Abstract
Objective
Postoperative nausea and vomiting (PONV) is a common problem associated with general anaesthesia. The incidence can be as high as 80% in high-risk patients. Our primary objective was to compare the efficacy of the combination of dexamethasone-ondansetron and dexamethasone-aprepitant in patients undergoing laparoscopic surgery.
Methods
Seventy 18 to 60 years old patients scheduled for laparoscopic surgery were included in the study. Sixty-seven patients completed the study. Patients in the dexamethasone-aprepitant group (group DA, n=35) received 40 mg of aprepitant orally 1–2 hours before the induction of anaesthesia and 2 mL saline intravenously (iv) within the last 30 minutes of surgery; patients in the dexamethasone-ondansetron group (group DO, n=35) received oral placebo identical to aprepitant 1–2 hours before the induction of anaesthesia and 4 mg ondansetron iv within the last 30 minutes of surgery. All patients received 8 mg dexamethasone iv after the induction of anaesthesia. The primary outcome was a complete response (no postoperative nausea, retching and vomiting and no need for rescue antiemetic); the secondary outcomes were the incidence of nausea, retching, vomiting, the need of rescue antiemetic and opioid consumption within 24 hours after surgery.
Results
A complete response was not significantly different between the groups (group DO: 67%, DA: 69%) at 24 hours (p=0.93). The incidence of PONV and postoperative opioid consumption was similar between the groups.
Conclusion
The study was designed to evaluate whether the combination of dexamethasone-aprepitant is better than the combination of dexamethasone-ondansetron regarding the complete response for PONV in patients undergoing laparoscopic surgery. The results however showed that dexamethasone-aprepitant has not improved the complete response for PONV compared to dexamethasone-ondansetron.
Keywords: Dexamethasone, ondansetron, aprepitant
Introduction
Postoperative nausea and vomiting (PONV) is one of the most common problems related to surgery and anaesthesia that occurs within 24 hours after surgery (1). In the absence of the pharmacological treatment, the incidence of PONV ranges between 20% and 30% in the general surgical population and increases up to 80% in high-risk surgical patients (2, 3).
The female gender, nonsmoking status, a history of PONV or motion sickness, the type of the surgery, a longer duration of surgery, the use of inhalational anaesthetic agents and nitrous oxide, reversal of the neuromuscular blockade, postoperative pain and the use of postoperative opioids can affect the incidence of PONV (4).
Various antiemetic drugs can be used for the treatment of PONV. Dexamethasone can decrease the incidence of PONV (5). However, some authors emphasise that the combination of antiemetic drugs can further reduce PONV compared to single-agent treatment (6, 7), especially for the high-risk patients (1). The dexamethasone-ondansetron combination effectively reduced the overall incidence of PONV for approximately 50% in high-risk and very high-risk patients compared to the control group (8).
Aprepitant is a neurokinin-1 (NK-1) receptor antagonist, and it has been recently defined as an alternative to prevent PONV (9). Some studies showed that aprepitant is significantly more effective than ondansetron, a serotonin (5-HT3) receptor antagonist, for the prevention of postoperative vomiting in open abdominal surgery (10, 11). However, there are no statistically significant differences in the nausea prevention (10, 11).
The dexamethasone-aprepitant combination was more effective than the dexamethasone-ondansetron combination for the prevention of postoperative vomiting in adults undergoing craniotomy under general anaesthesia (12). Accordingly, aprepitant can be combined with other antiemetic drugs to increase the antiemetic efficiency.
In this study, we examined PONV to evaluate whether the combination of aprepitant and dexamethasone is better than the combination of ondansetron and dexamethasone in patients undergoing laparoscopic surgery. The control group is structured according to the combination of ondansetron and dexamethasone, which is well known for its antiemetic efficacy.
Methods
This study was conducted with IRB approval and was registered with the http://www.clinicaltrials.gov protocol registration system (NCT02021851). An ethical approval (No: 125: 06/28/2011) was provided by the Ethics Committee of the Hospital, Istanbul, Turkey on June 29, 2011. After the approval by the institutional review board and written informed consent from each study participant, 70 American Society of Anaesthesiologist’s Class I or II patients, aged between 18 and 60 years, undergoing a laparoscopic gynaecologic surgical procedure or laparoscopic cholecystectomy under general anaesthesia were included in this double-blind, randomised, controlled trial. Patients were excluded if they were hypersensitive or had contraindication for the studied medications, received an antiemetic drug or steroid within 24 hours before anaesthesia, had a history of diabetes mellitus, or were pregnant and lactating. The patients were informed on how to use the patient-controlled analgesia device during the postoperative period. The smoking status was recorded for each patient.
Aprepitant, placebo identical to aprepitant, 4 mg ondansetron and saline solution were prepared by the pharmacy department and given to the blinded investigators. The patients, anaesthesiologists (except for the primary author), the statistician and observers were all blinded.
Patients were randomly assigned to two study groups of 35 patients, using a computer-generated random number table. Patients in the group DA received 40 mg aprepitant, and patients in the group DO received oral placebo, identical to aprepitant, orally 1–2 hours before the induction of anaesthesia.
All patients were premedicated with intravenous (iv) midazolam (1–2 mg). On the arrival to the operating room, standard anaesthetic monitors were applied. Anaesthesia was induced with iv propofol (2–3 mg kg−1) and fentanyl (1–1.5 μg kg−1). Tracheal intubation was facilitated with rocuronium (0.6 mg kg−1). After tracheal intubation all patients received iv 8 mg dexamethasone. The nasogastric tube was placed in all patients and removed at the end of the surgical procedure. Normocapnic mechanical ventilation was performed after intubation. General anaesthesia was maintained with sevoflurane (1 minimum alveolar concentration) in oxygen/air mixture and remifentanil (0.1–0.3 μg kg−1 min−1) infusion.
Intravenous saline was administered in the group DA, and ondansetron was administered in the group DO. All patients received tramadol (1.5 mg kg−1) and tenoxicam (20 mg) half an hour before emergence. Postoperative analgesia was provided with a patient-controlled analgesia system by using iv tramadol (5 mg mL−1) (2 mL bolus and 10 minutes lockout interval without basal infusion).
After the surgery, muscle relaxation was reversed by administering neostigmine (0.05 mg kg−1) and atropine (0.015 mg kg−1). Patients were extubated and transferred to the recovery unit.
Data collection
Nausea was defined as the subjectively unpleasant sensation associated with the awareness of the urge to vomit. Vomiting was defined as the forceful expulsion of gastric contents from the mouth. Retching was defined as an attempt to vomit, not productive of stomach contents. A complete response was defined as no postoperative nausea (VRS<4), retching or vomiting, and no need for rescue antiemetic. Nausea was rated on an 11-point verbal rating scale (VRS) with 0 equal to ‘no nausea’ and 10 equal to ‘nausea as bad as it could be.’ The nausea, vomiting and retching were assessed immediately on return to recovery room at 0, 30, 60, 90 and 120 minutes and 24 hours postoperatively. Complete response was recorded for 0–24 hours. Demographic data, risk factors for PONV, duration of the surgery, perioperative and postoperative use of tramadol and rescue antiemetic were recorded.
Rescue medication was offered to patients who requested it, had nausea lasting longer than 10 minutes, or had an episode of vomiting. All patients were treated postoperatively with 4 mg ondansetron iv to relieve the symptoms of PONV.
The primary outcome of our study was a complete response that is, no nausea (VRS<4), no retching, no vomiting and no rescue therapy from 0 to 24 hours after the surgery. The secondary outcomes were a decreasing incidence of nausea, retching, vomiting and reduced opioid consumption.
Statistical analyses
Statistical analyses were performed using the IBM Statistical Package for the Social Sciences (IBM SPSS Corp.; Armonk, NY, USA) software version 21. Descriptive analyses were presented as mean±standard deviation for continuous variables and as frequency and percentage for categorical variables. A one-sample Kolmogorov-Smirnov test was used to determine whether the variables were normally distributed. For normally distributed variables, two independent samples t-test was used to compare the mean difference between the two independent groups. For non-normally distributed data, the Mann-Whitney U test was used to compare the median difference between the two independent groups. The chi-squared test and Fisher’s exact test, where appropriate, was used to compare these proportions in different groups. The McNemar test was used to evaluate the change in the proportions between the 0–2-hour and 2–24-hour interms of nausea and retching. The Wilcoxon rank test was used to evaluate the 0–2-hour and 2–24-hour in terms of vomiting. P<0.05 was considered statistically significant between the groups.
The PASS 15 Power Analysis and Sample Size Software (2017) (NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass) was used to calculate the sample size. Based on a previous study, we assumed an approximate 50% complete response (no postoperative nausea [VRS<4], retching or vomiting and no need for rescue antiemetic) during the 0–24 h interval after surgery in the DO group as a control group (13). A sample size of 29 achieves 80.12% power to detect a complete response proportion difference in the DA group, that (P1-P0) of −0.2500 using a two-sided exact test with a significance level (alpha) of 0.05. These results assume that in the DO group, a complete response proportion under the null hypothesis (P0) is 0.5. The study size was set to 35 patients in each group to allow for 15% dropouts.
Results
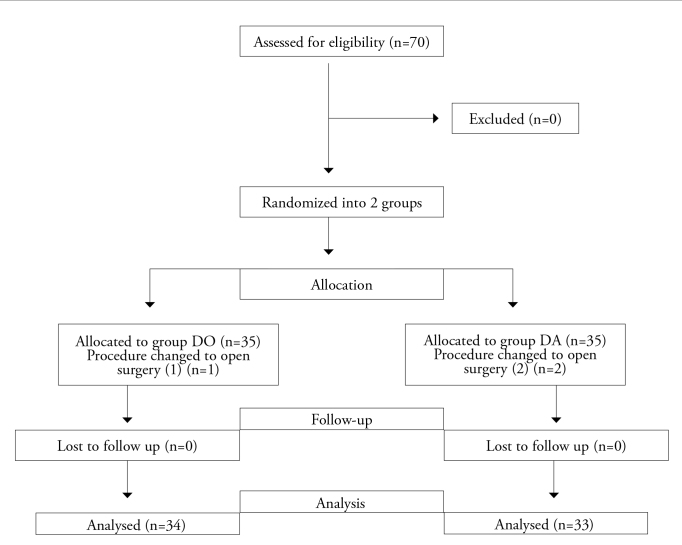
Seventy patients were included into this study. Three patients were excluded from the study due to changes in the surgical procedure from laparoscopy to laparotomy during the surgery. Therefore, 67 patients (in group DO [n=34], in group DA [n=33]) completed the study. Fifty-four of the patients who completed the study were female as opposed to 13 male patients (Figure 1).
Figure 1.
Study flow chart
There was no difference in patient demographics, and Apfel risk factors for PONV and duration of surgery between the two groups (Table 1).
Table 1.
Patient demographics, characteristics and intraoperative data
| Group DO (n=34) | Group DA (n=33) | |
|---|---|---|
| Age (year) | 35.3±7.9 | 40±10.9 |
| Weight (kg) | 66.8±14.3 | 66.9±13 |
| Height (cm) | 166.6±8 | 166.3±8 |
| Apfel’s risk score | ||
| 0 | 0 | 0 |
| 1 | 3 (9%) | 4 (12%) |
| 2 | 13 (38%) | 14 (43%) |
| 3 | 17 (50%) | 15 (45%) |
| 4 | 1 (3%) | 0 |
| Gender | ||
| Male | 5 (15%) | 8 (24%) |
| Female | 29 (85%) | 25 (76%) |
| Duration of surgery (min) | 67.1±24.5 | 74.8±29.4 |
Group DO: dexamethasone-ondansetron group; Group DA: dexamethasone-aprepitant group
Complete response was not different between the groups DO and DA (67% vs. 69%) for 24 hours (p=0.93) (Table 2).
Table 2.
Incidence of postoperative nausea, retching, vomiting, the use of rescue antiemetic, complete response and tramadol consumption in groups
| Group DO (n=34) | Group DA (n=33) | p | |
|---|---|---|---|
| Nausea (VRS≥4) | |||
| 0 min | 2 (6%) | 2 (6%) | 1 |
| 0–30 min | 4 (12%) | 6 (18%) | 0.46 |
| 30–60 min | 1 (3%) | 2 (6%) | 1 |
| 60–90 min | 2 (6%) | 1 (3%) | 1 |
| 90–120 min | 1 (3%) | 0 | 1 |
| 2–24 h | 4 (12%) | 3 (9%) | 1 |
| Retching | |||
| 0–2 h | 3 (9%) | 0 | 0.23 |
| 2–24 h | 1 (3%) | 0 | 0.16 |
| Vomiting | |||
| 0–2 h | 0 | 1 (3%) | 0.49 |
| 2–24 h | 1 (3%) | 1 (3%) | 1 |
| Rescue antiemetic | |||
| 0–24 h | 9 (26%) | 10 (30%) | 0.87 |
| Complete response | |||
| 0–24 h | 23 (67%) | 23 (69%) | 0.93 |
| Tramadol consumption | 252±94 | 255±100 | 0.90 |
Group DO: dexamethasone-ondansetron group; Group DA: dexamethasone-aprepitant group
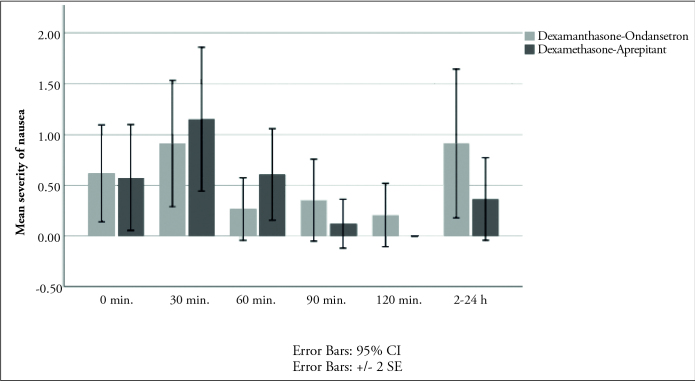
There were no statistically significant differences in the incidence of nausea, retching and vomiting between the two groups (Table 2). The incidence of nausea (VRS≥4) was not statistically significant among the groups at all measurement times (Table 2). The incidence of retching was 9% in the group DO, 0% in the group DA at 0–2h (p=0.23), 3% in the group DO and 0% in the group DA at 2–24h (p=0.16) (Table 2). The incidence of vomiting was 0% in the group DO, 3% in group DA at 0–2 h (p=0.49) and 3% in both groups at 2–24 h (p=1) (Table 2). There was no statistical difference in the severity of nausea between groups during 24 hours (Figure 2). There were no statistical differences between the change scores of nausea (p=0.749), retching (p=0.114) and vomiting (p=0.614) according to time in both groups.
Figure 2.
The severity of nausea scores in groups
Nine patients (26%) in group DO, and 10 patients (30%) in group DA received rescue antiemetic within 24 hours after surgery. This finding was not statistically significant (p=0.87) (Table 2).
There was no difference in tramadol consumption between the two groups (p=0.9) (Table 2).
Discussion
Postoperative nausea and vomiting is a well-described side effect related to the patient, anaesthetic and surgical factors. There are a lot of medications to prevent PONV, such as metoclopramide, dimenhydrinate, serotonin antagonists and dexamethasone. Although an antiemetic prophylaxis might not eliminate the risk of PONV, it can significantly reduce the incidence of nausea and vomiting (14). However, no single excellent medication or method is hitherto described.
Dexamethasone is well documented as an effective antiemetic. A single dose of dexamethasone administered perioperatively is rarely associated with significant side effects (15). Preoperative dexamethasone 8 mg significantly reduces PONV and the use of rescue antiemetic (16, 17). Karanicolas et al. (18) published a systematic review and meta-analysis of 17 randomised controlled trials that evaluated the impact of prophylactic corticosteroid administration on PONV. The authors concluded that prophylactic dexamethasone decreases the incidence of nausea and vomiting and that higher doses of dexamethasone (8–16 mg) are more effective than smaller doses (2–5 mg) in patients undergoing laparoscopic cholecystectomy (18). Therefore, we administered iv 8 mg dexamethasone to all patients in the study. The onset of the effect of dexamethasone usually takes a long time (19). Wang et al. (20) evaluated the effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for PONV. The prophylactic iv dexamethasone, when given immediately before the induction of anaesthesia, is more effective compared to administration at the end of the operation in preventing nausea and vomiting after major abdominal surgery (20). Therefore, dexamethasone is recommended to be administered before or after the induction of anaesthesia (19). Consequently, in this study we preferred to apply dexamethasone after tracheal intubation.
Prophylactic antiemetic therapy is effective, but combinations of antiemetics are recommended for patients who are at high risk of PONV (1, 21, 22). Moreover, patients with a moderate risk of PONV should receive antiemetic combinations with one or more prophylactic drugs from different classes (14). A combination of dexamethasone with other antiemetics is more effective than any single drug alone (19). Kawano et al. (23) concluded that dexamethasone and aprepitant combined were more effective than dexamethasone alone to prevent postoperative vomiting in patients at high-risk PONV. According to these data, we also preferred to combine an antiemetic therapy with dexamethasone for the prevention of PONV.
Ondansetron is a serotonin (5-HT3) receptor antagonist, and it can be used effectively in PONV. However, it might not eliminate PONV completely, probably because it acts through the blockage of one receptor (20). The efficacy of a dexamethasone-ondansetron combination is superior to a mono-therapy in PONV (20). For this reason, in this study, we preferred to apply the dexamethasone-ondansetron combination instead of a mono-therapy as a control group. White et al. (24) recommended combination drug therapy for routine antiemetic prophylaxis with a steroid and a 5-HT3 antagonist for high-risk patients. If a 5-HT3 antagonist is used, it should be given toward the end of the surgery (24). We also used ondansetron within the last 30 minutes of surgery. Kim et al. showed that the antiemetic prophylaxis with the dexamethasone-ondansetron combination is effective in reducing PONV in both high-risk and very high-risk patients (8). However, in this study, despite the prophylactic administration of the antiemetic drug in very high-risk patients, the incidence of PONV was around 30% (8).
Aprepitant is a relatively new selective NK-1 receptor antagonist antiemetic drug, able to alleviate the emetic effects of substance P (25). Some recent studies showed that it is effective in reducing the incidence of PONV in the first 48 hours after anaesthesia following the preoperative oral administration (26).
Kakuta et al. (27) showed that aprepitant can effectively decrease PONV and the amount of pain medication required by patients in laparoscopic gynaecological surgical procedures. In this study, patients received 80 mg of aprepitant orally. However, Dilorio et al. (28) concluded that a single preoperative oral aprepitant dose of 40 mg reduces the percentage of patients with PONV and the need for additional antiemetic drugs after total joint arthroplasty.
Gan et al. (10) published a multicentre, double-blind trial, involving 805 patients. In this study, even though the efficacy of aprepitant for nausea control, the need for rescue antiemetic and a complete response were similar, aprepitant was significantly more effective than ondansetron in the prevention of postoperative vomiting in the first 48 hours after open abdominal surgery. Hartrick et al. (29) found that even though aprepitant significantly reduced the incidence of PONV compared to a combination therapy, when it was used alone, it did not eliminate PONV. Same authors also indicated that to have an optimal prophylaxis for PONV, at least one other antiemetic agent should be added to aprepitant. Relying on the findings of these aforementioned researchers, we combined aprepitant with dexamethasone in this study.
Habib et al. (12) found prophylaxis with aprepitant and dexamethasone to be more effective than the combination of ondansetron and dexamethasone for the prevention of postoperative vomiting in adult patients undergoing craniotomy. However, the incidence or severity of nausea, the need for rescue antiemetic and a complete response did not differ between the groups. This result is similar to the Gan et al. (10) result. Habib et al. (12) found that the incidence of nausea was 65%, the need for rescue antiemetic was 61%, and a complete response was 28% in the aprepitant group at 24 hours. In accordance with the aforementioned study, we administered 40 mg aprepitant preoperatively or 4 mg ondansetron at the end of the surgery, and 8 mg dexamethasone to all patients during the induction period. The incidence or severity of nausea, the need for rescue antiemetic and a complete response did not differ between the groups as shown in the previous study (12). Also, the incidence of vomiting did not differ. In our study, the incidence of vomiting was 0% in group DO and 3% in group DA in the first 2 hours. This ratio was 3% in both groups within the 2–24 hours period. In our study, this incidence was lower than in the study of Habib et al. (12) (36% for the ondansetron group and 14% for the aprepitant group at 24 hours). The timing of the antiemetic therapy and antiemetic drugs were similar in both studies. The incidence of PONV is generally accepted to be 50%–80% after craniotomy (30), and 40%–80% after laparoscopic surgery (31). These incidences are similar. Nevertheless, PONV is directly related with the surgical area in neurosurgical patients (32), and Habib et al. (12) included patients undergoing infratentorial craniotomy with a high PONV incidence in their study.
Gan et al. (10) designed a multicentre study in which they found that complete response was similar among the 40 mg aprepitant (45%) and the 4 mg ondansetron (42%) treatment groups following the open abdominal surgery. In our study, a complete response was also similar among the DA (69%) and DO (67%) groups. Gan et al. (10) reported similar results for aprepitant and ondansetron. We also did not find a significant difference between our groups for a complete response. However, the percentage of complete result was much better in our study than Gan et al.’s study (10), and this finding could be related with the additive effect of the combined therapy. However, further studies are required to evaluate the additional effect of dexamethasone and aprepitant on PONV with or without the combination of these drugs.
Postoperative pain is another factor related to PONV. There was no statistically significant difference in perioperative analgesic consumption between our study groups.
Conclusion
We were unable to demonstrate that dexamethasone with aprepitant improved the complete response for PONV compared to dexamethasone with ondansetron following laparoscopic surgery. However, despite the fact that the complete response in our study was not different between the DO and DA groups for 24 hours, we have noted that our results were approximately 18% better for both groups in comparison to our reference study (i.e., approximately 50%, Gan et al. [13]). This brought us to the conclusion that further studies with larger series are needed for more specific results.
Acknowledgements
The authors thank Kutlughan Soyubol for editorial support.
Footnotes
a) This study was presented as a poster presentation at the European Society of Anaesthesiology (ESA) Euroanaesthesia, May 31–June 3, 2014, Stockholm, Sweden (Volume 31, June 2014, e-supplement 52) b) This study was presented as a poster presentation at 47th National Congress of the Turkish Anesthesiology and Reanimation Association, 20–24 November, 2013, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Yeditepe University Ethics Committee (No: 125: 28/06/2011).
Informed Consent: Written informed consent was obtained from participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.B.; Design - S.B.; Supervision - S.B.; Resources - N.K., M.H.; Materials - S.B.; Data Collection and/or Processing - S.B., M.H., G.Y., N.K.; Analysis and/or Interpretation - S.B., E.Ç.K.; Literature Search - S.B., G.Y., N.K.; Writing Manuscript - S.B.; Critical Review - Ö.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Golembiewski J, Tokumaru S. Pharmacological prophylaxis and management of adult postoperative/postdischarge nausea and vomiting. J Perianesth Nurs. 2006;21:38597. doi: 10.1016/j.jopan.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Tramèr MR. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part I. Efficacy and harm of antiemetic interventions, and methodological issues. Acta Anaesthesiol Scand. 2001;45:4–13. doi: 10.1034/j.1399-6576.2001.450102.x. [DOI] [PubMed] [Google Scholar]
- 4.Golembiewski JA, O’Brien D. A systematic approach to the management of postoperative nausea and vomiting. J Perianesth Nurs. 2002;17:364–76. doi: 10.1053/jpan.2002.36596. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Rodríguez PE, Fuentes-Orozco C, Gonzalez-Ojeda A. Effect of dexamethasone on postoperative symptoms in patients undergoing elective laparoscopic cholecystectomy: randomized clinical trial. A World J Surg. 2010;34:895–900. doi: 10.1007/s00268-010-0457-9. [DOI] [PubMed] [Google Scholar]
- 6.Singla NK, Singla SK, Chung F, Kutsogiannis DJ, Blackburn L, Lane SR, et al. Phase II study to evaluate the safety and efficacy of the oral neurokinin-1 receptor antagonist casopitant (GW679769) administered with ondansetron for the prevention of postoperative and postdischarge nausea and vomiting in high-risk patients. Anesthesiology. 2010;113:74–82. doi: 10.1097/ALN.0b013e3181d7b13a. [DOI] [PubMed] [Google Scholar]
- 7.Fujii Y. The utility of antiemetics in the prevention and treatment of postoperative nausea and vomiting in patients scheduled for laparoscopic cholecystectomy. Curr Pharm Des. 2005;11:3173–83. doi: 10.2174/1381612054864911. [DOI] [PubMed] [Google Scholar]
- 8.Kim EJ, Ko JS, Kim CS, Lee SM, Choi DH. Combination of antiemetics for the prevention of postoperative nausea and vomiting in high-risk patients. J Korean Med Sci. 2007;22:878–82. doi: 10.3346/jkms.2007.22.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apfel CC, Malhotra A, Leslie JB. The role of neurokinin-1 receptor antagonists for the management of postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2008;21:427–32. doi: 10.1097/ACO.0b013e328301831c. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. Aprepitant-PONV Study Group. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9. doi: 10.1213/01.ane.0000263277.35140.a3. [DOI] [PubMed] [Google Scholar]
- 11.Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, et al. Aprepitant-PONV Protocol 091 International Study Group. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99:202–11. doi: 10.1093/bja/aem133. [DOI] [PubMed] [Google Scholar]
- 12.Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011;112:813–8. doi: 10.1213/ANE.0b013e3181ff47e2. [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ, Coop A, Philip BK. A randomized, double-blind study of granisetron plus dexamethasone versus ondansetron plus dexamethasone to prevent postoperative nausea and vomiting in patients undergoing abdominal hysterectomy. Anesth Analg. 2005;101:1323–9. doi: 10.1213/01.ANE.0000180366.65267.F6. [DOI] [PubMed] [Google Scholar]
- 14.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Society for Ambulatory Anesthesia. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 15.Jo YY, Lee JW, Shim JK, Lee WK, Choi YS. Ramosetron, dexamethasone, and their combination for the prevention of postoperative nausea and vomiting in women undergoing laparoscopic cholecystectomy. Surg Endosc. 2012;26:2306–11. doi: 10.1007/s00464-012-2180-0. [DOI] [PubMed] [Google Scholar]
- 16.Tolver MA, Strandfelt P, Bryld EB, Rosenberg J, Bisgaard T. Randomized clinical trial of dexamethasone versus placebo in laparoscopic inguinal hernia repair. Br J Surg. 2012;99:1374–80. doi: 10.1002/bjs.8876. [DOI] [PubMed] [Google Scholar]
- 17.Bilgin TE, Birbicer H, Ozer Z, Doruk N, Tok E, Oral U. A comparative study of the antiemetic efficacy of dexamethasone, ondansetron, and metoclopramide in patients undergoing gynecological surgery. Med Sci Monit. 2010;16:336–41. [PubMed] [Google Scholar]
- 18.Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Ann Surg. 2008;248:751–62. doi: 10.1097/SLA.0b013e3181856024. [DOI] [PubMed] [Google Scholar]
- 19.Ho CM, Wu HL, Ho ST, Wang JJ. Dexamethasone prevents postoperative nausea and vomiting: benefit versus risk. Acta Anaesthesiol Taiwan. 2011;49:100–4. doi: 10.1016/j.aat.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91:136–9. doi: 10.1213/00000539-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 22.Kizilcik N, Bilgen S, Menda F, Türe H, Aydın B, Kaspar EC, Koner O. Comparison of Dexamethasone-Dimenhydrinate and Dexamethasone-Ondansetron in Prevention of Nausea and Vomiting in Postoperative Patients. Aesthetic Plast Surg. 2017;41:204–10. doi: 10.1007/s00266-016-0772-0. [DOI] [PubMed] [Google Scholar]
- 23.Kawano H, Matsumoto T, Hamaguchi E, Manabe S, Nakagawa M, Yamada A, et al. Antiemetic efficacy of combined aprepitant and dexamethasone in patients at high-risk of postoperative nausea and vomiting from epidural fentanyl analgesia. Minerva Anestesiologica. 2015;81:362–8. [PubMed] [Google Scholar]
- 24.White PF, Watcha MF. Postoperative nausea and vomiting: prophylaxis versus treatment. Anesth Analg. 1999;89:1337–9. doi: 10.1097/00000539-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Curran MP, Robinson DM. Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs. 2009;69:1853–78. doi: 10.2165/11203680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Jung WS, Kim YB, Park HY, Choi WJ, Yang HS. Oral administration of aprepitant to prevent postoperative nausea in highly susceptible patients after gynecological laparoscopy. J Anesth. 2013;27:396–401. doi: 10.1007/s00540-012-1529-9. [DOI] [PubMed] [Google Scholar]
- 27.Kakuta N, Tsutsumi YM, Horikawa YT, Kawano H, Kinoshita M, Tanaka K, et al. Neurokinin-1 receptor antagonism, aprepitant, effectively diminishes post-operative nausea and vomiting while increasing analgesic tolerance in laparoscopic gynecological procedures. J Med Invest. 2011;58:246–51. doi: 10.2152/jmi.58.246. [DOI] [PubMed] [Google Scholar]
- 28.DiIorio TM, Sharkey PF, Hewitt AM, Parvizi J. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res. 2010;468:2405–9. doi: 10.1007/s11999-010-1357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartrick CT, Tang YS, Hunstad D, Pappas J, Muir K, Pestano C, et al. Aprepitant vs. multimodal prophylaxis in the prevention of nausea and vomiting following extended-release epidural morphine. Pain Pract. 2010;10:245–8. doi: 10.1111/j.1533-2500.2010.00364.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergese S, Viloria A, Uribe A, Antor A, Fernandez S. Aprepitant versus ondansetron in preoperative triple-therapy treatment of nausea and vomiting in neurosurgery patients: study protocol for a randomized controlled trial. Trials. 2012;13:130. doi: 10.1186/1745-6215-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eriksson H, Korttila K. Recovery profile after desflurane with or without ondansetron compared with propofol in patients undergoing outpatient gynecological laparoscopy. Anesth Analg. 1996;82:533–8. doi: 10.1213/00000539-199603000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Audibert G, Vial V. Postoperative nausea and vomiting after neurosurgery (infratentorial and supratentorial surgery) Ann Fr Anesth Reanim. 2004;23:422–7. doi: 10.1016/j.annfar.2004.01.005. [DOI] [PubMed] [Google Scholar]