Abstract
Purpose:
Thyroid cancer is the most rapidly increasing cancer in the U.S, affects a young, mostly female population, and has high survival. The aim of this study was to determine if there is an increased risk of reproductive system adverse events or pregnancy complications among women diagnosed with thyroid cancer under the age of 50.
Methods:
Up to 5 female cancer-free individuals were matched to each female thyroid cancer survivor diagnosed before the age of 50 based on birth year, birth state, and follow-up time, within the Utah Population Database. Medical records were used to identify disease diagnoses stratified over three time periods: 0–1, >1–5, and >5–10 years after cancer diagnosis. Cox proportional hazards models were used to estimate hazard ratios (HR) with adjustment on matching factors, race, BMI, and Charlson Comorbidity Index.
Results:
There were 1,832 thyroid cancer survivors and 7,921 matched individuals. Thyroid cancer survivors had higher rates of having multiple health conditions associated with the gynecological system (15.4% vs. 9.4%) and pregnancy (14.3% vs 9.5%) >1–5 years after cancer diagnosis. Increased risks persisted >5–10 years after cancer diagnosis for menopausal disorders (HR=1.78, 99% CI=1.37, 2.33) and complications related to pregnancy (HR=2.13, 99% CI=1.14, 3.98). Stratified analyses showed these risks remained increased across different treatment types.
Conclusions:
There were significant risk increases in reproductive system and pregnancy complications among female thyroid cancer survivors within this study.
Impact for Cancer Survivors:
Although radiation has been linked to reproductive risks in previous studies, we found risks were increased in patients regardless of treatment.
Keywords: thyroid cancer survivors, reproductive risks, gynecological risks, pregnancy complications
Introduction
There are currently more than 630,000 thyroid cancer survivors in the United States.[1] With a five year survival rate of more than 98% and the incidence of thyroid cancer rising more rapidly than any other cancer in the United States, the health of thyroid cancer survivors is important to understand.[2] Thyroid cancer affects women more than men with nearly 75% of thyroid cancer cases occurring in women.[3] The median age for thyroid cancer diagnosis in women in 49.[4]
Thyroid cancer treatment typically includes a combination of surgery, radioactive iodine (RAI), and thyroid hormone replacement therapy. It is recommended that women who undergo RAI should wait at least 6 months to become pregnant to reduce the risk of congenital abnormalities, with some experts recommending waiting up to a year.[5, 6] Likely due to these recommendations, delayed time to pregnancy has been observed to be associated with patients who receive RAI.[7] Other reproductive effects that have been reported include early menopause, changes in menstrual cycles, and increased rates of spontaneous and induced abortions in the first year after RAI therapy.[8]
The aim of this study was to determine if there is an increased risk of reproductive disorders and/or pregnancy complications in female thyroid cancer survivors under the age of 50. We use a statewide sample of thyroid cancer survivors and matched cancer-free individuals who were linked to medical records, cancer registry data, and demographic data from the Utah Department of Health to examine these risks.
Methods
This cohort was established within the Utah Population Database (UPDB), which links data from the Utah Cancer Registry (UCR) (one of the original NCI SEER cancer registries), electronic medical records (EMR), statewide healthcare data, voter registration records, residential histories, family history records, and birth and death certificates.[9] The statewide healthcare data from UPDB includes ambulatory surgery and inpatient discharge data from the entire state of Utah (1996–2012) as well as linkage to EMR data from two of the largest healthcare providers in Utah, the University of Utah Health (1994–2015) and Intermountain Healthcare (1995–2015). Nearly 97% of the study population had medical records in at least one of these healthcare data sources with 85.6% having statewide ambulatory surgery and/or inpatient discharge data and 90.4% having University of Utah Health and/or Intermountain Healthcare EMR data.
First primary thyroid cancer cases identified through the Utah Cancer Registry between 1997–2012 were each matched to up to five cancer-free female controls who were living in Utah at the same time by birth year, birth state (Utah/not Utah), and follow-up time. The last followup date in UPDB is determined by the most recent among several data sources including driver licenses, voter registration, and vital statistics (birth and death certificates, etc.). Death dates are also captured nationwide using genealogy, the Social Security Death Index, and the Utah Cancer Registry records.
Thyroid cancer survivors who had previous cancers were not eligible for the study. Participants with thyroid cancer were excluded if the cancer was in situ (n=18) or the cancer stage was unknown/missing (n=101), they were not living in Utah when they were diagnosed with cancer (n=128), they had less than one year of follow-up time from cancer diagnosis (n=243), an eligible cancer-free individual could not be matched to them (n=217), they were male (n=821), or they were diagnosed at the age of 50 or older (n=1,053). Participants in the comparison group were not eligible if they had an invasive cancer diagnosis at any time.
All participants were linked to the available healthcare data in the UPDB. The Clinical Classification (CCS) for International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) created by the Healthcare Cost and Utilization Project (HCUP) was used to group the ICD-9 codes into clinically meaningful categories.[10] The CCS for ICD-9 CM categorizes the ICD-9 codes into four levels, with level 1 being the broadest (e.g. diseases of the genitourinary system) down to level 4 being the most specific (e.g. pelvic inflammatory disease). A total of 30 outcomes were analyzed which are associated with health conditions of female genital organs (n=10) and of pregnancy, childbirth, and the puerperium (n=20). Counts of unique health problems for both female genital organs and pregnancy, childbirth, and the puerperium were created for each time frame. Multiple health problems were counted as having two or more.
Follow-up time was calculated separately for each diagnosis. If the participant had an event for a particular condition, their follow-up time was calculated from cancer diagnosis date for thyroid cancer survivors or date of cancer diagnosis of thyroid cancer survivor they were matched to for the cancer-free individuals to date of diagnosis for that condition. If they were never diagnosed with that condition their follow-up time was calculated from thyroid cancer diagnosis date to their last date known in Utah or death date.
Statistical Analysis
Chi-squared tests were used to assess differences in the demographic characteristics between thyroid cancer survivors and the cancer free population. Demographics related only to cancer survivors (age at diagnosis, cancer stage, histology, diagnosis year, and treatment) were also reported. For analyses of disease risks, the diagnosis was categorized over time: zero to one year, one to five years and five to ten years from cancer diagnosis. Univariate and multivariate stratified Cox proportional hazards models were used to estimate hazard ratios (HR) and 99% confidence intervals (CIs) for all diagnoses across all three time periods. We adjusted for race, Charlson Comorbidity Index at baseline, and baseline BMI. The Charlson Comorbidity Index was calculated using all medical record data prior to the date of cancer diagnosis.[11] Proportional hazards assumptions were tested for all Cox proportional hazards models ran. If the proportional hazard assumption was not met, a flexible cubic spline model was used.
The most recent BMI measurement at least one year before cancer diagnosis was calculated to assess baseline BMI. Approximately 20% of subjects were missing BMI, thus we imputed BMI using cancer status, age at diagnosis, sex, race, and Charlson Comorbidity Index as predictors using multiple imputation. We compared Cox regression models including the original BMI variable and the imputed BMI variable to assure that our inferences did not change due to the imputed BMI.
Stratified analyses were performed by BMI (normal and overweight/obese), cancer stage (localized and regional/distance), and treatment (surgery only and surgery/RAI). Hazard ratios between stratified groups and follow-up periods were tested for significant differences. All analyses were conducted on SAS (version 9.4).
Results
The final cohort included 1,832 women diagnosed with thyroid cancer before the age of 50 with 7,921 matched individuals from a general population cohort. The thyroid cancer survivors were significantly more likely to be overweight or obese when compared to the general population cohort (Table 1). Women in the matched general population cohort were significantly more likely to have children after the date of cancer diagnosis (74.7% vs 70.9%, p=value=0.001), but not before that time (20.6% vs 20.9%, p-value=0.776).
Table 1:
Characteristics of young female thyroid cancer survivors and the matched general population cohort
| Thyroid cancer | Comparison group |
P-value | |||
|---|---|---|---|---|---|
| N=1832 | % | N=7921 | % | ||
| Birth year | |||||
| 1940–1959 | 282 | 15.4 | 1204 | 15.2 | 0.972 |
| 1960–1979 | 1316 | 71.8 | 5695 | 71.9 | |
| 1980–1994 | 234 | 12.8 | 1022 | 12.9 | |
| Race | |||||
| White | 1749 | 95.6 | 7375 | 95.1 | 0.333 |
| Non-White | 80 | 4.4 | 381 | 4.9 | |
| Unknown | 3 | 163 | |||
| Vital status | |||||
| Alive | 1805 | 98.5 | 7836 | 98.9 | 0.147 |
| Dead | 27 | 1.5 | 85 | 1.1 | |
| Body mass index at baseline* | |||||
| <18 kg/m2 | 49 | 2. 7 | 273 | 3. 5 | <0.0001 |
| 18–24.9 kg/m2 | 1157 | 63.2 | 5394 | 68.1 | |
| 25–29.9 kg/m2 | 397 | 21.7 | 1431 | 18.1 | |
| 30+ kg/m2 | 229 | 12.5 | 823 | 10.4 | |
| Had children | |||||
| Before cancer diagnosis | 382 | 20.9 | 1268 | 20.6 | 0.776 |
| After cancer diagnosis | 1299 | 70.9 | 5914 | 74.7 | 0.001 |
| Any time | 1419 | 77.5 | 6457 | 81.5 | <0.0001 |
| mean | std | mean | std | ||
| Average age at time of first child | 24.6 | 5.1 | 23.9 | 4.8 | <0.0001 |
| Overall average age of pregnancy | 27.6 | 4.6 | 26.9 | 4.4 | <0.0001 |
| Average number of children | 2.3 | 1.8 | 2.4 | 1.8 | 0.240 |
| Average number of children before cancer diagnosis | 0.4 | 0.8 | 0.3 | 0.8 | 0.319 |
| Average number of children after cancer diagnosis | 2.0 | 1.7 | 2.0 | 1.7 | 0.097 |
at least one year prior to cancer diagnosis for them or the cancer survivor they are matched to
The median age of cancer diagnosis was 36. The majority of thyroid cancer survivors had papillary cancer (94.2%) and localized cancer (75.1%) as shown in Table 2. Of the 1,806 who did have treatment data available, nearly all (99.7%) had a thyroidectomy (either hemi or total) and just over half (52.4%) received RAI. Frequencies for all outcomes across all three time periods are shown in Supplemental Table 1.
Table 2:
Clinical characteristics of young female thyroid cancer survivors
| Thyroid cancer | ||
|---|---|---|
| N=1832 | % | |
| Diagnosis year | ||
| 1997–2000 | 208 | 11.4 |
| 2001–2005 | 492 | 26.9 |
| 2006–2010 | 670 | 36.6 |
| 2011–2012 | 462 | 25.2 |
| Age at diagnosis | ||
| < 20 | 32 | 1. 8 |
| 20–29 | 399 | 21.8 |
| 30–39 | 706 | 38.5 |
| 40–49 | 695 | 37.9 |
| Median age = 36 | ||
| Cancer stage at diagnosis | ||
| Localized | 1376 | 75.1 |
| Regional | 428 | 23.4 |
| Distant | 28 | 1.5 |
| Histology | ||
| Papillary carcinoma | 1725 | 94.2 |
| Follicular carcinoma | 88 | 4.8 |
| Medullary carcinoma | 13 | 0.7 |
| Other | 6 | 0.3 |
| Treatment | ||
| Surgery only | 854 | 47.3 |
| Surgery and RAI | 947 | 52.4 |
| Other | 5 | 0.3 |
| Missing | 26 | |
RAI: Radioactive Iodine
Table 3 shows the overall hazard ratios across all three time periods. In both the first year and >1–5 years after cancer diagnosis, thyroid cancer survivors were significantly more likely to have one or multiple health conditions of female genital organs than the matched general population cohort. That risk was not observed >5–10 years after cancer diagnosis. There were also significant risk increases for multiple health conditions of pregnancy, childbirth, and the puerperium >1–5 years after cancer diagnosis (HR=1.56, 99% CI=1.28, 1.89). In the first year after cancer diagnosis, thyroid cancer survivors had significant risk increases for menstrual disorders (HR=2.04, 99% CI=1.53, 2.74), ovarian cysts (HR=1.83, 99% CI=1.08, 3.10), and menopausal disorders (HR=2.15, 99% CI=1.30, 3.56). The risks remained significantly increased through both >1–5 and >5–10 years from cancer diagnosis. As socioeconomic status is commonly associated with health outcomes, we ran these models with poverty rate at the ZIP code level as a potential confounder and there were no meaningful changes to the hazard ratios across all time periods. We performed a subset analysis on only those diagnosed with papillary thyroid cancer, as papillary is different from other histologies, and the results did not change.
Table 3:
Reproductive and pregnancy outcomes among thyroid cancer survivors compared to the general population cohort, by years since cancer diagnosis
| Diagnosis | 0–1 years after cancer diagnosis HR (99% CI) |
1–5 years after cancer diagnosis HR (99% CI) |
5–10 years after cancer diagnosis HR (99% CI) |
|---|---|---|---|
| Total number of health conditions of female genital organs | |||
| 1 | 1.81 (1.49,2.19) | 1.36 (1.18, 1.56) | 1.07 (0.76, 1.50) |
| 2+ | 1.81 (1.26, 2.59) | 1.63 (1.35, 1.96) | 1.12 (0.64, 1.97) |
| Total number of health conditions of pregnancy, childbirth, and the puerperium | |||
| 1 | 0.92 (0.65, 1.28) | 0.86 (0.67, 1.12) | 0.61 (0.21, 1.77) |
| 2+ | 0.93 (0.62, 1.38) | 1.56 (1.28, 1.89) | 1.35 (0.73,2.48) |
| Diseases of female genital organs | 1.83 (1.67, 2.00)† | 1.53 (1.42, 1.64)† | 1.49 (1.29, 1.71) |
| Inflammatory diseases of female pelvic organs | 1.27 (0.75, 2.15) | 1.55 (1.15, 2.09) | 1.48 (1.04, 2.12) |
| Cervicitis and endocervicitis | 1.41 (0.41, 4.91) | 2.10 (1.10, 3.99) | 2.61 (1.18, 5.73) |
| Menstrual disorders | 2.04 (1.53, 2.74) | 1.46 (1.21, 1.76) | 1.49 (1.17, 1.88) |
| Ovarian cyst | 1.83 (1.08, 3.10) | 1.63 (1.33, 1.94)† | 1.47 (1.02, 2.11) |
| Menopausal disorders | 2.06 (1.56, 2.55)† | 1.63 (1.24, 2.12) | 1.78 (1.37, 2.33) |
| Other female genital disorders | 1.69 (1.28, 2.22) | 1.50 (1.33, 1.66)† | 1.47 (1.18, 1.84) |
| Female genital pain and other symptoms | 1.63 (1.10, 2.41) | 1.49 (1.28, 1.71)† | 1.43 (1.08, 1.88) |
| Other and unspecified female genital disorders | 1.74 (1.23, 2.48) | 1.59 (1.28, 1.99) | 1.52 (1.13, 2.06) |
| Contraceptive and procreation management, not including sterilization | 1.81 (1.21, 2.71) | 1.31 (1.09, 1.54)† | 1.19 (0.83, 1.71) |
| Complications mainly related to pregnancy | 0.85 (0.50, 1.20)† | 1.63 (1.36, 1.97) | 1.32 (1.00, 1.73) |
| Other hemorrhage during pregnancy; childbirth and the puerperium | 0.90 (0.32, 2.56) | 1.60 (1.01, 2.53) | 2.13 (1.14, 3.98) |
| Diabetes or abnormal glucose tolerance | 1.09 (0.00, 2.28)† | 2.14 (1.23, 3.73) | 1.43 (0.57, 3.57) |
| Other complications of pregnancy | 1.08 (0.70, 1.46)† | 1.93 (1.59, 2.35) | 1.50 (1.12, 2.02) |
| Missed abortion | 0.81 (0.19, 3.53) | 2.16 (1.26, 3.73) | 1.20 (0.52, 2.77) |
| Other and unspecified complications of pregnancy | 1.33 (0.94, 1.73)† | 2.17 (1.76, 2.67) | 1.64 (1.21, 2.23) |
| Premature rupture of membranes | 1.18 (0.50, 2.78) | 3.13 (1.17, 8.37) | |
| Complications during labor | 0.56 (0.33, 0.94) | 1.23 (0.98, 1.55) | 1.19 (0.84, 1.68) |
All HR adjusted for baseline BMI, baseline Charlson Comorbidity Index, and race
Proportional hazard assumption not meant; flexible spline model used
The following outcomes were evaluated but no elevated risk was observed: abortion-related disorders, spontaneous abortions, induced abortions, ectopic pregnancy, hemorrhage during pregnancy; abruptio placenta; placenta previa, hypertension complicating pregnancy; childbirth and the puerperium, preeclampsia and eclampsia, early or threatened labor, prolonged pregnancy, fetal distress and abnormal forces of labor, normal delivery, and female infertility
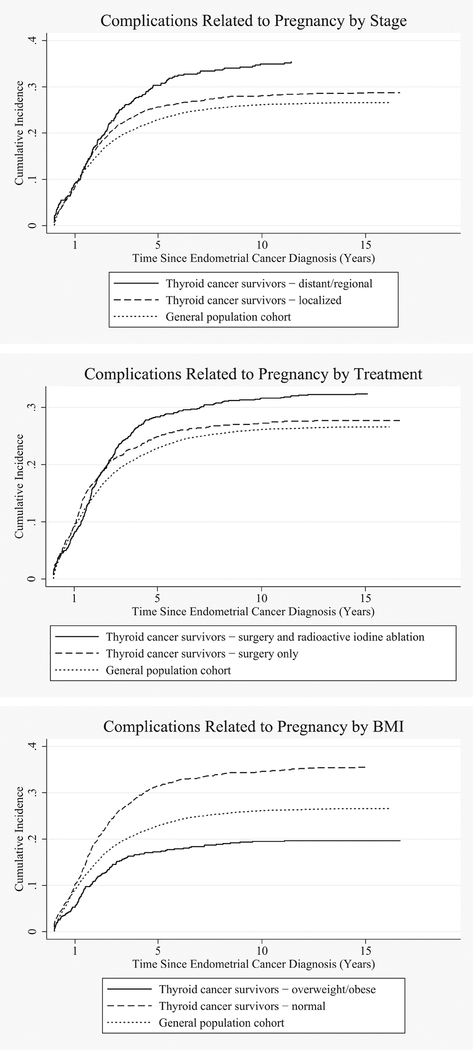
Risks for complications related to pregnancy were significantly increased both >1–5 and >5–10 years from cancer diagnosis (HR=1.63, 99% CI=1.36, 1.97 and HR=1.32, 99% CI=1.00, 1.73, respectively). These complications included increased risks for hemorrhage and diabetes or abnormal glucose tolerance during pregnancy, childbirth, and the puerperium. In these time periods, risks for cervicitis and endocervicitis were also significantly increased (HR=2.10, 99% CI=1.10, 3.99 and HR=2.61, 99% CI=1.18, 5.73, respectively). Figure 1 shows the incidence curves for complications during pregnancy for the thyroid cancer survivors stratified by treatment, localized and regional/distant thyroid cancer, and baseline BMI.
Fig. 1.
Cumulative incidence curves for selected outcomes by stage, cancer treatment, and baseline BMI for thyroid cancer survivors and the matched general population cohort.
Table 4 shows the hazard ratios stratified by localized and regional/distant thyroid cancer. Women diagnosed with localized thyroid cancer showed significantly increased risks for menstrual disorders, ovarian cysts, and menopausal disorders at both >1–5 and >5–10 years after cancer diagnosis; whereas women with regional or distant thyroid cancer did not show significant increased risks for these in either time period. Regional and distant thyroid cancer compared to localized thyroid cancer was associated with increased risks for cervicitis and endocervicitis >1–5 years after cancer diagnosis (HR=8.10, 99% CI=1.66, 38.71 vs HR=1.44, 99% CI=0.67, 3.12, respectively) and hemorrhage during pregnancy, childbirth, or the puerperium >5–10 years after cancer diagnosis (HR=3.23, 99% CI=1.10, 9.48 vs HR=1.79, 99% CI=0.79, 4.02, respectively).
Table 4:
Reproductive and pregnancy outcomes among thyroid cancer survivors compared to the general population cohort, by cancer stage and years since cancer diagnosis
| 1–5 years after cancer diagnosis | 5–10 years after cancer diagnosis | |||
|---|---|---|---|---|
| Localized | Regional/Distant | Localized | Regional/Distant | |
| HR (99% CI) | HR (99% CI) | HR (99% CI) | HR (99% CI) | |
| Total number of health conditions of female genital organs | ||||
| 1 | 1.30 (1.10, 1.53) | 1.57 (1.18, 2.09) | 0.92 (0.62, 1.35) | 1.79 (0.87, 3.70) |
| 2+ | 1.71 (1.37, 2.12) | 1.42 (0.97, 2.07) | 1.22 (0.64, 2.32) | 0.81 (0.25, 2.65) |
| Total number of health conditions of pregnancy, childbirth, and the puerperium | ||||
| 1 | 0.95 (0.70, 1.29) | 0.68 (0.41, 1.14) | 0.96 (0.28, 3.26) | 0.14 (0.01, 2.71) |
| 2+ | 1.59 (1.26, 2.01) | 1.49 (1.0 5, 2.11) | 1.18 (0.53, 2.61) | 1.55 (0.58, 4.10) |
| Diseases of female genital organs | 1.53 (1.42, 1.64)† | 1.57 (1.25, 1.99) | 1.53 (1.30, 1.80) | 1.37 (1.02, 1.84) |
| Inflammatory diseases of female pelvic organs | 1.45 (1.01, 2.06) | 1.82 (1.04, 3.18) | 1.42 (1.03, 1.81)† | 1.54 (0.74, 3.19) |
| Cervicitis and endocervicitis | 1.44 (0.67, 3.12) | 8.01 (1.66, 38.71)* | 2.61 (1.10, 6.18) | 3.39 (0.32, 36.08) |
| Menstrual disorders | 1.52 (1.22, 1.87) | 1.30 (0.88, 1.91) | 1.57 (1.20, 2.06) | 1.24 (0.76, 2.02) |
| Ovarian cyst | 1.63 (1.33, 1.94)† | 1.67 (1.11, 2.23)† | 1.53 (1.01, 2.32) | 1.25 (0.59, 2.64) |
| Menopausal disorders | 1.62 (1.20, 2.18) | 1.66 (0.90, 3.07) | 1.78 (1.32, 2.39) | 1.81 (0.97, 3.39) |
| Other female genital disorders | 1.50 (1.33, 1.66)† | 1.22 (0.85, 1.76) | 1.50 (1.17, 1.93) | 1.44 (0.90, 2.30) |
| Female genital pain and other symptoms | 1.49 (1.28, 1.71)† | 1.33 (0.84, 2.11) | 1.50 (1.10, 2.05) | 1.20 (0.66, 2.20) |
| Other and unspecified female genital disorders | 1.72 (1.34, 2.21) | 1.20 (0.73, 1.98) | 1.45 (1.03, 2.06) | 1.88 (1.02, 3.48) |
| Contraceptive and procreation management, not including sterilization | 1.56 (1.14, 2.12) | 1.47 (0.89, 2.42) | 1.24 (0.81, 1.88) | 1.06 (0.51, 2.21) |
| Complications mainly related to pregnancy | 1.70 (1.37, 2.12) | 1.48 (1.06, 2.08) | 1.24 (0.88, 1.75) | 1.47 (0.93, 2.33) |
| Other hemorrhage during pregnancy; childbirth and the puerperium | 1.92 (1.12, 3.28) | 1.07 (0.43, 2.68) | 1.79 (0.79, 4.02) | 3.23 (1.10, 9.48) |
| Diabetes or abnormal glucose tolerance | 1.96 (1.00, 3.84) | 2.57 (1.61, 3.53)† | 1.28 (0.41, 4.01) | 2.30 (0.44, 11.87) |
| Other complications of pregnancy | 2.01 (1.59, 2.55) | 1.77 (1.24, 2.54) | 1.44 (0.99, 2.08) | 1.67 (1.01, 2.77) |
| Missed abortion | 2.26 (1.21, 4.20) | 1.87 (0.59, 5.92) | 1.37 (0.52, 3.60) | 0.88 (0.16, 4.77) |
| Other and unspecified complications of pregnancy | 2.30 (1.79, 2.96) | 1.91 (1.32, 2.78) | 1.58 (1.08, 2.32) | 1.78 (1.06, 3.01) |
All HR adjusted for baseline BMI, baseline Charlson Comorbidity Index, and race
Hazard Ratios between age groups within follow-up periods are statistically significant p-value <0.05
Proportional hazard assumption not meant; flexible spline model used
The following outcomes were evaluated but no elevated risk was observed: abortion-related disorders, spontaneous abortions, induced abortions, female infertility, ectopic pregnancy, hemorrhage during pregnancy; abruptio placenta; placenta previa, hypertension complicating pregnancy; childbirth and the puerperium, preeclampsia and eclampsia, early or threatened labor, prolonged pregnancy, fetal distress and abnormal forces of labor, premature rupture of membranes, complications during labor, and normal delivery
Table 5 shows the results of the stratified analyses by treatment (surgery only and surgery with RAI). The risks for the number of health conditions were similar between the two treatment groups with women who had both surgery and RAI having slightly higher risks for health conditions of female genital organs and slightly lower risks for multiple health conditions of pregnancy, childbirth, and the puerperium. Thyroid cancer survivors who only had surgery had significant risk increases >1–5 years after cancer diagnosis for diabetes or abnormal glucose tolerance during pregnancy, childbirth, and the puerperium (HR=2.53, 99% CI=1.02, 6.31) and missed abortions (HR=3.08, 99% CI=1.36, 6.99) compared to the matched general population. Women who had both surgery and RAI did not have significant risk increases for either of these.
Table 5:
Reproductive and pregnancy outcomes among thyroid cancer survivors compared to the general population cohort, by treatment and years since cancer diagnosis
| 1–5 years after cancer diagnosis | 5–10 years after cancer diagnosis | |||
|---|---|---|---|---|
| Surgery Only | Surgery and RAI | Surgery Only | Surgery and RAI | |
| HR (99% CI) | HR (99% CI) | HR (99% CI) | HR (99% CI) | |
| Total number of health conditions of female genital organs | ||||
| 1 | 1.32 (1.07, 1.63) | 1.42 (1.17, 1.73) | 0.96 (0.59, 1.55) | 1.12 (0.68, 1.83) |
| 2+ | 1.60 (1.22, 2.10) | 1.69 (1.30, 2.20) | 1.31 (0.57, 3.03) | 0.97 (0.44, 2.11) |
| Total number of health conditions of pregnancy, childbirth, and the puerperium | ||||
| 1 | 0.78 (0.51, 1.19) | 0.94 (0.67, 1.31) | 0.68 (0.05, 9.46) | 0.60 (0.18, 1.99) |
| 2+ | 1.57 (1.16, 2.12) | 1.49 (1.15, 1.93) | 1.12 (0.42, 2.95) | 1.50 (0.64, 3.50) |
| Diseases of female genital organs | 1.43 (1.28, 1.59)† | 1.61 (1.37, 1.88) | 1.45 (1.19, 1.77) | 1.44 (1.26, 1.62)† |
| Inflammatory diseases of female pelvic organs | 1.63 (1.06, 2.50) | 1.50 (0.98, 2.29) | 1.53 (0.90, 2.59) | 1.40 (0.85, 2.31) |
| Cervicitis and endocervicitis | 2.44 (0.96, 6.18) | 1.83 (0.72, 4.66) | 2.93 (1.85, 4.02)† | 1.89 (0.64, 5.55) |
| Menstrual disorders | 1.51 (1.15, 1.99) | 1.45 (1.12, 1.87) | 1.47 (1.04, 2.08) | 1.56 (1.12, 2.16) |
| Ovarian cyst | 1.49 (1.03, 1.95)† | 1.84 (1.21, 2.78) | 1.33 (0.78, 2.26) | 1.51 (0.90, 2.51) |
| Menopausal disorders | 1.51 (1.04, 2.21) | 1.80 (1.22, 2.64) | 1.47 (1.00, 2.15) | 2.18 (1.48, 3.22) |
| Other female genital disorders | 1.48 (1.24, 1.71)† | 1.55 (1.21, 1.98) | 1.45 (1.06, 1.98) | 1.46 (1.07, 2.01) |
| Female genital pain and other symptoms | 1.56 (1.27, 1.86)† | 1.38 (1.00, 1.92) | 1.43 (0.98, 2.08) | 1.43 (0.95, 2.14) |
| Other and unspecified female genital disorders | 1.53 (1.10, 2.11) | 1.72 (1.26, 2.35) | 1.66 (1.07, 2.59) | 1.39 (0.91, 2.12) |
| Contraceptive and procreation management, not including sterilization | 1.16 (0.81, 1.50)† | 1.54 (1.09, 2.19) | 1.15 (0.66, 2.01) | 1.13 (0.68, 1.86) |
| Complications mainly related to pregnancy | 1.56 (1.17, 2.08) | 1.63 (1.27, 2.09) | 1.20 (0.78, 1.85) | 1.26 (0.86, 1.83) |
| Other hemorrhage during pregnancy; childbirth and the puerperium | 1.73 (0.87, 3.44) | 1.52 (0.80, 2.86) | 1.37 (0.48, 3.88) | 2.71 (1.16, 6.33) |
| Diabetes or abnormal glucose tolerance | 2.53 (1.02, 6.31) | 1.98 (0.97, 4.06) | 2.02 (0.60, 6.80) | 0.74 (0.14, 3.86) |
| Other complications of pregnancy | 1.90 (1.40, 2.57) | 1.90 (1.46, 2.48) | 1.39 (0.88, 2.21) | 1.43 (0.95, 2.15) |
| Missed abortion | 3.08 (1.36, 6.99) | 1.52 (0.70, 3.28) | 0.68 (0.13, 3.48) | 1.58 (0.59, 4.23) |
| Other and unspecified complications of pregnancy | 2.17 (1.57, 3.00) | 2.11 (1.60, 2.78) | 1.43 (0.89, 2.32) | 1.67 (1.10, 2.54) |
| Premature rupture of membranes | 1.72 (0.53, 5.64) | 0.81 (0.22, 2.93) | 2.13 (0.54, 8.34) | 5.17 (1.16, 22.99) |
All HR adjusted for baseline BMI, baseline Charlson Comorbidity Index, and race RAI: radioactive iodine
Proportional hazard assumption not meant; flexible spline model used
The following outcomes were evaluated but no elevated risk was observed: abortion-related disorders, spontaneous abortions, induced abortions, female infertility, ectopic pregnancy, hemorrhage during pregnancy; abruptio placenta; placenta previa, hypertension complicating pregnancy; childbirth and the puerperium, preeclampsia and eclampsia, early or threatened labor, prolonged pregnancy, fetal distress and abnormal forces of labor complications during labor, and normal delivery
The stratified hazard ratios by BMI are shown in Supplemental Table 2. Thyroid cancer survivors with normal BMI at baseline had significantly increased risks for cervicitis and endocervicitis (HR=3.05, 99% CI=1.38, 6.73) >1–5 years after cancer diagnosis, whereas the risk was not increased for those overweight or obese at baseline (HR=1.06, 99%=0.40, 2.84). Thyroid cancer survivors who were overweight or obese at baseline had significant risks for missed abortions (HR=3.93, 99% CI=1.44, 10.72) >1–5 years after cancer diagnosis and premature rupture of membranes during labor (HR=1.31, 99% CI=1.17, 90.49) >5–10 years after cancer diagnosis. These risks were not increased for women with normal BMI at baseline. Six of the outcomes were increased in risk for both thyroid cancer survivors who were normal BMI and overweight/obese in the 1–5 year time period. The total number of health conditions experienced by normal vs. overweight/obese thyroid cancer survivors were not statistically significantly different.
Discussion
Female thyroid cancer survivors diagnosed before the age of 50 had increased risks for health conditions associated with the reproductive system and pregnancy complications. The majority of the significant risk increases associated with pregnancy were observed >1–5 and >510 years from cancer diagnosis. There were very few pregnancies in the first year after cancer diagnosis for the thyroid cancer survivors. This may be indicative of patients responding to guidelines and waiting to become pregnant for at least a year after cancer treatment.[5] Thyroid cancer survivors wait longer to have another child after diagnosis, and therefore are older at the time of childbirth, which may account for some of the increases in pregnancy complications, although we did adjust for age to account for this issue.
Both menstrual and menopausal disorders have been previously reported as a late effect of RAI.[12, 13] Sioka et al. reported that 31.1% of 45 thyroid cancer survivors reported menstrual cycle irregularities compared to 14.5% of matched controls.[13] These numbers are higher than what we observed (15.1% and 10.3%, respectively, 1–5 years after cancer diagnosis), however we used ICD-9 codes, whereas Sioka et al. used medical records along with interviews asking detailed questions about menstrual irregularities.[13] Other studies have also reported similar rates of increased menstrual irregularities; however they often report them as transient with normal menstrual cycles after the first year.[14, 15] We observed significantly increased risks for menstrual disorders across all three time periods, though the risks appeared to be reduced after the first year.
These risks have generally been reported along with RAI and increased doses of RAI.[12, 14] RAI also been shown to be associated with an earlier age of menopause.[16, 14, 12] However, with the stratified treatment we observed increased risks for both menstrual and menopausal disorders regardless of treatment type during the first 1–5 years after cancer diagnosis. These risks appear to be elevated whether a woman received RAI or not, though the risks for menopausal disorders were elevated in women who had RAI compared to women who only had surgery. The risks for menstrual and menopausal disorders are also significant for women who had localized thyroid cancer, but not for women who had regional or distant thyroid cancer. Part of this may be due to power of the analysis as there were 1,376 women with localized thyroid cancer and 456 with either regional or distant thyroid cancer.
RAI has also been shown to be associated with increases in miscarriages and abortions; however the increase has not always been reported to statistically significant.[14, 17, 18] A literature review of pregnancy outcomes reported that while there was an increased risk of spontaneous and induced abortions in the first year after treatment, but there was little to no long term risk.[12] Our study observed that there were slightly elevated rates of spontaneous abortions (miscarriages), but there were no significant differences when compared to the general population cohort including in the stratified analyses across all time periods. The rates of induced abortions were very small as they would likely not be captured by our data unless they were medically necessary. The two licensed clinics in Utah that perform abortions are not in the network of data we received. Utah in general also has a low rate of abortions. This is lower than other studies have reported in both cases and controls, which may be due to the conservative nature of Utah. While spontaneous and induced abortions were not significantly different between the two cohorts, missed abortions were significantly increased >1–5 years after cancer diagnosis for the thyroid cancer survivors.
There was more than a two-fold increased risk of diabetes or abnormal glucose tolerance during pregnancy, childbirth, and the puerperium for thyroid cancer survivors. This includes complications of diabetes mellitus during pregnancy and gestational diabetes. When broken down, gestational diabetes accounted for nearly 90% of these cases in both populations. Subclinical hypothyroidism in early pregnancy has been to be associated with increased risk in gestational diabetes.[19] This could warrant the need for higher surveillance for thyroid cancer survivors during early pregnancy for gestational diabetes.
There was also a relationship between obesity and certain risks, including significantly increased risks for overweight/obese women for premature rupture of membranes (PROM). The relationship between obesity and thyroid cancer has been well-established.[20] Maternal obesity has been found to be associated with PROM and other maternal complication increases.[21, 22] As obesity is a potentially modifiable risk factor, it suggests a potential intervention in this survivor population to improve health, which may be particularly important for survivors contemplating pregnancy given these associations. It is also important to address the positive findings for thyroid cancer survivors. Overall thyroid cancer survivors were found to have no significant difference in the number of children, having normal deliveries, and hypertension complicating the pregnancy compared to the general population cohort.
There are several limitations to this study. First, the study population of Utah is less diverse racially than most areas of the country. However, this allowed for a more homogenous study population. Another limitation is the use of ICD-9 codes from medical record data. There are likely coding errors in these diagnoses codes. However, we would not expect these errors to be different between the thyroid cancer survivors compared to the general population cohort. It is also important to acknowledge that the thyroid cancer survivors were significantly less healthy at baseline than the general population cohort. However, we did adjust for baseline BMI and baseline CCI in all models to account for this as best possible. While there may be some residual confounding, it is important to acknowledge that this is a population with decreased health and therefore they may be more at risk for some of these outcomes.
Another limitation is there is no analysis on TSH suppression therapy. While the majority of the current literature correlates many genital and reproductive late effects with RAI, it is possible that TSH suppression therapy is also playing a role. We also did not have data on the dose of RAI. The most commonly used dosage of RAI during the time frame of this study ranged from 30 mCi for remnant ablation, and 100 – 150 mCi for therapeutic ablation for loco-regional and extensive disease. Inter-institution and practitioner variation of dose usage also ranged between 30 – 150 mCi. Based on these practice patterns, we estimate that about 50% of subjects were likely treated with 30 mCi and the rest with higher dosages. There is likely a dose effect relationship between RAI and long-term health effects that we were not able to parse out. Another limitation is that some of the outcomes had low event rates, which may have impacted the hazard ratios. However, many of the low event rates were in the first year after thyroid cancer diagnosis, which is important to include as the guidelines are targeted toward that year.
While we limited the thyroid cancer population to first primary cancer case, it may still be possible for some of the thyroid cancer patients to have been exposed to childhood head/neck irradiation, from nuclear accidents or from treatment of benign conditions such as ringworm or enlarged tonsils (performed 1940s-1960s).[23] However, if this affected any of the people in the study it is likely a small percentage.
The major strength of this study is the population based design with a large sample size of over 1,800 female thyroid cancer survivors of childbearing age. This large study population allows us to study both common and rare health effects diagnosed over several time periods. Another strength is the amount of medical record data. By having complete EMR data from two of the biggest medical care providers in the state of Utah as well as complete statewide ambulatory surgery and inpatient data, we were able to capture the majority of data available for those in the study. This study also does not rely on self-reported data as many previous studies on the reproductive and pregnancy health effects of thyroid cancer have, which gives the advantage of minimizing survival bias as well as recall errors in a cancer survivors cohort.[17, 13]
We may be more likely to capture the healthcare of the cancer survivors than the cancer free population as the major cancer treatment centers in Utah are within Intermountain Healthcare and University of Utah Health (including the Huntsman Cancer Institute). Additionally, the cancer survivors are under increased medical surveillance due to their cancer diagnosis and may be diagnosed earlier or more frequently with various diseases. We would expect this surveillance to be less intense 5+ years after cancer diagnosis. We observed increases in risk in these later follow-up times, suggesting that the associations observed in our study are not just due to increased medical surveillance in cancer patients. In addition, for the health outcomes associated with pregnancy, both thyroid cancer survivors and the women from the general population cohort would have similar medical surveillance during pregnancy.
There were significant risk increases in health outcomes associated with the reproductive system and pregnancy complications. Some of these increased risks such a menstrual disorders, menopausal disorders, and abortions have been documented previously but were mainly associated with RAI treatment. Future studies need to further assess the risks between TSH suppression therapy and genital and reproductive outcomes, in combination with other treatments, for female thyroid cancer survivors of childbearing age.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the NCI (R03 CA159357; R21 CA185811), the Huntsman Cancer Institute, Cancer Control and Population Sciences Program (HCI Cancer Center Support Grant P30CA042014), and the NCRR grant, “Sharing Statewide Health Data for Genetic Research” (R01 RR021746, G. Mineau, PI) with additional support from the Utah State Department of Health and the University of Utah. We thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database (UPDB). We thank the University of Utah Center for Clinical and Translational Science (CCTS) (funded by NIH Clinical and Translational Science Awards), the Pedigree and Population Resource, University of Utah Information Technology Services and Biomedical Informatics Core for establishing the Master Subject Index between the Utah Population Database, the University of Utah Health Sciences Center and Intermountain Health Care.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Society AC. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Cancer of the Thyroid - Cancer Stat Facts. 2017. https://seer.cancer.gov/statfacts/html/thyro.html. Accessed February 2, 2017.
- 3.Key Statistics for Thyroid Cancer. American Cancer Society. 2017. https://www.cancer.org/cancer/thyroid-cancer/about/key-statistics.html. Accessed February 21, 2017.
- 4.Cancer of the Thyroid - SEER Stat Fact Sheets. 2016. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed February 8, 2016.
- 5.McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet (London, England). 2013;381(9871):1046–57. doi: 10.1016/s01406736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 6.Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A et al. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nuclear medicine communications. 2014;35(8):808–17. doi: 10.1097/mnm.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 7.Wu JX, Young S, Ro K, Li N, Leung AM, Chiu HK et al. Reproductive outcomes and nononcologic complications after radioactive iodine ablation for well-differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2015;25(1):133–8. doi: 10.1089/thy.2014.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banach R, Bartes B, Farnell K, Rimmele H, Shey J, Singer S et al. Results of the Thyroid Cancer Alliance international patient/survivor survey: Psychosocial/informational support needs, treatment side effects and international differences in care. Hormones (Athens). 2013;12(3):42838. [DOI] [PubMed] [Google Scholar]
- 9.Overview - Utah Population Database - - Huntsman Cancer Institute - University of Utah Health Care - Salt Lake City, Utah. 2016. http://healthcare.utah.edu/huntsmancancerinstitute/research/updb/. Accessed May 10, 2016.
- 10.HCUP-US Tools & Software Page. 2016. https://www.hcupus.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed February 9, 2016.
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):37383. [DOI] [PubMed] [Google Scholar]
- 12.Sawka AM, Lakra DC, Lea J, Alshehri B, Tsang RW, Brierley JD et al. A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clinical endocrinology. 2008;69(3):479–90. doi: 10.1111/j.1365-2265.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 13.Sioka C, Kouraklis G, Zafirakis A, Manetou A, Dimakopoulos N. Menstrual cycle disorders after therapy with iodine-131. Fertility and sterility. 2006;86(3):625–8. doi: 10.1016/j.fertnstert.2006.02.081. [DOI] [PubMed] [Google Scholar]
- 14.Sioka C, Fotopoulos A. Effects of I-131 therapy on gonads and pregnancy outcome in patients with thyroid cancer. Fertility and sterility. 2011;95(5):1552–9. doi: 10.1016/j.fertnstert.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Souza Rosario PW, Alvarenga Fagundes T, Villas-Boas Fagundes AS, Barroso AL, Lamego Rezende L, Lanza Padrao E et al. Ovarian function after radioiodine therapy in patients with thyroid cancer. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2005;113(6):331–3. doi: 10.1055/s-2005-837666. [DOI] [PubMed] [Google Scholar]
- 16.Rosario PW, Fagundes TA, Fagundes AV, Barraso AL, Rezende LL, Padrao EL et al. Radioiodine therapy and age at menopause in patients with thyroid cancer. Clinical endocrinology. 2006;64(2):225–6. doi: 10.1111/j.1365-2265.2005.02413.x. [DOI] [PubMed] [Google Scholar]
- 17.Garsi JP, Schlumberger M, Rubino C, Ricard M, Labbe M, Ceccarelli C et al. Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(5):845–52. doi: 10.2967/jnumed.107.046599. [DOI] [PubMed] [Google Scholar]
- 18.Fard-Esfahani A, Hadifar M, Fallahi B, Beiki D, Eftekhari M, Saghari M et al. Radioiodine treatment complications to the mother and child in patients with differentiated thyroid carcinoma. Hellenic journal of nuclear medicine. 2009;12(1):37–40. [PubMed] [Google Scholar]
- 19.Ying H, Tang YP, Bao YR, Su XJ, Cai X, Li YH et al. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine. 2016;54(3):742–50. doi: 10.1007/s12020-016-1022-6. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Medical science monitor : international medical journal of experimental and clinical research. 2015;21:283–91. doi: 10.12659/msm.892035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadley EE, Discacciati A, Costantine MM, Munn MB, Pacheco LD, Saade GR et al. Maternal obesity is associated with chorioamnionitis and earlier indicated preterm delivery among expectantly managed women with preterm premature rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2017:1–8. doi: 10.1080/14767058.2017.1378329. [DOI] [PubMed] [Google Scholar]
- 22.Madan J, Chen M, Goodman E, Davis J, Allan W, Dammann O. Maternal obesity, gestational hypertension, and preterm delivery. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(1):82–8. doi: 10.3109/14767050903258738. [DOI] [PubMed] [Google Scholar]
- 23.Childhood Head & Neck Irradiation. American Thyroid Association 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.