Fossils from Utah show large flowering trees evolved at least 15 million years earlier than previously thought.
Abstract
The diversification of flowering plants and marked turnover in vertebrate faunas during the mid-Cretaceous transformed terrestrial communities, but the transition is obscured by reduced terrestrial deposition attributable to high sea levels. We report a new fossil assemblage from multiple localities in the Upper Cretaceous Ferron Sandstone Member of the Mancos Shale Formation in Utah. The fossils date to the Turonian, a severely underrepresented interval in the terrestrial fossil record of North America. A large silicified log (maximum preserved diameter, 1.8 m; estimated height, ca. 50 m) is assigned to the genus Paraphyllanthoxylon; it is the largest known pre-Campanian angiosperm and the earliest documented occurrence of an angiosperm tree more than 1.0 m in diameter. Foliage and palynomorphs of ferns, conifers, and angiosperms confirm the presence of mixed forest or woodland vegetation. Previously known terrestrial vertebrate remains from the Ferron Sandstone Member include fish teeth, two short dinosaur trackways, and a pterosaur; we report the first turtle and crocodilian remains and an ornithopod sacrum. Previous studies indicate that angiosperm trees were present by the Cenomanian, but this discovery demonstrates that angiosperm trees approaching 2 m in diameter were part of the forest canopies across southern North America by the Turonian (~92 million years ago), nearly 15 million years earlier than previously thought.
INTRODUCTION
Terrestrial communities underwent marked changes associated with the diversification of flowering plants during the mid-Cretaceous (1). Unfortunately, globally high sea levels and reduced terrestrial deposition limit our understanding of these communities. An especially underrepresented time is the Turonian [ca. 94 to 90 million years (Ma) ago] for which few localities are known across western North America for plant and terrestrial vertebrate fossils (2). The combination of a sparse fossil record in western North America and marked ecological changes means that each new discovery has the potential to substantially influence our understanding of the ecological changes that took place during this important interval.
Today, flowering plants range in size from minute herbs to enormous trees. How quickly did they come to occupy this range of morphospace? Ancestral state reconstructions suggest that the common ancestor of crown-group angiosperms produced small amounts of wood (3). Direct evidence from fossils shows that small herbs evolved early (4, 5) but it may have taken millions of years for flowering plants to attain sizes rivaling the tallest living tropical lowland emergent trees. The fossil record of angiosperm woods is informative in part because trunk diameter is related to tree height and standing biomass (6). Fossil angiosperm wood is rare in pre-Campanian deposits, and most are small fragments (<0.1 m in diameter); there are fewer than 100 published occurrences worldwide (7, 8). These attributes contributed to the hypothesis that angiosperms were subordinate to gymnosperm trees in many habitats (9, 10); however, occurrences of moderately large angiosperm wood fragments (0.1 to 1 m in diameter) from Albian-Turonian deposits (11–14) suggest that sampling biases might mask angiosperm physiognomic diversity and abundance in mid-Cretaceous deposits (15), as they do for several Cretaceous terrestrial vertebrate groups (2). Under this hypothesis, large angiosperm trees would have been an important component of poorly sampled mid-Cretaceous communities (15).
Here, we bring a new discovery to bear on the question of when angiosperm trees attained very large size (>1 m in diameter at breast height). By placing our findings in the broader context for fossil angiosperm woods, we show that large angiosperm trees were part of forest canopies across southern North America (Appalachia to the east and Laramidia to the west) by the Turonian. In addition to the fossil wood, we report the first leaf fossils and new terrestrial vertebrate fossils from the Ferron Sandstone Member of the Mancos Shale. Our findings highlight the current severity of geologic biases in the Upper Cretaceous North American fossil record while simultaneously opening a new fossiliferous horizon for remedying those biases.
RESULTS
Our fieldwork expands the floral and vertebrate records in the Ferron Sandstone Member of the Mancos Shale, adding angiosperm macrofossils and turtle, crocodylian, and ornithopod body fossils to already known chondrichthyan (16) and pycnodont teeth (17), pterosaur body fossils (18), and trackways of ornithopod (19) and therizinosaur (20) dinosaurs. The fossils described herein are from the top of parasequence 6 (that is, sixth from the top of the Ferron Sandstone) in sequence 2 (that is, second from the top) of Zhu et al. (21). Bentonites above and below parasequence 6, in sequences 1 and 2 of Zhu et al. (21), constrain the age of the vertebrate and plant body fossils and vertebrate ichnofossils to between 90.64 ± 0.25 Ma and 90.69 ± 0.34 Ma and are correlated to the Prionocyclus macombi ammonite zone of Cobban et al. (22).
Angiosperm wood
A large decorticated log, ~11 m in preserved length with a maximum diameter estimate of 1.8 m, was found at 38.2°N, 110.8°W (more precise location is on file with the Bureau of Land Management). This is approximately double the size of previously documented angiosperm woods from pre-Campanian deposits worldwide (Fig. 1). We did not collect the entire specimen (Fig. 2A), but hand samples and thin sections are archived at the University of Florida (UF 19462-69143). A 3D surface model created through photogrammetry is available on Morphobank (http://morphobank.org/permalink/?P3218). Preservation of the trunk is variable. The core is more than 1.6 m in diameter, and following the partial excavation and careful examination of the trunk, we estimate the diameter at 1.8 m; however, it does not appear to have undergone significant taphonomic distortion on macro- or microscopic scales that would make the diameter appear larger. We estimated the stem length (tree height) at 50.8 m based on the 1.8 m in diameter estimate (at approximately breast height) using the allometric scaling equation developed from the cannel compendium data (23) by Niklas and Spatz (24) and 53.6 m using the pantropical model of height-diameter relationships developed from direct measurements of tropical dicot trees by Feldpausch et al. (25). The diameter at breast height value of 1.8 m is beyond the range of values in both data sets, precluding the use of a prediction interval; however, we defend our initial estimate because the empirical data used to develop these models fit a mechanistic predictive model (24).
Fig. 1. Cretaceous woods.
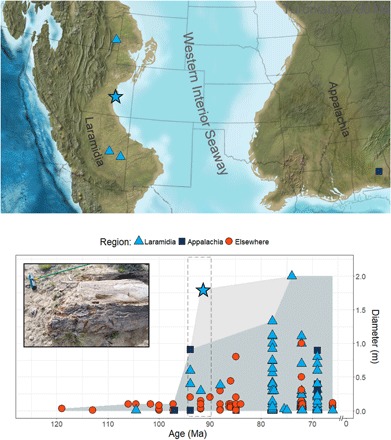
Map of Turonian localities in western North America with angiosperm woods >10 cm in diameter and stacked area curve showing the contribution of this discovery (indicated by star) to the global record of Cretaceous angiosperm woods. Ages are midpoint estimates. The gray area indicates the maximum observed angiosperm diameter through the Cretaceous. Dashed box indicates Turonian occurrences shown in the map above. Inset shows the new angiosperm log in the field (Photo Credit: M.D. D’Emic, Adelphi University). During much of the Late Cretaceous, the Western Interior Seaway divided North America into Appalachia in the east and Laramidia in the west. Map modified from Blakey (38).
Fig. 2. P. cf. alabamense UF 19462-69143.
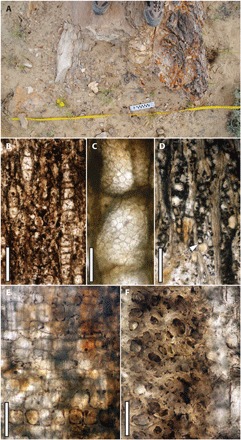
(A) Photograph of the log in the field. (B) Transverse section (XS) showing diffuse porous wood with vessels in short radial multiples of 2 to 11, growth rings absent, axial parenchyma rare, radial bands of fibers, and rays roughly the same width. (C) Tangential longitudinal section (TLS) showing crowded, hexagonal pits on the vessel wall. (D) TLS showing closely spaced lens-shaped 2- to 4-seriate rays among elongate fibers. (E) Radial longitudinal section showing rows of procumbent and upright ray parenchyma cells. (F) XS showing thin-walled ray cells (at left) and medium thick-walled fibers (at right). Scale bars, 500 μm (B), 100 μm (C), 250 μm (D), 200 μm (E), and 50 μm (F). (Photo Credits: A: M.D. D’Emic, Adelphi University; B to F: N.A. Jud, University of Florida)
We studied the anatomy of the angiosperm wood in transverse, radial longitudinal, and TLS from a hand sample taken from the outermost portion of the log. The wood lacks distinct growth rings and is diffuse-porous. The vessels are solitary (30%) and in radial multiples of up to 11, with a mean tangential diameter of 137 μm (n = 25; SD, 30; range, 92 to 181 μm) and a frequency of 6.0 vessels mm−2 (Fig. 2B). The vessel elements have simple perforation plates, abundant tyloses, and crowded alternate intervessel pits 11 and 12 μm across (Fig. 2C). Axial parenchyma is scanty paratracheal (Fig 2B). The rays are heterocellular and mostly 2 to 4 seriate, with 1 to 2 rows of marginal upright cells (Fig. 2, D and E). Mean ray height is 645 μm (n = 25; SD, 299; range, 204 to 1449 μm), and ray frequency is 7.1 mm−1. Poor preservation obscures the vessel-ray parenchyma pits. Fibers are medium thick-walled (Fig. 2F), septate, and without distinctly bordered pits. We did not observe crystals, storied structure, canals, nor cambial variants.
The fossil belongs to the genus Paraphyllanthoxylon Bailey. In nearly all features observed, this specimen conforms to Paraphyllanthoxylon alabamense Cahoon (table S1); however, we did not observe the vessel-ray parenchyma pits, and it has vessels that are arranged in longer radial multiples than observed in the specimens from Alabama (13). It is our judgment that these differences do not warrant the recognition of a new species, so we refer the Ferron log to P. cf. alabamense. The largest reported diameter from the type locality for that species is 0.9 m (13), although anecdotal evidence suggests that there were also specimens more than 1 m in diameter (26).
Leaf assemblage
Approximately 11 km northwest of the large angiosperm log, we made a small collection of leaf fossils from brownish gray shales (38.39°N, 110.88°W), near an accumulation of Rosselia trace fossils erroneously identified as sauropod dinosaur footprints in a conference abstract (27). The leaves co-occur with impressions of bivalves in a fluvial and distributary channel that is part of a broader fresh-to-brackish water lower delta plain environment. The assemblage includes isolated shoots of Elatides curvifolia (Dunker) Nathorst (Fig. 3, A and B), and fragmentary remains of nonmonocot angiosperm leaves. The most complete angiosperm leaf is the lower portion of a notophyllous leaf with an attached petiole (Fig. 3C). We also found small fern fragments (Fig. 3D).
Fig. 3. Plant compression fossils from the Ferron Sandstone of Utah.

(A) Leafy shoot of E. curvifolia (Dunker) Nathorst; UF 19523-70170. (B) Close-up of (A). (C) Indeterminate angiosperm leaf; UF 19523-70171. (D) Isolated fern pinnule; UF 19523-70170. Scale bars, 5 mm (A to C) and 3 mm (D). (Photo Credits: N.A. Jud, University of Florida)
Vertebrate remains
Vertebrate body fossils occur primarily at the base of fluvial and distributary channels about 10 km northwest of the large angiosperm log and were deposited in a fresh-to-brackish water lower delta plain environment. A single shark tooth [Burpee Museum of Natural History (BMRP) 2017.8.1] is attributable to Cretodus crassidens (Fig. 4A), a common lamniform shark in Turonian Western Interior Seaway deposits (16, 28). We also recovered crocodyliform teeth (BMRP 2017.8.5, BMRP 2017.8.4, and BMRP 2017.8.3; Fig. 4B), a large (~9 cm long and ~1 cm thick) turtle scute (BMRP 2017.8.6; Fig. 4C), and a partial ornithopod sacrum (BMRP 2017.8.2; Fig. 4D).
Fig. 4. Vertebrate fossils from the Ferron Sandstone of Utah.
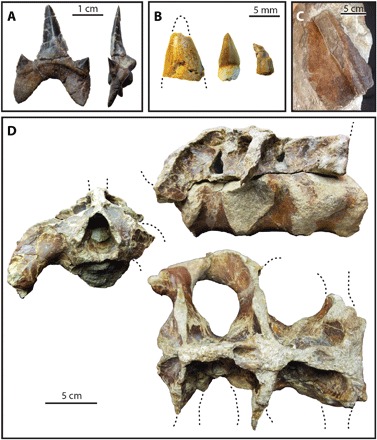
(A) Tooth of C. crassidens BMRP 2017.8.1. (B) Crocodyliform teeth 2017.8.5 (left), 2017.8.4 (center), BMRP 2017.8.3 (right). (C) Dorsal turtle scute BRMP 2017.8.6. (D) Ornithopod sacrum BMRP 2017.8.2. (Photo Credits: S.A. Williams, Burpee Museum of Natural History)
DISCUSSION
The Ferron Sandstone Member preserves fossil wood, leaves, and both terrestrial and marine vertebrate body fossils. The occurrence of C. crassidens and associated radiometrically dated bentonite beds confirms the Turonian age for the fossils. The Turonian vertebrate fossil record is sparse (2), limiting our understanding of the origin of exceedingly diverse and geographically heterogeneous (29) Campanian-Maastrichtian communities. These findings begin to fill the gap in our knowledge of mid-Cretaceous terrestrial communities in North America.
Until now, the only plant fossils known from the Ferron Sandstone Member were palynomorphs obtained within 10 km of where the log reported herein is located (30). The mixture of fungal, algal, and fern spores, taxodiaceous conifer pollen, and palm pollen are indicative of a warm (frost-free), ever-wet climate (30). It is surprising that in the same formation where an enormous angiosperm tree was found, no nonmonocot angiosperm pollen types have yet been reported (30); however, in at least some instances, Paraphyllanthoxylon woods are attributable to Lauraceae (31), a family with a notoriously poor dispersed pollen record (32). The log described here is substantially larger than all previously published reports of angiosperm wood from Cretaceous deposits, except for some stumps from the upper Campanian of New Mexico (33). Collectively, these fossils represent the largest known Cretaceous angiosperms (Fig. 1).
Despite considerable evidence for high species richness among angiosperms since the Albian-Cenomanian (1), the angiosperm contribution to canopy cover before the Campanian-Maastrichtian (that is, during the mid-Cretaceous) remains obscure (10, 15). Only a handful of plant assemblages with fossilized woods are known from the Turonian of North America, and none of those specimens approach the size of the Ferron log (data file S1). The largest of the previously documented mid-Cretaceous woods are P. arizonense logs >0.6 m in diameter from the Cenomanian-Turonian of Arizona (8), and P. alabamense logs >0.9 m in diameter from the Cenomanian-Turonian of Alabama (13, 26). This discovery demonstrates that angiosperm trees approaching 2 m in diameter were part of the canopy by the Turonian nearly 15 Ma earlier than previously thought.
We report new and unexpected floral and faunal occurrences from a severely underrepresented time in the terrestrial geologic record of North America. Among these newly reported occurrences are chondrichthyan, testudine, crocodyliform, and ornithopod remains and fern, conifer, and angiosperm megafossils. This Paraphyllanthoxylon is the earliest documented occurrence of an angiosperm tree more than 1.0 m in preserved diameter. Our findings demonstrate that by the Turonian, flowering plants diversified to effectively fill the full range of heights available to land plants.
MATERIALS AND METHODS
We used traditional survey and excavation methods to recover vertebrate and plant fossils from the study area. The study horizon is the Ferron Sandstone Member of the Mancos Shale Formation in Utah, which records relatively brief intervals of eastward fluvio-deltaic deposition into the then-extensive Western Interior Seaway (21). Plant and vertebrate fossils were collected on lands administered by the Bureau of Land Management under permits UT13-013S and UT13-014E. The fossil log was discovered in a tidal deposit, indicating some degree of transport from the site of growth. We used Agisoft Photoscan (34) to produce 3D models of the log using photogrammetry. Thin sections of transverse, radial, and tangential planes were produced using standard petrographic techniques (35). Thin sections were examined with light microscopy. The description of the wood anatomy follows the International Association of Wood Anatomists guidelines (36). Photos were taken with a Canon Electro-Optical System digital camera, mounted onto a Nikon microscope for microscopic images. The images were processed in Photoshop using only whole-image manipulations to improve contrast. We compared the specimen with other Cretaceous angiosperm woods described in the literature and with fossil specimens at the Florida Museum of Natural History, including those of P. alabamense Cahoon (13). We evaluated the significance of the size of the specimen by comparing it with other Cretaceous occurrences from around the world for which we could obtain diameter estimates. We augmented the data set of North American occurrences presented by Jud et al. (37) with updated diameter estimates, and we included occurrences from other continents.
Supplementary Material
Acknowledgments
We thank M. Becker (William Paterson University) for discussion, B. Britt and R. Sheetz (Brigham Young University) for collections access, the Bureau of Land Management (J. Reay, S. Fivecoat, R. Hunt-Foster, and S. Foss), field assistants S. Lande, M. Yaravitz, D. Boudreau, and M. Giles, S. M. DeWitt for comments and assistance with figures, C. Gee, and an anonymous reviewer. Funding: This project was supported by the National Geographic Society’s Committee for Research and Exploration (grant 9252-13), Stony Brook University, and Adelphi University. Author contributions: Study design by N.A.J., M.D.D., and S.A.W. N.A.J. and M.D.D. drafted the manuscript. Field work and collection by M.D.D., S.A.W., J.C.M., and K.M.T. Geologic context provided by J.B. Vertebrate descriptions and identifications by M.D.D., S.A.W., and J.C.M. N.A.J. described and identified plant remains. All authors gave final approval for the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data and results supporting this paper have been uploaded as part of the online electronic Supplementary Material and will also be available on Morphobank (http://morphobank.org/permalink/?P3218). Other data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaar8568/DC1
Table S1. Comparison of the Cretaceous Paraphyllanthoxylon species and the anatomically similar genus Aplectotremas.
Data file S1. Cretaceous angiosperm wood fossils used to create Fig. 1 (Excel file).
REFERENCES AND NOTES
- 1.Lidgard S., Crane P. R., Angiosperm diversification and Cretaceous floristic trends: A comparison of palynofloras and leaf macrofloras. Paleobiology 16, 77–93 (1990). [Google Scholar]
- 2.Benson R. B. J., Mannion P. D., Butler R. J., Upchurch P., Goswami A., Evans S. E., Cretaceous tetrapod fossil record sampling and faunal turnover: Implications for biogeography and the rise of modern clades. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 88–107 (2013). [Google Scholar]
- 3.Feild T. S., Arens N. C., Form, function and environments of the early angiosperms: Merging extant phylogeny and ecophysiology with fossils. New Phytol. 166, 383–408 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Taylor D. W., Hickey L. J., An Aptian plant with attached leaves and flowers: Implications for angiosperm origin. Science 247, 702–704 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Jud N. A., Fossil evidence for a herbaceous diversification of early eudicot angiosperms during the Early Cretaceous. Proc. R. Soc. B Biol. Sci. 282, 20151045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enquist B. J., Niklas K. J., Invariant scaling relations across tree-dominated communities. Nature 410, 655–660 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Philippe M., Gomez B., Girard V., Coiffard C., Daviero-Gomez V., Thevenard F., Billon-Bruyat J.-P., Guiomar M., Latil J.-L., Le loeuff J., Néraudeau D., Olivero D., Schlögl J., Woody or not woody? Evidence for early angiosperm habit from the Early Cretaceous fossil wood record of Europe. Palaeoworld 17, 142–152 (2008). [Google Scholar]
- 8.Wheeler E. A., Lehman T. M., New Late Cretaceous and Paleocene dicot woods of Big Bend National Park, Texas and review of Cretaceous wood characteristics. IAWA J. 30, 293–318 (2009). [Google Scholar]
- 9.C. A. Hollick, E. C. Jeffrey, Studies of Cretaceous coniferous remains from Kreischerville, New York, in Memoirs of the New York Botanical Garden (New York Botanical Garden, 1909), p. 220. [Google Scholar]
- 10.Wing S. L., Boucher L. D., Ecological aspects of the Cretaceous flowering plant radiation. Annu. Rev. Earth Planet. Sci. 26, 379–421 (1998). [Google Scholar]
- 11.Bailey I. W., The problem of identifying the wood of Cretaceous and later dicotyledons: Paraphyllanthoxylon arizonense. Ann. Bot. os-38, 439–451 (1924). [Google Scholar]
- 12.Spackman W., Jr, A dicotyledonous wood found associated with the Idaho Tempskyas. Ann. Mo. Bot. Gard. 35, 107–115 (1948). [Google Scholar]
- 13.Cahoon E. J., Paraphyllanthoxylon alabamense—A new species of fossil dicotyledonous wood. Am. J. Bot. 59, 5–11 (1972). [Google Scholar]
- 14.Thayne G. F., Tidwell W. D., Stokes W. L., Flora of the Lower Cretaceous Cedar Mountain Formation of Utah and Colorado, Part I. Paraphyllanthoxylon utahense. Great Basin Nat. 43, 394–402 (1983). [Google Scholar]
- 15.Wolfe J. A., Upchurch G. R. Jr, North American nonmarine climates and vegetation during the Late Cretaceous. Palaeogeogr. Palaeoclimatol. Palaeoecol. 61, 33–77 (1987). [Google Scholar]
- 16.Becker M. A., Wellner R. W., Mallery C. S. Jr, Chamberlain J. A. Jr, Chondrichthyans from the Lower Ferron Sandstone Member of the Mancos Shale (Upper Cretaceous: Middle Turonian) of Emery and Carbon Counties, Utah, USA. J. Paleontol. 84, 248–266 (2010). [Google Scholar]
- 17.Becker M. A., Maisch H. M. IV, Chamberlain J. A. Jr, Pycnodonts from the Lower Ferron Sandstone Member of the Upper Cretaceous Mancos Shale (Middle Turonian), Emery and Carbon counties, Utah. Mt. Geol. 49, 101–114 (2012). [Google Scholar]
- 18.S. C. Bennett, A large pterodactyloid pterosaur from the Late Cretaceous Ferron Sandstone of Utah, in New Perspectives on Pterosaur Palaeobiology, D. W. E. Hone, M. P. Witton, D. M. Martill, Eds. (Geological Society, 2017), vol. 455. [Google Scholar]
- 19.R. Jones, Dinosaur trackway from the Ferron Sandstone Member of the Mancos Shale Formation (Upper Cretaceous) of central Utah, in Proceedings of the 6th Fossil Resource Conference. Lakewood, National Park Service, Geological Resources Division (Radiological Health Department, University of Utah, 2001), pp. 48–51. [Google Scholar]
- 20.G. Gierliński, M. Lockley, A trackmaker for Saurexallopus: Ichnological evidence for oviraptorosaurian tracks from the Upper Cretaceous of Western North America, in At the Top of the Grand Staircase, A. L. Titus, M. A. Loewen, Eds. (Indiana University Press, 2013), pp. 530–535. [Google Scholar]
- 21.Zhu Y., Bhattacharya J. P., Li W., Lapen T. J., Jicha B. R., Singer B. S., Milankovitch-scale sequence stratigraphy and stepped forced regressions of the Turonian Ferron Notom Deltaic Complex, south-central Utah, USA. J. Sediment. Res. 82, 723–746 (2012). [Google Scholar]
- 22.W. A. Cobban, I. Walaszczyk, J. D. Obradovich, K. C. McKinney, “A USGS zonal table for the Upper Cretaceous Middle Cenomanian-Maastrichtian of the Western Interior of the United States based on ammonites, inoceramids, and radiometric ages” U.S. Geological Survey Open-File Report 2006-1250 (2006), 46 pp.
- 23.M. G. R. Cannell, World forest biomass and primary production data (1982); http://agris.fao.org/agris-search/search.do?recordID=XF2015022134.
- 24.Niklas K. J., Spatz H.-C., Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. Proc. Natl. Acad. Sci. U.S.A. 101, 15661–15663 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldpausch T. R., Banin L., Phillips O. L., Baker T. R., Lewis S. L., Quesada C. A., Affum-Baffoe K., Arets E. J. M. M., Berry N. J., Bird M., Brondizio E. S., de Camargo P., Chave J., Djagbletey G., Domingues T. F., Drescher M., Fearnside P. M., França M. B., Fyllas N. M., Lopez-Gonzalez G., Hladik A., Higuchi N., Hunter M. O., Iida Y., Salim K. A., Kassim A. R., Keller M., Kemp J., King D. A., Lovett J. C., Marimon B. S., Marimon-Junior B. H., Lenza E., Marshall A. R., Metcalfe D. J., Mitchard E. T. A., Moran E. F., Nelson B. W., Nilus R., Nogueira E. M., Palace M., Patiño S., Peh K. S.-H., Raventos M. T., Reitsma J. M., Saiz G., Schrodt F., Sonké B., Taedoumg H. E., Tan S., White L., Wöll H., Lloyd J., Height-diameter allometry of tropical forest trees. Biogeosciences 8, 1081–1106 (2011). [Google Scholar]
- 26.Beals H. O., Smoot B. C. Jr, Paleobotany in Alabama. Ala. Acad. Sci. J. 35, 5–6 (1964). [Google Scholar]
- 27.S. A. Williams, M. D. D’emic, S. C. Bennett, J. C. Mathews, K. M. Tremaine, J. P. Bhattacharya, “A new terrestrial vertebrate fauna from the Late Cretaceous Ferron Sandstone of North America,” Journal of Vertebrate Paleontology, Program and Abstracts, 237 (2015).
- 28.K. Shimada, M. Everhart, B. Reilly, C. Rigsby, “First associated specimen of the Late Cretaceous shark Cretodus (Elasmobranchii: Lamniformes)” Journal of Vertebrate Paleontology, Program and Abstracts, 31, 194 (2011).
- 29.Gates T. A., Prieto-Márquez A., Zanno L. E., Mountain building triggered Late Cretaceous North American megaherbivore dinosaur radiation. PLOS ONE 7, e42135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akyuz I., Warny S., Famubode O., Bhattacharya J. P., Palynology of the Upper Cretaceous (Turonian) Ferron Sandstone Member, Utah, USA: Identification of marine flooding surfaces and Milankovitch cycles in subtropical, ever-wet, paralic to non-marine palaeoenvironments. Palynology 40, 122–136 (2016). [Google Scholar]
- 31.Herendeen P. S., Lauraceous wood from the mid-Cretaceous Potomac group of eastern North America: Paraphyllanthoxylon marylandense sp. nov. Rev. Palaeobot. Palynol. 69, 277–290 (1991). [Google Scholar]
- 32.Herendeen P. S., Crepet W. L., Nixon K. C., Fossil flowers and pollen of Lauraceae from the Upper Cretaceous of New Jersey. Plant Syst. Evol. 189, 29–40 (1994). [Google Scholar]
- 33.J. Parrott, G. R. Upchurch, E. A. Wheeler, G. H. Mack, Forest of Giants: An in situ angiosperm forest of Late Cretaceous south-central New Mexico, Botany 2013: Annual Meeting of the Botanical Society of America in New Orleans, LA (2013); http://www.2013.botanyconference.org/engine/search/index.php?func=detail&aid=451 [accessed 01 July 2018]. [Google Scholar]
- 34.AgiSoft PhotoScan Professional (AgiSoft LLC., 2015).
- 35.H. Haas, N. P. Rowe, Thin sections and wafering, in Fossil Plants and Spores: Modern Techniques, T. P. Jones, N. P. Rowe, Eds. (Geological Society of London, 1999), pp. 76–81. [Google Scholar]
- 36.IAWA Committee , IAWA list of microscopic features for hardwood identification. IAWA J. 10 suppl. 3, 219–332 (1989). [Google Scholar]
- 37.Jud N. A., Wheeler E. A., Rothwell G. W., Stockey R. A., Angiosperm wood from the Upper Cretaceous (Coniacian) of British Columbia, Canada. IAWA J. 38, 141–161 (2017). [Google Scholar]
- 38.R. Blakey, Middle Turonian (Pryonocyclus Hyattii) – 90.9 Ma. Deep Time Maps, http://deeptimemaps.com [accessed 04 August 2018].
- 39.Mädel E., Die fossilen Euphorbiaceen-Holzer mit besonderer Berucksichtigung neuer Funde aus der Oberkreide Sud-Afrikas. Senckenberg. Lethaea. 43, 283–321 (1962). [Google Scholar]
- 40.Petrescu I., Ianoliu C., Dragos I., Prezenta genului Paraphyllanthoxylon (Euphorbiaceae) in cretacicul din Bazinul Hateg. Contr. Bot. Cluj. 227–234 (1978). [Google Scholar]
- 41.Serlin B. S., An early Cretaceous fossil flora from northwest Texas: Its composition and implications. Palaeontogr. B. 182, 52–86 (1982). [Google Scholar]
- 42.Wheeler E. F., Lee M., Matten L. C., Dicotyledonous woods from the Upper Cretaceous of southern Illinois. Bot. J. Linn. Soc. 95, 77–100 (1987). [Google Scholar]
- 43.Wheeler E. A., Paleocene dicotyledonous trees from Big Bend National Park, Texas: Variability in wood types common in the Late Cretaceous and early Tertiary, and ecological inferences. Am. J. Bot. 78, 658–671 (1991). [Google Scholar]
- 44.Cevallos-Ferriz S. R. S., Weber R., Dicotyledonous wood from the Upper Cretaceous (Maastrichtian) of Coahuila. Rev. Mex. Cienc. Geológicas. 10, 65–70 (1992). [Google Scholar]
- 45.Wheeler E. A., McClammer J., LaPasha C. A., Similarities and differences in dicotyledonous woods of the Cretaceous and Paleocene. San Juan Basin, New Mexico, USA. IAWA J. 16, 223–254 (1995). [Google Scholar]
- 46.Iamandei E., Iamandei S., Paraphyllanthoxylon bacense n. sp. (Euphorbiaceae) in the Upper Maastrichtian-Lower Palaeocene from Bacea and Techereu, Apuseni (Metalliferous) Mts. Acta Horti Bot. Bucurestiensis. 28, 409–418 (2000). [Google Scholar]
- 47.Meijer J. J. F., Fossil woods from the Late Cretaceous Aachen Formation. Rev. Palaeobot. Palynol. 112, 297–336 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Wheeler E. A., Lehman T. M., Late Cretaceous woody dicots from the Aguja and Javelina Formations, Big Bend National Park, Texas, USA. IAWA J. 21, 83–120 (2000). [Google Scholar]
- 49.Takahashi K., Suzuki M., Dicotyledonous fossil wood flora and early evolution of wood characters in the Cretaceous of Hokkaido, Japan. IAWA J. 24, 269–309 (2003). [Google Scholar]
- 50.Gryc V., Vavrčík H., Sakala J., Cenomanian angiosperm wood from the Bohemian Cretaceous Basin, Czech Republic. IAWA J. 30, 319–329 (2009). [Google Scholar]
- 51.Estrada-Ruiz E., Upchurch G. R. Jr, Wheeler E. A., Mack G. H., Late Cretaceous angiosperm woods from the Crevasse Canyon and McRae Formations, south-central New Mexico, USA: Part 1. Int. J. Plant Sci. 173, 412–428 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaar8568/DC1
Table S1. Comparison of the Cretaceous Paraphyllanthoxylon species and the anatomically similar genus Aplectotremas.
Data file S1. Cretaceous angiosperm wood fossils used to create Fig. 1 (Excel file).


