Abstract
Background:
Negative pressure wound therapy was developed for treating wounds associated with unfavorable healing factors. The principles of the negative pressure wound therapy applied on clean and closed surgical incision originate the closed incision negative pressure therapy (ciNPT). We evaluated the use of ciNPT in the setting of oncological breast surgery.
Methods:
From January 1, 2015, to June 31, 2015, we prospectively selected 37 patients undergoing oncological breast surgery with a minimum of 4 risk factors. Seventeen patients (25 surgeries) voluntary tested ciNPT (ciNPT sample), whereas the remaining 20 (22 surgeries) chose conventional postsurgery dressing (Standard Care sample). Follow-up controls to evaluate postsurgical complications were performed on days 7, 14, 30, and 90. At 12 months, the quality of life, scar, and overall aesthetic outcomes were evaluated with specific questionnaires filled in by surgeon and patient. The Standard Care sample was investigated on risk factors associated with poor healing.
Results:
The ciNPT sample showed a significant prevalence of high risk factors, especially extensive undermining and bilateral surgeries, and a predominance of women under 65 years; only 1/25 (4%) surgical procedures was followed by complications. In the Standard Care sample, 10 of 22 surgeries (45%) were followed by complications. The difference in complication rate between the 2 samples was significant. The BIS (Body Image Scale) scores suggested that most patients were satisfied with their body image regardless of the type of dressing. All other questionnaire scores clearly vouched for a significant superiority of the ciNPT. Previous surgery ≤ 30 days emerged as the surgery-related high risk factor most frequently associated with postsurgery complications.
Conclusion:
The results of our study support the use of ciNPT in oncological breast surgery: it showed to be a well-tolerated, adaptable, and reliable dressing capable of reducing postsurgical complications and improving scar outcomes in patients presenting with high risk factors.
INTRODUCTION
Oncologic breast surgery, employing techniques ranging from more conservative breast-conserving surgery (BCS) to intermediate oncoplastic surgery1–3 (OPS) to more radical ones (mastectomies, with or without tissue sparing and reconstructions), is an expanding and increasingly demanding field of surgery. The literature reports overall complication rates up to 35% for BCS cases,4,5 50% for breast reconstruction,6,7 and 30% for OPS.8 Postsurgical complications affect the quality of life, increase the costs of the health system, and may delay the beginning of adjuvant therapies.9
Negative pressure wound therapy was developed for treating wounds associated with unfavorable healing factors.10 It proved to be effective in the treatment of many chronic11 and surgical wounds,12 including breast surgery.13 The principles of the negative pressure wound therapy applied on clean and closed surgical incision originate the closed incision negative pressure therapy (ciNPT). A 2016 international consensus conference stated that the use of ciNPT in surgical procedures on high-risk patients appears to have the potential for reducing surgical incision complications and health care costs.14
In our institution, ciNPT with Prevena (KCI, an Acelity company, Sant Antonio, Tex.) is currently being used on abdominal wall reconstruction incisions of high-risk patients and on pathological scar revisions of severely burned patients, with good results in terms of suture dehiscence rate and scar features.15 Our hypothesis was that ciNPT with Prevena could give better results than the conventional dressing also in patients undergoing complex oncological breast surgeries and reconstructions. We thus performed a small-size study including the presurgery evaluation of patient- and surgery-related risk factors and the postsurgery estimation of wound complications and aesthetic outcomes. Our aim was to obtain an indication on 2 issues: (1) efficacy of ciNPT compared with Standard Care; (2) risk factors associated with a poor outcome in the Standard Care sample that could be considered strong advocates of the use of ciNPT.
MATERIALS AND METHODS
Study Population
Our institution made available 25 Prevena to be tested in breast surgeries. The candidates for their application were drawn from the surgeries scheduled at our Breast Unit from January 1, 2015, to June 31, 2015.
The first step was an estimation of preoperative risk factors for each planned surgery. Risk factors, selected after a literature review, were divided between patient-related factors: age (≥ 65 years), body mass index (≥ 30 kg/m2), breast conformation (size, ptosis), smoking, diabetes, hypertension, use of corticosteroids, peripheral artery and liver diseases, neo-adjuvant/chemotherapy and radiation therapy; and surgery-related factors: previous surgery (≤ 30 days or > 30 days), extensive undermining (level-2 oncoplastic procedures, nipple-sparing mastectomy), type of reconstruction (1 or 2 stages implant-based reconstruction), use of acellular dermal matrices (ADM) and autologous reconstruction.16,17 Among these, obesity, large and ptotic breasts, smoking, radiation therapy, use of corticosteroids, previous surgeries within 30 days, extensive undermining, 1-stage reconstruction and use of ADM were to be considered at higher risk for complications.18,19 Based on a previously described grading system for the selection of patients for the use of Prevena in orthopedic surgeries,20 we set a threshold of a minimum of 4 risk factors (with at least 1 high risk).
Ineligibility criteria were: (1) documented allergy to acrylic glue, silver ionic, and polyester; (2) any type of surgical-site infection (SSI) as for the Antibiotic Prophylaxis for Preventing SSI Consensus Conference definition21 and antibiotics within 14 days of surgery.
Forty-seven surgical procedures satisfied all criteria. The patients involved were extensively informed about the different aspects of a ciNPT device, stressing that the postsurgical follow-up would have been the same independent of the dressing. Following the indication of our Ethical Committee, the choice was demanded to each patient, after consultation with her general practitioner.
The first 25 surgeries on patients who elected to be treated with Prevena constituted the ciNPT sample; the remaining 22 formed the Standard Care sample.
All patients signed an informed consent form, including a consent for the taking of image records, and the study was conducted in good clinical practice according to the Helsinki Declaration of 1975 and subsequent modifications.
Postsurgery Protocol
All surgeries were performed by the same surgical team (general and plastic surgeon) employing the same techniques: BCS, OPS, tissue sparing (nipple-sparing and skin-sparing), and simple mastectomies were performed based on each patient’s oncological and reconstructive treatment goals.
The Prevena incision management system was placed on the closed surgical incision in a customized fashion (Customisable kit), providing a continuous −125 mm Hg pressure for 7 days. Following the Prevena removal, a skin adhesive closure (Steri-strip, 3M, St. Paul, Minn.) was applied over each incision for further 7 days. The drain(s) was(were) always left outside of the Prevena film, which secured the dressing to the application site (Fig. 1).
Fig. 1.
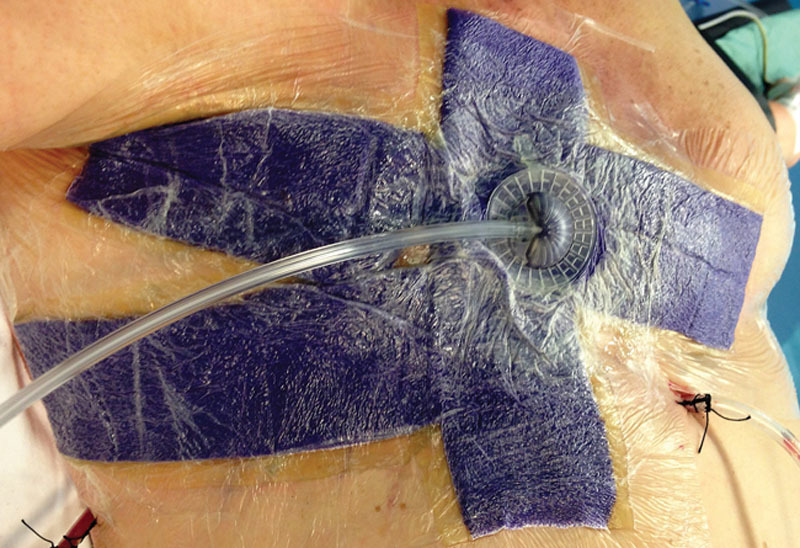
The Prevena incision management system is placed in a customized fashion.
The standard care procedure involved Steri-strip skin adhesive closure for 14 days, changed after 7 days.
All other treatments were the same for the 2 groups: preoperative weight-based antibiotics with appropriate intraoperative redosing, ChloraPrep (CareFusion Corporation, San Diego, Calif.) skin prepping, and oral antibiotics continued postoperatively until drain(s) removal.
A team formed by a general surgeon, plastic surgeon, and breast nurse assessed the conditions of all patients on the follow-up visits (FU) on days 7, 14, 30, 90, and 12 months postoperatively.
The postsurgical complications evaluated were infection, hematoma, seroma, and skin necrosis. Infection was defined as: (1) purulent drainage from the incision; (2) positive culture swab; (3) signs or symptoms of systemic infection.22,23 Hematoma and seroma were considered only when aspiration was necessary. Skin necrosis was divided into minor, defined as partial-thickness skin flap necrosis requiring local wound management and major, defined as full-thickness skin flap necrosis requiring surgical intervention.24 Suction drains were removed once the output was less than 30 ml, not hematic over 24 hours.
The quality of life, scar, and overall aesthetic outcomes were evaluated with specific questionnaires filled in by surgeon and patient.
Statistical Analysis
Continuous variables did not meet the normality requirements of the Shapiro-Wilks W test and were thus expressed as median (first and third quartiles) and compared with the nonparametric Mann-Whitney test.
Categorical variables were expressed as counts (percentages) and studied with the chi-square test with Yates correction, or, when appropriate, with Fisher’s exact test. The requirement for significance was P < 0.05. Calculations were run on Statplus:Mac version v6 (AnalystSoft, Walnut, Calif.) and on Openepi version 3.01.25
RESULTS
Surgical Procedures Risk Factors
Table 1 shows the risk factors characterizing the surgical procedures in the 2 samples: ciNPT (25 surgeries on 17 patients) and Standard Care (22 surgeries on 20 patients).
Table 1.
Risk Factors in the 47 Surgical Procedures
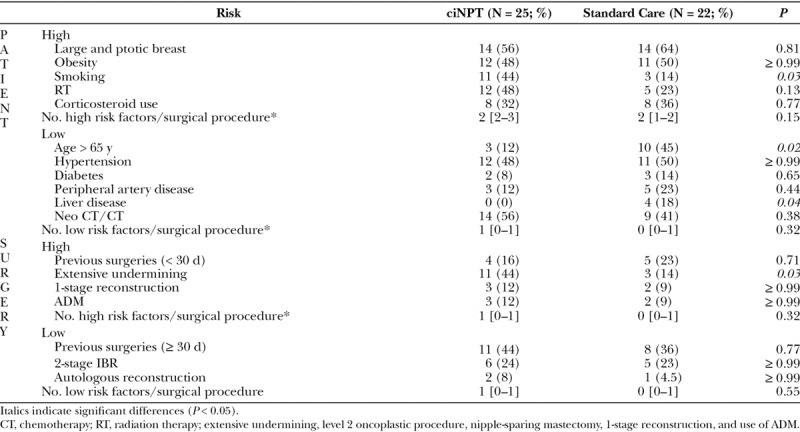
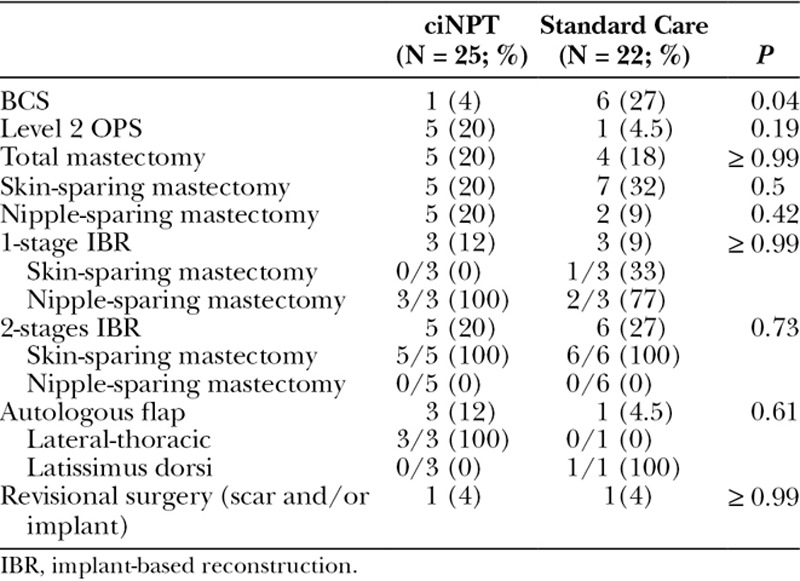
The ciNPT sample shows a significant prevalence of high risk factors (P = 0.04), especially extensive undermining (P = 0.03) and bilateral surgeries (8 of 17 versus 2 of 20; P = 0.02), not to forget the age factor, with predominance of women under 65 years (88% versus 55%; P = 0.02), for whom the aesthetic issues are more important. Table 2 details the surgeries and reconstructions performed over the breasts in the two groups.
Table 2.
Surgical Procedures (Breast Surgeries and Reconstructions)
Outcomes at Follow-up
At the 30 days FU, the postsurgery evolution was quite different for the 2 samples. In the ciNPT sample, only 1 of 25 (4%) surgical procedure was followed by complications: a seroma and a major skin necrosis. The patient, a 79-year-old woman with 3 high and 4 low risk factors underwent a partial thickness skin graft, and the wound was completely healed by the 90-days FU.
In the Standard Care group, 10 of 22 surgeries (45%) were followed by complications: 3 had 2 complications each (2 seroma and skin necrosis and 1 hematoma and skin necrosis), whereas 8 had 1 complication (4 skin necrosis, 3 seromas, and 1 hematoma). Four skin necrosis were major and underwent surgical closure, being completely healed by the 90 days FU; 3 were minor and were healed by secondary intention within 30 days.
The average Prevena placement time was 1 week. No adverse event such as blister formation, as reported by Howell et al.26 was observed.
The drain placement time was 17 (15–21) days for ciNPT and 19.5 (15–27) days for Standard Care (P = 0.70).
The difference in complication rate between the 2 samples was significant: 4% for ciNPT versus 45% for Standard Care (P = 0.001). Skin necrosis incidence was 1 of 25 (4%) in the ciNPT sample and 7 of 22 (32%) in the Standard Care group (P = 0.02).
At the 7 days FU, no adverse events, patient discomfort and ciNPT device and/or dressing malfunction were observed.
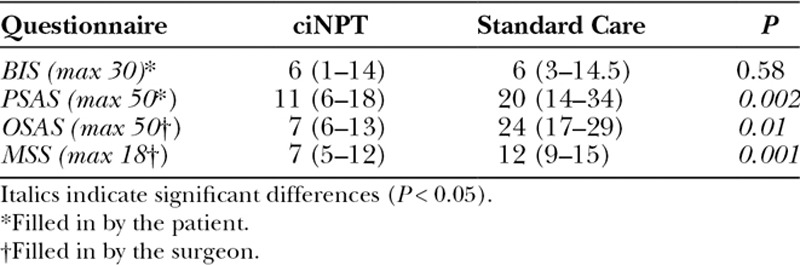
The quantitative evaluation of the postoperative body image and scar features was performed by means of questionnaires. The plastic surgeon filled in the Observer Scar Assessment Scale27 and the Manchester Scar Scale28. Two questionnaires on the personal level of satisfaction with the outcome were filled in by the patient: Body Image Scale (BIS)29 and Patient Scar Assessment Scale27. For all tests, the higher the scores, the lower the level of satisfaction and quality of the scar. The BIS scores suggested that most patients, whether treated with ciNPT or Standard Care, were satisfied with their body image. All other questionnaire scores clearly vouched for a significant superiority of the ciNPT postsurgery approach (Table 3).
Table 3.
Outcome of Questionnaires on the Level of Satisfaction
Risk Factors Associated with Complication in the Standard Care Sample
We investigated the Standard Care group for clues on the risk factors associated with poor healing. The comparison of surgeries with and without complications (10 and 12 cases, respectively) evidenced the significant role of the surgery-related high risk factors. Only 1 of the 12 cases without complications had 1 surgery-related high risk factor (extensive undermining), whereas 7 of the 10 cases with complications had at least 1 (8% versus 70%; P = 0.006). Two of the 7 cases were characterized by the overlapping of 3 high risks (extensive undermining, 1-stage reconstruction and ADM); the remaining 5 only by previous surgery within the last 30 days. Recent previous surgery thus emerged as the surgery-related high risk factor most frequently associated with postsurgery complications (5 of 10 versus 0 of 12; P = 0.01). Previous surgery ≤ 30 days was present also in the only patient in the ciNPT sample who presented a major skin necrosis. No difference was observed instead for the patient-related high risks (P = 0.92), in particular neither smoke (P = 0.86) nor radiation therapy (P ≥ 0.99).
DISCUSSION
Preventing complications in oncological breast surgery can be achieved through the understanding of which factors may cause them. Some studies evaluated the risk factors associated with postsurgical complications in breast reconstruction.24,30 We were the first, to the best of our knowledge, to classify risk factors between patient- and surgery-related and to subclassify those considered to be at higher risk18,19 to obtain indications on when to use ciNPT in oncological breast surgeries.
The comparison of the postsurgery outcomes in the ciNPT sample (25 surgeries on 17 patients) and the Standard Care sample (22 surgeries on 20 patients) evidenced a significantly lower rate of complications for the former: 1 of 25 (4%) versus 10 of 22 (45%), P = 0.001. In particular, skin necrosis incidence was significantly lower for ciNPT sample than for Standard Care: 1 of 25 (4%) versus 7 of 22 (32%; P = 0.02). It is worth noticing that the number of high risk factors was significantly higher for ciNPT than for Standard Care (P = 0.04): P = 0.03 for extensive undermining and P = 0.02 for bilateral surgeries. This unbalance, penalizing ciNPT, lends more importance to the statistically significant better postsurgery outcome of this sample.
The significant difference in the rate of complications between the 2 samples cannot be attributed to a poor outcome of the Standard Care one: the postoperative complications and suture dehiscence rates observed in this group agree with those reported by Sullivan et al.31 for breast reconstruction and by Harvey et al.32 for OPS.
At the 1-year follow-up, the scar features were similar for the 2 groups. However, the questionnaires filled in by the plastic surgeon and those on the level of satisfaction with the outcome filled in by the patients clearly vouched for a significant superiority of the ciNPT postsurgery approach. This result agrees with other studies that examined the effect of ciNPT on the scar features and aesthetic outcome of the surgery.33–35
Our results on the positive effect of ciNPT are consistent with the latest literature on this subject. The first reports regard the ciNPT with PICO (Smith & Nephew Wound Management, London, United Kingdom). Pellino et al.36 reported a lower SSI rate in 25 patients undergoing breast surgery treated with ciNPT compared with the contralateral side with conventional dressing (8% versus 36%). Holt and Murphy37 reported a reduction in wound breakdown in 24 patient undergoing OPS treated with ciNPT, compared with the contralateral side where a reduction mammaplasty was performed (4.2% versus 16.7%).
Gabriel et al.38 were the first to report on the use of Prevena in 13 patients undergoing immediate postmastectomy breast reconstruction. They quoted an overall complication rate of 18% complications/breast, 12% suture dehiscence, and 4% flap necrosis. A direct comparison with our 4% complication rate is not straightforward. The number of risk factors per patients were higher in our study (4–7 versus 1–2), and some risk factors and surgical techniques in our study were associated with an increased transudate formation. This could explain our longer drain duration time (17 versus 8.2 days).
Kim et al.24 evaluated flap necrosis after immediate expander-based breast reconstruction, reporting an overall complication rate of 11% for ciNPT versus 28% for conventional dressing (P = 0.02) and an overall skin necrosis incidence of 9% versus 24% (P = 0.03). These figures well compare with our results: 4% versus 45% (P = 0.001) for the complication rate and 4% versus 32% (P = 0.02) for skin necrosis.
One of the outcome of our study was the greater impact of surgery-related risk factors over the patient-related risk factors: 8 of the 11 surgical procedures with complications had surgery-related high risks, against 13 of the 36 surgical procedures without complications, P = 0.04. Instead no difference for the patient-related high risks was observed. This result is consistent with the recommendation of the 2016 international consensus conference14 for patients undergoing high-risk procedures.
CONCLUSIONS
The results of our study support the use of ciNPT in oncological breast surgery. It suffers from several limitations: it is underpowered to significantly identify all existing differences between the 2 samples, it was not a randomized case-control study and the patients in the 2 groups could not be stratified according to the risk factors.
Footnotes
Published online 15 June 2018.
Presented at the Italian Society of Plastic, Recosntructive and Aesthetic Surgery National Congress 2016 in Turin, Italy.
Statement of conforming to the Declaration of Helsinki: All patients signed an informed consent form before surgery, and the study was conducted in good clinical practice according to the Helsinki Declaration of 1975 and subsequent modifications.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspect Plast Surg. 1998;11:71. [Google Scholar]
- 2.Petit JY, Rietjens M, Garusi C, et al. Integration of plastic surgery in the course of breast-conserving surgery for cancer to improve cosmetic results and radicality of tumor excision. Recent Results Cancer Res. 1998;152:202. [DOI] [PubMed] [Google Scholar]
- 3.Clough KB, Nos C, Salmon RJ, et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg. 1995;96:363. [DOI] [PubMed] [Google Scholar]
- 4.Vitung AF, Newman NA. Complications in breast surgery. Surg Clin N Am. 2007;87:431. [DOI] [PubMed] [Google Scholar]
- 5.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698. [DOI] [PubMed] [Google Scholar]
- 6.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1. [DOI] [PubMed] [Google Scholar]
- 7.Vinton AL, Traverso LW, Jolly PC. Wound complications after modified radical mastectomy compared with tylectomy with axillary lymph node dissection. Am J Surg. 1991;161:584. [DOI] [PubMed] [Google Scholar]
- 8.Peled AW, Sbitany H, Foster RD, et al. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast J. 2014;20:302. [DOI] [PubMed] [Google Scholar]
- 9.Harvey J, Henderson J, Patel L, et al. Therapeutic mammaplasty—impact on the delivery of chemotherapy. Int J Surg. 2014;12:51. [DOI] [PubMed] [Google Scholar]
- 10.Morykwas MJ, Argenta LC. Vacuum-assisted closure: a new method for wound control and treatment. Ann Plast Surg. 1997;38:553. [DOI] [PubMed] [Google Scholar]
- 11.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553. [DOI] [PubMed] [Google Scholar]
- 12.Willy C. The Theory and Practice of Vacuum Therapy: Scientific Basis, Indications for Use, Case Reports, Practical Advice. 20062nd ed Ulm, Germany: Lindqvist Book Publishing. [Google Scholar]
- 13.Kostaras EK, Tansarli GS, Falagas ME. Use of negative-pressure wound therapy in breast tissues: evaluation of the literature. Surg Infect (Larchmt). 2014;15:679. [DOI] [PubMed] [Google Scholar]
- 14.Willy C, Agarwal A, Andersen CA, et al. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2017. Apr;14(2):385. doi: 10.1111/iwj.12612. Epub 2016 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollero D, Malvasio V, Catalano F, et al. Negative pressure surgical management after pathological scar surgical excision: a first report. Int Wound J. 2015;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gfrerer L, Mattos D, Mastroianni M, et al. Assessment of patient factors, surgeons, and surgeon teams in immediate implant-based breast reconstruction outcomes. Plast Reconstr Surg. 2015;135:245e. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Park SJ, Bang SI, et al. Does the use of incisional negative-pressure wound therapy prevent mastectomy flap necrosis in immediate expander-based breast reconstruction? Plast Reconstr Surg. 2016;138:558. [DOI] [PubMed] [Google Scholar]
- 18.de Blacam C, Ogunleye AA, Momoh AO, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012;255:551. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen LT, Hørby J, Friis E, et al. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28:815. [DOI] [PubMed] [Google Scholar]
- 20.Stannard JP, Atkins BZ, O’Malley D, et al. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage. 2009;55:58. [PubMed] [Google Scholar]
- 21.Ariyan S, Martin J, Lal A, et al. Antibiotic prophylaxis for preventing surgical site infection in plastic surgery: an evidence-based consensus conference statement from the American Association of Plastic Surgeons. Plast Reconstr Surg. 2015;135:1723. doi: 10.1097/PRS.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg. 2013;132:1057. [DOI] [PubMed] [Google Scholar]
- 23.Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg. 2014;72:503. [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Park SJ, Bang SI, et al. Does the use of incisional negative-pressure wound therapy prevent mastectomy flap necrosis in immediate expander-based breast reconstruction? Plast Reconstr Surg. 2016;138:558. [DOI] [PubMed] [Google Scholar]
- 25.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available at www.OpenEpi.com, updated 2013/04/06.
- 26.Howell RD, Hadley S, Strauss E, et al. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Pract. 2011;22:176. [Google Scholar]
- 27.Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960; discussion 1966. [DOI] [PubMed] [Google Scholar]
- 28.Beausang E, Floyd H, Dunn KW, et al. A new quantitative scale for clinical scar assessment. Plast Reconstr Surg. 1998;102:1954. [DOI] [PubMed] [Google Scholar]
- 29.Hopwood P, Fletcher I, Lee A, et al. A body image scale for use with cancer patients. Eur J Cancer. 2001;37:189. [DOI] [PubMed] [Google Scholar]
- 30.Semsarzadeh NN, Tadisina KK, Maddox J, et al. Closed incision negative-pressure therapy is associated with decreased surgical-site infections: a meta-analysis. Plast Reconstr Surg. 2015;136:592. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan SR, Fletcher DR, Isom CD, et al. True incidence of all complications following immediate and delayed breast reconstruction. Plast Reconstr Surg. 2008;122:19. [DOI] [PubMed] [Google Scholar]
- 32.Harvey J, Henderson J, Patel L, et al. Therapeutic mammaplasty—impact on the delivery of chemotherapy. Int J Surg. 2014;12:51. [DOI] [PubMed] [Google Scholar]
- 33.Glaser DA, Farnsworth CL, Varley ES, et al. Negative pressure therapy for closed spine incisions: a pilot study. Wounds. 2012;24:308. [PubMed] [Google Scholar]
- 34.Galiano R, Djhoan R, Shin J, et al. The effects of a single use canister-free negative pressure wound therapy (NPWT) system on the prevention of postsurgical wound complications in patients undergoing bilateral breast reduction surgery. Aesthetic Surgery of the Breast Symposium, December 10–13, 2014Milan, Italy. [Google Scholar]
- 35.Scalise A, Tartaglione C, Bolletta E, et al. The enhanced healing of a high-risk, clean, sutured surgical incision by prophylactic negative pressure wound therapy as delivered by Prevena™ Customizable™: cosmetic and therapeutic results. Int Wound J. 2015;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellino G, Sciaudone G, Candilio G. Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg. 2014;12:S64. doi: 10.1016/j.ijsu.2014.08.378. Epub 2014 Aug 23. [DOI] [PubMed] [Google Scholar]
- 37.Holt R, Murphy J. PICO™ incision closure in oncoplastic breast surgery: a case series. Br J Hosp Med (Lond). 2015;76:217. [DOI] [PubMed] [Google Scholar]
- 38.Gabriel A, Sigalove SR, Maxwell GP. Initial experience using closed incision negative pressure therapy after immediate postmastectomy breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e819. [DOI] [PMC free article] [PubMed] [Google Scholar]


