Abstract
The homeobox gene Gsx2 has previously been shown to inhibit oligodendroglial specification in dorsal lateral ganglionic eminence (dLGE) progenitors of the ventral telencephalon. The precocious specification of oligodendrocyte progenitor cells (OPCs) observed in Gsx2 mutants, however, is transient and begins to normalize by late stages of embryogenesis. Interestingly, this normalization correlates with the expansion of Gsx1, a close homolog of Gsx2, in a subset of progenitors in the Gsx2 mutant LGE. Here, we interrogated the mechanisms underlying oligodendroglial specification in Gsx2 mutants in relation to Gsx1. We found that Gsx1/2 double mutant embryos exhibit a more robust expansion of Olig2+ cells (i.e. OPCs) in the subventricular zone (SVZ) of the dLGE than Gsx2 mutants. Moreover, misexpression of Gsx1 throughout telencephalic VZ progenitors from E15 and onward resulted in a significant reduction of cortical OPCs. These results demonstrate redundant roles of Gsx1 and Gsx2 in suppressing early OPC specification in LGE VZ progenitors. However, Gsx1/2 mutants did not show a significant increase in adjacent cortical OPCs at later stages compared to Gsx2 mutants. This is likely due to reduced proliferation of OPCs within the SVZ of the Gsx1/2 double mutant LGE, suggesting a novel role for Gsx1 in expansion of migrating OPCs in the ventral telencephalon. We further investigated the glial specification mechanisms downstream of Gsx2 by generating Olig2/Gsx2 double mutants. Consistent with the known essential role for Olig2 in OPC specification, ectopic production of cortical OPCs observed in Gsx2 mutants disappeared in Olig2/Gsx2 double mutants. These mutants, however, maintained the expanded expression of gliogenic markers Zbtb20 and Bcan in the VZ of the LGE similarly to Gsx2 single mutants, suggesting that Gsx2 suppresses gliogenesis via Olig2-dependent and -independent mechanisms.
Keywords: Gliogenesis, Neurogenesis, Oligodendrocyte progenitor cell (OPC), Telencephalon
1. Introduction
During mammalian central nervous system (CNS) development, neural progenitors first give rise to neurons and subsequently generate glial cells, including oligodendrocytes and astrocytes (Bayer and Altman, 1991). This neuronal to glial switch occurs in a ventral-to-dorsal manner and has been shown in anterior (telencephalon) and posterior (hindbrain and spinal cord) CNS regions (Kessaris et al., 2001; Tekki-Kessaris et al., 2001; Fogarty et al., 2005; Vallstedt et al., 2005; Kessaris et al., 2006). In the telencephalon, a regional fate-mapping approach revealed that embryonic oligodendrocyte progenitor cells (OPCs) are first generated from ventral progenitor regions, including the medial ganglionic eminence (MGE) at approximately E12.5 and lateral ganglionic eminence (LGE) at approximately E15, while postnatal OPCs are largely generated from progenitors derived from the dorsal telencephalon (i.e. cortex) (Kessaris et al., 2006). However, the mechanisms controlling the temporal dynamics of the neuronal to glial fate change in VZ progenitor cells of the telencephalon remain elusive.
Gsx2 is one factor which has been shown to play a role in controlling the switch from neurogenesis to gliogenesis (Chapman et al., 2013). Gsx2 is expressed in a gradient within early ventricular zone (VZ) progenitors of the LGE and MGE, with the highest levels in the dorsal (d)LGE and lowest levels within the MGE. However, by late stages of embryogenesis, Gsx2 has downregulated within most cells of the MGE and ventral (v)LGE, and high level expression is limited to dLGE progenitors (Corbin et al., 2000; Toresson et al., 2000; Yun et al., 2001; Waclaw et al., 2009). In accordance with this expression pattern, Gsx2 both inhibits oligodendroglial specification and promotes the specification of LGE-derived neuronal fates in a temporal manner (Chapman et al., 2013; Waclaw et al., 2009). Specifically, striatal projection neurons are generated from the ventral vLGE during early stages of neurogenesis (E9–11), and olfactory bulb interneurons from the dLGE at late stages of neurogenesis (E12 onward) (Waclaw et al., 2009). Parallel to this, Gsx2 begins to downregulate in MGE and vLGE progenitors, which corresponds with the timing of the ventral (early) to dorsal (late) generation of OPCs in these regions (Kessaris et al., 2006).
The homoebox gene Gsx1 is closely related to Gsx2, sharing 97% identity between their homeodomains (Valerius et al., 1995). However, its expression pattern is in a gradient largely opposite to that of Gsx2 expression, with high levels of Gsx1 expression within the MGE and much lower levels within the vLGE (Valerius et al., 1995; Pei et al., 2011; Qin et al., 2017). In the absence of Gsx2, Gsx1 expands dorsally, encompassing the entire extent of the LGE by E16.5 (Toresson and Campbell, 2001; Yun et al., 2003; Wang et al., 2003). Corresponding to this dorsal expansion of Gsx1 in Gsx2 mutant LGE progenitors, the severe depletion of LGE- derived neuronal subtypes begins to recover by later embryonic stages (Szucsik et al., 1997; Corbin et al., 2000; Toresson et al., 2000; Yun et al., 2001; Stenman et al., 2003; Chapman et al., 2013). Thus, Gsx1 is thought to specify progenitors towards LGE neuronal fates in a manner similar to Gsx2, and therefore its dorsal expansion has a compensatory effect and is responsible for the partial restoration of LGE neuronal fates in Gsx2 mutants (Toresson and Campbell, 2001; Yun et al., 2003; Pei et al., 2011; Chapman et al., 2013). The loss of Gsx1 alone, however, does not result in a robust phenotype in the LGE, likely due to the remaining Gsx2 expression within VZ progenitors which correctly specifies these cells (Li et al., 1996; Toresson and Campbell; 2001; Yun et al., 2003; Wang et al., 2013).
Here we explored the molecular mechanisms underlying the OPC phenotype in the Gsx2 mutant LGE. The precocious oligodendroglial specification in the dLGE of Gsx2 mutants begins to recover at late embryonic stages, which temporally correlates with the expansion of Gsx1 (Pei et al., 2011; Chapman et al., 2013). By analyzing OPCs within Gsx1/2 double mutants as well as Gsx1 over-expressing embryos, we demonstrate that Gsx1 negatively regulates the specification of OPCs in telencephalic VZ progenitors similar to Gsx2 and is required for the partial normalization of neuronal fates within Gsx2 mutants. Specifically, Gsx1/2 double mutants display further increased OPC specification than Gsx2 mutants that persists throughout late stages of embryogenesis. These expanded OPCs, however, exhibit defective proliferation. As a result, the numbers of LGE-derived OPCs in the adjacent cortex of Gsx1/2 mutants are not as large as those in the Gsx2 mutants. Complementing these results, misexpression of Gsx1 throughout late-stage (i.e. E15 onward) telencephalic VZ progenitors suppresses oligodendroglial specification, leading to reductions of OPCs in surrounding mantle regions. Despite that Gsx1 expression expands throughout the Gsx2 mutant LGE, it is not expressed throughout the apical-basal extent of the mutant VZ, as is the case for Gsx2 (Wang et al., 2009). Instead, Gsx1 expression marks the VZ/SVZ boundary of the Gsx2 mutant LGE. In contrast, factors associated with glial cell development (Olig2, Bcan, and Zbtb20) are expanded in the Gsx2 mutant LGE and are expressed in more apical VZ cells as compared to the expanded Gsx1 expression. Moreover, analyzing Olig2/Gsx2 double mutants revealed that Olig2 is crucial for the expansion of the OPCs in the Gsx2 mutant cortex. However, the glial associated markers (Bcan and Zbtb20) remain expanded in Olig2/Gsx2 double mutant VZ progenitors similar to Gsx2 mutants. Altogether, these studies reveal that Gsx1 plays a repressive role on embryonic oligodendroglial specification within LGE VZ progenitors, comparable to that of Gsx2 and Olig2-dependent and -independent mechanisms exist downstream of Gsx factors in the specification of glial progenitors.
2. Materials and methods
2.1. Animals
Olig2cre/+ mice (Dessaud et al., 2007) were used in combination with Gsx2EGFP/+ (Wang et al., 2009) for the generation of Gsx2;Olig2 double mutant embryos (Gsx2EGFP/EGFP;Olig2cre/cre). Gsx2EGFP/+ mice were crossed with Gsx2RA/+ mice (“RA” refers to the germline recombined allele from the Gsx2 conditional mice, resulting in a null allele) (Waclaw et al., 2009) to generate G.sx2EGFP/RA mutant embryos. Gsx1+/- mice (Li et al., 1996) were genotyped according to previous studies (Toresson and Campbell, 2001). Gsx1+/- and Gsx2EGFP/+ heterozygous mice were crossed, as well as Gsx1+/- and Gsx2RA/+ heterozygous mice, resulting in Gsx2EGFP/+; Gsx1+/- and Gsx2RA/+; Gsx1+/- double heterozygotes. These double heterozygotes were then cross-bred with each other to generate Gsx2EGFP/RA; Gsx1-/- (Gsx1/2) double mutant embryos. Foxg1tTA/+ (Hanashima et al., 2002), and tet-O-Gsx1-IRES-EGFP(IE) (Pei et al., 2011) mice were genotyped as previously described (Waclaw et al., 2009; Pei et al., 2011). Foxg1Tta/+ mice were crossed with tet-O-Gsx1-IRES-EGFP(IE) mice to obtain Foxg1tTA/+; tet-O-Gsx1-IRES- EGFP(IE) double transgenic embryos. 0.02 mg/ml of doxycycline hyclate (Dox, Sigma) was added to the drinking water of pregnant females beginning on E7 and taken away 4 days later (i.e. E11), which results in repression of the transgene until approximately E15 (Chapman et al., 2013).
For staging of embryos, the morning of vaginal plug detection was designated as embryonic day (E)0.5. For every stage and marker used at least three embryos of each genotype were analyzed. Animal protocols were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee in accordance with NIH guidelines.
2.2. Histological analysis
Embryo heads were fixed in 4% paraformaldehyde at 4C overnight, followed by at least 3 washes in PBS. They were then placed into 30% sucrose over multiple days for cryoprotection, prior to sectioning on a cryostat at 12μm thickness onto positively-charged slides. Fluorescent immunohistochemical staining was performed on slide-mounted sections, with first a one hour blocking incubation in 10% normal donkey serum (Jackson ImmunoResearch), followed by an overnight incubation in a primary antibody used at the following respective concentrations: chick anti-GFP (1:1000 Aves), goat anti-GFP (1:3000, Abcam), rabbit anti-GFP (1:1000, Invitrogen), guinea pig anti-Gsx1 (1:4000, Qin et al., 2017), rabbit anti-Gsx2 (1:5000, Toresson et al., 2000), rabbit anti-Ki67 (1:1000, Abcam), rabbit anti-01ig2 (1:2000, Millipore), rabbit anti-PDGFRα (1:200, Santa Cruz), goat anti-Sox10 (1:200, Santa Cruz), goat anti-Sp8 (1:8000, Santa Cruz), rabbit anti-Zbtb20 (1:333, Sigma). Subsequently, a two hour incubation in the corresponding fluorescent secondary antibodies was performed using: donkey anti-goat antibodies conjugated to Cy2, Cy3, or Cy5 (1:200, Jackson Immunoresearch) and donkey anti-rabbit antibodies conjugated to Cy2, Cy3, or Cy5 (1:200, Jackson Immunoresearch). To detect EGFP protein expressed from the Gsx2EGFP allele, a tyramide amplification kit (ThermoFisher Scientific, T20932) was utilized. Double staining for Olig2 and Gsx2 was done using the tyramide amplification kit to visualize rabbit anti-01ig2 (1:20,000, Millipore) and to quench the first antibody followed by Gsx2 immunostaining as described above.
2.3. In Situ Hybridization
In situ hybridization was essentially carried out as described (Toresson et al., 1999; Kohli et al., 2017). A Bcan cDNA fragment (1089 bp) was amplified from E14.5 mouse embryo forebrain cDNA with the following primer pair containing Sal1 and Spe1 sites: CCGTCGACCTTCAATGTCTACTGCTTCC and CCACTAGTAGATGCTGGTCAGATGAG.
The Bcan cDNA fragment was cloned into Spe1/Sal1 sites of pBluescript KS. Digoxigenin- labeled antisense probes against Bcan were generated by linearizing with Sal1 (New England Biolabs) and using T7 RNA polymerase (Roche). Combination situ hybridization/IHC was carried out as previously described (Kohli et al., 2017).
2.4. Quantification
To quantify PDGFRα, 01ig2, and Sp8 expression within the dLGE of E15.5 embryos, thresholding and measuring tools were utilized in ImageJ to determine the area of immunopositive cells in three adjacent rostral to caudal sections. PDGFRα expression was quantified in the VZ/SVZ region due to the cytoplasmic nature of the staining that leads to labeling of the radial glia and robust staining in the VZ/SVZ regions. PDGFRα and Sp8 expression were quantified in the same manner within the E18.5 dLGE, however Olig2 expression within this region is sparse enough for cellular resolution, and thus was quantified manually with a cell counter. For the quantification of E18.5 cortical OPCs, two high-powered images were taken within the cortex of three adjacent rostral-caudal sections, and Sox 10, Olig2 and PDGFRα OPCs were quantified manually using a cell counter. To account for any variation in cortical size, the volumes of regions quantified were calculated using ImageJ and OPC numbers were normalized by comparing cells per millimeter squared (mm2). OPCs double labeled with EGFP and PDGFRα or Olig2 or Sox 10 were quantified in the same manner and as described in Chapman et al. (2013). For the quantification of Olig2 and Ki67 at E15.5, three images were taken in the dLGE SVZ region and quantified for Olig2 positive cells and Olig2;Ki67 double positive cells per field. At least three embryos were analyzed for each genotype.
3. Results
3.1. Increased oligodendroglial specification in Gsx1/2 double mutants
In the absence of Gsx2, dLGE progenitors are precociously directed towards an oligodendroglial fate at mid-embryonic (i.e. E15) stages, but this expansion of OPCs is transient and improves by birth (Chapman et al., 2013). Gsx1 is known to expand in the absence of Gsx2 in a subset of LGE progenitors by mid-embryonic stages (Toresson et al., 2001; Yun et al., 2003; Wang et al., 2009; Pei et al., 2011). As previously described (Wang et al., 2009; Pei et al., 2011), expanded Gsx1 gene expression in the Gsx2 mutant LGE marks the VZ/SVZ boundary and does not occupy the apical half of the mutant LGE VZ, where the most primitive progenitors exist. Using a new Gsx1 specific antibody (Qin et al, 2017), we observe the same staining pattern (Suppl. Fig. 1A-D). To determine if the dorsal expansion of Gsx1 in the LGE, observed in Gsx2 mutants (Suppl. Fig. 1C-D; see also Toresson et al., 2001; Yun and Rubenstein, 2003), plays a role in the normalization of OPC specification in Gsx2 mutants, we generated Gsx1/2 double mutant embryos (Gsx2EGFP/RA; Gsx1-/- embryos). The dorsal expansion of Gsx1 along the VZ/SVZ border reaches the vLGE by E12.5 and is fully extended throughout the dLGE prior to E16.5 (Wang et al., 2009; Suppl. Fig. 1A-D’). Thus, to determine any compensatory effect of Gsx1 on OPC specification, we examined the expression of the OPC markers PDGFRα and Olig2 within the dLGE of Gsx1/2 double mutants at E15.5. The dLGE SVZ normally contains Sp8-positive olfactory bulb and amygdalar interneuron progenitors (Fig. 1C) (Waclaw et al., 2006; Waclaw et al., 2010). In fact, the dLGE SVZ of control embryos completely lacks PDGFRα staining (Fig. 1A) and contains only a very small number of Olig2-positive cells that do not overlap with Sp8 positive cells in the same region (Fig. 1B, 1D). In Gsx2 mutants, however, the stream of Sp8- positive neuroblasts is drastically decreased and replaced with PDGFRα- and Olig2-positive OPCs (Fig. 1E-H) (Waclaw et al., 2006; Chapman et al., 2013). Interestingly, Sp8 expression in Gsx1/2 double mutants is more severely diminished and largely absent from the dLGE SVZ as compared to Gsx2 mutants (compare Fig. 1G to K; see also Fig. 1S). Complementing these results, Gsx1/2 double mutant embryos display a greater increase in PDGFRα and Olig2 cells within the VZ and SVZ (Fig. 1I,J) as compared to Gsx2 mutants (Fig. 1E,F). These findings suggest that Gsx1, similar to Gsx2, negatively regulates OPC specification of LGE progenitors. Indeed, the loss of both genes leads to a further increase in precocious OPCs within the double mutant LGE (Fig. 1Q,R).
Fig. 1. Gsx1/2 double mutants display a greater increase in OPC specification from dLGE progenitors than Gsx2 mutants.
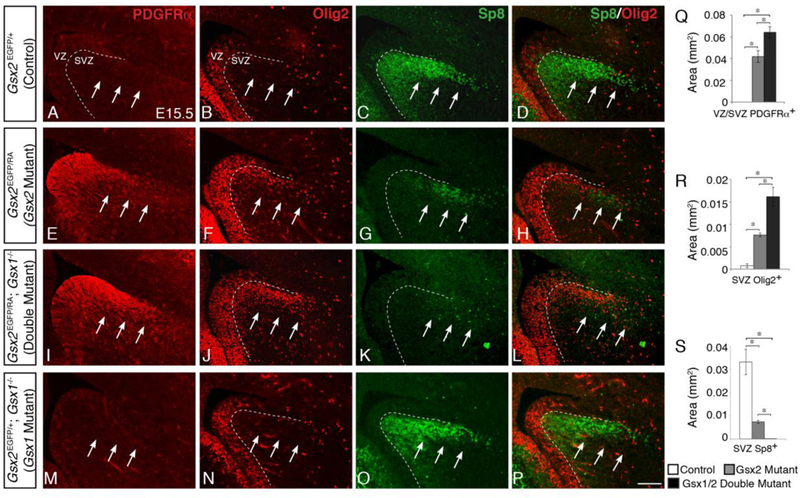
At E15.5, control dLGE progenitors generate many Sp8- positve neuroblasts (C) and very few OPCs, as indicated by lack of PDGFRα expression (A) and only scattered Olig2 expression within the SVZ (B). Gsx2 mutants conversely express much lower levels of Sp8 (G) and instead ectopically express high levels of PDGFRα (E) and Olig2 (F) within the dLGE. This loss of Sp8 corresponds with the expanded OPCs, indicated in an overlay of Sp8 and Olig2 (H, compared to D). Gsx1/2 double mutants display even higher levels of PDGFRa (I) and Olig2 (J,L) expression within the dLGE. Accordingly, only a few cells express Sp8 within this region of double mutants (K,L). Quantification of these cells indicate that Gsx1/2 double mutants have significantly more PDGFRα expression within the VZ/SVZ and Olig2 expression within the SVZ than Gsx2 mutants, which have significantly more than control (Q,R). Correspondingly, Gsx1/2 double mutants have significantly fewer Sp8-positive cells within the dLGE SVZ than Gsx2 mutants, which have substantially fewer than control embryos (S). Gsx1 mutant embryos display no defects in oligodendroglial or neuronal specification, and resemble control embryos with no PDGFRα expression (M), very little Olig2 expression (N,P), and normal levels of Sp8 (Ο,Ρ) within the dLGE. Data represent the mean ±SEM. *p<0.01, as determined by a one-way ANOVA followed by a Tukey HSD post-hoc test. Scale bar: P = 100μΜ
Gsx1 has been shown to regulate the specification of LGE neuronal subtypes in a similar manner as Gsx2 (Pei et al., 2011; Toresson and Campbell, 2001; Yun et al., 2003), however, the loss of Gsx1 alone has no apparent effect on LGE neuronal specification. This is not surprising given the expression pattern of Gsx1, displaying highest levels of expression within the MGE and only scattered cells within the vLGE along the VZ/SVZ border in wild type embryos (Suppl. Fig. 1A-D’). Furthermore, Gsx1 mutants still have functioning Gsx2 within the VZ, and even slightly increased levels in the vLGE at late stages of neurogenesis (E16.5-E18.5), which is therefore able to correctly specify these progenitors (Pei et al., 2011). Accordingly, Gsx1 mutants display no differences in embryonic oligodendroglial specification, and resemble control embryos with a stream of Sp8-positive cells and little to no PDGFRα or Olig2 expression within the dLGE SVZ (Fig. 1M-P).
By late embryonic stages, the molecular identity of LGE progenitors (i.e. Dlx, Isl1, Ascl1, Er81 expression) in Gsx2 mutants has begun to normalize (Toresson et al., 2000; Yun et al., 2001). This recovery is largely dependent on the dorsal expansion of Gsx1 (see Supp. Fig. 1), which is evidenced by the fact that Gsx1/2 double mutants have more severe misspecification of LGE progenitors than Gsx2 mutants (Stenman et al., 2003; Toresson and Campbell, 2001; Yun et al., 2003). The expression of Sp8 in the dLGE SVZ of Gsx2 mutants is improved by E18.5, however, not to the same level as observed in controls (Fig. 2F) (Chapman et al., 2013). Conversely, in Gsx1/2 double mutants, only a few scattered cells express Sp8 in this region, further supporting that this recovery is largely dependent on Gsx1 (Fig. 2I,L). To determine if Gsx1 is similarly required for the normalization of precocious OPC specification seen in Gsx2 mutants, we examined the OPC markers PDGFRα and Olig2 in E18.5 Gsx1/2 double mutants.
Fig. 2. Expanded OPC specification from dLGE progenitors persists up until birth in Gsx1/2 double mutants, despite recovering in Gsx2 mutants.
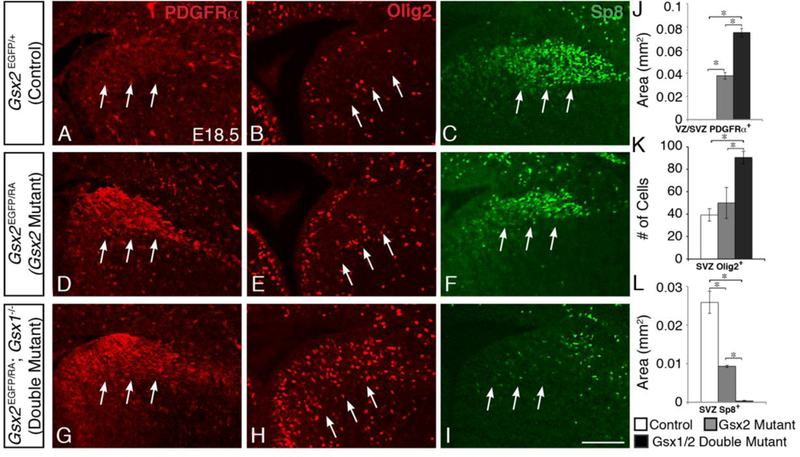
By E18.5, the increased oligodendroglial specification from the dLGE is beginning to recover in Gsx2 mutants, with only slightly increased PDGFRα expression in the VZ/SVZ (D, compared to A) and no difference in Olig2-positive cells (E, compared to B) within the SVZ. Accordingly, Sp8 expression is also returning to the dLGE (F, compared to C). In contrast, Gsx1/2 double mutants continue to express highly increased levels of PDGFRα (G) and 01ig2 (H), while only few scattered Sp8- positive cells remain within the dLGE (I). The number of Olig2-positive cells as well as the area of PDGFRα- and Sp8-immunostained cells within the dLGE are quantified in J-L. Data represent the mean ±SEM. *p<0.01, as determined by a one-way ANOVA followed by a Tukey HSD post-hoc test. Scale bar: I = 100μΜ
As expected, Gsx1/2 double mutants express increased PDGFRα and Olig2 expression within the VZ/SVZ of the dLGE (Fig. 2G,H,J,K) compared to Gsx2 mutants (Fig. 2D,E). This indicates that in Gsx2 mutants, the dorsal expansion of Gsx1 into the LGE not only restores neurogenesis but also prevents ectopic misspecification of OPCs.
3.2. OPCs in adjacent mantle regions are not further expanded in Gsx1/2 double mutants due to reduced LGE SVZ proliferation
Although the precocious specification of dLGE progenitors toward an oligodendroglial fate in Gsx2 mutants is transient, this still leads to increased numbers of LGE-derived OPCs in surrounding cortical mantle regions at E18.5 (Fig. 3C,D) (Chapman et al., 2013). Given that Gsx1/2 double mutants display an even further increase in precocious OPC specification throughout late embryonic stages, as compared to Gsx2 mutants, we expected these embryos to exhibit a larger expansion of OPCs in the adjacent cortex. Surprisingly, Gsx1/2 double mutants (Fig. 3E,F) generate significantly fewer OPCs within the cortex than Gsx2 mutants (Fig. 3C,D), however, they still exhibit significantly more cortical OPCs than in control embryos (Fig. 3A,B). While Gsx2 mutants exhibited a 43% and 59% increase in Sox10- and PDGFRα-positive cells in the cortex compared to control embryos, Gsx1/2 double mutants only displayed a 27% and 37% increase in Sox10- and PDGFRα-positive cells, respectively (Fig. 3G). Using the EGFP knock- in allele of Gsx2 to follow G.sx2-expressing progenitors into surrounding mantle regions, we found that the increased cortical OPCs in Gsx2 mutants are largely, if not exclusively, derived from Gsx2 mutant progenitors (Chapman et al., 2013). Considering only the EGFP-positive population of OPCs, we again observed a much larger increase, 120% and 108%, in Gsx2- derived Sox 10-positive and PDGFRα- positive OPCs, respectively, verifying that the increased cortical OPCs are largely derived from Gsx2-mutant progenitors (Fig. 3H). When examining the EGFP positive cells in the cortex of Gsx1/2 double mutants, we found extensive co-labeling of EGFP/Sox10 and EGFP/PDGFRα (Fig. 3E,F) and a much larger increase than total OPCs, however there are still fewer than in Gsx2 mutant embryos. Gsx1/2 double mutants displayed a 80% increase in EGFP/Sox10-positive and an 83% increase in EGFP/PDGFRα-positive OPCs (Fig. 3H). This suggests that, similar to Gsx2 mutants, the overall increase in OPCs in Gsx1/2 double mutants is largely derived from Gsx1/2-mutant progenitors.
Fig. 3. Increased oligodendroglial specification in Gsx1/2 double mutants does not lead to a further expansion in adjacent cortical OPCs as compared to Gsx2 mutants.
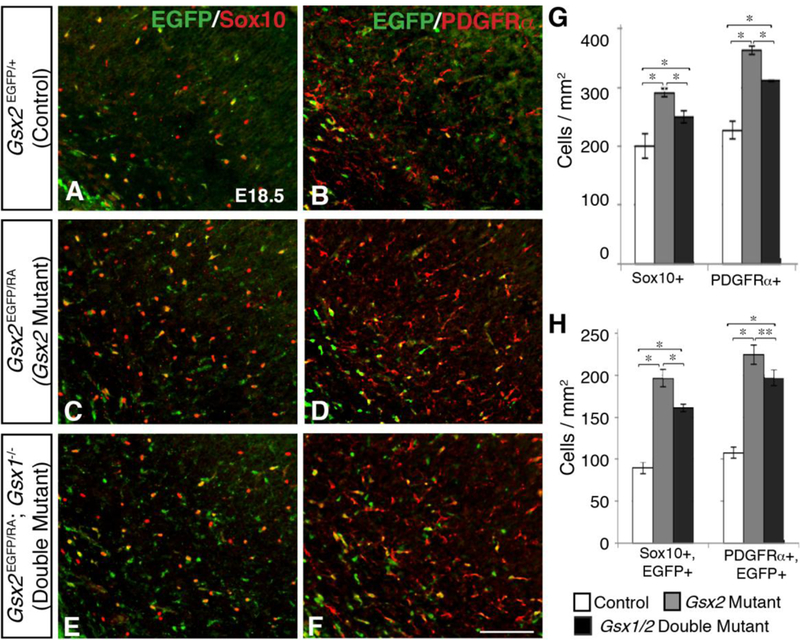
Gsx2 mutants display increased Sox10 (control 203.2±28.7; mutant 290.3±6.4 cells/mm2) and PDGFRa (control 227.4±15.2; mutant 362.6±7.0 cells/mm2) OPCs within the cortex at E18.5 (C-D compared to A-B; G). Gsx1/2 double mutants also exhibit increased cortical Sox10 (258.7±7.8) and PDGFRα (310.8±1.1) OPCs compared to controls (E-F), however Gsx1/2 double mutants have significantly fewer OPCs than Gsx2 mutants (G). When considering only cortical OPCs that originated from G.vx2-expressing progenitors (co-expressing EGFP), Gsx1/2 double mutants again generated significantly more Sox10 (control 89.5±6.9; double mutant 161.0±4.5 cells/mm2) and PDGFRα (control 107.8±6.6; double mutant 196.8±9.3 cells/mm2) OPCs than controls, yet significantly fewer than Gsx2 mutants (196.6±10.5 Sox10+ cells/mm2; 224.5±11.6 PDGFRα+ cells/mm2) (H). Both Gsx2 mutants and Gs1/2 double mutants contained significantly more Gsx2-derived OPCs within the cortex, indicating that the vast majority of increased OPCs arose from Gsx2 mutant progenitors (H). Data represent the mean ±SEM. *p<0.01 and **p<0.05, as determined by a one-way ANOVA followed by a Tukey HSD post- hoc test. Scale bar: F = 100μM
Although Gsx1/2 double mutants exhibit a significant upregulation in both G.sx2-derived OPCs as well as total number of OPCs within the cortex compared to control embryos, there are still significantly more OPCs within the cortex of Gsx2 mutants (Fig. 3G,H). This was unexpected, since Gsx1/2 double mutants have increased PDGFRα- and Olig2-expressing cells within the dLGE SVZ, as compared to Gsx2 mutants (see Figures 1 and 2). One possibility could be that the increased OPCs in Gsx1/2 double mutants are migrating and expanding into different regions of the telencephalon instead of the cortex. To determine if these OPCs were migrating ventrally instead, we analyzed the OPC markers Olig2, PDGFRα, and Sox 10 within mantle regions of the subpallium, however no significant increases were detected (data not shown). We also examined Plp1 expression at E18.5 to address OPC differentiation. No precocious expression of Plp1 was found in the embryonic cortex of Gsx1/2 double mutants (data not shown). Moreover, since Gsx1/2 double mutants are lethal after P0, we could not address OPC differentiation in the postnatal cortex. Another possibility could be that Gsx1 plays additional roles outside of the VZ/SVZ of the LGE similar to Ascl1, which plays a role in migrating OPCs (Nakatani et al., 2013) and is required for the expansion and/or maintenance of OPCs in the Gsx2 mutant cortex (Chapman et al., 2013). In line with this, a recent gene expression catalog of early perinatal cortex cell types showed Gsx1 as one of several new transcription factors expressed in OPCs (Zhang et al., 2014).
Gsx2 has been shown to play a key role in proliferation in the embryonic progenitors and postnatal neural stem cells from telencephalic regions in vivo and in vitro (Pei et al., 2011; Mendez-Gomez and Vicario-Abejon, 2012; Lopez-Juarez et al., 2013). In fact, Gsx2 mutants have reduced proliferation within SVZ progenitors at E12.5, which, similar to the molecular identity of the LGE, has largely recovered by E16.5 (Toresson et al., 2000; Yun et al., 2001), suggesting a role for Gsx1/2 in progenitor proliferation. This recovery is also dependent on Gsx1, as Gsx1/2 double mutants have been shown to have decreased SVZ proliferation persisting throughout E16.5 (Toresson and Campbell, 2001). OPCs are also highly proliferative, and thus we analyzed the expression of the cell cycle marker Ki67 (Schlüter et al., 1993) within the dLGE SVZ together with 01ig2 at E15.5 in order to determine whether the increased OPCs in Gsx1/2 double mutants exhibit defective proliferation. By E15.5, Ki67 expression has recovered in Gsx2 mutant SVZ progenitors (Fig. 4C), appearing similar to control embryos (Fig. 4A). Consistent with previous studies (Toresson and Campbell, 2001), Gsx1/2 double mutant embryos continue to display reduced Ki67 expression within SVZ progenitors (Fig. 4E). Control embryos contain only a few scattered Olig2+ OPCs within the dLGE SVZ, and some of these co-express Ki67 indicating they are actively proliferating (arrows point towards SVZ region in Fig. 4B and4G).
Fig. 4. Defective proliferation of OPCs within the dLGE of Gsx1/2 double mutant embryos.
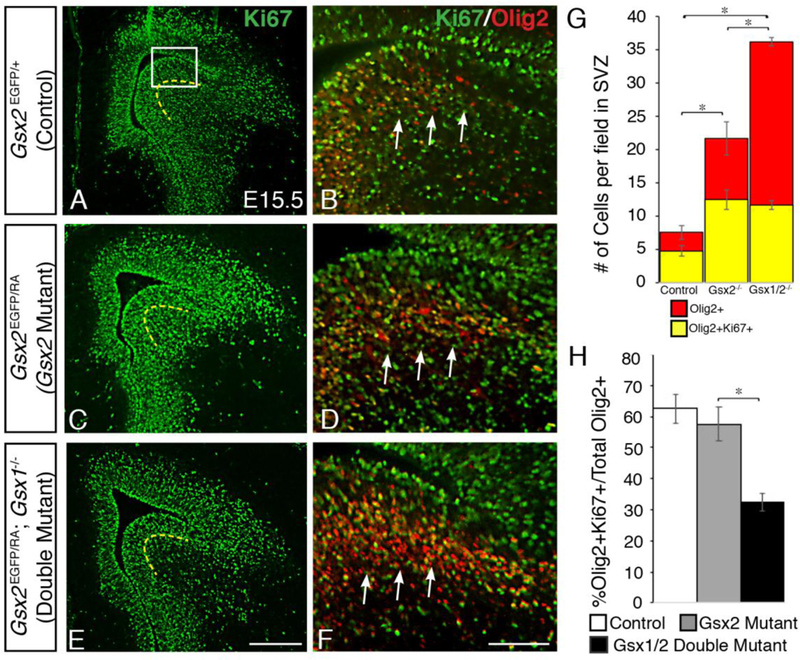
Gsx2 mutants have defective proliferation within the SVZ of the LGE at early stages of neurogenesis, however this begins to recover by E15.5 as expression of cell cycle marker Ki67 appears within the SVZ (denoted by dashed line) and looks rather normal in the Gsx2 mutant (C) compared to controls (A). In contrast, Gsx1/2 double mutants continue to display reduced levels of proliferation within the SVZ at E15.5 (E). When analyzing the OPC marker Olig2 in combination with Ki67 in high-powered images of the dLGE, there are very few Olig2+ cells within the dLGE SVZ of control embryos and some express Ki67 (B) (4.8±0.8 cells per field).In Gsx2 mutants, many of the expanded O1ig2-positive cells within the SVZ are proliferative, as demonstrated by co-expression with Ki67 (D) (12.5±1.5 cells per field). Gsx1/2 double mutants show similar numbers of Olig2+Ki67+ cells in this region (11.7±0.7 cells per field) compared to Gsx2 mutants. However, Gsx1/2 double mutants show increased total Olig2-positive cells within this region (36.2±0.6 cells per field) compared to control (7.6±1.0 cells per field) and Gsx2 mutants (21.7±2.5 cells per field) (F,G). When considering the percentage of 01ig2+Ki67+ cells to total 01ig2+ cells, Gsx1/2 double mutants (32.3%±2.8) showed reduced numbers compared to control (63.2%±4.7) and Gsx2 mutants (57.6%±5.6) (H). Data represent the mean ±SEM. *p<0.01 in G and *p<0.05 in H as determined by a one-way ANOVA followed by a Tukey HSD post-hoc test. Scale bar: E = 200μΜ, F = 100μΜ
In contrast, Gsx2 mutants have significantly more Olig2-positive cells within the SVZ, many of which co-label with Ki67 (Fig. 4D and 4G). Gsx1/2 double mutant embryos contain even more Olig2-positive cells within the dLGE SVZ, however, a significantly smaller fraction of the total Olig2 population co-labeled with Ki67 compared to Gsx2 mutants (Fig. 4F-H), which is in line with the reduced Ki67 in the SVZ of the LGE (Fig. 4E and Toresson and Campbell, 2001). In fact, Gsx1/2 double mutants show only 32% of Olig2 positive cells are Olig2;Ki67 double positive cells compared to the 58% observed in Gsx2 mutants (Fig. 4G-H). This suggests that although Gsx1/2 double mutants have an overall increase in OPC specification from dLGE progenitors (Olig2-positive cells), a smaller percentage of the total Olig2 positive cells are actively dividing within the SVZ than those in Gsx2 single mutants, which may contribute to less of an expansion of OPCs in surrounding cortical regions as compared to Gsx2 mutants (see Fig. 3).
3.3. Gsx1 is sufficient to repress the specification of OPCs in late telencephalic progenitors
To over-express Gsx1 at distinct stages of development, we utilized a binary transgenic system that can be temporally regulated with doxycycline (Dox). This system utilizes a Foxg1tTA/+ knock-in mouse that expresses the tetracycline transactivator in the Foxg1 lineage (Hanashima et al., 2002) and tetO-Gsx1-IE transgenic mice which express Gsx1 only after tTA expression (Pei et al., 2011). We analyzed Foxg1tTA; tetO-Gsx1-IE double transgenic (Gsx1 DT) embryos at E18.5 following an E7–11 Dox treatment, which has been shown to delay Dox responsive transgene expression to E15.5 onward in the telencephalon (Chapman et al., 2013). To evaluate transgene activation after DOX treatment, we analyzed GFP expression from the Gsx1 transgene (tetO-Gsx1-IRES-eGFP). We detect GFP expression in both progenitor and mantle zone areas of the telencephalon at E18.5 (Fig. 5C,F). However, we observe stronger GFP expression in the VZ/SVZ of the dorsal telencephalon compared to the VZ/SVZ of the LGE in the ventral telencephalon and almost no transgene activation in medial/septal regions (Fig. 5C,F). Delayed over-expression of Gsx1 results in the respecification of progenitors to a dLGE neuronal identity (Fig. 5), similar to what was previously observed with delayed over-expression of Gsx2 (Chapman et al., 2013). Both Ascl1 (Fig. 5B,C) and Sp8 (Fig. 5E,F) are upregulated within the dorsal telencephalon (i.e. cortex) of these Gsx1 DT embryos at E18.5. This misspecification, though similar to the Gsx2 DT, appears less robust, which is consistent with results from previous early-stage Gsx1 over-expression studies (Pei et al., 2011).
Fig. 5. Over-expression of Gsx1 from E15 on results in specification of VZ progenitors toward a dLGE fate.
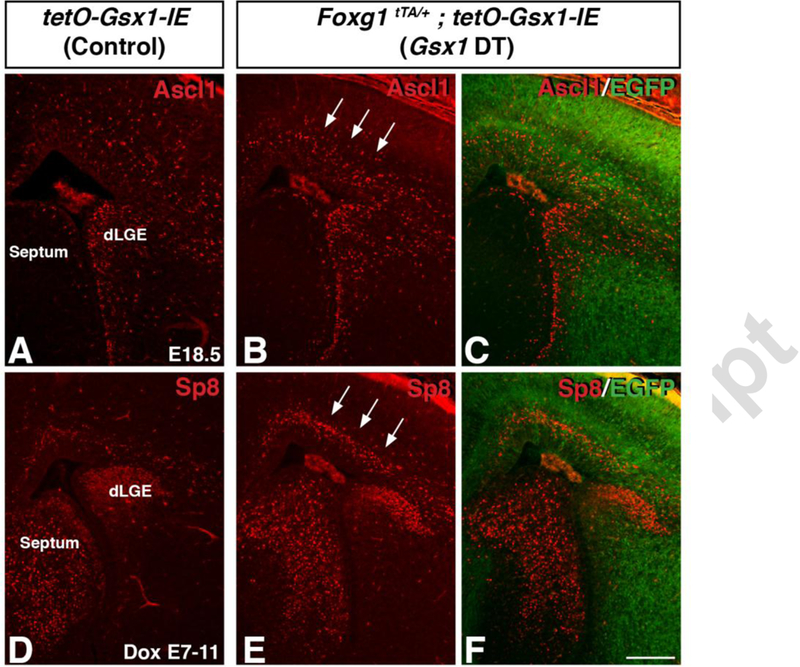
Misexpression of Gsx1 from El5 onward promotes dLGE neurogenesis, as indicated by the ectopic upregulation of Ascl1 (B,C, arrows) as well as the dLGE marker Sp8 (E,F, arrows) throughout the SVZ of the dorsal telencephalon, as compared to control embryos (A, D). Scale bar: F = 200μΜ
Gsx2 DT embryos with delayed Dox treatment from E7–11 resulted in a significant reduction of OPCs within the cortex at E18.5 (Chapman et al., 2013). To determine whether Gsx1, like Gsx2, is sufficient to suppress oligodendroglial specification in LGE progenitors, we analyzed OPCs in the cortex of Foxg1tTA; tetO-Gsx1-IE DT embryos with the same delayed Dox treatment from E7–11. Both Gsx1 and Gsx2 DT embryos have cortical morphological defects that are improved with Dox treatment (Waclaw et al., 2009, Pei et al., 2011, and Chapman et al., 2013). However, Gsx1 DT embryos still show an enlarged ventricle and slightly thinner cortex (Fig. 5 and 6), which is similar to Gsx2 DT embryos (Chapman et al., 2013). Gsx1 DT embryos showed a significant reduction in OPCs within the cortex as indicated by Sox10, PDGFRα, and Olig2 expression (Fig. 6D-F) as compared to controls (Fig. 6A-C). Specifically, there was a 39% reduction in Sox 10-expressing cells, a 43% reduction in PDGFRα-expressing cells, and a 30% reduction in Olig2-expressing cells (Fig. 6G). Consistent with the reduced ability of Gsx1 to specify progenitors towards neuronal fates as compared to Gsx2, this suppression of OPCs is slightly less robust than in Gsx2 DT embryos, which had a 46%, 47%, and 37% decrease in Sox10-, PDGFRα-, and Olig2-expressing OPCs, respectively (Chapman et al., 2013). When examining EGFP expression (a surrogate of Gsx1 overexpression) with these OPC markers, we observed little to no co-localization (Fig. 6H-J), indicating that the remaining OPCs were not generated from progenitors that over-express Gsx1. Overall, these results indicate that Gsx1 is able to inhibit oligodendroglial specification within late (i.e. E15 onward) LGE progenitors, analogous to the role of Gsx2 in OPC specification.
Fig. 6. Gsx1 is sufficient to repress the specification of OPCs within telencephalic VZ progenitors.
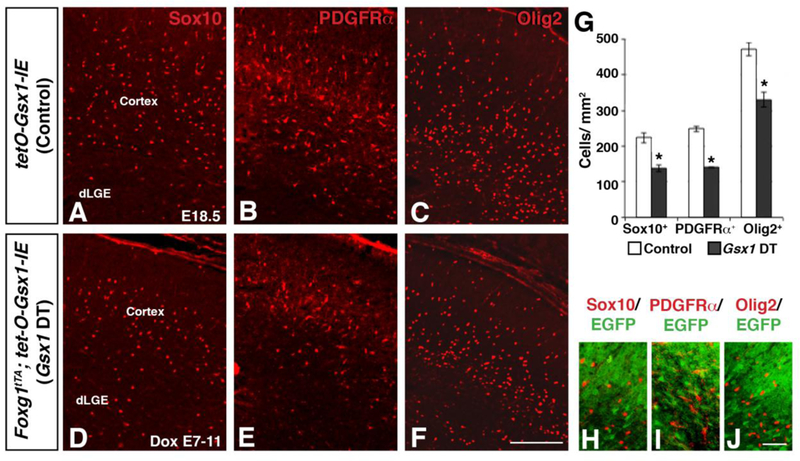
The over-expression of Gsx1 in telencephalic progenitors results in severely reduced Sox10 (control 223.5±14.4; DT 137.2±9.8 cells/mm2), PDGFRα (control 248.3±7.9; DT 140.6±1.9 cells/mm2), and Olig2 (control 471.8±19.0; DT 330.8±21.6 cells/mm2) OPCs within the cortex (D-F) as compared to control embryos (A-C; quantification in G). Immunostaining for EGFP in combination with OPC markers (H-J), revealed little to no co-expression, suggesting that Gsx1 is sufficient to inhibit OPC specification. Data in G represent the mean ±SEM. *p<0.005, significance determined by Student’s t-test. Scale bar: F = 100μΜ, J = 50μΜ
3.4. Increased expression of glial markers in LGE progenitors of Gsx2 mutant
Gsx2 has been shown to negatively regulate OPC generation from LGE progenitors (Chapman et al., 2013). In particular, dLGE progenitors in the SVZ of Gsx2 mutants were shown to be precociously misspecified to an oligodendroglial fate (i.e. Olig2+) at mid-embryonic stages (e.g. E15.5) (Chapman et al., 2013). We analyzed the normal expression of Olig2 in the VZ of the LGE at E13.5 and compared it to the high dorsal to low ventral gradient of Gsx2 expression. Interestingly, Olig2 appears to be expressed in an opposing gradient to Gsx2 with very few Olig2 positive cells present in the high Gsx2-expressing dLGE domain (Fig. 7A-B). Further examination of Gsx2 mutants reveals that expanded Olig2 expression is also observed in VZ progenitors throughout the apical-basal extent of the LGE at E16.5 (compare Suppl. Fig. 1F to E). Notably, the expression of Olig2 is observed in the more apical VZ cells where the patterning and specification of glial cells takes place compared to increased Gsx1 positive cells at the VZ/SVZ boundary in the Gsx2 mutant LGE (Suppl. Fig. 1G-J).
Fig. 7. Candidate glial progenitor genes, Zbtb20 and Bcan are increased in VZ progenitors of Gsx2 mutants.
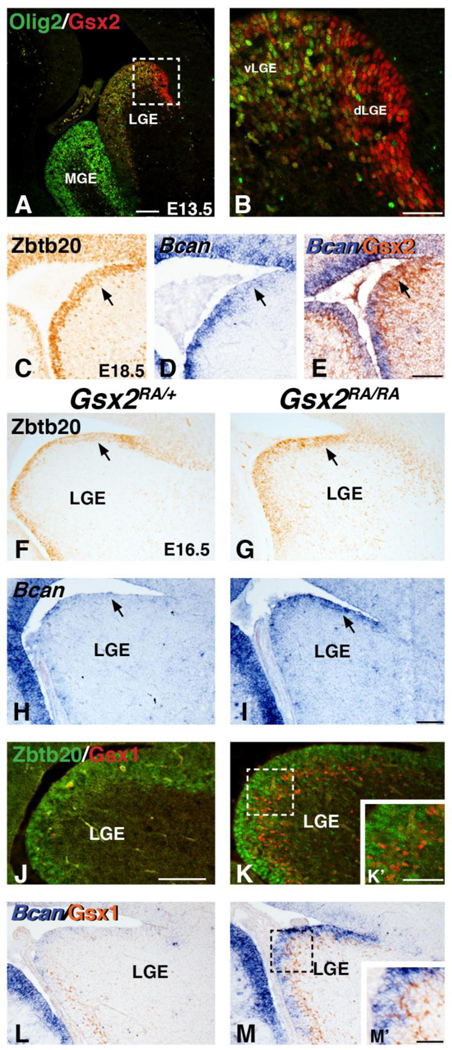
Immunostaining for 01ig2 and Gsx2 reveals opposing patterns of expression in the E13 LGE (A-B). Dashed box in A depicts the high magnification view of the LGE in B. Gsx2 positive cells in the dorsal LGE region (dLGE) are largely Olig2 negative, whereas double positive cells are throughout the ventral LGE (vLGE). Immunostaining for Zbtb20 (C) and in situ staining for Bcan (D) show weak expression in the dLGE at E18.5 (arrows in C-D). Double labeling with in situ for Bean and IHC for Gsx2 show the dLGE is high for Gsx2 and weak for Bcan (arrow in E). Gsx2 mutants display increased Zbtb20 (compare arrows in G to F) and Bcan (compare arrows in I to H) expression in the dLGE region at E16.5. Immunostaining for Zbtb20 and Gsx1 show the increased Zbtb20 is distinct from the expanded Gsx1 positive cells in VZ progenitors of Gsx2 mutants (compare K to J, see also inset K’). Double labeling with in situ for Bcan and IHC for Gsx1 (L) also shows little overlap in the Gsx2 mutant LGE progenitors (M and M’). Scale bar: A = 200μΜ, B, K% Μ’ = 50μΜ, E 100μΜ for C-E, I = 100μΜ for F- I,L-M, J = 100μΜ for J-K
Olig2 and PDGFRα represent markers of early stage OPCs (reviewed in Rowitch and Kriegstein, 2010; Emory and Lu, 2015) and are ectopically and robustly expressed in the Gsx2 mutant LGE progenitors of both the VZ and SVZ from E12.5 onward (Chapman et al., 2013; Fig. 1). However, other OPC markers like Sox10 were not detected in mutant LGE progenitors but only in the migrating OPCs located within the brain parenchyma (i.e. cortex) of Gsx2 mutants (Chapman et al., 2013). To better assess the extent of glial specification of VZ progenitors in the Gsx2 mutant LGE, we identified additional candidate glial markers previously associated with gliogenesis or astrogliogenesis that are increased in Gsx2 mutant LGE progenitors. We found that the glial associated transcription factor Zbtb20 (Nagao et al., 2016) and proteoglycan Brevican/Bcan (Jaworski et al., 1995) display somewhat complimentary expression to Gsx2 in the developing LGE of wild type embryos (Fig. 7C-E). In fact, in situ hybridization for Bcan and IHC for Gsx2 show that the high Gsx2 domain in the dLGE is weak for Bcan which is more robustly expressed in vLGE at E18.5 (Fig. 7D-E). Importantly, Gsx2 mutants show increased Zbtb20 (Compare Fig. 7G to F) and Bcan (compare Fig. 7I to H) in the dLGE region of the VZ at E16.5. Moreover, it appears that the increased expression of these glial markers is found in VZ cells that are closer to the apical surface (Fig. 7J-M) than the expanded Gsx1 positive cells located basally at the VZ/SVZ boundary in the Gsx2 mutant LGE (Fig. 7K’,M’). The increase of Zbtb20 and Bcan at E16.5 is quite interesting given that these are genes originally associated with gliogenesis and that they are also expressed in the LGE at times where the LGE is suggested to be both neurogenic and gliogenic. Moreover, the pallial genes (Pax6/Ngn2) that are abnormally expressed in Gsx2 mutant LGE are largely recovered after E14.5 when Gsx1 has expanded into the dorsal regions of the LGE (Corbin et al., 2000; Toresson and Campbell, 2000; Toresson et al., 2001; Yun et al., 2001 and 2000). Therefore, it is possible that in the absence of Gsx2, dLGE VZ progenitors acquire molecular identity associated with glial specification at a stage earlier than normal, which leads to precocious generation of OPCs (until E15, before the compensatory expansion of Gsx1 expression into the dLGE).
3.5. Role of Olig2 in regulating glial identity of VZ progenitors in the Gsx2 mutant LGE
Olig2 is required for the generation of OPCs throughout the CNS (Lu et al., 2002; Zhou and Anderson, 2002). To determine a role for Olig2 in regulating glial specification of VZ progenitors in Gsx2 mutants, we generated Gsx2;Olig2 double mutant embryos (Gsx2EGFP/EGFP;Olig2cre/cre). Since germline Olig2 mutants are not viable at postnatal stages (Lu et al., 2002; Zhou and Anderson, 2002), we analyzed embryos at E18.5 when Gsx2 mutants show a robust increase in PDGFRα positive OPCs in the brain parenchyma (cortex) (Fig. 3) as well as in VZ progenitors of the mutant LGE (compare Fig. 8G to A). Predictably, the increased cortical PDGFRα expression observed in Gsx2 mutants is absent in Gsx2;Olig2 double mutants (compare asterisk in Fig. 8J to G). In fact, the apparent loss of OPCs is identical to the phenotype observed in the Olig2 single mutant cortex (compare cortex in Fig. 8J,D to A). Interestingly, however, the ectopic PDGFRα expression in VZ progenitors of the Gsx2 mutant LGE remained in Gsx2;Olig2 double mutants (compare arrows in Fig. 8J to G) suggesting that the Gsx2 mutant VZ progenitors are still biased towards an oligodendroglial cell fate despite the loss of Olig2. Complementing this result, two other glial progenitor markers Zbtb20 and Bcan also remain expanded throughout the LGE VZ in Gsx2;Olig2 double mutants which is indistinguishable from Gsx2 mutants at E18.5 (compare arrows in Fig. 8K,L to H,I) and in contrast to the weak or absent expression detected in the dLGE VZ of both WT (Fig. 8B,C) and Olig2 mutants (Fig.8E-F). Collectively, these data suggest that Olig2 is required for the OPC expansion phenotype in the LGE SVZ and cortex of Gsx2 mutants but the upregulation of a set of glial markers (e.g. PDGFRα, Zbtb20, and Bcan) in VZ progenitors of the Gsx2 mutant LGE is independent of Olig2 function. Moreover, we detect ectopic Bcan in Gsx1/2 double mutants (data not shown) identical to Gsx2 mutants, further supporting the idea that the ectopic expression of Bcan/Zbtb20 in the apical VZ is independent or upstream of the compensatory Gsx1 expression in the VZ/SVZ boundary of the Gsx2 mutant LGE. These results suggest that Gsx factors inhibits gliogenesis in VZ progenitors via two separate mechanisms; one through the suppression of Olig2 and a second manner through the inhibition of other gliogenic factors.
Fig. 8. Gsx2;Olig2 double mutants display expanded glial progenitor marker in the dLGE region.
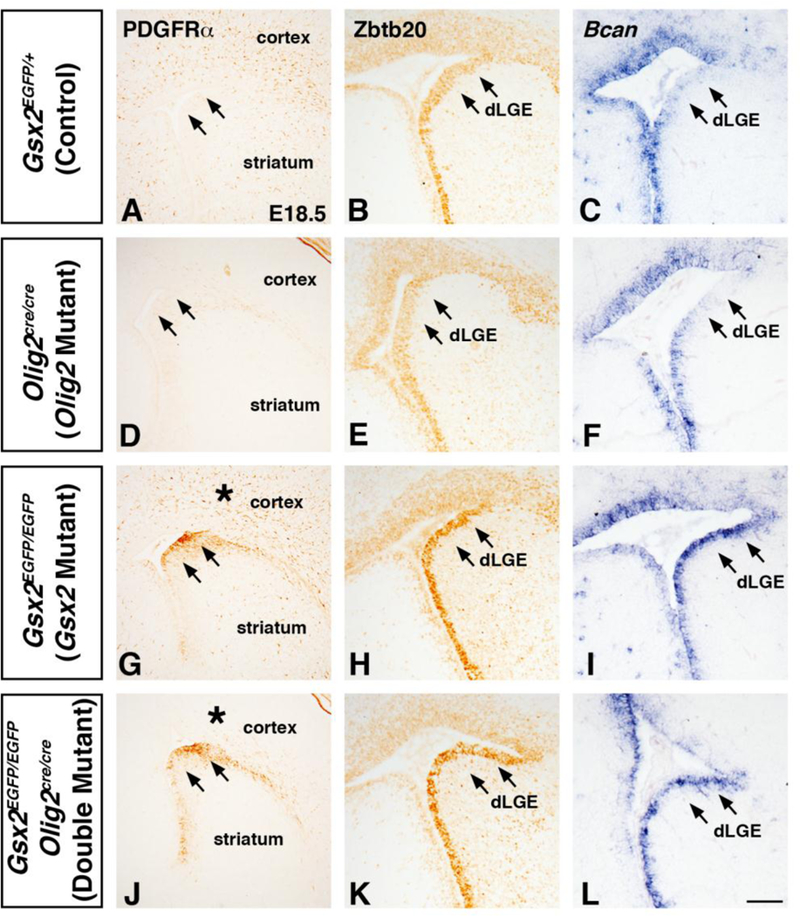
At El8.5, Gsx2 mutants show increased PDGFRα positive cells in the cortex and LGE (compare G to A). Gsx2;Olig2 double mutants show a loss of PDGFRα expression in the cortex (compare asterisk in J to G) which is similar to Olig2 mutants (compare J to D). However, PDGFRα expression remains expanded in the LGE of Gsx2;Olig2 double mutants similar to Gsx2 mutants (compare arrows in J to G). Both Zbtb20 and Bean remain increased in the dLGE of Gsx2;Olig2 double mutants similar to Gsx2 mutants (compare arrows in K,L to H,I). Olig2 mutants do not show changes in the LGE expression gradient (low in dLGE) of Zbtb20 (compare E to B) or Bcan (compare F to C). Scale bar: L = 100μΜ.
4. Discussion
The complex molecular mechanisms underlying the neurogenic to gliogenic fate change in neural progenitors are currently being elucidated and involve specific spatial and temporal regulations. Gsx2 has been implicated in regulating the timing of the neuronal to glial switch within LGE progenitors by simultaneously promoting neuronal fates and suppressing oligodendroglial fates in the developing ventral telencephalon (Waclaw et al., 2009; Chapman et al., 2013). Gsx1 has been shown to specify progenitors towards LGE neuronal fates in a manner similar to Gsx2 (Pei et al., 2011), however it is unknown whether Gsx1 is also able to repress the specification of OPCs from embryonic VZ progenitors. Analyzing oligodendroglial markers within Gsx1/2 double mutants as well as Gsx1 gain-of-function embryos, we have demonstrated that Gsx1 acts in the same manner as Gsx2 in repressing embryonic OPC specification within LGE VZ progenitors. We also show complementary domains of expression within the LGE between Gsx2 and the glial progenitor genes (Olig2, Zbtb20, and Bcan). In addition, we show that Gsx2 is required to restrict the expression of these genes in more apical VZ progenitors compared to the compensatory Gsx1 expression in the Gsx2 mutants at the VZ/SVZ boundary in the dLGE region. Moreover, despite that Olig2 has been shown to be a major regulator of OPC generation, its increased expression in the LGE VZ progenitors of Gsx2 mutants, is only required for the increased expansion of OPCs in the LGE SVZ and surrounding parenchymal regions observed in Gsx2 mutants but not for the abnormal expression of glial markers in primary VZ progenitors seen in these mutants.
Gsx1 is normally expressed in progenitors along the VZ/SVZ boundary in a gradient of expression with highest levels in cells of the MGE compared to only scattered cells within the vLGE and limited expression in cells of the dLGE (Toresson and Campbell, 2001; Yun et al., 2003; Pei et al., 2011; see also Suppl. Fig. 1). The loss of Gsx1 alone does not overtly affect the specification of LGE progenitors. In contrast, in the absence of Gsx2, Gsx1 expands throughout the dorsal extent of the LGE at the VZ/SVZ boundary and is then required for much of the normalization of gene expression that occurs in late stage mutant LGE progenitors (Toresson and Campbell, 2001; Yun et al., 2003). In fact, Gsx2-expressing progenitors normally generate striatal projection neurons and olfactory bulb interneurons from the vLGE and dLGE, respectively (Waclaw et al., 2009). In Gsx2 mutants, these neuronal subtypes are severely diminished, but begin to recover at later stages (E14.5 onward). This restoration of neurogenesis is due to the dorsal expansion of Gsx1 and its ability to specify LGE progenitors in a similar manner to Gsx2 (Toresson and Campbell, 2001; Yun et al., 2003; Pei et al., 2011).
Gsx2 mutants also display a precocious increase in oligodendroglial specification within dLGE SVZ progenitors at E15.5, however by E18.5, this misspecification begins to normalize (Chapman et al., 2013). Our results indicate that, similar to the partial restoration of LGE neuronal specification, this recovery in precocious oligodendroglial specification is dependent on Gsx1. At E15.5, Gsx1/2 double mutants exhibit a more robust increase in OPC specification within progenitors of the dLGE SVZ, as compared to Gsx2 mutants. Fittingly, this expansion of OPCs is in more ventral regions of the double mutant dLGE (Fig. 1I,J compared to E,F), which is in accordance with the dorsal expansion of Gsx1 approaching from the vLGE. Additionally, Gsx1/2 double mutants continue to display increased levels of OPC markers in dLGE SVZ progenitors at E18.5, which is distinct from the recovery observed at this time in Gsx2 single mutants. This suggests that the dorsal expansion of Gsx1 in Gsx2 mutants not only restores neurogenesis, but also negatively regulates the specification of OPCs to inhibit precocious oligodendrogenesis.
We were surprised to find, however, that even though Gsx1/2 double mutants have increased oligodendroglial specification within SVZ progenitors of the dLGE as compared to Gsx2 mutants, it does not lead to more of an expansion of OPCs in surrounding mantle regions as seen in Gsx2 mutants. In fact, there are significantly fewer OPCs within the cortex of Gsx1/2 double mutants than in Gsx2 mutants. Nevertheless, Gsx1/2 double mutants still generate significantly more cortical OPCs than control embryos. This discrepancy in numbers of OPCs between the LGE SVZ and cortical mantle zone can be partially explained by defective proliferation of OPCs within the SVZ of Gsx1/2 double mutants (Toresson and Campbell, 2001). Thus, even though there are more cells exhibiting OPC characteristics (e.g. Olig2) within the dLGE, a reduced percentage of these are dividing, resulting in fewer OPCs within the adjacent mantle regions. Another partial explanation for the fewer cortical OPCs could simply be due to the severe disruption in the morphology and patterning of the double mutant telencephalon (Toresson and Campbell, 2001; Yun et al., 2003). Gsx1/2 double mutants have a much smaller LGE/ striatal size than the Gsx2 mutant, which is also smaller than controls, and accordingly double mutants have fewer VZ progenitor cells surrounding the lateral ventricle. Perhaps these decreased numbers of early VZ progenitors in combination with more severe molecular alterations also contribute to the lack of further expansion of OPCs into adjacent mantle regions. In line with this possibility, we cannot rule out a change in fate of the Gsx lineage in the cortical cells originating from the LGE/MGE. However, previous studies have shown that no abnormalities in the generation of cortical interneurons were detected in Gsx1/2 double mutants (Yun et al., 2003). A final possibility could be that Gsx1 may play additional roles beyond embryonic LGE VZ progenitors, more specifically in the postnatal oligodendrocyte lineage. In fact, Gsx1 was recently identified as one of several novel markers in a gene expression catalog of different stages of oligodendrocyte development in the perinatal cortex (Zhang et al., 2014). In any case, Gsx1 is required for the full expansion potential of the precocious dLGE-derived OPCs generated in the Gsx2 mutant. Given that both Gsx2 and Gsx1/2 mutants are lethal at birth before OPC differentiation, our future experiments will utilize conditional mutagenesis to overcome the lethality and address mutant OPC expansion, differentiation, and myelination.
To complement these loss-of-function studies, we also over-expressed Gsx1 from E15 onward throughout telencephalic progenitors and found significant reductions in OPCs within the adjacent cortex three days later, at E18.5. Very few, if any of the progenitors that overexpressed Gsx1, gave rise to OPCs, suggesting that Gsx1, like Gsx2, is sufficient to suppress oligodendroglial specification in embryonic telencephalic VZ progenitors. However, we did not observe a complete loss of OPCs in the cortex at E18.5 after delayed misexpression of Gsx1. In fact, nearly all of the remaining OPCs in the cortex were negative for GFP, a surrogate for Gsx1 transgene expression. One possible explanation could be the mosaic and weaker expression of the transgene in LGE progenitors compared to pallial progenitors after DOX treatment. In fact, it is proposed the LGE is the progenitor source for OPCs after E14 (Kessaris et al., 2006), which is in line with the transgene activation after E7-E11 DOX treatment (Chapman et al., 2013). These delayed over-expression studies confirm a similar temporal effect of Gsx1 to Gsx2 in promoting LGE molecular identity (Pei et al., 2011) since this delayed over-expression of Gsx1 also results in misspecification of progenitors toward dLGE neuronal fates, as indicated by ectopic upregulation of Sp8 and Ascl1 within the cortex.
It is interesting to note that despite the ability of Gsx1 to specify tel encephalic progenitors in the same manner as Gsx2, it does so in a less robust way. This is consistent with results from previous early-stage Gsx1 over-expression studies (Pei et al., 2011). We discovered that this was the case, in regard to both neuronal and oligodendroglial specification, with increased cortical Ascl1 and Sp8 expression and fewer OPCs in the cortex in Gsx2 DT embryos as compared to Gsx1 DT embryos. This may not be unexpected, considering Gsx1 is normally only expressed in scattered cells at the VZ/SVZ boundary of the vLGE and very sparingly in the dLGE.
Combined fate mapping approaches with Gsx2-cre and Nkx2.1 cre have suggested that the LGE produces OPCs at midgestation stages (around E15.5) (Kessaris et al., 2006). However, it remains unknown if the entire span of the LGE VZ or a specific dorsal or ventral subdomain within it is responsible for gliogenesis. In the absence of Gsx2, the dLGE SVZ, which is normally occupied by the neurogenic markers (Sp8 and Er81), ectopically expresses OPC markers (PDGFRα and Olig2), suggesting that Gsx2 expression in the dLGE VZ promotes neurogenic genes and also controls the timing of OPC generation from the LGE (Chapman et al., 2013). We show here that Gsx2 and Olig2 are expressed in largely opposing gradients in the LGE VZ with highest expression of Gsx2 in the dLGE and Olig2 largely absent in the dLGE with higher levels in the vLGE. In line with the role of Gsx2 controlling OPC timing, the absence of Gsx2 results in an expansion of Olig2 and other candidate gliogenic markers (Zbtb20 and Bcan) into the dLGE region in the apical part of the VZ. This is in contrast to the compensatory expansion of Gsx1 that is found at the VZ/SVZ boundary region of the Gsx2 mutant LGE (Pei et al., 2011 and Suppl. Fig. 1). Despite the role of 01ig2 as a major regulator of oligodendrocyte specification and differentiation (Lu et al., 2002; Zhou and Anderson, 2002; Yue et al., 2006; Zhu et al., 2012; Yu et al., 2013), some gliogenic genes such as Zbtb20, Bcan, and PDGFRα remain expressed in the dLGE VZ of Olig2;Gsx2 double mutants (Figure 8), indicating that Olig2 is not required for their expression in the LGE VZ.
Altogether, our results indicate a novel role for Gsx1 in specifying LGE progenitors in Gsx2 mutants. In addition to promoting LGE neuronal subtypes, Gsx1 is also able to suppress oligodendroglial specification from LGE VZ progenitors. This results in a compensatory effect of Gsx1 that is able to partially restore neurogenesis and repress precocious oligodendroglial specification that occurs in the dLGE of Gsx2 mutants. Our findings in Gsx1/2 mutants suggest that Gsx genes are required for the full expansion potential of LGE-derived OPCs. Furthermore, our results suggest Gsx factors suppresses gliogenesis via Olig2-dependent mechanisms in the expansion of cortical OPCs and Olig2-independent mechanisms in expansion of glial specification markers in the apical VZ upstream of the VZ/SVZ expression of Gsx1.
Supplementary Material
Olig2 and Gsx1 expression in telencephalic VZ progenitors of control and Gsx2 mutant embryos. Adjacent section comparison of Gsx1 specific antibody staining to Gsx1 gene expression in control and Gsx2 mutants at E16.5 (A-D). Boxes in B and D indicate high power images shown in B’ and D’ that show the increased Gsx1 gene and protein expression in the basal region of the VZ in the Gsx2 mutant LGE (B,D) that is not observed in controls (A,C). Olig2 expression is increased in the Gsx2 mutant LGE ventricular zone (compare arrow in F to E). Dashed boxes in E and F represent high power image in G-H and I-J respectively. Gsx1 (G, I) and Olig2/Gsx1 double stains (H, J) show that Olig2 is expressed in more apical cells in the LGE compared to Gsx1 in Gsx2 mutants (I, J). Scale bar: B, B’, F = 100μΜ, J= 50μΜ
Highlights:
Loss of Gsx1 and Gsx2 results in increased OPC specification in LGE progenitors.
Misexpression of Gsx1 in telencephalic VZ progenitors resulted in a reduction of cortical OPCs.
Candidate gliogenesis genes (Olig2, Bcan, and Zbtb20) are expanded in the Gsx2 mutant LGE and expressed in more apical VZ cells as compared to Gsx1 expression.
Olig2-dependent and -independent mechanisms exist downstream of Gsx factors in the specification of glial progenitors in the LGE.
Acknowledgements
We thank Jane Johnson for providing the Ascl1 antibody. We thank Drs. Tom Jessell and Ben Novitch for providing the Olig2Cre/+ mice and Dr. Steven Potter for the Gsx1+/- mice. This work was supported by the NIH grants R01 NS044080 to KC, R01 NS069893 to MN and KC, and R01 NS088529 to RRW. HC was supported by the NIH training grant T32 ES007051.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayer SA and Altman J (1991). Neocortical development. Raven Press. [Google Scholar]
- 2.Chapman H, Waclaw RR, Pei Z, Nakafuku M, Campbell K, 2013. The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 140, 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G, 2000. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 127, 5007–5020. [DOI] [PubMed] [Google Scholar]
- 4.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J, 2007. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717–720. [DOI] [PubMed] [Google Scholar]
- 5.Fogarty M, Richardson WD, Kessaris N, 2005. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 132, 1951–1959. [DOI] [PubMed] [Google Scholar]
- 6.Hanashima C, Shen L, Li SC, Lai E, 2002. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci 22, 6526–6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaworski DM, Kelly GM, Hockfield S, 1995. The CNS-specific hyaluronan-binding protein BEHAB is expressed in ventricular zones coincident with gliogenesis. J Neurosci 15, 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD, 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessaris N, Pringle N, Richardson WD, 2001. Ventral neurogenesis and the neuron-glial switch. Neuron 31, 677–680. [DOI] [PubMed] [Google Scholar]
- 10.Kohli V, Nardini D, Ehrman LA, Waclaw RR, 2017. Characterization of Glcci1 expression in a subpopulation of lateral ganglionic eminence progenitors in the mouse telencephalon. Dev Dyn. Epub ahead of print , July 26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, Campbell K, Nakafuku M, 2013. Gsx2 controls region- specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes & development 27, 1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Zeitler PS, Valerius MT, Small K, Potter SS, 1996. Gsh-1, an orphan Hox gene, is required for normal pituitary development. EMBO J 15, 714–724. [PMC free article] [PubMed] [Google Scholar]
- 13.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH, 2002. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86. [DOI] [PubMed] [Google Scholar]
- 14.Mendez-Gomez HR, Vicario-Abejon C, 2012. The homeobox gene Gsx2 regulates the self-renewal and differentiation of neural stem cells and the cell fate of postnatal progenitors. PLoS One 7, e29799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagao M, Ogata T, Sawada Y, Gotoh Y, 2016. Zbtb20 promotes astrocytogenesis during neocortical development. Nat Commun 7, 11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakatani H, Martin E, Hassani H, Clavairoly A, Maire CL, Viadieu A, Kerninon C, Delmasure A, Frah M, Weber M, Nakafuku M, Zalc B, Thomas JL, Guillemot F, Nait- Oumesmar B, Parras C, 2013. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci 33, 9752–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei Z, Wang B, Chen G, Nagao M, Nakafuku M, Campbell K, 2011. Homeobox genes Gsx1 and Gsx2 differentially regulate telencephalic progenitor maturation. Proc Natl Acad Sci U S A 108, 1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S, Ware SM, Waclaw RR, Campbell K, 2017. Septal contributions to olfactory bulb interneuron diversity in the embryonic mouse telencephalon: role of the homeobox gene Gsx2. Neural Dev 12, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schluter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J, 1993. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle- maintaining proteins. J Cell Biol 123, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stenman J, Toresson H, Campbell K, 2003. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci 23, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szucsik JC, Witte DP, Li H, Pixley SK, Small KM, Potter SS, 1997. Altered forebrain and hindbrain development in mice mutant for the Gsh-2 homeobox gene. Dev Biol 191, 230–242. [DOI] [PubMed] [Google Scholar]
- 22.Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD, 2001. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 128, 2545–2554. [DOI] [PubMed] [Google Scholar]
- 23.Toresson H, Campbell K, 2001. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development 128, 4769–4780. [DOI] [PubMed] [Google Scholar]
- 24.Toresson H, Mata de Urquiza A, Fagerstrom C, Perlmann T, Campbell K, 1999. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development 126, 1317–1326. [DOI] [PubMed] [Google Scholar]
- 25.Toresson H, Potter SS, Campbell K, 2000. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127, 4361–4371. [DOI] [PubMed] [Google Scholar]
- 26.Valerius MT, Li H, Stock JL, Weinstein M, Kaur S, Singh G, Potter SS, 1995. Gsh-1: a novel murine homeobox gene expressed in the central nervous system. Dev Dyn 203, 337–351. [DOI] [PubMed] [Google Scholar]
- 27.Vallstedt A, Klos JM, Ericson J, 2005. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45, 55–67. [DOI] [PubMed] [Google Scholar]
- 28.Waclaw RR, Allen ZJ 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K, 2006. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron 49, 503–516. [DOI] [PubMed] [Google Scholar]
- 29.Waclaw RR, Ehrman LA, Pierani A, Campbell K, 2010. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci 30, 6944–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K, 2009. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron 63, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL, 2013. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol 521, 1561–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Waclaw RR, Allen ZJ 2nd, Guillemot F, Campbell K, 2009. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Chen Y, Kim B, Wang H, Zhao C, He X, Liu L, Liu W, Wu LM, Mao M, Chan JR, Wu J, Lu QR, 2013. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, Lu QR, 2006. A critical role for dorsal progenitors in cortical myelination. J Neurosci 26, 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun K, Garel S, Fischman S, Rubenstein JL, 2003. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol 461, 151–165. [DOI] [PubMed] [Google Scholar]
- 36.Yun K, Potter S, Rubenstein JL, 2001. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128, 193–205. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Anderson DJ, 2002. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A, 2012. Olig2-dependent developmental fate switch of NG2 cells. Development 139, 2299–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Olig2 and Gsx1 expression in telencephalic VZ progenitors of control and Gsx2 mutant embryos. Adjacent section comparison of Gsx1 specific antibody staining to Gsx1 gene expression in control and Gsx2 mutants at E16.5 (A-D). Boxes in B and D indicate high power images shown in B’ and D’ that show the increased Gsx1 gene and protein expression in the basal region of the VZ in the Gsx2 mutant LGE (B,D) that is not observed in controls (A,C). Olig2 expression is increased in the Gsx2 mutant LGE ventricular zone (compare arrow in F to E). Dashed boxes in E and F represent high power image in G-H and I-J respectively. Gsx1 (G, I) and Olig2/Gsx1 double stains (H, J) show that Olig2 is expressed in more apical cells in the LGE compared to Gsx1 in Gsx2 mutants (I, J). Scale bar: B, B’, F = 100μΜ, J= 50μΜ


