Summary:
This is the first case report of long-term follow-up after applying the autologous cultured dermal substitute to establish the wound bed before split skin graft. The results suggest that application of autologous cultured cultured dermal substitute contributes to establish the high-quality wound bed for skin graft. Split-thickness skin grafts (STSGs) are the gold standard for the treatment of burn scar contracture. Young patients in particular may require additional skin grafts as they grow, and donor site for skin grafts may be limited. We applied autologous cultured dermal substitutes (CDSs) that are expected to establish a high-quality wound bed to allow thin STSGs. This is the first report of follow-up after application of autologous CDS combined with thin STSG. A male neonate suffered third-degree burns (20% of the total body surface area) on the back. After 2 years, scar contracture of the gluteal regions were released and autologous CDS were applied. Five days after the treatment, a super thin (4–6/1,000 per inch) skin grafting was performed. After 3 years, scar contracture of the back was released and autologous CDS was applied for 2 weeks. Then a split-thick graft was harvested from the same donor site. Ten years after the last operation, the width of the skin graft on his back has extended from 5–8 cm. The contour of the grafted skin is soft, smooth, and can be pinched. This long-term result shows the autologous CDS can be expected to establish the high-quality wound bed that allows thin STSG.
Skin grafting is now accepted as the gold standard for the treatment of second- or third-degree burns.1 Young patients in particular may require additional skin grafts as they grow, and donor site for skin grafts may be limited. In 2006, Fujimori et al.2 reported managing an extensive burn scar by combining autologous cultured dermal substitutes (CDSs) and super-thin STSGs. They noted that the donor site of the super-thin STSG did not cause hypertrophic scarring. There were concerns, however, that super-thin STSGs containing small amounts of dermis caused marked contracture. The aim of this case report was to describe the long-term clinical outcome after applying an autologous CDS in combination with super-thin STSGs to treat extensive burn scar contracture.
MATERIALS AND METHODS
The clinical evaluation of autologous CDS was conducted in compliance with the ethical guidelines of Osaka Medical College Hospital. The autologous CDS was prepared using the method described previously.3 Briefly, approximately, 1 × 2 cm of normal skin was harvested from the donor-patients. The fibroblasts were extracted via enzyme treatment and cultivated to establish a cell bank. The cultured fibroblasts were seeded onto 2-layered sponges composed of hyaluronic acid and atelocollagen. The number of fibroblasts was adjusted to approximately 1.0 × 105 cells/cm2. The autologous CDS was ready after 1 week of quarantine.4–6
CASE REPORT
A male neonate suffered extensive third-degree burns (20% of the total body surface area) on his back caused by a hot-water bag in the nursery (Fig. 1). Eighteen days after the burn injury, he underwent treatment with a combination of small pieces of autologous skin grafts harvested from the posterior surface of his lower leg and meshed allogeneic skin grafts. Six months after treatment, the scar contracture of his back was released by Z-plasty, and 1 × 2 cm of normal skin was taken from the neighboring area to create an autologous CDS. After 2 years, scar contracture of the gluteal regions was released, and autologous CDS was applied to the defect. Five days later, a super-thin (0.004–0.006 inch) STSG was applied. After 3 years, another contracture on his back was released, and autologous CDS was applied for 2 weeks (Fig. 2). STSG was then harvested from the same donor site used when he was 2 years old (Fig. 3). After this last operation, the patient no longer requires additional treatment as the range of his forward-bending position has been adequate. Furthermore, the width of the skin graft on his back has extended from 5 cm to 8 cm in the span of 10 years. The contour of the grafted skin is soft and smooth enough that it can be pinched (Fig. 4).
Fig. 1.

A male neonate has burns on his back that affects 20% of his total body surface area.
Fig. 2.
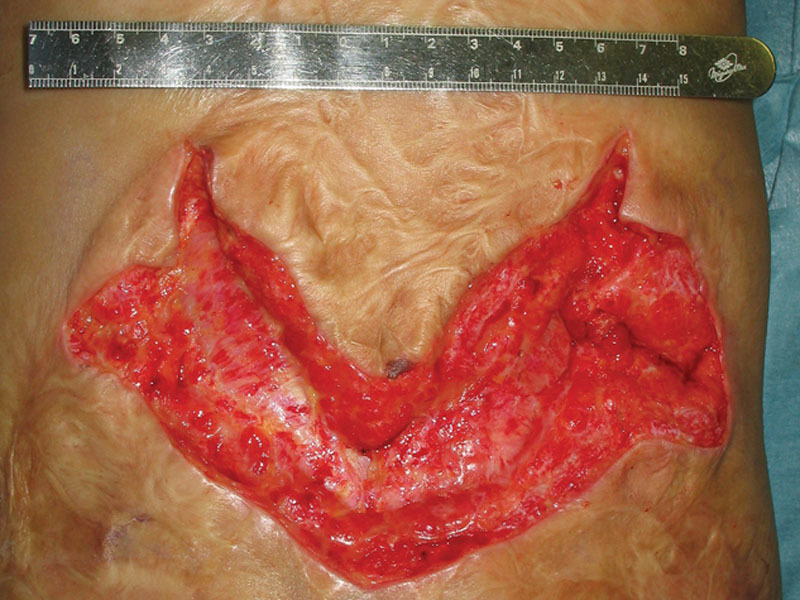
After 3 years, the scar contracture, which involved the deep fascial layer on his back was released. A wound bed was established by applying autologous cultured dermal substitutes for 2 weeks.
Fig. 3.

The donor site of a previous super-thin skin graft did not involve hypertrophic scarring.
Fig. 4.

Ten years after the patient’s last scar-releasing surgery, the skin graft has expanded along with the patient’s natural growth. The contour of the grafted skin is soft, smooth, and can be pinched.
DISCUSSION
Postburn scar contraction can cause functional impairment. Young patient in particular releasing of the contracture and additional skin grafting may be required as they grow. On the other hand, donor sites of the STSG often cause the hypertrophic scarring, and skin grafts cannot be harvested from the same donor site.7 Therefore, donor sites for skin grafts may become limited during treatments, and additional iatrogenic scarring may become a concern. Hypertrophic scarring at donor sites develop after injuries that involve a deep layer of dermis; therefore, thin STSG, that is, 4–6/1,000 inches is suitable considering its donor morbidity. However, thin skin grafts containing small amounts of dermis is prone to contracture. Contraction of thin skin grafts is caused by contracture of myofibroblasts of the wound bed. Recently, dermal regeneration template (Integra Life Sciences, Plainsboro, N.J) has been accepted as an option for contracture release procedures. They provide the necessary scaffold to induce fibroblast invasion and capillary growth to form neo-dermis. However, they cannot reduce myofibroblasts, and young patients who have undergone contracture release using dermal regeneration templates may require additional treatments they grow.8 In 2000, Lamme et al.9 did a study about the effect of dermal substitutes seeded with autologous fibroblasts. Their study indicated that cultured autologous fibroblasts significantly reduced the contracture of the STSG by reducing the myofibroblasts. Furthermore, mature collagen bundles and elastins were significantly higher in the regenerated tissue treated with the dermal substitute seeded with autologous fibroblasts.
Allogenic CDS was first described by Kuroyanagi et al.3 who showed that CDSs have the ability to release cytokines. The presence of these cytokines, known to be key factors, are expected to improve wound healing. Furthermore, autologous CDS is supposed to reduce scar contracture. In 2006, Fujimori et al.2 involved the management of an extensive burn scar by combining autologous CDS and super thin STSG. Young patients with extensive burn scars often require several surgical treatments as they grow. Furthermore, hypertrophic scarring of STSG donor sites makes it difficult to harvest skin grafts from the same donor sites. 2 As a result, donor sites for STSG are limited, and iatrogenic scar formation becomes an inevitable complication. Ideally, additional scarring should be avoided or minimized. To fulfill this condition, super-thin STSGs are expected to reduce hypertrophic scarring at the donor sites. Thin STSGs, however, are associated with the risk of secondary contracture. Our long-term result suggests that autologous CDS can be used to establish high-quality wound beds that allow thin skin grafts.
Footnotes
Published online 19 June 2018.
This study has been approved by the institutional review board of Osaka Medical College. Registration Number: 200.
The cultured dermal substitutes were provided by R&D Center for Artificial Skin, School of Allied Health Sciences, Kitasato University, Kanagawa, Japan, under the support of the Regenerating Medical Millennium Project of the Ministry of Health, Labour, and Welfare of Japan. This study was conducted as a clinical study of the application of CDS in compliance with the ethical guidelines of Osaka Medical College. Therefore, the CDS was produced and donated without any charge.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.McDonald WS, Deitch EA. Hypertrophic skin grafts in burned patients: a prospective analysis of variables. J Trauma. 1987;27:147. [DOI] [PubMed] [Google Scholar]
- 2.Fujimori Y, Ueda K, Fumimoto H, et al. Skin regeneration for children with burn scar contracture using autologous cultured dermal substitutes and superthin auto-skin grafts: preliminary clinical study. Ann Plast Surg. 2006;57:408. [DOI] [PubMed] [Google Scholar]
- 3.Kuroyanagi Y, Kubo K, Matsui H, et al. Establishment of banking system for allogeneic cultured dermal substitute. Artif Organs. 2004;28:13. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa T, Suga Y, Mizoguchi M, et al. Clinical trial of allogeneic cultured dermal substitute for the treatment of intractable skin ulcers in 3 patients with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2004;50:803. [DOI] [PubMed] [Google Scholar]
- 5.Kubo K, Kuroyanagi Y. Development of a cultured dermal substitute composed of a spongy matrix of hyaluronic acid and atelo-collagen combined with fibroblasts: cryopreservation. Artif Organs. 2004;28:182. [DOI] [PubMed] [Google Scholar]
- 6.Yamada N, Uchinuma E, Kuroyanagi Y. Clinical trial of allogeneic cultured dermal substitutes for intractable skin ulcers. J Artif Organs. 2012;15:193. [DOI] [PubMed] [Google Scholar]
- 7.Hinshaw JR, Miller ER. Histology of healing split-thickness, full-thickness autogenous skin grafts and donor sites. Arch Surg. 1965;91:658. [DOI] [PubMed] [Google Scholar]
- 8.Stiefel D, Schiestl C, Meuli M. Integra artificial skin for burn scar revision in adolescents and children. Burns. 2010;36:114. [DOI] [PubMed] [Google Scholar]
- 9.Lamme EN, Van Leeuwen RT, Brandsma K, et al. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol. 2000;190:595. [DOI] [PubMed] [Google Scholar]


