Abstract
Background
Malignant glioma is refractory to conventional treatment, highlighting a need to develop novel efficacious therapies. Biguanides, a class of oral antidiabetic drug, have been thought to inhibit proliferation and metastasis in a variety of cancers.
Purpose
The objective of this study was to investigate the affections of biguanides, phenformin (Phen) and metformin (Met), on growth and migration of glioma cells LN229 in vitro and in vivo.
Methods
Glioma cells LN229 were treated with Phen or Met, then cell proliferation and death were evaluated by MTT assay and PI stain, and cell cycle were evaluated using flow cytometric analysis, meantime wound healing assay and transwell migration assay were performed to detect cell migration ability. In addition, LN229 were injected in thigh of nude mice, and the mice were treated with Phen or Met to detect the effect of Phen and Met in vivo.
Results
Phen and Met could significantly inhibit cell growth through inhibiting cell proliferation, promoting cell death and disturbing cell cycle, and these drugs also could inhibit cell colony formation in glioma cells LN229 in vitro. Meanwhile, both Phen and Met could significantly inhibit cell migration of LN229 in vitro, through effecting the expression of E-cadherin and Vimentin. In addition, both Phen and Met inhibited the growth and migration of LN229 in a tumor xenograft model. Furthermore, Phen and Met were associated with the increased level of ROS of cell mitochondrial, and ROS inhibitor NAC could significantly rescue the cell death induced by Phen and Met.
Conclusion
Phen and Met displayed powerful antitumor effects of LN229, and our findings powerfully suggest the possibility of Phen and Met being used as an adjuvant agent in the treatment of glioma patients.
Keywords: phenformin, metformin, glioma, LN229, proliferation, migration
Introduction
Malignant glioma, the most common central nervous system tumor, compriseŝ80% of intracranial malignant tumors.1,2 Even with aggressive treatment using a combination of surgery, chemotherapy, and radiation therapy, the median survival time is only 12–15 months.3,4 Although research on glioma treatment has made considerable progress, the findings did not significantly improve patients’ outcome.5 Therefore, it is pressing to find new therapeutic agents based on biologic characteristics, and signal pathways are required to improve the outcome of glioma patients.
Biguanides, including phenformin (Phen), metformin (Met), and so on, have been widely used throughout the world to treat type II diabetes.6,7 These agents exert an anti-tumor effect on many cancers, including glioma, according to recent epidemiological surveys and laboratory studies.8,9 To reveal the mechanism of antitumor activity of biguanides, several potential mechanisms have been investigated. These studies all showed that biguanides played an important role in activating the AMP-activated protein kinase (AMPK) signaling pathway.10,11 The primary molecular role of Met is inhibiting mitochondrial respiratory complex I, namely, reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase, which could reduce cellular synthesis of ATP and induce reactive oxygen species (ROS) imbalance.12 In fact, some studies have indicated that Met could increase the level of ROS in lung cancer and breast cancer,13,14 but no report has shown the association of biguanides and ROS in glioma cells.
Meanwhile, because of Phen-associated lactic acidosis in elderly patients with renal failure, compared with Met, Phen use has been limited to relatively few countries.15 However, some studies have found that Phen is more active against tumor cells than Met.16 Nevertheless, very few studies have reported the role of Phen on glioma; one study has shown that Phen inhibited the self-renewal of glioma stem cells, as well as decreased the expression of stemness and mesenchymal markers. In this study, the effects of Phen and Met on glioma cells were examined in vitro and in vivo, and the mechanism of action of biguanides in glioma cells was determined, especially the role of ROS in biguanide inhibition of glioma cells.
Materials and methods
Cell culture and agents
LN229, a human glioblastoma cell line, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in 1640 medium supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and 100 U/mL each of penicillin and streptomycin (Thermo Fisher Scientific) in 5% CO2 at 37°C. Phen, Met, N-acetylcysteine (NAC), and dorsomorphin were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
MTT assay was used to determine IC50 and cell proliferation
In this assay, 5×103 cells were seeded in 96-well plates and incubated for 24 h inside an incubator containing 5% CO2 at 37°C. Then, different concentrations of Phen (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM) and Met (0, 10, 20, 40, 60, 80, and 100 mM) were added to 96-well plates on the following day and incubated for 48 h, respectively. At the end of incubation, cells were analyzed using the MTT assay kit (Sigma-Aldrich Co.). The IC50 of the 48 h values was determined from the dose–response curves. In addition, cells were incubated with lower concentrations of Phen (0.1 mM) or Met (10 mM) for 1, 2, 3, 4, and 5 d to detect cell proliferation using the MTT assay. The absorbance at a wavelength of 490 nm was measured with a microplate reader. All experiments were performed at least three times.
Cell cycle analysis
LN229 cells were plated in six-well plates (400,000 cells per well) and incubated overnight following treatment with Phen (0.1 mM) or Met (10 mM). After 48 h, the cells were trypsinized and fixed with 70% ice-cold ethanol. After fixation, the cells were washed with PBS and stained with a solution containing 50 µg/mL propidium iodide (PI; Sigma-Aldrich Co.), 200 µg RNase A (Sigma-Aldrich Co.), and 0.05% Triton X-100 (Sigma-Aldrich Co.) by incubation at 37°C for 30 min. The stained cells were immediately analyzed (BD Company, Franklin Lakes, NJ, USA). All experiments were performed at least three times.
PI staining was used to detect cell death
LN229 cells were treated with Phen (0.1 mM) or Met (10 mM) for 24 h, following which the treated cells were incubated with PI (1 µg/mL) for 3–5 min at 37°C and 5% CO2. These cells were then observed under a fluorescence microscope, 100 cells were counted in ten randomly selected fields per experimental condition, and values of PI-positive (dead) cells were expressed as percentage of positive cells.
Two-dimensional clonogenic survival assay
LN229 cells 1×103 were seeded on six-well plates and incubated for 10 d in medium containing Phen (0.1 mM) or Met (10 mM) or in blank medium. Then, the colonies were washed with PBS, fixed with methanol, and stained with gentian violet. Pictures were taken, and colonies containing >50 cells were scored as surviving cells.
Soft agar colony formation assay
For this assay, 1×104 LN229 cells were plated in 0.4% agarose on top of a 1% agarose base supplemented with complete medium containing Phen (0.1 mM) or Met (10 mM) or in blank medium. Cells in agarose were allowed to grow for 4 weeks in 5% CO2 at 37°C, and total colonies were counted. Pictures were taken, and the number of colonies was counted by microscope.
Wound-healing assay
About 5×105 LN229 cells were seeded in one well of six-well plates and grown overnight; the cell monolayer was wounded by scratching with a 20 µL pipette tip, followed by washing three times with PBS. Then, the cells were incubated in serum-free culture medium with added Phen (0.1 mM) or Met (10 mM) or in blank medium. For each well, images of the scratch were taken, and the distances between the lesion edges were calculated using an inverted microscope at 0 and 24 h. The relative migrating distance of cells was measured as the distance of cell migration/the distance measured at 0 h.
Cell migration assay
First transwell filters (pore size, 8 µm; Falcon; BD Biosciences) were placed on a 24-well plate containing 500 mL 1640 medium plus either Phen (0.1 mM) or Met (10 mM), or in blank medium; then, about 1×105 LN229 cells were added to the upper compartment of a transwell chamber and allowed to migrate for 24 h at 37°C. Then, the cells were harvested, and cells that had migrated to the bottom surface of the filter membrane were stained with 0.5% crystal violet solution and photographed in five preset fields per insert.
Western blot
Cells were lysed in buffer (20 mM Tris–HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 2.5 mM sodium pyrophosphate, 1% Triton X-100, 1 mM sodium vanadate, 1 mM beta-glycerophosphate, 1 mM phenylmethylsulfonylfluoride, and 1 mg/mL leupeptin). The protein concentration was measured using the Bradford method, and proteins were resolved on a denaturing 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) apparatus. The primary antibodies used in the western blot were as follows: rabbit anti-cyclin D1 (Proteintech, Chicago, IL, USA), rabbit anti-caspase 3 (Abcam, Cambridge, MA, USA), Bcl-2 (Abcam), Bcl-xL (Abcam), vimentin (Proteintech), E-cadherin (Proteintech), and mouse anti-β-actin (Proteintech). Western blot detection was conducted using a Li-Cor Odyssey image reader, and ImageJ (National Institutes of Health) was used to quantify the western blots. The anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG secondary antibodies were from Li-Cor.
Animal experiments
Male BALB/c-nude mice (4–6 weeks of age) were purchased from the Experimental Animal Center of Nanjing Medical. Mice were administered standard feed, housed, and maintained in a pathogen-free house in a 12:12 h light–dark cycle. Temperature and humidity were maintained at 24°C±2°C and 50%±5%, respectively. All mice were injected subcutaneously in the flank region with 100 µL suspension (5×106) of LN229 cells. Once tumors reached ~0.5–0.6 cm3, all the mice were divided into three groups, one group (n=5) was treated with Phen (40 mg/kg/d) by intraperitoneal injection, one group (n=5) was treated with Met (1 mg/kg/d) by intraperitoneal injection, and another group (n=5) was used as control. The size of the tumor was measured twice a week with calipers, and the volume of tumor was determined using the simplified formula of a rotational ellipsoid (length × width2 × 0.5). All animal studies followed an approved protocol by Tianjin Medical University, in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cellular ATP detection
Cellular ATP was detected using CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega Corporation, Fitchburg, WI, USA) according to the manual’s instructions. LN229 cells were plated in 12-well plates (1×105 cells per well) and incubated overnight following treatment with Phen (0.1 mM) or Met (10 mM). After 24 h, the culture medium was discarded, and 400 µL luciferase was added, shaken for 5 min, and the liquid was poured into Eppendorf tubes. ATP was detected using a fluorescence detector.
Intracellular ROS measurement
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) (Thermo Fisher Scientific) was used to measure intracellular ROS, which was oxidized to fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. LN229 cells were plated in 12-well plates (1×105 cells per well) and incubated overnight following treatment with Phen (0.1 mM) or Met (10 mM). After 24 h, the culture medium was discarded, cells were washed with PBS two times after treatment with reagents and were incubated with 20 µM of H2DCFDA at 37°C for 30 min. To remove excess probe, the cells were washed with PBS again, and the fluorescence intensity was measured at an excitation wavelength of 495 nm and emission wavelength of 527 nm. Fluorescence intensity was calculated with ImageJ software (1.47) by analyzing these pictures.
Statistical methods
SPSS 16.0 was used to evaluate the data, and the data are presented as mean values ± SD. The standard two-tailed independent samples t-test was used to compare the differences between the two groups. The significance level was defined as p<0.05 (* indicates p<0.05, ** indicates p<0.01). Each assay was performed in triplicate in at least two independent experiments.
Results
Phen and Met inhibited cell proliferation of LN229
To evaluate the antitumor effects of Phen, cell proliferation of LN229 was determined using MTT in medium containing different concentrations of Phen (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM) or Met (0, 10, 20, 40, 60, 80, and 100 mM). Following exposure to Phen or Met, LN229 cell viability decreased in a dose-dependent manner, and the IC50 value of Phen was about 0.6 mM in 48 h (Figure 1A), and the IC50 value of Met was about 60 mM (Figure 1B). Meanwhile, cell proliferation at a low concentration of Phen (0.1 mM) or Met (10 mM) was also determined, and the results showed that cell proliferation was also inhibited (Figure 1C and D). In addition, cell cycle analysis by flow cytometry was performed following exposure of LN229 to Phen (0.1 mM) or Met (10 mM) for 48 h, and the results revealed that there was G1 phase arrest, and that more cells were accumulated in the G1 phase after treatment with Phen or Met for 48 h (Figure 1E and F). Furthermore, both Phen (0.1 mM) and Met (10 mM) induced cell death successfully, detected by PI stain, in 48 h (Figure 1G and H). To further explore the mechanism of action of Phen and Met in inhibiting cell proliferation and inducing cell death, western blot was utilized to detect the expression levels of proteins cyclin D1, caspase 3, Bcl-2, and Bcl-xL, and we found that the level of cyclin D1 was increased, but the levels of caspase 3, Bcl-2, and Bcl-xL were decreased significantly (Figure 1I).
Figure 1.
Phen and Met inhibited cell proliferation and arrested cell cycle of LN229 glioma cells.
Notes: (A) The survival of LN229 cells treated with different concentrations of Phen (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM) for 48 h, determined using the MTT assay. (B) The survival of LN229 cells treated with different concentrations of Met (0, 10, 20, 40, 60, 80, and 100 mM) for 48 h, determined using the MTT assay. (C) The survival of LN229 cells treated with lower concentration of Phen (0.1 mM) for 48 h, determined using the MTT assay. (D) The survival of LN229 cells treated with lower concentration of Met (10 mM) for 48 h, determined using the MTT assay. (E) The cell cycle fraction of LN229 cells after treatment with Phen (0.1 mM) for 48 h. (F) The cell cycle fraction of LN229 cells after treatment with Met (10 mM) for 48 h. (G) The death of LN229 cells after treatment with Phen (0.1 mM) for 48 h. (H) The death of LN229 cells after treatment with Met (10 mM) for 48 h. (I) The expression levels of cyclin D1, caspase 3, Bcl-2, and Bcl-xL after treatment with Phen (0.1 mM) or Met (10 mM). (J) The relative expression level of cyclin D1, caspase3, Bcl-2 and Bcl-xL. *p<0.05, **p<0.01.
Abbreviations: Ctrl, control; Met, metformin; Phen, phenformin.
Phen and Met inhibited colony formation of LN229
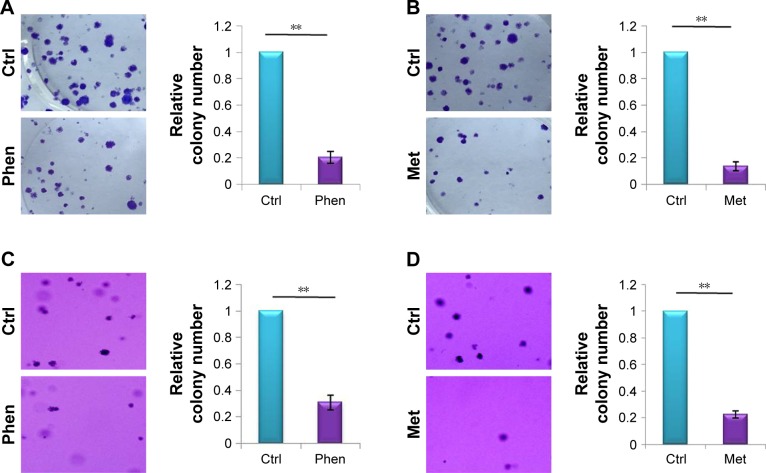
Colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. In this study, we utilized two-dimensional clonogenic survival assay and soft agar colony formation assay to detect the cells’ ability for colony formation after Phen (0.1 mM) or Met (10 mM) treatment. The results showed that both Phen and Met could inhibit colony formation significantly, not just in two-dimensional clonogenic survival assay (Figure 2A and B) but also in soft agar colony formation assay (Figure 2C and D).
Figure 2.
Phen and Met inhibited colony formation of LN229 cells.
Notes: (A) The effect of Phen (0.1 mM) on two-dimensional colony formation. (B) The effect of Met (10 mM) on two-dimensional colony formation. (C) The effect of Phen (0.1 mM) on suspension colony formation, determined by soft agar colony formation assay. (D) The effect of Met (10 mM) on suspension colony formation, determined by soft agar colony formation assay. **p<0.01.
Abbreviations: Ctrl, control; Met, metformin; Phen, phenformin.
Phen and Met inhibited cell migration ability of LN229
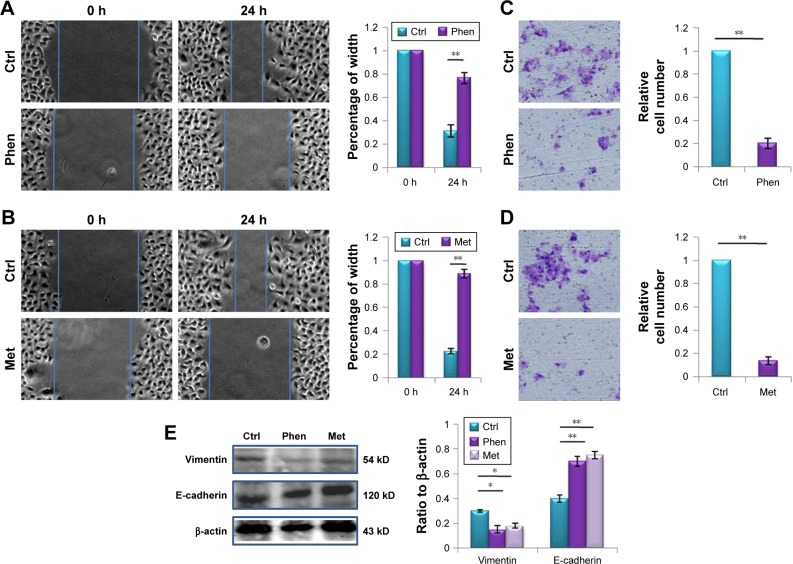
To explore the effect of Phen and Met on cell metastasis of LN229, the wound-healing assay and transwell migration assay were performed. Thus, 0.1 mM Phen or 10 mM Met was used to treat LN229 cells, and the results showed that both Phen and Met significantly inhibited cell migration in both wound-healing assay (Figure 3A and B) and transwell migration assay (Figure 3C and D). The proteins E-cadherin and vimentin were also detected by western blot, and we found that the level of E-cadherin was decreased and vimentin level was increased after treatment with Phen and Met (Figure 3E).
Figure 3.
Phen and Met inhibited migration of LN229 cells.
Notes: (A) The effect of 0.1 mM Phen on cell migration, detected by the wound-healing assay at 24 h. (B) The effect of 10 mM Met on cell migration, detected by the wound-healing assay at 24 h. (C) The effect of 0.1 mM Phen on cell migration, detected by the transwell assay at 24 h. (D) The effect of 10 mM Met on cell migration, detected by the transwell assay at 24 h. (E) The expression levels of E-cadherin and vimentin after treatment with Phen or Met, detected by western blot. *p<0.05, **p<0.01.
Abbreviations: Ctrl, control; Met, metformin; Phen, phenformin.
Phen and Met inhibited tumor growth and metastasis of LN229 in vivo
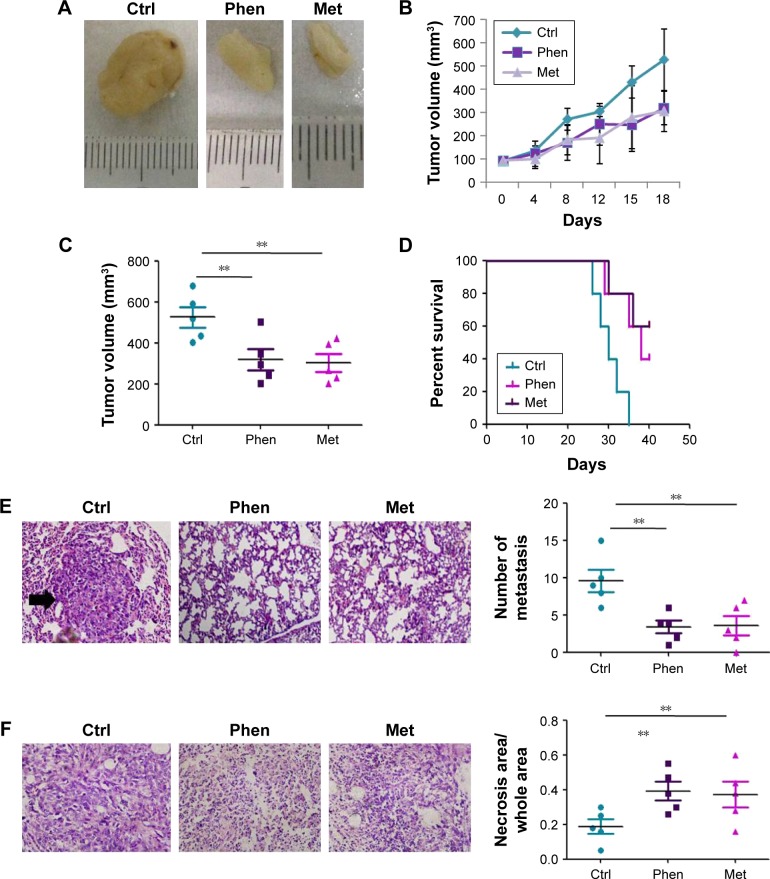
To determine whether Phen and Met could inhibit tumor growth and metastasis in vivo, BALB/c nude mice were injected subcutaneously in the flanks with LN229 cells and treated with Phen (40 mg/kg/d for 18 d, intraperitoneal injection) or Met (1 mg/kg/d for 18 d, intraperitoneal injection). We found that both Phen and Met significantly inhibited the growth of tumors in BALB/c nude mice (Figure 4A and B), and the tumor volumes of the Phen-treated mice and the Met-treated mice were significantly smaller than those of the control group (Figure 4C). Meanwhile, the survival rate of Phen-treated mice and Met-treated mice was increased significantly compared with the control (Figure 4D). In addition, both Phen and Met could significantly inhibit tumor metastasis in lung tissues (Figure 4E). Furthermore, compared with the control group, the area ratio of necrosis in LN229 xenografts was insignificantly increased in the Phen-treated mice and the Met-treated mice (Figure 4F).
Figure 4.
Phen and Met inhibited tumor growth and metastasis of LN229 cells in an in vivo xenograft.
Notes: (A) The present xenograft tumor of mice treated with Phen, Met, or Control. (B) Phen and Met inhibited the growth of LN229 xenograft tumor. (C) The tumor volumes of the three groups. (D) The survival rate of the mice of the three groups. (E) The number of metastatic foci in the lungs of the three groups. The arrow indicates metastasis foci. (F) The area ratio of necrosis in the tissues of the three groups. **p<0.01.
Abbreviations: Ctrl, control; Met, metformin; Phen, phenformin.
Phen and Met induced cell death through ROS imbalance
Several reports have found that biguanides play an important role in activating AMPK signaling pathway, which inhibits cancer cell growth.17 To determine whether biguanides could activate AMPK in LN229 glioma cells, we detected p-AMPK level by western blot. In LN229 cancer cells, both Phen and Met could activate AMPK and increase the level of p-AMPK (Figure 5A). However, the AMPK inhibitor, dorsomorphin, could not rescue the cell death induced by Phen or Met (Figure 5B), which suggested that Phen- or Met-induced cell death was not through activating AMPK in LN229. To further explore the mechanism of biguanide induction of cell death, we determined the cellular ATP level after treatment with biguanides; we found that Phen or Met did not change the level of ATP (Figure 5C). Because some authors propose that biguanides could affect the lactate production in cell, we also determined the level of lactate.18 The results showed that both Phen and Met could increase the level of lactate (Figure 5D), but just the lactate could not induce cell death when we added different doses of lactate (5, 10, and 20 mM) to the cell medium (Figure 5E).
Figure 5.
The effects of Phen and Met on LN229 cells in vitro.
Notes: (A) The expression levels of AMPK and p-AMPK after treatment with Phen or Met in LN229 cells. (B) The cell death of LN229 after treatment with Phen, Met, or in combination with dorsomorphin. (C) The levels of ATP in LN229 cells after treatment with Phen or Met. (D) The levels of lactate in LN229 cells after treatment with Phen or Met. (E) The death of LN229 cells after treatment with Phen, Met, or lactate. *p<0.05, **p<0.01.
Abbreviations: Ctrl, control; Met, metformin; NS, not significant; Phen, phenformin; Com.C, compound C.
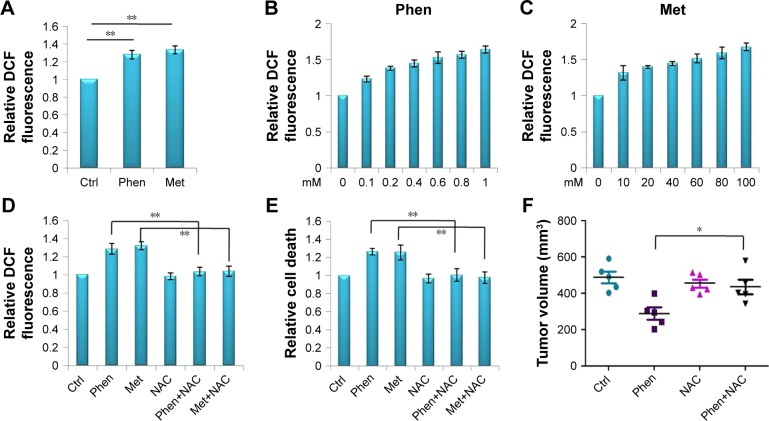
The primary molecular role of biguanides is inhibiting mitochondrial respiratory complex I, which could induce ROS imbalance. So, in this study, we determined the level of ROS in cell mitochondria by DCF fluorescence, and the results showed that both Phen and Met could increase ROS significantly in a dose-dependent manner (Figure 6A–C). Furthermore, NAC,19 the ROS inhibitor, could successfully decrease the level of ROS induced by Phen and Met (Figure 6D) and rescue the cell death induced by Phen and Met (Figure 6E). Meanwhile, we also determined the role of NAC when combined with biguanides in vivo, and we found that NAC could rescue glioma cells in vivo significantly (Figure 6F). All the results suggest that the antitumor role of Phen and Met is mainly through increasing the level of ROS in LN229 cells.
Figure 6.
Phen and Met induced cell death through ROS imbalance.
Notes: (A) The level of ROS in mitochondria after treatment with Phen or Met. (B) The level of ROS in mitochondria after treatment with different doses of Phen (0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM). (C) The level of ROS in mitochondria after treatment with different doses of Met (0, 10, 20, 40, 60, 80, and 100 mM). (D) The levels of ROS in cells after treatment with Phen and Met, or when combined with NAC. (E) The survival of cells after treatment with Phen, Met, or when combined with NAC. (F) NAC could rescue the tumor growth inhibited by Phen in a xenograft model. *p<0.05, **p<0.01.
Abbreviations: Ctrl, control; DCF, 2′,7′-dichlorofluorescein; Met, metformin; NAC, N-acetylcysteine; Phen, phenformin; ROS, reactive oxygen species.
Discussion
In this study, we demonstrated that both Phen and Met could significantly inhibit growth and migration of the glioma cell line LN229 in vitro and in vivo, with ROS playing an important role in the outcome. Phen and Met could prominently increase the level of ROS of cell mitochondria, and the ROS inhibitor NAC could significantly rescue the cell death induced by Phen or Met. Our findings strongly suggest the possibility of Phen and Met being used as an adjuvant agent in the treatment of glioma patients.
Biguanides are a class of agents widely used to treat type II diabetes. Phen and Met belong to biguanides and receive attention again because of their antitumor effect on many cancers, including hepatocellular, colorectal, pulmonary, and pancreatic carcinomas.20–27 Many studies also reported the powerful antitumor activity of biguanides in animal models and cell lines.28,29 Several reports also showed that Met could inhibit the growth of glioma cells or glioma stem cells in vitro and in vivo,30–33 and one study found that Phen inhibited the self-renewal of glioma stem cells and enhanced the treatment effect of temozolomide.34 In this study, we demonstrated that both Phen and Met could significantly inhibit cell growth and colony formation in LN229 glioma cells in vitro. Meanwhile, both Phen and Met could significantly inhibit cell migration of LN229 in vitro and inhibit the growth and migration of LN229 in a tumor xenograft model.
Previous studies have shown that biguanides played a role in activating AMPK-mammalian target of rapamycin (mTOR) signaling pathway, which is important in regulating cancer cell survival, proliferation, and apoptosis, as well as the process of epithelial-to-mesenchymal cell transition (EMT) phenotype.17,35 Meanwhile, 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR), similar to biguanides, also could activate AMPK and enhance the efficacy of rapamycin in human cancer cells.36 To reveal the mechanism of biguanides in the antitumor effect against glioma, we determined the level of p-AMPK, and we found that both Phen and Met could activate AMPK and increase the level of p-AMPK in our study, but an AMPK inhibitor could not rescue the cell death induced by Phen or Met, which suggested that the Phen- or Met-induced cell death in LN229 was not through activation of AMPK. However, we think that LN229 just represents a portion of gliomas, and Phen or Met could not activate AMPK in these gliomas.
The primary molecular role of biguanides is to inhibit mitochondrial respiratory complex I, which could induce ROS imbalance. So, in this study, we determined the level of ROS in cell mitochondria and found that both Phen and Met increased the level of ROS in LN229. Furthermore, NAC, the ROS inhibitor, could successfully counteract the inhibiting effect of Phen and Met for LN229. Meanwhile, the role of NAC when combined with biguanides in vivo was also determined, and we found that NAC could rescue glioma cells in vivo significantly. Actually, some studies have indicated that Met could increase the level of ROS, which promoted cell death in lung cancer and breast cancer.13,14 However, one report showed that Met-induced decrease in cell survival is associated with reduced ROS in pancreatic cancer cells.37 These results suggested that biguanides could affect the level of ROS in cancer cells, which in turn is associated with cell function. However, further studies should be performed to explore the mechanism of antitumor action of biguanides and to evaluate the effect of biguanides on clinical glioma.
Conclusion
Taken together, our results showed that both Phen and Met displayed powerful antitumor effects on LN229 glioma cells in vitro and in vivo, and these agents may be potential adjuvant antitumor drugs for glioma.
Acknowledgments
We sincerely appreciate all volunteers who participated in this study. We also sincerely thank the Tianjin Medical University for their assistance in experiments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Leu S, von Felten S, Frank S, Boulay JL, Mariani L. IDH mutation is associated with higher risk of malignant transformation in low-grade glioma. J Neurooncol. 2016;127(2):363–372. doi: 10.1007/s11060-015-2048-y. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Ahuja A, Ali J, Baboota S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016;23(1):214–229. doi: 10.3109/10717544.2014.909906. [DOI] [PubMed] [Google Scholar]
- 4.Kaidar-Person O, Darawshe F, Tzuk-Shina T, Eran A. The clinical significance of ependymal enhancement at presentation in patients with malignant glioma. Rambam Maimonides Med J. 2015;6(4) doi: 10.5041/RMMJ.10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suryadevara CM, Verla T, Sanchez-Perez L, et al. Immunotherapy for malignant glioma. Surg Neurol Int. 2015;6(suppl 1):S68–S77. doi: 10.4103/2152-7806.151341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbilas D, Martineau LC, Harris CS, et al. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: part II. Can J Physiol Pharmacol. 2009;87(6):479–492. doi: 10.1139/y09-029. [DOI] [PubMed] [Google Scholar]
- 7.Abbas SY, Basyouni WM, El-Bayouk KAM, et al. New biguanides as anti-diabetic agents part I: synthesis and evaluation of 1-substituted biguanide derivatives as anti-diabetic agents of type II diabetes insulin resistant. Drug Res. 2017;67(10):557–563. doi: 10.1055/s-0043-102692. [DOI] [PubMed] [Google Scholar]
- 8.Velez J, Pan R, Lee JT, et al. Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget. 2016;7(32):51435–51449. doi: 10.18632/oncotarget.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song IS, Han J, Lee HK. Metformin as an anticancer drug: a commentary on the metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. J Diabetes Investig. 2015;6(5):516–518. doi: 10.1111/jdi.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourron O, Daval M, Hainault I, et al. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 2010;53(4):768–778. doi: 10.1007/s00125-009-1639-6. [DOI] [PubMed] [Google Scholar]
- 11.Bridges HR, Sirvio VA, Agip AN, Hirst J. Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase. BMC Biol. 2016;14:65. doi: 10.1186/s12915-016-0287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamichhane S, Bastola T, Pariyar R, et al. ROS production and ERK activity are involved in the effects of d-beta-hydroxybutyrate and metformin in a glucose deficient condition. Int J Mol Sci. 2017;18(3):E674. doi: 10.3390/ijms18030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou XB, Li TH, Ren ZP, Liu Y. Combination of 2-deoxy d-glucose and metformin for synergistic inhibition of non-small cell lung cancer: a reactive oxygen species and P-p38 mediated mechanism. Biomed Pharmacother. 2016;84:1575–1584. doi: 10.1016/j.biopha.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Saeki K, Watanabe M, Tsuboi M, et al. Anti-tumour effect of metformin in canine mammary gland tumour cells. Vet J. 2015;205(2):297–304. doi: 10.1016/j.tvjl.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Fimognari FL, Corsonello A, Pastorelli R, Antonelli Incalzi R. Older age and phenformin therapy: a dangerous association. Intern Emerg Med. 2008;3(4):401–403. doi: 10.1007/s11739-008-0154-y. [DOI] [PubMed] [Google Scholar]
- 16.Orecchioni S, Reggiani F, Talarico G, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer. 2015;136(6):E534–E544. doi: 10.1002/ijc.29193. [DOI] [PubMed] [Google Scholar]
- 17.Meng S, Cao J, He Q, et al. Metformin activates AMP-activated protein kinase by promoting formation of the alphabetagamma heterotrimeric complex. J Biol Chem. 2015;290(6):3793–3802. doi: 10.1074/jbc.M114.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137(1):25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 19.Jia D, Koonce NA, Griffin RJ, Jackson C, Corry PM. Prevention and mitigation of acute death of mice after abdominal irradiation by the antioxidant N-acetyl-cysteine (NAC) Radiat Res. 2010;173(5):579–589. doi: 10.1667/RR2030.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Souza A, Khawaja KI, Masud F, Saif MW. Metformin and pancreatic cancer: is there a role? Cancer Chemother Pharmacol. 2016;77(2):235–242. doi: 10.1007/s00280-015-2948-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HH, Guo XL. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol. 2016;78(1):13–26. doi: 10.1007/s00280-016-3037-3. [DOI] [PubMed] [Google Scholar]
- 22.Menamin UC, Cardwell CR, Hughes CM, Murray LM. Metformin use and survival from lung cancer: a population-based cohort study. Lung Cancer. 2016;94:35–39. doi: 10.1016/j.lungcan.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Medairos RA, Clark J, Holoubek S, et al. Metformin exposure is associated with improved progression-free survival in diabetic patients after resection for early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2016;152(1):55.e1–61.e1. doi: 10.1016/j.jtcvs.2016.03.094. [DOI] [PubMed] [Google Scholar]
- 24.Nie Z, Zhu H, Gu M. Reduced colorectal cancer incidence in type 2 diabetic patients treated with metformin: a meta-analysis. Pharm Biol. 2016;54(11):2636–2642. doi: 10.1080/13880209.2016.1176057. [DOI] [PubMed] [Google Scholar]
- 25.Ridler C. Therapy: metformin protective against colorectal cancer? Nat Rev Gastroenterol Hepatol. 2016;13(5):250. doi: 10.1038/nrgastro.2016.61. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Oncotarget. 2017;8(9):16017–16026. doi: 10.18632/oncotarget.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casadei Gardini A, Faloppi L, De Matteis S, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: validation study and biological rationale. Eur J Cancer. 2017;86:106–114. doi: 10.1016/j.ejca.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, He Z, Jia Z, Xu C. Inhibitory effect of metformin combined with gemcitabine on pancreatic cancer cells in vitro and in vivo. Mol Med Rep. 2016;14(4):2921–2928. doi: 10.3892/mmr.2016.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang HH, Zhang Y, Cheng YN, et al. Metformin in combination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog. 2018;57(1):44–56. doi: 10.1002/mc.22718. [DOI] [PubMed] [Google Scholar]
- 30.Kim EH, Lee JH, Oh Y, et al. Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro Oncol. 2017;19(2):197–207. doi: 10.1093/neuonc/now174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Wang X, Zheng J, Wang H, Liu J. Effects of metformin treatment on glioma-induced brain edema. Am J Transl Res. 2016;8(8):3351–3363. [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SH, Li S, Lu G, et al. Metformin treatment reduces temozolomide resistance of glioblastoma cells. Oncotarget. 2016;7(48):78787–78803. doi: 10.18632/oncotarget.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leidgens V, Proske J, Rauer L, et al. Stattic and metformin inhibit brain tumor initiating cells by reducing STAT3-phosphorylation. Oncotarget. 2017;8(5):8250–8263. doi: 10.18632/oncotarget.14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang W, Finniss S, Cazacu S, et al. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget. 2016;7(35):56456–56470. doi: 10.18632/oncotarget.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther. 2015;16(1):77–87. doi: 10.4161/15384047.2014.987021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay S, Chatterjee A, Kogan D, Patel D, Foster DA. 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle. 2015;14(20):3331–3339. doi: 10.1080/15384101.2015.1087623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Lanza-Jacoby S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: role of NOX4. Biochem Biophys Res Commun. 2015;465(1):41–46. doi: 10.1016/j.bbrc.2015.07.118. [DOI] [PubMed] [Google Scholar]