent than was the case for Mg vs. Ca salts, with all the data having closely overlapping confidence intervals.
Figure 9.
Isobolograms for 48-h LC50s from binary mixture experiments of Na and Ca salts. Upper panel (A) provides LC50s on the basis of added salt concentration, while lower panel (B) uses exposure metrics of osmolarity and Ca activity. Primary colors denote different anions (yellow=Cl, red=SO4), and intermediate colors denote graded mixtures of anions (indicated by arrows on legend).
While this common relationship is very compelling and will be useful for risk assessments, the interpretation of it in terms of similar vs dissimilar action is not clear. It does not show the pattern of dissimilar action with ameliorative interaction illustrated in the theoretical plot in Figure 1D and demonstrated for osmolarity vs. K activity in Figure 2F, as it lacks a horizontal plateau. It more resembles similar action with ameliorative interaction (Figure 1B and 8B), but this interpretation also poses some problems. First, it would require an explanation of why Ca is additive with both Mg and Na, but Mg is clearly independent of Na. Second, and more important, the expected additive contribution of the Ca salts to osmolarity is already accounted in Figure 9B and for this isobologram to represent additivity requires a second, additive contribution of Ca to the overall toxicity. Although the simple interactions illustrated in Figure 1 seem sufficient for the other salt interactions, these Na vs Ca salt interactions suggest more complexity. Given the multiple roles of both Na and Ca in ionoregulation [9], the possibility of complicated responses to these ions shouldn’t be surprising; in fact, the surprise might be that the relatively simple relationships described in Figure 1 are effective in describing so many of the mixtures tested. In the end, it must be realized that any mechanisms inferred from these data are approximations and that any mathematical relationships developed from these data will be empirical to some degree.
Binary mixtures of mannitol with NaCl, MgCl2, and CaCl2
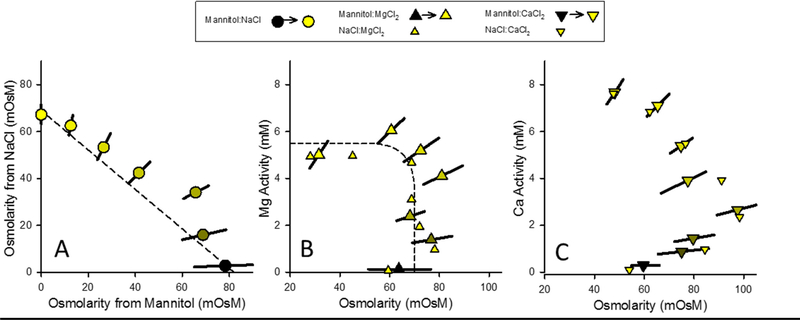
In our previous work [1], mannitol was used as a means to increase external osmolarity without adding any of the major ions under study, and was shown to have the same toxicity as Na salts when LC50s are based on osmolarity. Binary mixtures of mannitol with NaCl, MgCl2, and CaCl2 were tested in the present study to further evaluate the similarity of action of mannitol and NaCl and an expected dissimilarity of action between mannitol and MgCl2 or CaCl2 (Figure 10).
Figure 10.
Isobolograms for 48-h LC50s from binary mixture experiments of mannitol with NaCl, MgCl2, and CaCl2 on basis of exposure metrics of osmolarity, Mg activity, and Ca activity. Small triangles provide LC50s for NaCl×MgCl2and NaCl×CaCl2 experiments for comparison to relationships with mannitol. Dashed lines denote approximate isoboles for simple concentration addition (panel A) and simple independent action (panel B). Gradation from black to yellow indicates gradation of mixtures from mannitol to pure Cl salt.
For mannitol×NaCl, the isobole (Figure 10A) shows approximate linearity, although one point significantly deviates from the line. We have no definitive explanation for this deviation, but it is not greater than some of the deviations from linearity observed for other experiments (e.g., Figure 7B) and is relatively small (1.3 TU based on additive toxicity of the pure compounds), so might just reflect test uncertainty. However, because mannitol and NaCl would differ somewhat in their effects on ion exchange, their specific toxicological impacts could also differ somewhat, even if their toxicities are both largely driven by osmolarity. In fact, the slopes of the concentration-effect relationships for mannitol, although steep, are less so than those for NaCl, suggesting some such differences (for 3 mannitol-only tests, the log standard deviation of the estimated tolerance distribution ranged from 0.06 to 0.22, averaging 0.13, whereas for 10 NaCl-only tests, it ranged from <0.03 to 0.07, with the “less-than” values indicating a very steep slope for which a point estimate for the standard deviation could not be estimated). Nonetheless, the close agreement between NaCl and mannitol toxicities on the basis of osmolarity is notable; even the minor difference in Figure 10A between the osmolarities at the x-intercept (78 mOsm/L) and y-intercept (67 mOsm/L) is consistent with differences in Ca activities, which were estimated to be 0.17 mM for the NaCl-only test and 0.28 mM for the mannitol-only test (Supplemental Data Table S1).
The similarities between NaCl and mannitol toxicities are even more evident in their interactions with MgCl2 and CaCl2. For mannitol×MgCl2 (Figure 10B), LC50s expressed as Mg activity vs osmolarity show a pattern of independent action very close to NaCl×MgCl2. Even more notable is the similarity of mannitol×CaCl2 (Figure 10C) to NaCl×CaCl2, where the data track each other very well, with the same ameliorative effect of Ca and the same shape at high Ca activity as discussed regarding Figure 9. Overall, the data in Figure 10 indicate a very similar toxicological action of NaCl and mannitol for acute toxicity to C. dubia, and support the interpretation that there is a non-specific ion toxicity that is closely correlated to osmolarity.
SUMMARY AND IMPLICATIONS
Addressing the toxicity of the major geochemical ions considered in the present study involves 7 separate toxicants, multiple mechanisms of action, diverse interactions among the ions, and the need to address ion mixtures with widely varying compositions. Previous work regarding the acute toxicity of single salts of these ions to C. dubia ([1] and references therein) quantified some noteworthy interactions, such as the dependence of the toxicities of Na and Mg salts on Ca concentrations and of the toxicities of K salts on Na concentration. Soucek et al. [3] demonstrated some degree of additive toxicity for NaCl and Na2SO4. Mount et al. [2] provided evidence for both additive and non-additive interactions for different salt combinations; consequently, although their model for mixture toxicity was premised on additive toxicity, it included an interaction term to reflect non-additive interactions between different cations. Our previous work [1] also provided evidence of additivity among ions as part of an inferred nonspecific ion toxicity. By evaluating interactions of pairs of salts in mixture studies, the present study has substantiated these mechanisms and better defined these relationships, providing a good foundation for subsequent work on risk assessment methods for ion mixtures, as follows.
Non-specific ion toxicity
A mechanism of non-specific ion toxicity to which all ions additively contribute and which is correlated to osmolarity is supported by the results of several of the experiments in the present study. LC50s from binary mixture tests with different Na salts show simple concentration addition. Adjusted for its dependence on Ca concentration, this toxicity is much better correlated with osmolarity (or other measures of total ion concentration) than with any specific ion(s).
By looking at the interactions of Na salts with the salts of other cations, it was also demonstrated that these other cations can contribute to this non-specific ion toxicity when they are at concentrations below that needed to induce cation-specific toxicity. Non-specific ion toxicity related to osmolarity was especially supported by 1) a mixture experiment of NaCl and mannitol, which showed approximate additivity and consistent osmolarity-based LC50s, and 2) mixture experiments of both NaCl and mannitol with both MgCl2 and CaCl2, which showed virtually identical isobolograms when osmolarity was used as an exposure metric.
K-specific toxicity
The existence of K-specific toxicity independent of other ions was clearly demonstrated in binary mixture experiments of KCl with the Cl salts of the other cations. For the experiments with MgCl2 and CaCl2, the isobolograms showed simple independent action, especially when cation activities were used for the exposure metrics. There was a small reduction of KCl toxicity from moderate increases of Ca above that in our standard dilution water; although of limited practical consequence, this emphasizes how care is needed in applying the results of single salt tests to more complex mixtures in which other ions are unlikely to be at low background values.
The need for such care was especially evident in the KCl×NaCl mixture experiment. This mixture experiment showed a complex isobologram because of both 1) an ameliorative effect of Na on the K-specific toxicity and 2) the contribution of KCl to osmolarity, and thus to the nonspecific ion toxicity as well as K-specific toxicity. As such, even for field situations with high K concentrations that might appear toxic based on toxicity in typical laboratory waters, K-specific toxicity might not occur because of sufficiently high concentrations of Na and other ions. It is thus important to not just consider the toxicity of a salt per se, but its contributions to multiple mechanisms in the context of more complicated and field-relevant mixtures.
Mg-specific toxicity
Binary mixture experiments involving Mg salts further demonstrated the existence of Mg-specific toxicity inferred in [1]. Experiments with different Mg salts showed simple additive toxicity that was best correlated with Mg activity, and had little or no dependence on anions. As noted above regarding K-specific toxicity, the KCl×MgCl2 experiment showed clear independence of the cations. Experiments with Mg and Na salts also showed independent action, provided that the contribution of the Mg salt to general ion toxicity was accounted for by using osmolarity as one of the exposure metrics in the isobologram, and that Mg activity was used as the other metric.
The binary experiments with Mg and Na salts also showed some ameliorative effect on Na salt toxicity from moderate increases of Mg above our standard dilution water. Although minor, these effects further underscore the need to consider uncertainties that arise in applying salt toxicities determined in dilution waters in which other ions are low, to more complex and typical ion mixtures.
Ca-specific toxicity
Previous work with CaCl2 toxicity [1] suggested a Ca-specific toxicity, and results from the present study support this conclusion. The CaCl2×CaSO4 experiment showed additive toxicity with constant LC50s based on Ca activity, which also matched LC50s based on Caactivity from [1] for Ca gluconate and CaCl2 in various dilution waters. As noted above regarding K-specific toxicity, the KCl×CaCl2 mixture experiment clearly showed independent action of K and Ca. However, the mixture experiments of Ca and Mg salts suggested additive toxicity between Ca and Mg in addition to the ameliorative effect of Ca on Mg toxicity at lower Ca concentrations; thus, the Ca-specific and Mg-specific toxicities might reflect a shared mechanism.
When LC50s are expressed on the basis of osmolarity and Ca concentrations, the mixture experiments with Na and Ca salts showed a consistent isobologram that was independent of the anions used. However, interaction based on these exposure metrics was not independent, but also not additive, and suggests more complex relationships than the present study could resolve. Nonetheless, it provides an excellent and relatively simple empirical relationship that can form the basis for developing and evaluating risk assessment methodologies.
Practical implications and future work
The mechanism-related exposure metrics and other interactions defined in the present study and our previous work [1] provide a good foundation for formulating relationships for describing the acute toxicity of ion mixtures to C. dubia. The data in Figures 7E and 8B would support relationships for assessing what combinations of Mg and Ca concentrations would cause toxicity, independent of other mechanisms. Similarly, the data in Figures 6F and 9B would support evaluating the toxicity of total ion concentration as a function of Ca concentration, again independent of other mechanisms. Combined, such relationships could be used to assess the acute toxicity of any ion mixture to C. dubia. Using the metrics of osmolarity and cation activities entails some complicated calculations, but simpler relationships based directly on total concentrations could be evaluated regarding how well they approximate, over a given scope of applicability, the more rigorous relationships.
A subsequent article will describe the formulation of such relationships and evaluation of their efficacy for describing toxicity in complex ion mixtures patterned after actual field exposures, including a comparison to the model of Mount et al. [2]. It should be emphasized that this will pertain just to acute toxicity to C. dubia. Ongoing work by us and others will extend these efforts to other species and endpoints.
Supplementary Material
Acknowledgements:
S. Wisniewski and T.D. Dawson supervised the culture of test organisms used in this research and provided additional input to the experiments. J. Nichols provided a very useful review of this manuscript.
Footnotes
Disclaimer: The views expressed in the present article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Data availability: Data are available through the U.S. Environmental Protection Agency Environmental Dataset Gateway (https://edg.epa.gov/metadata/catalog/main/home.page) or by request from the corresponding author (erickson.russell@epa.gov).
SUPPLEMENTAL DATA
Table S-1. 48-h median lethal added salt concentrations (LC50, with 95% confidence limits) and estimated ion concentrations, ion activities, pH, and osmolarities at the LC50 for exposures of Ceriodaphnia dubia to various binary salt mixtures.
REFERENCES
- 1.Mount DR, Erickson RJ, Highland TL, Hockett JR, Hoff DJ, Jenson CT, Norberg-King TJ, Peterson KN, Wisniewski S. 2016. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environ Toxicol Chem doi: 10.1002/etc.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ Toxicol Chem 16:2009–2019. [Google Scholar]
- 3.Soucek DJ. 2007. Comparison of hardness‐and chloride‐regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ Toxicol Chem 26:773–779. [DOI] [PubMed] [Google Scholar]
- 4.Plackett RL, Hewlett PS. 1952. Quantal responses to mixtures of poisons. J Royal Stat Soc B 14:141–163. [Google Scholar]
- 5.Anderson PD, Weber LJ. 1975. The toxicity to aquatic populations of mixtures containing certain heavy metals. pp. 933–953. In: Proceedings of the International Conference on Heavy Metals in the Environment Institute of Environmental Studies, University of Toronto, Toronto, Canada. [Google Scholar]
- 6.Könemann H 1981. Fish toxicity tests with mixtures of more than two chemicals: A proposal for a quantitative approach and experimental results. Toxicol 19:229–238. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Environmental Protection Agency. 2002. Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms. Fourth ed. EPA/821/R-02/013. Washington, DC. [Google Scholar]
- 8.Meyer JS, Rogers SI. 2016. Chronic toxicity of a high-TDS effluent to Ceriodaphnia dubia:TDS, conductivity, ion concentrations, or ion ratios. Presentation, 4th Aquatic Toxicology Symposium, Bar Harbor, ME, USA. [Google Scholar]
- 9.Bradley T 2009. Animal Osmoregulation. Oxford University Press, New York, NY, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.