Abstract
Background:
A nanocrystal intravenous (IV) formulation of meloxicam is being studied with the aim of providing postoperative analgesia.
Methods:
This randomized, multicenter, double-blind, placebo-controlled trial evaluated meloxicam IV 30 mg or placebo (≤ 3 doses) in 219 subjects undergoing abdominoplasty. The primary endpoint was the summed pain intensity difference over 24 hours postdose (SPID24).
Results:
Meloxicam IV–treated subjects had a statistically significant reduction in the least squares mean of SPID24 compared with placebo-treated subjects (−4,262.1 versus −3,535.7; P = 0.0145). Meloxicam IV was associated with statistically significant differences over placebo on several other secondary endpoints, including other SPID intervals (ie, SPID12, SPID48, and SPID24–48), achievement of perceptible pain relief, the proportion of subjects with a ≥ 30% improvement in the first 24 hours, and Patient Global Assessment of pain at hour 48. Meloxicam IV was also associated with a reduction in the number of subjects receiving opioid rescue medication during hours 24–48 and the total number of doses of opioid rescue analgesia. Meloxicam IV was generally well tolerated, with the numbers and frequencies of adverse events similar to that of the placebo group. There was no evidence of an increased risk of adverse events commonly associated with nonsteroidal anti-inflammatory drugs including bleeding, thrombotic, cardiovascular, renal, hepatic, cardiovascular, injection site, and wound healing events.
Conclusion:
Meloxicam IV provided sustained pain relief and generally was well tolerated in subjects with moderate-to-severe pain following abdominoplasty.
INTRODUCTION
Abdominoplasty is 1 of the most frequently performed cosmetic surgeries in the United States, and the number of procedures has substantially increased in recent years from 62,713 in 2000 to 127,633 in 2016.1 For such procedures, safe and effective pain control is an important component of postoperative patient care.2 Postoperative pain reduction can lead to earlier mobilization, shortened hospital stays, reduced hospital costs, quicker return to normal activities, and increased patient satisfaction.3,4
Although opioids, in combination with other forms of analgesia, have been a mainstay of perioperative pain control, there has been an increased emphasis on the use of alternative medications, given the potential risks associated with these agents (eg, adverse events [AEs] such as nausea/vomiting, constipation, pruritus, sedation, respiratory depression, development of dependence).5–8 Multimodal pain management approaches to the treatment of postoperative pain (eg, use of synergistic combinations of analgesics) are gaining acceptance in a variety of surgical procedures.7–9 Nonsteroidal anti-inflammatory drugs (NSAIDs) are considered an important component of such regimens.7,8
Meloxicam is a long-acting preferential cyclo-oxygenase-2 (COX-2) inhibitor with analgesic, antipyretic, and anti-inflammatory properties.10,11 It has been shown to have more favorable gastrointestinal tolerability compared with nonselective NSAIDs.12 Oral formulations of meloxicam have demonstrated effectiveness for reducing symptoms associated with chronic conditions such as rheumatoid arthritis and osteoarthritis.11,13 However, oral formulations of meloxicam have poor solubility and are not rapidly absorbed, with peak plasma concentrations 9–11 hours after an oral dose of 30 mg12,14; as such, oral meloxicam is not indicated for the management of acute pain. An intravenous nanocrystal formulation of meloxicam has recently been developed for administration as a bolus injection that in phase 2 trials has provided onset of analgesia as early as 10 minutes postdose, with maintenance of analgesic throughout the 24-hour dosing period.15–17 The primary objective of the present study (NCT02678286) was to evaluate the analgesic efficacy of intravenous (IV) meloxicam versus placebo in subjects with moderate-to-severe pain following abdominoplasty.
METHODS
Study Design
This phase 3, randomized, multicenter, double-blind, placebo-controlled trial was conducted at 4 clinical trial sites in the United States. All subjects remained at study site throughout the treatment phase. The study was conducted according to Good Clinical Practices as referenced in the International Conference on Harmonisation Guidance for Industry and the principles of the Declaration of Helsinki. The protocol was reviewed and approved by the institutional review board, and all subjects provided written informed consent.
Dosing
Subjects were screened for participation within 28 days before study drug administration. Once subjects met postoperative randomization eligibility criteria (see Key Eligibility Requirements), they were randomized (1:1) to treatment with meloxicam IV 30 mg or IV placebo, administered as an IV bolus over 15–30 seconds every 24 hours for up to 3 doses. Subjects with inadequately controlled pain could receive rescue medication (oxycodone 5 mg orally) after first study drug administration and then could be given rescue medication every 2 hours as requested. The third dose of study medication could be administered at the discretion of the investigator at 48 hours (before discharge).
Surgical technique was standardized across all sites. During surgery, subjects received an anesthetic regimen using fentanyl and propofol, with or without volatile anesthetics or muscle relaxants. No local anesthetics were employed. The subjects underwent a standardized surgical procedure consisting of a low transverse abdominal incision, infra-umbilical fascial plication and bilateral drain placement. Patients requiring more extensive surgical procedures such as liposuction or dissection above the umbilicus were excluded from the study. During the immediate postoperative period, fentanyl (25 μg IV bolus) was administered as necessary for postoperative pain. Analgesic medications other than those prespecified for postoperative use (eg, opioids, acetaminophen, NSAIDs [other than aspirin for cardiovascular indications]) were prohibited during the treatment period (through hour 48).
Key Eligibility Criteria
Men and nonpregnant, nonlactating women 18–75 years of age (inclusive) with a body mass index ≤ 35 kg/m2 scheduled to undergo abdominoplasty without collateral procedures were eligible for participation. Subjects also had to meet the following postoperative randomization criteria: (1) moderate or severe pain on a 4-point categorical pain rating scale and a score ≥ 4 on the 11-point numeric pain rating scale within 3 hours of the end of surgery (last suture) on day 1; (2) ability to answer questions and follow commands and participate in pain assessment evaluations; (3) undergoing a surgical procedure lasting ≤ 3 hours; and (4) having no significant deviations from surgical protocol, anesthetic protocol, or specified pretreatment analgesic regimen. Subjects must also have had no evidence of the following during or following surgery: evidence of respiratory insufficiency, clinically significant hypotension, bradycardia, or any other abnormality that, in the investigator’s opinion, significantly increased the risks of study drug administration.
Exclusion criteria included allergy to meloxicam or other NSAIDs or excipients, elevated aminotransferases; history of HIV, hepatitis B, or hepatitis C; significant renal, hepatic, cardiovascular, metabolic, neurologic, psychiatric conditions; history of migraine (within past 12 months); and myocardial infarction or coronary artery bypass graft surgery within 12 months. Subjects with active or recent bleeding (within 6 months), gastrointestinal ulceration or bleeding, or a known bleeding disorder or taking agents affecting coagulation were also ineligible.
Assessments
Pain intensity (PI) was assessed using the 11-point numeric pain rating scale (0–10; 0 equated to no pain, and 10 equated to the worst pain imaginable) at predose and at 0.25, 0.5, 0.75, 1, 2, 4, and 6 hours postdose 1, and then at 2-hour intervals until hour 48. The performance of the study medication was assessed at hours 24 and 48 using a Patient Global Assessment of pain control (PGA; 0–4 [0 = poor, 1 = fair, 2 = good, 3 = very good, or 4 = excellent]).
Endpoints
The primary efficacy endpoint was the summed PI difference from hour 0 to hour 24 (SPID24). SPID is a measure that combines relief magnitude and duration of pain into a single score; it is calculated by the sum of the time-weighted PI difference (difference between current pain and pain at baseline) multiplied by the interval between ratings. To address the impact of rescue medication on treatment response to PI during each interval, the prerescue PI score from a rescue was carried forward to replace the scheduled PI scores collected (or to be collected) within 2 hours following the use of rescue.
Secondary efficacy endpoints included SPID at other time points (SPID6, SPID12, SPID48, and SPID24–48); time to perceptible and meaningful pain relief as measured by 2-stopwatch technique; the proportion of subjects with improvement (ie, pain reduction) ≥ 30% and ≥ 50% within 6 hours following the first study dose; PGA of pain control at hours 24 and 48; and time to administration of first dose of rescue analgesia and number of times rescue analgesia was used during 0–24, 24–48, and 0–48 hours. Additional exploratory endpoints evaluated efficacy responses during the second 12 hours (ie, 12–24 hours and 36–48 hours) and the last 6 hours (18–24 hours and 42–48 hours) of the dosing interval and included SPID, proportion of subjects requiring rescue analgesia, and total number of doses of rescue analgesia per subject. Safety endpoints include the incidence of AEs and serious AEs, laboratory tests, vital signs, 12-lead electrocardiogram findings, and AEs of special interest (ie, bleeding, cardiovascular, hepatic, injection site reactions, renal, thrombotic, wound healing events).
Statistical Analysis
The intent-to-treat population includes all subjects randomized and was the primary efficacy set. The sample size was selected with the assumption that the effect size for SPID24 between meloxicam IV and placebo is at least 0.40. Using a 2-group t test with a 0.05 two-sided significance level, this study was designed to have greater than 80% power to detect the difference between treatment groups.
Analysis of covariance was used to assess the difference between treatment groups for SPID. The analysis of covariance model included mean effects of treatment and investigational site and a covariate of baseline PI score. The difference between groups was assessed using a 2-sample t test of least-squares (LS) means values.
RESULTS
Subjects
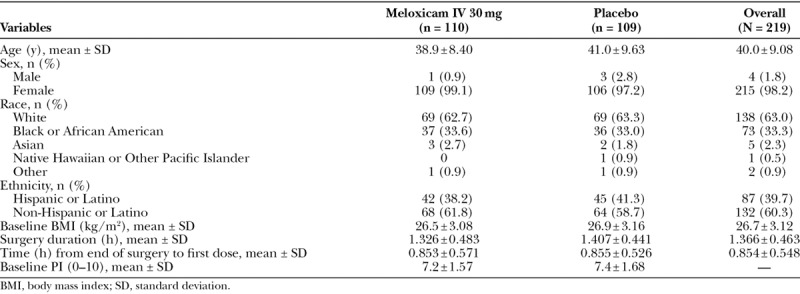
A total of 219 subjects (215 female, 4 male) were enrolled, and all were randomized to treatment. Treatment groups were comparable with respect to demographic and baseline characteristics (Table 1). Most subjects received 3 doses of study medication (n = 87 [87.1%] meloxicam IV; n = 86 [78.9%] placebo).
Table 1.
Summary of Subject Demographics and Disposition
Efficacy
For the primary endpoint (ie, SPID24) subjects who received meloxicam IV had a statistically significant greater reduction in PI compared with subjects who received placebo. The LS mean reductions in SPID24 were −4,262.1 ± 214.19 and −3,535.7 ± 215.05, respectively, in meloxicam IV and placebo groups (P = 0.0145; Fig. 1).
Fig. 1.
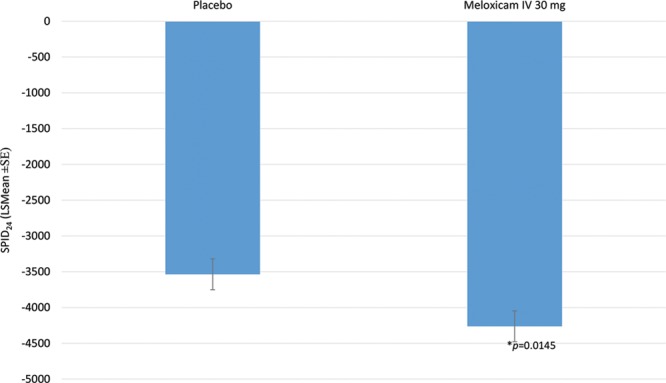
Primary endpoint: SPID24.
For the secondary efficacy endpoints, meloxicam IV was associated with statistically significant differences from placebo in most, but not all outcome measures. Meloxicam IV demonstrated a statistically significant effect on pain reduction compared with placebo in SPID12, SPID48, and SPID24–48, but not at SPID6 (Table 2). PI differences (PIDs) from baseline for each treatment group over time (ie, from 0 to 48 hours) are illustrated in Fig. 2. As illustrated, the meloxicam IV group had numerically lower PID values compared with the placebo group at every study time point after 30 minutes. In the meloxicam IV group, there was a statistically significant difference in time to perceptible pain relief as compared with the placebo group (median 0.76 versus 1.28 hours; P = 0.0050); however, there was no statistically significant difference between the meloxicam and placebo groups for time to meaningful pain relief (median ~ 3 hours in both groups; P = 0.5096). Significantly more subjects who received meloxicam IV had a ≥ 30% improvement in the first 24 hours compared with placebo (71.8% versus 56.9%; P = 0.0178). Although a higher proportion of meloxicam-treated subjects had ≥ 50% improvement in the first 24 hours, the difference between groups was not statistically significant (28.2% versus 18.3%; P = 0.0788). During the first 6 hours after treatment, there were no statistically significant differences between treatment groups in the proportion of subjects with ≥ 30% (36.4% versus 31.2%) and ≥ 50% (13.6% versus 8.3%) improvement in pain. Regarding PGA, there was a statistically significant difference between groups favoring meloxicam IV at hour 48 (P = 0.0027) but not at hour 24. Overall, the proportion of subjects reporting good or better (2+ or greater) pain control on the PGA was numerically better for meloxicam IV–treated subjects than for placebo at hour 24 (84.3% versus 67.3%) and hour 48 (88.8% versus 83.4%).
Table 2.
Summary of LS Mean (SE) SPID Data at Secondary Time Points
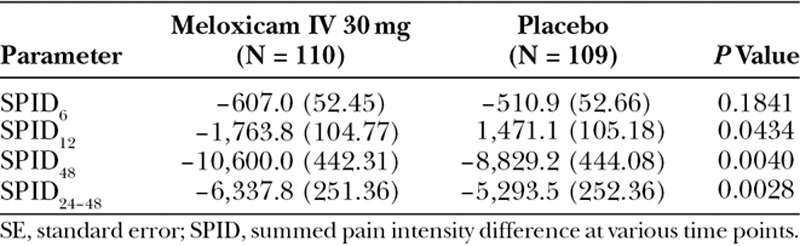
Fig. 2.
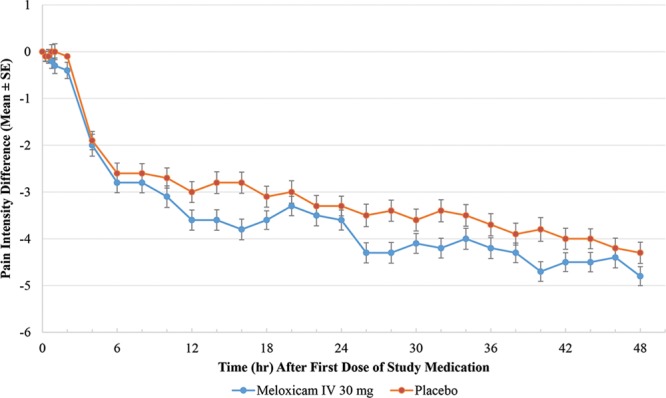
Mean PID values (± standard error) from hours 0–48.
The vast majority of subjects in both treatment arms used ≥ 1 dose of rescue medication (oxycodone 5 mg every 2 hours as needed) in the first 24 hours (88.2% versus 89.9%; P = 0.6559). There was no statistically significant difference in the time to first dose of rescue medication (2.6 versus 2.45 hours). However, significantly fewer meloxicam IV-treated subjects received rescue medication during hours 24–48 (55.6% versus 75.7%; P = 0.0014) and the number of doses of rescue analgesia per subject was also significantly lower among meloxicam IV-treated subjects during hours 0–24 (3.66 versus 4.38; P = 0.0275), hours 24–48 (1.75 versus 2.72; P = 0.0009), and hours 0–48 (5.38 versus 7.07; P = 0.0027).
Exploratory efficacy analyses showed that subjects treated with meloxicam IV had greater pain reduction, fewer subjects requiring rescue (Fig. 3), and a lower mean number of rescue analgesic doses per subject (Fig. 4) than placebo-treated subjects in the second 12 hours and last 6 hours of the dosing interval. Although the magnitude of difference in pain reduction between treatment groups as measured by SPID was smaller in the last 6 hours of the dosing interval, the meloxicam IV group maintained a greater reduction in PI compared with the placebo group. The differences in SPIDs were statistically significant in favor of meloxicam IV at all intervals (SPID12–24, [P ≤ 0.0115]; SPID36–48, [P ≤ 0.0119]; SPID42–48, [P ≤ 0.0370]) with the exception of SPID18–24 (P ≤ 0.1559), where the direction of effect was maintained.
Fig. 3.
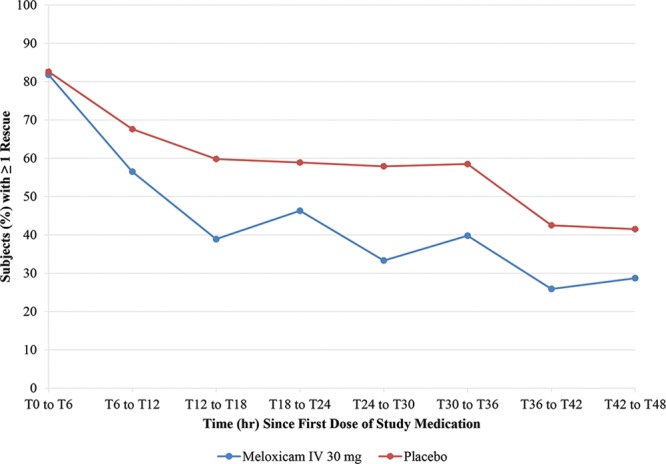
Proportion of subjects with ≥ 1 rescue analgesia in 6-hour intervals.
Fig. 4.
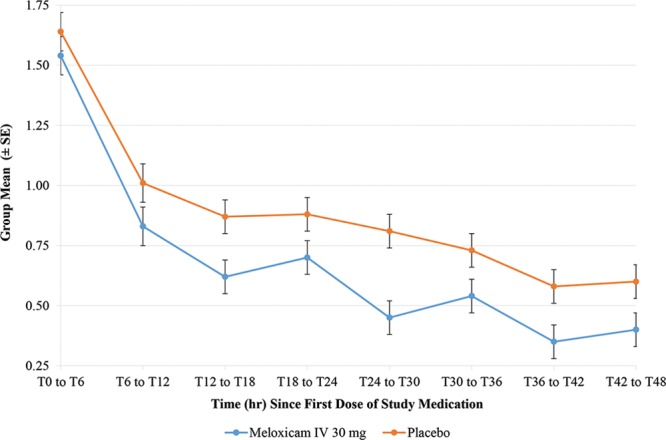
Mean number of rescue analgesia doses per subject in 6-hour intervals.
Safety
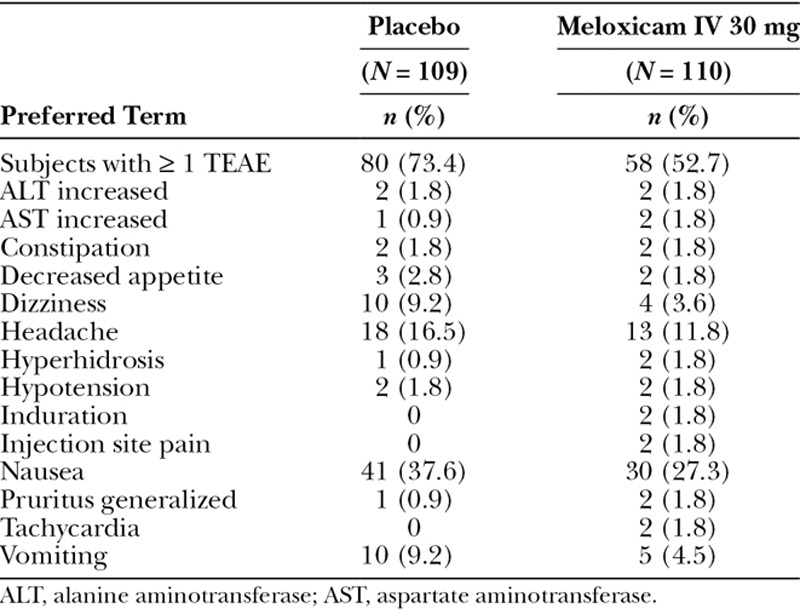
Meloxicam IV 30 mg was well tolerated with no deaths during the study. In addition, no subjects discontinued from the meloxicam IV group due to an AE. The most common treatment-emergent adverse events (TEAEs) are summarized in Table 3. Nausea, headache, vomiting, and dizziness were the most common events in the meloxicam IV group, which were observed at lower incidences than in the placebo group. All events in meloxicam-treated subjects were mild to moderate in intensity and most were considered either not related (39%) or only possibly related (59%) to treatment.
Table 3.
TEAEs Occurring in ≥ 1% of Meloxicam IV-Treated Subjects
Four subjects, 3 (2.8%) in the placebo group and 1 (0.9%) in the meloxicam IV group, experienced a serious AE. Two serious AEs related to bleeding (postprocedural hemorrhage) were reported (1 in each group). One subject (placebo group) discontinued treatment due to this event. The 2 other serious AEs in the placebo group were pulmonary embolism and postoperative wound infection.
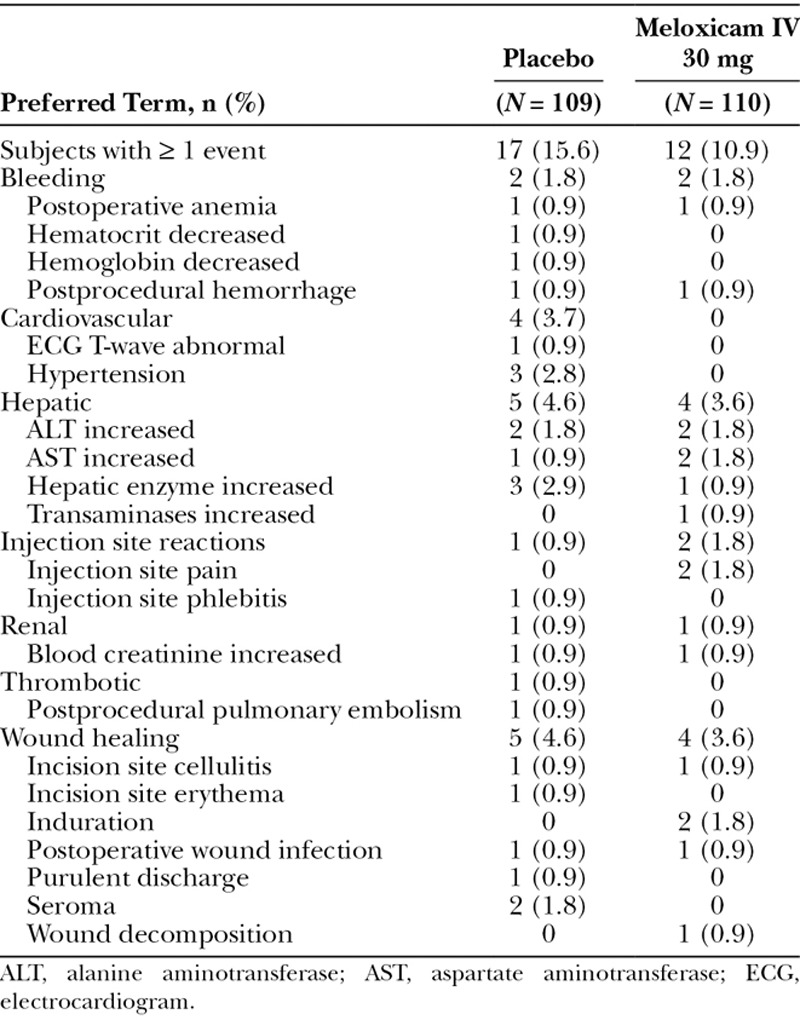
The overall incidence of AEs of special interest was similar between the meloxicam IV and placebo groups (10.9% and 15.6%, respectively) as summarized in Table 4. In particular, wound healing assessments were generally consistent with investigator expectations as reflected by a high level of investigator satisfaction with wound healing and a similar incidence of clinically significant wound status observations in the meloxicam IV group and placebo groups (3.6% and 4.6%, respectively).
Table 4.
AEs of Special Interest Occurring in ≥ 1 Subject
Few clinically meaningful vital sign changes or electrocardiogram results were reported during the study. In addition, while there were shifts in individual clinical laboratory parameters over time, the shifts were generally similar between meloxicam IV- and placebo-treated subjects. None of these variations were suggestive of a clinically significant trend across subjects within any treatment group.
DISCUSSION
Abdominoplasty is a relatively new model of moderate to severe postsurgical pain lasting > 24 hours postprocedure.18,19 This model was thought to be a good choice for this study because (1) the soft tissue dissection employed for abdominoplasty is quite extensive and as such represents a clinical scenario that can be extrapolated to other large/major soft-tissue surgeries; (2) recruitment into the model is relatively rapid; (3) rigid experimental controls can be employed to reduce patient-to-patient and site-to-site variability without sacrificing subject safety; and (4) the surgery has a low complication rate and a high patient satisfaction rate.20,21
In this study, meloxicam IV 30 mg provided sustained pain relief when administered to subjects with moderate-to-severe pain following abdominoplasty. In particular, the primary endpoint (SPID24) was met, demonstrating significant reduction in pain from baseline with a single dose. In addition, various secondary efficacy endpoints were met, including other SPID intervals (SPID12, SPID48, SPID24–48), time to perceptible and meaningful pain relief, proportion of subjects with pain reduction from baseline, PGA of pain control at 48 hours, and the use of rescue medications.
In this study, subjects were required to have moderate-to-severe pain before they were eligible to receive study medication. It is known that acute pain is more difficult to manage if permitted to become severe.22 In clinical practice, subjects would most likely receive meloxicam IV after surgery and before PI reaches the moderate-to-severe threshold, when it would be more difficult to control and when the need for rescue analgesia would be increased. This may explain why during the earliest time interval (0–6 hours, SPID6), reductions in PI did not reach statistical significance for some secondary endpoints (ie, SPID and PID).
Meloxicam IV was also associated with a reduction in opioid rescue medication use, including reductions in both the number of subjects receiving rescue medication during hours 24–48 and the number of rescue doses throughout the 48-hour postsurgery period. The observed decrease in opioid rescue medication may be particularly noteworthy given the known opioid-related AEs (GI effects [nausea/vomiting, constipation], pruritus, sedation, respiratory depression) and the risk of opioid dependency with opioid use for postoperative pain.5–7 Although statistical comparisons were not performed for AEs, subjects in the meloxicam IV group tended to have lower rates of nausea and vomiting compared with placebo-treated subjects.
There is no obvious reason why there was no difference between the meloxicam IV and placebo groups in time to first rescue analgesic use and in the proportion of subjects who used ≥ 1 dose of rescue medication during the first 24 hours. The high proportion of placebo subjects who used rescue medications in this study contrasts with 2 recent studies that evaluated acute pain after abdominal surgery.18,19 In the 2 studies, approximately 64% of placebo subjects used ≥ 1 dose of rescue medication compared with 89.9% of placebo subjects in the current study.
Some of the factors that may have influenced the outcome of rescue medication use endpoints in this study could be study design, including time between the end of surgery and the time a subject is eligible for their first dose of study drug, the subject’s expectation about the timing and duration of rescue medication effect, and/or the subject’s ability to tolerate any degree of pain or any worsening of pain.
Overall, the end-of-dosing interval analyses demonstrated that, over the 24-hour dosing period, subjects treated with meloxicam IV had greater pain reductions (SPID and PID), a lower proportion of subjects requiring rescue, and a lower number of rescue medication doses per subject compared with the placebo group.
Meloxicam was generally well tolerated with no increased risk of AEs compared with those receiving placebo. The occurrence of specific AEs known to be associated with NSAIDs8,23 were generally similar to or lower in meloxicam-treated subjects compared with those receiving placebo. Wound healing complications, which have been reported to be significantly increased in patients undergoing abdominoplasty,21 were not different between treatment groups in our study.
A limitation of this study is the variance from normal clinical practice, which includes a prolonged (3-day) stay in the clinic following abdominoplasty to allow for extended safety and efficacy assessments, limited subject access to multimodal analgesia, and delayed administration of analgesia until pain reached the threshold of moderate to severe. The model is relatively new and may evolve as more data regarding the postoperative pain profile become available. Based on the results of this study, future use of this model may need to evaluate immediate postoperative pain management, similar to that utilized in bunionectomy studies, to achieve a more stable baseline score and less rescue use in the first hour following study medication administration.
CONCLUSIONS
This study demonstrated that meloxicam IV 30 mg provided significant pain relief in subjects with moderate-to-severe pain following abdominoplasty surgery. Once daily dosing with meloxicam IV maintained analgesia over the 24-hour dosing interval and reduced postoperative rescue opioid use. Furthermore, meloxicam IV administered as bolus injections was generally well tolerated with a safety profile comparable with placebo. Findings from this study support further investigation of the efficacy and safety of once-daily administration of meloxicam IV within the context of a perioperative multimodal approach for managing moderate-to-severe pain.
ACKNOWLEDGMENTS
Assistance with article preparation was provided by Bret Fulton, RPh, and Susan Martin, PhD, of The Medicine Group (New Hope, PA).
Footnotes
Published online 19 June 2018.
Supported by Recro Pharma, Inc., Malvern, Penn.
Presented at Plastic Surgery the Meeting (ASPS) 2017 in Orlando, Fla.
Disclosure: Dr. Neil Singla and Dr. Sonia Singla are employees of Lotus Research, which conducted this trial. Dr. Matthew Bindewald is an employee of MGB Plastic Surgery Associates of San Antonio, who conducted this trial. Dr. David Leiman is an employee of HD Research, who conducted this trial. Dr. Harold Minkowitz is an employee of Research Concepts, who conducted this trial. Dr. Wei Du received consultancy fees from Recro Pharma, Inc., Malvern, Penn. Randall J. Mack, Dr. Stewart W. McCallum, and Dr. Alex Freyer are employees and security holders of Recro Pharma, Inc., Malvern, Penn. Dr. Rosemary Keller was an employee of Recro Pharma at the time of this study. The Article Processing Charge was paid for by Recro Pharma.
Trial Registration: ClinicalTrials.gov Identifier: NCT02678286. Name of Registry: Evaluation of N1539 Following Abdominoplasty Surgery.
Plain Language Summary: Meloxicam is a long-acting NSAID with preferential COX-2 inhibition and possesses sustained anti-inflammatory, analgesic, and antipyretic activities. An intravenous (IV) nanocrystal formulation of meloxicam is being investigated for the management of moderate-to-severe pain. The primary objective of this article was to report the findings from clinical trial NCT02678286 on the analgesic efficacy and safety of meloxicam IV compared with placebo in subjects with acute moderate-to-severe pain following abdominoplasty surgery. Based on the results from this clinical trial, meloxicam IV provided pain relief over the 24-hour dosing interval and reduced postoperative rescue opioid use. Furthermore, meloxicam IV was generally well tolerated, with a safety profile comparable with placebo.
REFERENCES
- 1.The American Society of Plastic Surgeons National Clearinghouse of Plastic Surgery Procedural Statistics. 2016 Plastic surgery statistics report. 2017. Available at https://www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed September 7, 2017.
- 2.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248. [DOI] [PubMed] [Google Scholar]
- 3.Feng LJ. Painless abdominoplasty: the efficacy of combined intercostal and pararectus blocks in reducing postoperative pain and recovery time. Plast Reconstr Surg. 2010;126:1723. [DOI] [PubMed] [Google Scholar]
- 4.Morales R, Jr, Mentz H, 3rd, Newall G, et al. Use of abdominal field block injections with liposomal bupivicaine to control postoperative pain after abdominoplasty. Aesthet Surg J. 2013;33:1148. [DOI] [PubMed] [Google Scholar]
- 5.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105. [PubMed] [Google Scholar]
- 6.Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95:301. [DOI] [PubMed] [Google Scholar]
- 7.White PF. What are the advantages of non-opioid analgesic techniques in the management of acute and chronic pain? Expert Opin Pharmacother. 2017;18:329. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131. [DOI] [PubMed] [Google Scholar]
- 9.Pogatzki-Zahn E, Chandrasena C, Schug SA. Nonopioid analgesics for postoperative pain management. Curr Opin Anaesthesiol. 2014;27:513. [DOI] [PubMed] [Google Scholar]
- 10.Degner F, Türck D, Pairet M. Pharmacological, pharmacokinetic and clinical profile of meloxicam. Drugs Today. 1997;33:739. [Google Scholar]
- 11.Mobic [package insert]. 2016Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.. [Google Scholar]
- 12.Del Tacca M, Colucci R, Fornai M, et al. Efficacy and tolerability of meloxicam, a COX-2 preferential nonsteroidal anti-inflammatory drug. Clin Drug Investig. 2002;22:799. [Google Scholar]
- 13.Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1. [DOI] [PubMed] [Google Scholar]
- 14.Davies NM, Skjodt NM. Clinical pharmacokinetics of meloxicam. A cyclo-oxygenase-2 preferential nonsteroidal anti-inflammatory drug. Clin Pharmacokinet. 1999;36:115. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb IJ, Tunick DR, Mack RJ, et al. Evaluation of the safety and efficacy of an intravenous nanocrystal formulation of meloxicam in the management of moderate-to-severe pain after bunionectomy. J Pain Res. 2018;11:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen SE, Cooper SA, Mack RJ, et al. A randomized double-blind controlled trial of intravenous meloxicam in the treatment of pain following dental impaction surgery. J Clin Pharmacol. 2018;58:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack RJ, Du W, Freyer A. An evaluation of the efficacy and safety of N1539, a novel intravenous formulation of NanoCrystal meloxicam, in subjects with moderate to severe pain following hysterectomy. American Pain Society Annual Scientific Meeting; May 11–14, 2016; Austin, TX. [Google Scholar]
- 18.Minkowitz HS, Leiman D, Melson T, et al. Sufentanil sublingual tablet 30 mcg for the management of pain following abdominal surgery: a randomized, placebo-controlled, phase-3 study. Pain Pract. 2017;17:848. [DOI] [PubMed] [Google Scholar]
- 19.Singla N, Minkowitz HS, Soergel DG, et al. A randomized, phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res. 2017;10:2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staalesen T, Elander A, Strandell A, et al. A systematic review of outcomes of abdominoplasty. J Plast Surg Hand Surg. 2012;46:139. [DOI] [PubMed] [Google Scholar]
- 21.Hensel JM, Lehman JA, Jr, Tantri MP, et al. An outcomes analysis and satisfaction survey of 199 consecutive abdominoplasties. Ann Plast Surg. 2001;46:357. [DOI] [PubMed] [Google Scholar]
- 22.Berry P, et al.; National Pharmaceutical Council (NPC). Management of acute pain and chronic noncancer pain. In: Pain: Current Understanding of Assessment, Management, and Treatments. Reston, Va.: NPC:60. Available at americanpainsociety.org/uploads/education/npc.pdf. Accessed February 68, 2018. [Google Scholar]
- 23.McCarberg B, Gibofsky A. Need to develop new nonsteroidal anti-inflammatory drug formulations. Clin Ther. 2012;34:1954. [DOI] [PubMed] [Google Scholar]


