Abstract
Background
Osteopontin (OPN), a member of the small integrin binding ligand N-linked glycoprotein family, has been analyzed in numerous types of human malignancy.
Purpose
The present study detected the expression levels of OPN and evaluated its role in tumor progression in patients with non-small cell lung cancer (NSCLC).
Patients and methods
OPN expression levels were detected using immunohistochemistry in 101 NSCLC tumors. The mRNA and protein levels have significant difference between advanced NSCLC and stage I/II NSCLC. The drug resistance, invasive ability and lactate production of NSCLC cancer cell lines (A549 and SK-MES-1) were detected in cancer cells with the disturbance of OPN.
Results
Immunostaining indicated that OPN was primarily expressed in the cytoplasm of NSCLC cells. Moreover, OPN correlates with NSCLC clinical traits. The results demonstrated that OPN expression levels significantly correlated with cancer differentiation, distant metastasis and the efficacy of platinum-based treatment. Notably, the results identified OPN expression levels as a potential factor for predicting the response of cells to first-line platinum-based chemotherapy using multivariate analysis, as well as predicting cancer differentiation and distant metastasis. Additionally, the abrogation of OPN levels reduced lactate production in NSCLC cells and occurred along side with the downregulation of lactate dehydrogenase A (LDHA).
Conclusion
The results of the current study suggest that OPN may be able to predict poor prognosis and cisplatin resistance in patients.
Keywords: osteopontin, non-small-cell lung cancer, clinical outcome, drug resistance, invasion, lactate production
Introduction
Lung cancer is the most frequent cause of cancer-associated mortality worldwide.1,2 Non-small-cell lung cancer (NSCLC) accounts for ~85% of lung cancer cases and has an overall 5-year survival rate of <20.0%.1 This low rate may occur as a result of the majority of cases being diagnosed as late-stage cancer and therefore being unsuitable for curative surgery.3
A number of clinical trials have suggested that adjuvant chemotherapy is the optimal standard treatment for patients with NSCLC at present.1,4,5 However, 70%–80% of patients who receive cisplatin-based chemotherapy develop drug resistance.6 Therefore, predicting tumor drug resistance using novel prognostic biomarkers is important for the treatment of patients with NSCLC. Novel biomarkers may be useful to stratify patients with NSCLC in order to select patients who may benefit from receiving aggressive adjuvant chemotherapy.
Osteopontin (OPN) is expressed by macrophages, fibroblasts, and lymphocytes, as well as endothelial, smooth muscle, and epithelial cells. It is involved in multiple physiological processes including cell adhesion, wound healing, bone resorption, angiogenesis, immune system responses, and tissue remodeling.7,8 OPN is overexpressed in numerous types of cancer including breast, lung, colorectal, stomach, and ovarian cancer as well as melanoma.9,10 Proteomic analysis of tumor tissue has identified OPN as an effective biomarker for numerous types of cancer and a number of studies on clinical specimens have correlated OPN expression levels with high-grade malignancies.7,8,11,12 High levels of OPN expression in lung cancer positively correlate with reduced patient survival rate.13 Additionally, highly expressed OPN in tumor cells is reported to be associated with a poor survival rate of patients with stage I14,15 and aggressive16,17 NSCLC. OPN levels in the circulating plasma from 158 patients with NSCLC were significantly higher compared with patients with benign pulmonary disease and 25 healthy control groups.17 Taken together, previous reports suggest that OPN may aid multiple pathological processes involved in NSCLC, including cancer cell proliferation, invasion, tumor progression, and metastasis. However, the correlation between OPN expression levels and chemotherapeutic efficacy in patients with NSCLC remains to be elucidated.
A key checkpoint of anaerobic glycolysis is the conversion of pyruvate to lactate and this is catalyzed by lactate dehydrogenase A (LDHA).18 Numerous types of cancer exhibit elevated LDHA and this has been associated with tumor cell growth, survival, and invasion.19–21 In a previous study, high levels of LDHA expression were identified to be correlated with drug resistance and a decreased survival time in numerous types of myeloma.22 It remains to be elucidated whether there is a correlation between OPN and LDHA expression levels.
The aim of the present study was to assess OPN expression levels in 101 consecutive patients with NSCLC and correlate its expression levels with the response to platinum-based chemotherapy and clinicopathological parameters. The current study also examined the effect of OPN expression levels on NSCLC cell drug resistance, invasion, and lactate production.
Materials and methods
NSCLC patients and tissue samples
Between March 2010 and August 2016, a total of 73 patients with advanced NSCLC treated with chemotherapy, 28 patients with stage I and II NSCLC, from the Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Soochow University (Suzhou, China) were selected to participate in the current study. The definition of NSCLC stage was formulated following reference instructions.23 Patients were diagnosed with NSCLC following a bronchoscopic lung biopsy and advanced-stage NSCLC included patients with stage III and IV NSCLC that were considered inoperable. Patients underwent imaging studies prior to treatment and received first-line chemotherapy regimens with platinum-based reagents. Treatment response evaluation was performed following the fourth cycle of chemotherapy. The present study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Soochow University. Written informed consent was obtained from all patients.
Immunohistochemistry (IHC)
Paraffin tissue sections were formalin fixed in preparation for IHC. The tissues were cut into 4-µm sections, dewaxed, and rehydrated and, to inactivate the endogenous peroxidase, the tissues were incubated in 0.3% (v/v) hydrogen peroxide and 0.01 M PBS (pH 7.2) for 20 minutes. Antigen retrieval was performed under high pressure for 2 minutes using 0.01 M sodium citrate buffer (pH 6.0). The sections were immunostained with 2 µg/mL anti-OPN primary antibody (Bioworld Technology Co, Ltd, Nanjing, China) overnight at 4°C and stained with a horseradish peroxidase (HRP)/fragment antigen binding polymer-conjugated secondary antibody (OriGene Technologies, Rockville, MD, USA) for 30 minutes at room temperature. Finally, diaminobenzidine was used to activate the HRP-conjugated secondary antibody at room temperature for 1 minute and the tissue was counterstained for 15 minutes with hematoxylin. At least five fields were randomly selected for each section, which were examined and scored by two independent investigators with no knowledge of the clinical details of the patients. The immunostaining was scored as previously described.24 A score of high expression was assigned when ≥30% of the cancer cells were positive for OPN expression and low expression was scored when <30% of the cells were negative for OPN expression. This scoring method was used, as it was preferred to multiple cutoffs that were examined (0%, 20%, 50%, and 100%). The subcellular localization of the staining was also evaluated.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA from the cells was extracted with Trizol® (Thermo Fisher Scientific, Inc, Waltham, MA, USA). The expression levels of hmgb3 were detected using RT-qPCR. The primers used were as follows: sense, 5′-GCCACAGCATCTGGGTATTT-3′; antisense, 5′-GTGATTTGCTTTTGCCTCCT-3′. β-Actin was used as an internal control and the primers for β-actin were: sense, 5′-AGCGAGCATCCCCCAAAGTT-3′; antisense, 5′-GGGCACGAAGGCTCATCATT-3′. RT-qPCR was performed using the SYBR® Green (Takara Biotechnology Co, Ltd, Japan) dye detection method on an Applied Biosystems StepOne™ Sequence Detection System (Thermo Fisher Scientific, Inc).
Cell lines, small interfering RNAs (siRNAs), and OPN-overexpressed plasmids transfection
A549, SK-MES-1, and H1703 human NSCLC cell lines and a normal lung bronchial epithelial cell line 16HBE were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in an atmosphere of 5% CO2 and 95% air. The siRNAs for opn (siOPN-1: 5′-guGAUUGAUAGUCAGGAACUUuc-3′; siOPN - 2 : 5′ - guGAACGACUCUGAUGAUGUAUUuc-3′) and the negative control siRNA (siNC: 5′-guACUCUAUCUGCACGCUGACuc-3′) were synthesized by Guangzhou RiboBio Co, Ltd (Guangzhou, China). The full-length OPN sequence was synthesized and subcloned into pcDNA3.1 vector (Thermo Fisher Scientific, Inc). The plasmids or siRNAs were then transfected into cells separately using Lipofectamine 3000 reagent (Thermo Fisher Scientific, Inc). Transfection was performed as previously described.25
Western blot analysis
A total of 20 µg of protein was electrophoresed on a 10% SDS-PAGE gel, transferred onto polyvinylidene difluoride membrane, blocked, and incubated with primary antibodies (OPN; Bioworld Technology Co, Ltd; 1:1,000 dilution; LDHA; Bioworld Technology Co, Ltd; 1:5,000 dilution). β-Actin (Bioworld Technology Co, Ltd; 1:1,000 dilution) was used as a loading control. The membrane was incubated with a HRP-conjugated secondary antibody at room temperature for 2 hours. Protein bands were detected on a G:Box Chemi-Imager (Syngene, Frederick, MD, USA) using a SuperSignal West Pico Chemiluminescent Detection System (Thermo Fisher Scientific, Inc).
MTT assay
A549, SK-MES-1, and H1703 cells were plated at a density of 1×103 cells/well in 96-well plates in 100 µL medium. Cells were transfected with plasmids or siRNAs for 24 hours prior to cisplatin (CDDP; Sigma-Aldrich Co, St Louis, MO, USA; doses of 0, 0.5, 1, 5, 10, or 50 µg/mL), lactate (Sigma-Aldrich Co), or sodium oxamate (Sigma-Aldrich Co) being added to the medium. At the end of the test, 10 µL MTT was added to each well and the mixture was incubated at 37°C for 4 hours in the dark. The media and MTT were removed and 100 µL dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The absorption value was recorded at a wavelength of 490 nm.
Transwell invasion assay
A transwell invasion assay was performed using a 24-well transwell chamber with a pore size of 8 mm (Costar®; Corning Incorporated, Corning, NY, USA). NSCLC cells (2×103) were transfected with siOPN-1/siOPN-2 or siNC and were seeded in serum-free medium into the insert chambers, which were coated with Matrigel (BD Biosciences, San Jose, CA, USA). Medium supplemented with 10% fetal bovine serum was added to the lower chamber as a chemoattractant. Following incubation for 24 hours, the invaded cells were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and quantified from five microscopic fields.
Lactate production measurement
NSCLC cells were transfected with siOPN-1/siOPN-2 or siNC for 48 hours and were seeded (1×105 cells/well) into 35-mm dishes and incubated at 37°C. Following incubation for 36 hours, the cells were separated using centrifugation. Lactate production levels in the culture media were detected using the Lactate Assay Kit (BioVision, Inc, Milpitas, CA, USA) according to the manufacturer’s protocol.
Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA). For continuous and categorical variables, a two-sample t-test for independent samples and Pearson chi-square test, respectively, were performed. One-way analysis of variance was performed to compare the means of two or more independent groups. P<0.05 was considered to indicate a statistically significant difference.
Results
OPN is overexpressed in tissue from patients with advanced NSCLC
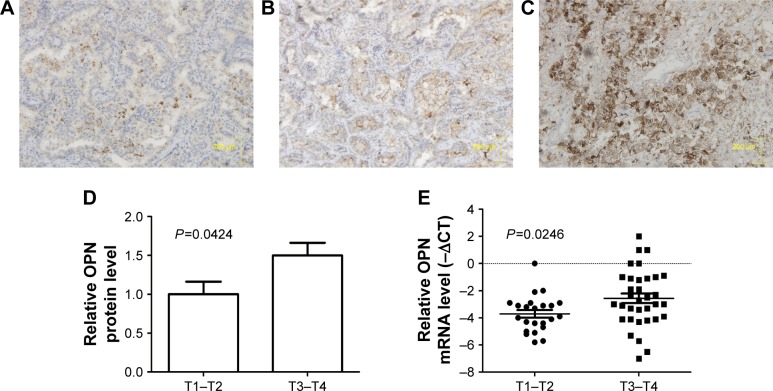
The expression levels of OPN were detected in tissue samples from 28 patients with stage I/II and 73 patients with advanced-stage NSCLC. OPN was predominantly detected in the cytoplasm (Figure 1A–C). Analysis of the IHC scores indicated that OPN was significantly upregulated in advanced NSCLC cases (P=0.0424; Figure 1D). RT-qPCR was performed to evaluate OPN expression levels in tissues collected from 22 patients with stage I/II and 31 patients with advanced-stage NSCLC. These results suggested that OPN expression was significantly upregulated in tissues from patients with advanced-stage NSCLC, compared with those from stage I/II NSCLC group (P=0.0246; Figure 1E).
Figure 1.
Expression levels of OPN in NSCLC tissue.
Notes: (A) IHC staining of adjacent normal lung tissue, (B) a stage I NSCLC tumor with low levels of OPN expression, and (C) a stage IV NSCLC tumor with high expression levels of OPN, stained with OPN antibody and hematoxylin. (D) Histogram showing the analysis from the IHC staining of OPN protein in low (T1–T2) and advanced (T3–T4) stages of NSCLC tumors. (E) RT-qPCR analysis of OPN mRNA expression levels in low (T1–T2) and advanced (T3–T4) stages of NSCLC tumors.
Abbreviations: IHC, immunohistochemistry; NSCLC, non-small-cell lung cancer; OPN, osteopontin; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; T1–T2, stage I/II tumors; T3–T4, stage III/IV tumors.
OPN expression levels correlate with clinicopathological parameters in patients with advanced NSCLC
The correlation between OPN expression levels and the clinicopathological characteristics of patients with advanced NSCLC was analyzed (Table 1).
Table 1.
OPN protein expression and clinicopathological factors in advanced NSCLC patients
| Characteristics | Number of patients | OPN protein expression
|
P-valuea | |
|---|---|---|---|---|
| Low | High | |||
| Gender | 0.432 | |||
| Male | 42 | 18 | 24 | |
| Female | 31 | 13 | 18 | |
| Age (years) | 0.417 | |||
| <65 | 47 | 19 | 28 | |
| ≥65 | 26 | 12 | 14 | |
| Pathologic type | 0.356 | |||
| SCC | 39 | 17 | 22 | |
| AC | 34 | 14 | 20 | |
| Smoking history | 0.281 | |||
| Yes | 58 | 26 | 32 | |
| No | 15 | 5 | 10 | |
| Differentiation | 0.031b | |||
| Low | 2 | 1 | 1 | |
| Moderate | 55 | 26 | 29 | |
| High | 16 | 4 | 12 | |
| Metastasis | 0.019b | |||
| M0 | 32 | 21 | 11 | |
| M1 | 41 | 11 | 30 | |
| Response | 0.038b | |||
| PR | 27 | 12 | 15 | |
| SD | 38 | 18 | 20 | |
| PD | 8 | 2 | 6 | |
Notes:
Pearson chi-square test;
significant.
Abbreviations: AC, adenocarcinoma; NSCLC, non-small-cell lung cancer; PD, progressive disease; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease; OPN, osteopontin.
A total of 42 (57.53%) patients were placed in the high-OPN expression group, with the cytoplasm of >30% of tumor cells exhibiting positive staining. Table 1 demonstrates that OPN expression levels were significantly correlated with tumor differentiation (P=0.031), metastatic status (P=0.019), and response to platinum-based chemotherapy (P=0.038).
OPN expression levels are associated with the efficacy of platinum-based therapeutic agents in patients with advanced NSCLC
Table 1 indicates that OPN expression levels were significantly correlated with the patients’ response to platinum-based therapeutic regimens (P=0.038). Following corrections for age, gender, smoking history, pathological type, grade of metastasis, and differentiation, logistic regression analysis indicated that OPN expression levels (P=0.005), grade of metastasis (P=0.021), and level of tumor cell differentiation (P=0.031) may be independent predictors of the tumor response to first-line platinum-based chemotherapy (Table 2).
Table 2.
Multivariate analyses of variables related to response to platinum-based first-line chemotherapy in advanced NSCLC patients
| Factors | HR (95% CI) | P-valuea |
|---|---|---|
| Age (≥65/<65 years) | 0.785 (0.437–1.039) | 0.178 |
| Gender (female/male) | 0.689 (0.351–1.353) | 0.279 |
| Smoking history (yes/no) | 2.344 (0.754–4.537) | 0.743 |
| Pathologic type (SCC/AC) | 0.845 (0.337–1.303) | 0.291 |
| Metastasis (M0/M1) | 1.814 (1.421–2.365) | 0.021b |
| Differentiation (low + moderate/high) | 1.667 (1.138–2.115) | 0.031b |
| OPN expression (high/low) | 2.326 (1.721–2.616) | 0.005b |
Notes:
Pearson chi-square test;
significant.
Abbreviations: AC, adenocarcinoma; NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma.
Downregulation of OPN expression decreases NSCLC cell drug resistance and invasive ability
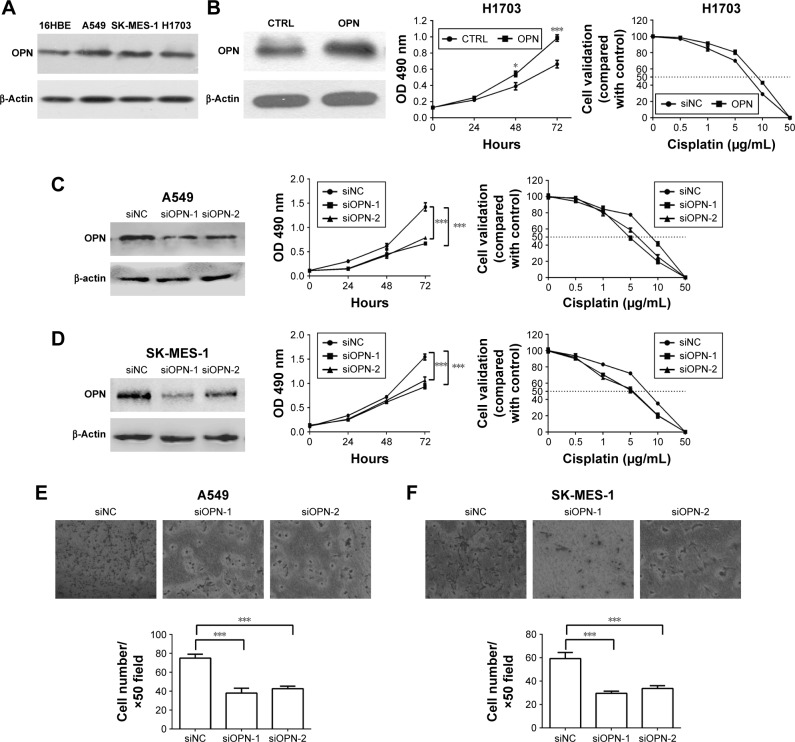
To evaluate the effect of OPN levels on NSCLC cell progression, the expression of OPN was firstly detected in NSCLC cell lines and immortalized human normal bronchial epithelial cells (16HBE). The protein level of OPN was higher in NSCLC cells compared with 16HBE (Figure 2A). Next, knockdown of OPN using RNA interference was performed on A549 and SK-MES-1 cell lines, and overexpressed in H1703 cells. The results revealed that overexpression of OPN significantly increased the proliferation of H1703 cells and decreased the sensitivity of cells to CDDP (Figure 2B). The decrease of OPN protein attenuated the proliferation of A549 and SK-MES-1 cells, and increased the sensitivity of NSCLC cancer cells to CDDP (Figure 2C and D). The MTT assay demonstrated that the CDDP IC50 values of siOPN-1/siOPN-2-transfected A549 and SK-MES-1 cells were 5.07/5.12 and 4.87/5.00 µg/mL, respectively, which was lower compared with the control cells (A549, 8.58 µg/mL; SK-MES-1, 7.56 µg/mL; Figure 2C and D). The transwell assay evaluated the cell invasion of NSCLC cells following siOPN or siNC treatment. Figure 2E and F reveals that A549 and SK-MES-1 cell lines exhibited decreased invasive abilities when OPN expression levels were abrogated using siOPN-1/siOPN-2.
Figure 2.
Effects of OPN levels on cisplatin resistance and invasive abilities of NSCLC cells.
Notes: (A) Western blot analysis was used to detect OPN protein levels in NSCLC cell lines (A549, SK-MES-1, and H1703) and immortalized human normal bronchial epithelial cells (16HBE). (B) MTT assay detects the effect of overexpressed OPN on H1703 cells proliferation and resistance to CDDP. MTT assay was used to investigate the viability of A549 (C) and SK-MES-1 (D) cells following treatment with siOPN-1/siOPN-2 or siNC and multiple doses of cisplatin (0, 0.5, 1, 5, 10, and 50 µg/mL). The IC50 value for CDDP is indicated by a horizontal dashed line. A transwell assay was performed to examine the invasive ability of NSCLC cell lines following treatment with siOPN-1/siOPN-2 or siNC in (E) A549 and (F) SK-MES-1 cells. Migratory cells were imaged and counted. *P<0.05; ***P<0.0001.
Abbreviations: CDDP, cisplatin; NSCLC, non-small-cell lung cancer; OPN, osteopontin; siNC, small interfering RNA negative control; siOPN, small interfering RNA targeting OPN; CTRL, control.
Reduced OPN levels correlate with a decrease in LDHA expression levels and lactate production
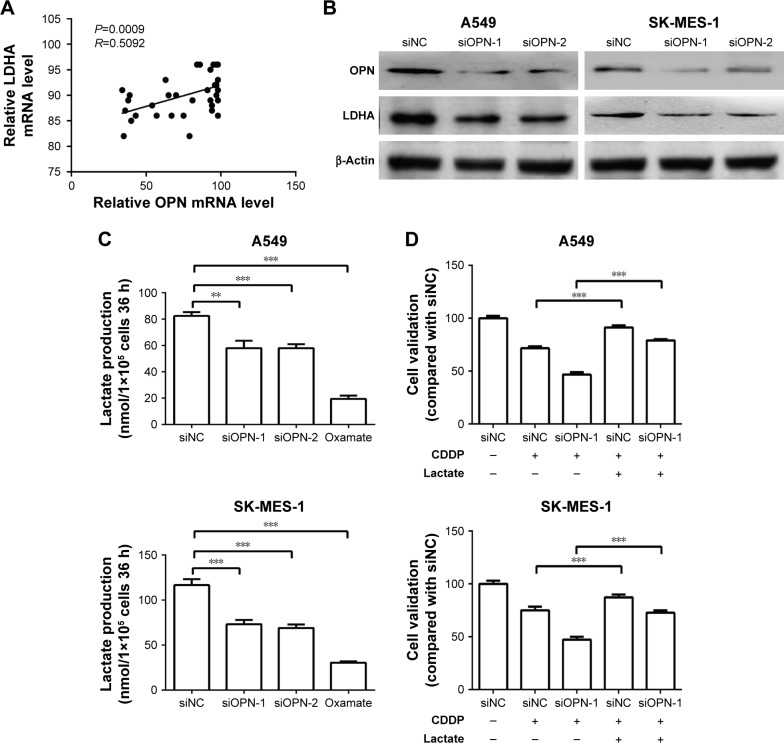
To identify the underlying mechanisms of the effects of OPN, the cisplatin resistance of NSCLC cells, Gene Expression Omnibus repository DataSet Record GDS1650 was screened for correlation between OPN expression levels and drug resistance. Notably, as seen in Figure 3A, OPN mRNA levels significantly correlated with LDHA, which has been associated with the response to chemotherapy and tumor invasion.19,20 Additionally, the knockdown of OPN using siOPN-1/siOPN-2 decreased LDHA protein levels (Figure 3B) and lactate production (Figure 3C) in NSCLC cell lines compared with cells treated with siNC. Finally, to verify lactate contributes to OPN caused CDDP resistance in NSCLC cells, the lactate treatment (5 mM, 24 hours) was found to increase NSCLC cells CDDP (5 µg/mL, 24 hours) resistance and partial rescue OPN-deficiency led CDDP sensitivity (Figure 3D). This suggests that OPN levels may impact the efficacy of cisplatin-resistance ability of NSCLC cells through the regulation of LDHA.
Figure 3.
OPN levels correlate with LDHA expression levels and lactate production in NSCLC cell lines.
Notes: (A) Data from the Gene Expression Omnibus repository DataSet Record GDS1650 indicated that the mRNA expression levels of OPN correlate with LDHA mRNA expression levels. (B) Western blot analysis showing OPN and LDHA protein levels following OPN knockdown using siRNAs. (C) Lactate production of A549 and SK-MES-1 cells was measured following transfection with siOPN-1/siOPN-2 or siNC; oxamate (lactate dehydrogenase inhibitor) treatment was used as control. (D) CDDP resistance of A549 and SK-MES-1 cells when treated with siRNAs, CDDP (5 µg/mL, 24 hours), or lactate (5 mM, 24 hours). **P<0.001, ***P<0.0001.
Abbreviations: CDDP, cisplatin; LDHA, lactate dehydrogenase A; NSCLC, non-small-cell lung cancer; OPN, osteopontin; oxamate, sodium oxamate; siNC, small interfering RNA negative control; siOPN, small interfering RNA targeting OPN; siRNA, small interfering RNA.
Discussion
OPN is a secreted chemokine that has been reported to exhibit overexpression in numerous types of cancer, including breast, lung, colorectal, stomach, and ovarian cancer as well as melanoma.9 In lung cancer, OPN is reported to be overexpressed in tumor tissues and correlates with poor clinical outcomes,13–15,26–28 and also in the plasma of patients with NSCLC.29 Cui et al30 demonstrated that the interaction between OPN and αvβ3 integrin may possess an important role in the tumor growth of human lung cancer cells when injected into mice. OPN may also promote the growth of metastatic tumors in a preclinical model of NSCLC.31 These previous studies suggest that OPN may serve as an important oncoprotein in the growth and progression of NSCLC.
In the present study, the expression levels of OPN were detected in NSCLC using IHC and RT-qPCR. Compared with stage I and II specimens, the advanced-stage NSCLC tissue exhibited an increased level of expression of OPN mRNA and protein. Therefore, it was hypothesized that OPN may contribute to NSCLC progression. Inconsistent with previous reports,32–34 we found no significant difference of OPN between stage I/II NSCLC tumors and adjacent normal lung tissues (data not shown); this may have been caused by the small sample size in our study (28 stage I/II NSCLC tumors vs 28 adjacent normal lung tissues). The analysis of OPN expression levels in 73 advanced NSCLC patients indicated that OPN overexpression was significantly associated with tumor differentiation (P=0.031), metastasis status (P=0.019), and the response to platinum-based chemotherapy (P=0.038). Combined with the reports of Shojaei et al and Li et al,35,36 OPN levels were correlated with the progression of NSCLC and the efficacy of platinum-based chemotherapy in patients with NSCLC. However, the underlying molecular phenomenon is rarely uncovered. Here, we emphasized on OPN-mediated NSCLC tumor invasion and CDDP resistance was found to be correlated with NSCLC cells, aerobic glycolysis (lactate production).
A metabolic alteration exhibited by cancer cells is the Warburg effect, which is associated with malignant progression and drug resistance.37 LDHA, a key enzyme in the glycolytic pathway, correlates with resistance to chemotherapy and cancer progression in multiple types of tumors.19 The current study indicates that OPN expression levels correlate with LDHA, affecting its protein levels and, therefore, lactate production. This suggests that the overexpression of OPN in NSCLC may promote platinum resistance and invasion through LDHA upregulation.
In conclusion, the increase in OPN expression levels was associated with poor outcome in patients with NSCLC in the current study. OPN may be a predictor of the tumor response to first-line platinum-based chemotherapy. The downregulation of OPN expression decreased the resistance of NSCLC cells to cisplatin and also reduced the invasive and lactate producing abilities of tumor cells. These results suggest that OPN expression levels may be used as a bio-marker for patients with advanced NSCLC and a potential therapeutic target for increasing the efficacy of platinum in NSCLC tumors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81602636) and Nanjing Medical Science and Technology Development Project (ZKX15049).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378(9804):1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 4.Cheong KA, Chrystal K, Harper PG. Adjuvant chemotherapy in non-small cell lung cancer. Int J Clin Pract. 2007;61(1):143–146. doi: 10.1111/j.1742-1241.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- 5.Qi L, Li Y, Qin Y, et al. An individualised signature for predicting response with concordant survival benefit for lung adenocarcinoma patients receiving platinum-based chemotherapy. Br J Cancer. 2016;115(12):1513–1519. doi: 10.1038/bjc.2016.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allingham-Hawkins D, Lea A, Levine S. ERCC1 expression analysis to guide therapy in non-small cell lung cancer. PLoS Curr. 2010;2:RRN1202. doi: 10.1371/currents.RRN1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18(8):883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M, Behera R, Chakraborty G, et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011;15(9):1113–1126. doi: 10.1517/14728222.2011.594438. [DOI] [PubMed] [Google Scholar]
- 9.Coppola D, Szabo M, Boulware D, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10(1 Pt 1):184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 10.Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18(8):883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- 11.Fedarko NS, Jain A, Karadag A, van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7(12):4060–4066. [PubMed] [Google Scholar]
- 12.Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103(6):861–869. doi: 10.1038/sj.bjc.6605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers AF, Wilson SM, Kerkvliet N, O’Malley FP, Harris JF, Casson AG. Osteopontin expression in lung cancer. Lung Cancer. 1996;15(3):311–323. doi: 10.1016/0169-5002(95)00595-1. [DOI] [PubMed] [Google Scholar]
- 14.Donati V, Boldrini L, Dell’Omodarme M, et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res. 2005;11(18):6459–6465. doi: 10.1158/1078-0432.CCR-05-0541. [DOI] [PubMed] [Google Scholar]
- 15.Boldrini L, Donati V, Dell’Omodarme M, et al. Prognostic significance of osteopontin expression in early-stage non-small-cell lung cancer. Br J Cancer. 2005;93(4):453–457. doi: 10.1038/sj.bjc.6602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu TT, Han ZG, Shan L, et al. Expression of osteopontin in non-small cell lung cancer and correlative relation with microvascular density. Asian Pac J Cancer Prev. 2014;15(1):29–32. doi: 10.7314/apjcp.2014.15.1.29. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005;11(13):4646–4652. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 18.Nair VS, Gevaert O, Davidzon G, Plevritis SK, West R. NF-κB protein expression associates with (18)F-FDG PET tumor uptake in non-small cell lung cancer: a radiogenomics validation study to understand tumor metabolism. Lung Cancer. 2014;83(2):189–196. doi: 10.1016/j.lungcan.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Huang X, Xu Z, et al. LDHA promotes tumor metastasis by facilitating epithelial–mesenchymal transition in renal cell carcinoma. Mol Med Rep. 2017;16(6):8335–8344. doi: 10.3892/mmr.2017.7637. [DOI] [PubMed] [Google Scholar]
- 21.Lei W, Kang W, Nan Y, et al. The downregulation of miR-200c promotes lactate dehydrogenase A expression and non-small cell lung cancer progression. Oncol Res. 2018. Jan 10, Epub. [DOI] [PMC free article] [PubMed]
- 22.Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med. 1991;115(12):931–935. doi: 10.7326/0003-4819-115-12-931. [DOI] [PubMed] [Google Scholar]
- 23.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378(9804):1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 24.Hong LZ, Wei XW, Chen JF, Shi Y. Overexpression of periostin predicts poor prognosis in non-small cell lung cancer. Oncol Lett. 2013;6(6):1595–1603. doi: 10.3892/ol.2013.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang B, Zhou H, Lang X, Liu Z. siRNA-induced ABCE1 silencing inhibits proliferation and invasion of breast cancer cells. Mol Med Rep. 2014;10(4):1685–1690. doi: 10.3892/mmr.2014.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Zhang DM, Zhao D, et al. The prognostic value of osteopontin expression in non-small cell lung cancer: a meta-analysis. J Mol Histol. 2014;45(5):533–540. doi: 10.1007/s10735-014-9574-3. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Zhou J, Deng Z, et al. [Expression of FGF-2 and osteopontin in non-small cell lung cancer] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(11):1114–1119. Chinese. [PubMed] [Google Scholar]
- 28.Ostheimer C, Evers C, Bache M, Reese T, Vordermark D. Prognostic implications of the co-detection of the urokinase plasminogen activator system and osteopontin in patients with non-small-cell lung cancer undergoing radiotherapy and correlation with gross tumor volume. Strahlenther Onkol. 2018;194(6):539–551. doi: 10.1007/s00066-017-1255-1. [DOI] [PubMed] [Google Scholar]
- 29.Chang YS, Kim HJ, Chang J, Ahn CM, Kim SK, Kim SK. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007;57(3):373–380. doi: 10.1016/j.lungcan.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Cui R, Takahashi F, Ohashi R, et al. Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer. 2007;57(3):302–310. doi: 10.1016/j.lungcan.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Shojaei F, Scott N, Kang X, et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012;31(1):26. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Q, Guo L, Lin G, et al. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015;39(4):539–544. doi: 10.1016/j.canep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Tong DY, Tang LY, et al. Expressions of osteopontin (OPN), ανβ3 and Pim-1 associated with poor prognosis in non-small cell lung cancer (NSCLC) Chin J Cancer Res. 2012;24(2):103–108. doi: 10.1007/s11670-012-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Cheng D, Li X, Fang X. [Expression and clinicopathological significance of OPN and CD44v6 in lung cancer] Zhongguo Fei Ai Za Zhi. 2007;10(2):98–101. doi: 10.3779/j.issn.1009-3419.2007.02.04. Chinese. [DOI] [PubMed] [Google Scholar]
- 35.Shojaei F, Scott N, Kang X, et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012;31:26. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Jiang Z, Li X, Zhang X. SIRT1 overexpression protects non-small cell lung cancer cells against osteopontin-induced epithelial– mesenchymal transition by suppressing NF-κB signaling. Onco Targets Ther. 2018;11:1157–1171. doi: 10.2147/OTT.S137146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kritikou E. Cancer biology: Warburg effect revisited. Nat Rev Mol Cell Biol. 2008;9(4):264. [Google Scholar]