Abstract
Purpose of Review
Stem cells have been proposed as sources for tissue replacement when healing does not occur. These cells could contribute directly to skin structures via differentiation, or via producing trophic factors that would ‘educate’ the micro-environment to encourage tissue repair. Studies in animals have supported both mechanisms, but translation to humans has been challenged by poor cell survival after transplantation. However, the improvement noted with even transient existence suggests another new possibility, that of suppressing the inflammatory response that limits regenerative healing. Herein, we will propose that this immunomodulatory aspect holds promise for promoting skin healing.
Recent Findings
We have found that stem cell transplantation into wounds can dampen both acute and chronic inflammation, leading to more regenerative-like healing and diminished scarring.
Summary
Wound healing could be improved by dampening inflammation both initially to allow for tissue replacement to proceed and late to reduce scarring.
Keywords: Multipotential stromal cells/mesenchymal stem cells, inflammation, wound healing, wound matrix, immunomodulation
Introduction
Wound healing is at its simplest the replacement of damaged tissue. Repaired tissues approximate many of the functions of pre-wounded tissue, but can possess some degree of functional deficit or scarring in adults; defectiveness is often related to the severity of the injury. For instance, superficial first degree wounds and even small third degree wounds are repaired with minimal or no noticeable defects. However, for larger and more extensive wounds the replacement tissue is functionally limited by significant scarring (1). Impaired healing is exacerbated by comorbidities, such as metabolic syndromes, and nerve (neuropathy) and vascular diseases. Even advanced age can compromise healing to the degree that it may never occur due to a stalled wound response (2).
Non-healing wounds often lack responsive parenchymal cells (mainly, keratinocytes, fibroblasts and endothelial cells) that constitute most of the mature tissue; in these situations, stem cells have been proposed as precursors to, or stimulators of cellular replacement (3). In animal models, resident and transplanted stem cells appear to contribute to the skin wound healing by differentiating into the parenchymal subtypes. However, the situation in humans is more nuanced and complex, despite the presence of tissue-resident stem cells in many human organs (4). Stem cells and primary cells show limited survival when engrafted onto human wounds. The cell lifespan can be extended by embedding cells in engineered skin substitutes (ESS). This appears to provide sufficient time to enable replacement by endogenous cells and tissue (such as in burns). However, in the absence of replacement by endogenous cells (such as chronic wounds), the eventual failure of the cells to survive in the ESS is limiting.
Interestingly, the one area that has been relatively understudied in stem cell contributions to skin wound healing is the one aspect for which stem cells are routinely used clinically (albeit in different clinical indications), that of dampening inflammation. In both excessive scarring and deficient wound closure, one notes a continued innate inflammatory response that further aggravates the tissue. Additionally, both acute and chronic inflammation impede wound healing; a chronic inflammatory milieu will drive hypertrophic scarring and suboptimal repair despite closure. Eliminating or suppressing microbial overgrowth does not revert this non-healing towards successful closure until the inflammation is also eliminated. Herein, we propose that this aspect of stem cell function warrants trials for treating non-healing wounds.
Wound Healing viewed through Inflammation
Normal wound healing proceeds through phases that involve inflammatory milieus. In the immediate aftermath of excisional skin wound healing, the platelet plug not only provides for hemostasis, but also traps cells of the innate immune response (polymorphonuclear cells, macrophages, and lymphocytes including CD3+ T cells) (5). Furthermore, the degranulated platelets are reported to secrete up to 300 bioactive molecules from intracellular granules (6); these soluble factors contain numerous inflammatory cytokines and chemokines that attract additional innate cells and promote inflammation. The teleological purpose for this level of inflammation is to destroy invading microbes. Microbial invasion through the disrupted external epithelial barrier unmet by a strong inflammatory response may develop into sepsis to the detriment or death of the individual. Thus, the initial actions must ensure a relatively sterile wound bed for subsequent phases of tissue replacement.
Fortunately, in the absence of ongoing infection, initial inflammation is dampened through the course of normal wound healing by the in-migrating fibroblasts, endothelial angiogenesis, and M2 (pro-reparative) macrophages to collectively suppress inflammation in the wound bed (7,8). The emerging inflammation suppressive network also harnesses the resident T-regulatory cells to limit the CD3+ T-cells and reduce pro-apoptotic cytokines release. The phenotypic switch in macrophages with a depletion of the initial inflammatory load of matrix debris and cells of the innate immune system, creates a pro-synthesis environment in which newly recruited fibroblasts produce provisional extracellular matrix proteins (i.e. immature collagens III/IV, fibronectin, Tenascins, etc.).
A maturing tissue then proceeds to produce signals that limit the production of matrix and prunes the over-exuberant neo-angiogenesis of the repair and proliferation phase. This occurs mainly, but not solely, through the expression of CXCR3 ligands from maturing endothelial cells and re-differentiated keratinocytes (9). While this signaling axis, and others including angiopoietin-2, largely revert the wound bed to its pre-existing quiescent state, the response is incomplete and some of the excess, but disorganized matrix that was formed in the earlier stages persist (10).
Inflammation: Stalling Healing or Inducing Scarring
Inflammation remains a key protective event in wound healing in the post-natal period. However, fetal healing is relatively devoid of inflammation and healing is regenerative and ‘scarless’ rather than the reparative healing noted in adult tissue (1, 11). Of course, the sterile fetal environment does not necessitate a robust inflammatory defense against microbes for survival in case of injury. This difference between fetal and post-natal healing highlights that there is a downside to the acute inflammation.
The main downside of this rapid inflammatory response is the production of proteases (mainly by neutrophils and M1 macrophages) that generate matrix fragments, particularly collagen fibrils that drive innate infiltration in a feed-forward fashion. Thus, proteases hamper a functional provisional matrix from supporting fibroblast migration and neo-vessels, all while churning out pro-inflammatory peptides to fuel the pathological inflammatory process. In most circumstances, the acute inflammation runs its course and is suppressed for healing, but a number of perturbations allow for this inflammatory milieu to persist. These include not just infection, but simply extensive colonization with microbes that further activate cells of the innate immune system. Further, situations in which endogenous parenchymal cells (fibroblasts and endothelial cells, and their precursors) are deficient or defective also prevent the suppression of this inflammation; advanced aged and metabolic syndromes are the two most common situations undergirding these pathologies.
On the other hand, even if the acute inflammation is curtailed and wound closure occurs, chronic inflammation can also perturb wound healing. Hypertrophic scarring appears to represent a sterile chronic inflammatory situation (9). The failure to end the tissue replacement phase of healing means a persistent matrix turnover that generates the chemotactic fragments that further recruit macrophages and naïve lymphocytes to the tissue, though at lower densities than that which is noted in the early inflammatory stage of healing. These cells reinforce the matrix immaturity, which in turn maintains the fibroblasts in the synthetic state. Thus, the result is not breakdown of the matrix and tissue, but rather excessive deposition of relatively immature matrix.
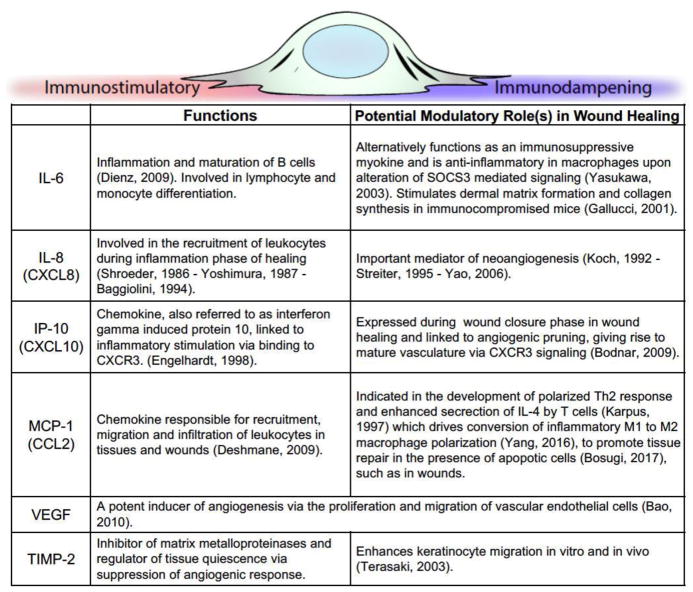
It must be noted that the inflammation is regulated by a plethora of cytokines and chemokines released into the wound bed during the process of healing. A few of the major ones are highlighted in Figure 1. These include both pro-inflammatory molecules such as IL-8 (CXCL8), but also those that may serve dual roles. For instance, MCP-1 (CCL2) recruits leukocytes to the wound, while at the same time driving macrophages to a more suppressive M2 phenotype. The chemokine ligands for CXCR3, with IP-10 (CXCL10) at the forefront as a chemoattractant for leukocytes, also serve as stop signals to in-migration of the formed cells. As much has been reviewed elsewhere on the cytokine milieu in wounds (12, 13), these specifics serve only as an entry to such studies. (Figure 1)
Figure 1.
Key inflammation modulating signals during wound healing.
Figure CITATIONS
Dienz O., Eaton SM., Bond JP. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp. Med. 2009;206:69–78.
Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien KR., Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;(4):551–556.
Gallucci RM., Sugawara T., Yucesoy B., Berryann K., Simeonova PP., Matheson JM., Luster MI. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21(8):603–609.
Schroeder JM., Christophers E. Identification of C5a des arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest. Dermatol. 1986;87:53–58.
Yoshimura T., Matsushima K., Oppenheim JJ, Leonard EJ Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987;139:788–793.
Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179.
Koch AE., Polverini PJ, Kunkel SL., Harlow LA., DiPietro LA., Elner VM., Elner SG., Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801.
Strieter RM., Polverini PJ, Kunkel SL., Arenberg DA., Burdick MD., Kasper J, Dzuiba J, Damme JV., Walz A., Marriott D. The functional role of the ‘ELR’ motif in CXC chemokine-mediated angiogenesis. J Biol. Chem. 1995;270:27348–27357.
Yao M., Zhou RH., Petreaca M., Zheng L., Shyy J, Martins-Green M. Activation of sterol regulatory element-binding proteins (SREBPs) is critical in IL-8-induced angiogenesis. J Leukoc. Biol. 2006;80:608.
Terasaki K., Kanzaki T., Aoki T., Iwata K., Saiki I. Effects of recombinant human tissue inhibitor of metalloproteinases-2 (rhTIMP-2) on migration of epidermal keratinocytes in vitro and wound healing in vivo. J Dermatol. 2003;30(3):165–172.
Deshmane SL., Kremlev S., Amini S., Sawaya BE. Monocyte Chemoattractant Protein (MCP-1): An Overview. J Interferon Cytokine Res. 2009;29(6):313–326
Yang M., Ma B., Shao H., Clark AM., Wells A. Macrophage phenotypic subtypes diametrically regulate epithelial- mesenchymal plasticity in breast cancer cells. BMC Cancer. 2016;16:419.
Bosurgi L., Cao YG., Cabeza-Cabrerizo M., Tucci A., Hughes LD., Kong Y., Weinstein JS., Licona-Limon P., Schmid ET., Pelorosso F., Gagliani N., Craft JE., Flavell RA., Ghosh S., Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342): 1072–1076.
Bao P., Kodra A., Tomic-Canic M., Golinko MS., Ehrlich HP., Brem H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J Surg. Res. 2009;152(2):347–358
Engelhardt E., Toksoy A., Goebeler M., Debus S., Bröcker EB., Gillitzer R. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig Are Sequentially and Differentially Expressed During Phase-Specific Infiltration of Leukocyte Subsets in Human Wound Healing. Am. J Pathol. 1998;53(6):1849–1860.
Bodnar RJ, Yates CC., Rodgers ME., Du X., Wells A. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122(Pt 12):2064.
Stem Cells Augment Healing by Dampening Inflammation
The key to more regenerative healing may instead be modulating inherent inflammation to minimize detrimental effects. Global suppression of inflammation has been tried, but does not aid in wound healing (14). Moreover, genetic ablation of myeloid-derived neutrophils and macrophages in mice resulted in impaired angiogenesis and myofibroblast differentiation, delaying wound contraction during the inflammatory phase (15). However, this failure may have been caused by the approaches not accounting for the changing nature of the inflammatory response throughout healing. As noted above, the inflammation progresses from one of acute M1 macrophage dominated microbicidal events, to an M2 macrophage regulated suppressive milieu late in healing. Eliminating specific cell classes in a spatiotemporal fashion will disrupt this continuum. For instance, diphtheria toxin induced ablation of macrophages did not significantly alter tissue or scar formation in mice during late healing (15), and PU.1 null mice deficient in neutrophils are reported to heal comparably with macrophage bearing mice, but exhibit scarless healing (16). Therefore, we posit that inflammation needs to diminish in a manner responsive to the changing situation of the healing wound.
This could be accomplished by timing sequential interventions, but this would work only in small wounds where the bed is essentially synchronized. However, in large and complex wounds, disparate areas of the wound reside in different stages of progression. Thus, the intervention needs to be tailored locally and not globally (or systemically). This would be best accomplished by bio-responsive interventions.
Stem cells fit the bill as both immunosuppressive and responsive to their local environment. Under different conditions, the mesenchymal stem cells/multipotent stromal cells (MSCs) produce distinct patterns of cytokines (17, 18). In situations of negligible oxygen and nutrient starvation, such as before neo-vessel sprouting, the stem cells express factors that promote angiogenesis by attracting the endothelial cells (and macrophages) and providing for matrix turnover (19–21). One of the main proangiogenic factors secreted by MSC is VEGF-A, a key player regulating endothelial cell health and function during neovascularization (tissue replacement phase) (22). VEGF-A also can act as an immunosuppressive agent by inhibiting dendritic cell maturation and function via its down regulation of NF-κB on hematopoietic progenitor cells (23), while also increasing the indoleamine 2,3-dioxygenase (IDO) secretion from dendritic cells subsequently suppressing lymphocyte proliferation (24). MSCs are also immunosuppressive, secreting IL-10 but with little TNFα produced (25). As oxygen tension increases and the wound enters the tissue replacement phase, the inflammatory cytokines IL-1b, IL-6 and IL-15 increase. MSCs secretion of IL-6 is particularly important as this has been shown to directly suppress monocyte differentiation into dendritic cells, while also inhibiting the proliferation of CD4+ T cells (26). MSC IL-6 secretion also resulted in higher levels of IL-10 secretion through either autocrine processes or through interaction with dendritic cells (27, 28). Increases in fibrogenic growth factors TGF-beta and basic FGF (18) also occur, allowing for recruitment of pericytes to prune and connect vascular conduits (29), while promoting the transition from collagen III to collagen-I rich matrix. Interestingly, collagen I fibrils are suppressive to cell migration and cell proliferation, so this would drive the wounds towards maturation, and paradoxically could lead to lower levels of immune infiltration. This is what is seen late in wound healing, with a reduction in CD3+ lymphocytes in the face of MSC transplanted into acute wounds (5).
The presence of MSC through the resolution phase of wound healing also serves to dampen the chronic inflammatory response and suppress hypertrophic scarring (9–10). In fact, transplantation of MSC can be dominant in a genetically engineered model of scarring in mice. In the absence of CXCR3 signaling, the wound undergoes a chronic inflammatory phase starting months after initial wound closure, leading to a hypertrophic scar. Introduction of MSC early on while the wound is open reduces the mononuclear cell load (both monocytes/macrophages and lymphocytes) resulting in near regenerative healing. The signals from the MSC are still being defined, but the matrix is matured and that is thought to be the key educating aspect of the wound bed. In addition, MSC have been shown to modulate the extracellular matrix microenvironment around them by binding and activating exogenous proMMP-2 and proMMP-13, or secreting high levels of soluble TIMP-2 to inhibit activity of high perivascular activities of MMP-2 and MMP-9 (30, 31). In both scenarios, added ECM modulation through direct interaction or indirect interaction via a controlled inflammatory response is key to achieving proper wound resolution.
MSC Survival in the Wound Bed
As MSC transplantation improves healing the question is how to achieve this clinically. Most all of the impact of MSC appears to occur by modulating the wound bed, rather than contributing to tissue as ‘building blocks’. This is assumed as the improvement is noted even in xenografts into immune-competent mice (5, 32). In many other studies, it is quite difficult to note transplanted cells after as short as 3 days; researchers have documented the lack of MSC survival in a variety of tissues including the heart (33), brain (34), and kidney (35). With results showing only a 5% survival rate within a two-week period post myocardial infarction (MI) treatment (33), or a 1% cell survival rate one hour after injection into an ischemic kidney model (35). Even when using an immunodeficient mouse model, Toma et al reported having less than 0.44% survival of MSCs 4 days post MI (36). We feel that it is for this reason many studies fail to show improvement of healing.
We posit that true wound improvement necessitates the MSC being present during all three stages of repair – initial inflammation, tissue replacement, and resolution. These stages take over two weeks at the minimum with resolution stretching for months. Thus, MSC must be reintroduced repeatedly, or provided with a survival advantage. Multiple applications are unlikely to be either practical or effective as cell death and the signals that are presented upon death initiate innate inflammation, and thus would work counter to the beneficial immunosuppressive effects.
Signaling of the Epidermal Growth Factor Receptor (EGFR) tyrosine kinase from the cell membrane promotes cell survival without driving proliferation (37, 38). This provides for a mechanism to prolong the presence of non-autologous MSC in order to suppress inflammation and ‘educate’ the wound bed in para- and matricrine fashion. This can be accomplished by covalently tethering EGF to a substratum (38) or matrix (39). However, as the tight binding of EGF to its receptor leads to tonic signaling, titering the density of tethered ligands would be a challenge as the different cells in the wound bed present highly varied levels EGFR. Rather a low affinity, high avidity ligand would be preferential. Such a situation is presented physiologically by the EGF like repeats of Tenascin C (40) and Laminin V (41). Tenascin C is present during organogenesis and the tissue replacement phase of wound healing (and perversely at the invasive fronts of aggressive tumors (42). These are times and regions in which cells are migrating but also confronted by stressors and death signals. These repeats, ultralow affinity ligands for EGFR, can confer survival advantages to a variety of cells including MSC (38, 39). Incorporation of Tenascin C with MSC in the wound bed leads to improved healing over either component alone (5, 32).
Conclusion
Aberrant wound repair, leading to either chronic ulceration or scarring, presents multiple derangements. However, chief among these is dysregulation of inflammation. Suppression of such augmented innate immunity, either in level or persistence, should aid in more regenerative repair and healing. MSC can provide such a boost, even if eventually cleared from the wound bed as non-autologous. MSC function as plastic and responsive immune suppressive signalers that also direct the resident cells to produce matrix appropriate to the stage of wound healing. However, for the MSC to function, they must survive during the stages of wound repair; this requires survival signaling to counter death cytokines and other stressors. The EGF-like repeats of the matricellular proteins tenascin C and laminin V can be used in such a manner. The combination of MSC in a pro-reparative hydrogel containing these survival signals provides for a novel approach promoting near regenerative healing.
Acknowledgments
This work was supported by grants from the National Institute of General Medical Sciences (NIH, USA) (GM063569 and GM069668). A.B. is supported on a NIH T32 CATER fellowship (EB001026). We thank the members of the Wells laboratory for helpful discussions and suggestions.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Andrew Bradshaw and Kyle Sylakowski declare that they have no conflict of interest.
Dr. Wells has a patent Owned by Univ Pittsburgh, pending; this patent is not licensed.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Research. 2012;96:325–333. doi: 10.1002/bdrc.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology. 2013;14(6):573–590. doi: 10.1007/s10522-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Zhao Y, Hao H, Han W, Fu X. Mesenchymal stem cell-based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regen. 2015;23:465–482. doi: 10.1111/wrr.12304. [DOI] [PubMed] [Google Scholar]
- 4.Klimczak A, Kozlowska U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016 doi: 10.1155/2016/4285215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor D, Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with tenascin-C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017;26:103–113. doi: 10.3727/096368916X692249. This study demonstrates that stem cells can reduce scarring in wounds, and links the effect to suppression of inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Investig. 2011;131:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates CC, Whaley D, Hooda S, Hebda PA, Bodnar RJ, Wells A. Delayed re-epithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair and Regen. 2009;17:34–41. doi: 10.1111/j.1524-475X.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevent dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplant. 2012;21:919–931. doi: 10.3727/096368911X623817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2012;126(4):1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair and Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 13.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochem Biophys Acta. 2013;1832:1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bootun R. Effects of immunosuppressive therapy on wound healing. Int Wound J. 2013;10(1):98–104. doi: 10.1111/j.1742-481X.2012.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 16.Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. Wound Healing in the PU.1 Null Mouse—Tissue Repair Is Not Dependent on Inflammatory Cells. Curr Biol. 2003;13(13):1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 17.Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine Secretion Profiling of Human Mesenchymal Stem Cells by Antibody Array. Int J Stem Cells. 2009;2(1):59–68. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong HT, Redmond SL, Marano RJ, Atlas MD, von Unge M, Aabel P, Dilley RJ. Paracrine Activity from Adipose-Derived Stem Cells on In Vitro Wound Healing in Human Tympanic Membrane Keratinocytes. Stem Cells Dev. 2017;26(6):405–418. doi: 10.1089/scd.2016.0204. [DOI] [PubMed] [Google Scholar]
- 20.Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kim DI. Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol Int. 2016;40(1):27–35. doi: 10.1002/cbin.10519. [DOI] [PubMed] [Google Scholar]
- 21*.Paquet J, Deschepper M, Moya A, Logeart-Avramoglou D, Boisson-Vidal C, Petite H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl Med. 2015;4(7):809–821. doi: 10.5966/sctm.2014-0180. This study provides for the basis of changes in stem cell functions during distinct phases of wound healing based on the availability of blood flow and oxygenation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular Endothelial Growth Factor (VEGF)-Mediated Angiogenesis Is Associated with Enhanced Endothelial Cell Survival and Induction of Bcl-2 Expression. Am J Path. 1999;154(2):375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 24.Marti LC, Pavon L, Severino P, Sibov T, Guilhen D, Moreira-Filho CA. Vascular endothelial growth factor-A enhances indoleamine 2,3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Mem Inst Oswaldo Cruz. 2014;109:70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Sivanathan KN, Gronthos S, Grey ST, Rojas-Canales D, Coates PT. Immunodepletion and Hypoxia Preconditioning of Mouse Compact Bone Cells as a Novel Protocol to Isolate Highly Immunosuppressive Mesenchymal Stem Cells. Stem Cells Dev. 2017;26(7):512–527. doi: 10.1089/scd.2016.0180. This reports on the responsiveness of stem cells to the external environment alters the communication with other endogenous cells via paracrine signaling. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10:410–415. doi: 10.1016/j.autrev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World Journal of Stem Cells. 2014;6(5):552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodnar RJ, Rodgers ME, Chen W, Wells A. Pericyte regulation of vascular remodeling through the CXC Receptor 3. Arterioscler Thromb Vasc Biol. 2013;33:2818–2829. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozito TP, Jackson WM, Nesti LJ, Tuan RS. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biology. 2014;34:132–143. doi: 10.1016/j.matbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226:385–396. doi: 10.1002/jcp.22344. [DOI] [PubMed] [Google Scholar]
- 32*.Yates CC, Rodrigues M, Nuschke A, Johnson Z, Whaley D, Stolz D, Wells A. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther. 2017;8:193. doi: 10.1186/s13287-017-0644-9. These finding demonstrate that stem cells can act primarily to educate other endogenous cells to drive healing, rather than being necessary as precursors to the tissue replacement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Lacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19(9):1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 35.Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14(7):277–285. doi: 10.1016/j.molmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues M, Blair H, Stockdale L, Griffith L, Wells A. Surface tethered epidermal growth factor protects proliferating and differentiating multipotential stromal cells from FasL induced apoptosis. Stem Cells. 2013;31:104–116. doi: 10.1002/stem.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuschke A, Rodrigues M, Rivera J, Yates-Binder C, Whaley D, Stolz D, Wells A. EGF tethered to β-tricalcium phosphate bone scaffolds via a high affinity binding peptide enhances survival of human mesenchymal stem cells/multipotent stromal cells (MSC) in an immune-competent parafascial implantation assay in mice. Stem Cells Transl Med. 2016;5:1580–1586. doi: 10.5966/sctm.2015-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues M, Yates C, Nuschke A, Griffith L, Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Engineering Part A. 2013;19(17–18):1972–1983. doi: 10.1089/ten.tea.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swindle CS, Tran K, Johnson TD, Banerjee P, Mayes AM, Griffith LG, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154(2):459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grahovac J, Becker D, Wells A. Melanoma cell invasiveness is regulated at least in part by the epidermal growth factor-like repeats of tenascin-C. J Invest Dermatol. 2013;133:210–220. doi: 10.1038/jid.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]