Abstract
Objectives
To assess the prevalence of Rome IV nonerosive esophageal phenotypes in children using multichannel intraluminal impedance testing and to describe the rates of proton pump inhibitor (PPI) responsiveness and the frequency of microscopic esophagitis in these patients.
Study design
We conducted a retrospective review of all children ≥5 years of age who underwent esophagogastroduodenoscopy and multichannel intraluminal impedance testing off PPI therapy for evaluation of typical gastroesophageal reflux symptoms. Only children with symptoms during the multichannel intraluminal impedance testing were included. Children were categorized into the following nonerosive esophageal phenotypes using Rome IV criteria: nonerosive reflux disease, reflux hypersensitivity, and functional heartburn. Rates of esophagitis and responsiveness to acid suppression therapy were assessed.
Results
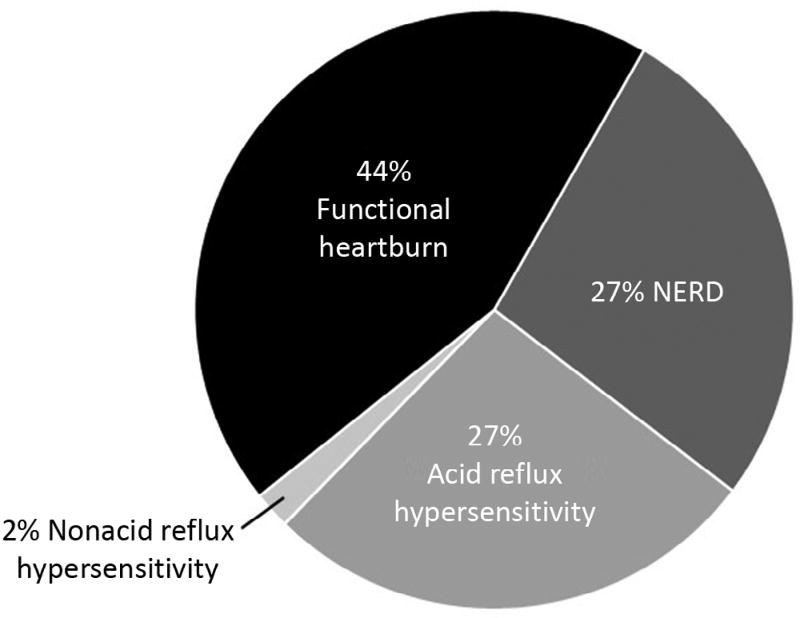
Forty-five children were included: 27% were categorized as having nonerosive reflux disease, 29% with reflux hypersensitivity (27% acid and 2% nonacid), and 44% with functional heartburn. Older children reported significantly more heartburn (P < .001) than younger children, whereas younger children were more likely to report nonspecific pain (P = .047). There were no differences between groups in other reflux symptoms, rates of responsiveness to PPIs, or the presence of microscopic esophagitis on biopsy.
Conclusions
Functional heartburn is the most common Rome IV nonerosive esophageal phenotype in children. Neither microscopic esophagitis nor PPI responsiveness can predict phenotype in pediatric patients.
Gastroesophageal reflux disease (GERD) is defined as the presence of bothersome symptoms resulting from the movement of gastric contents into the esophagus.1 Because testing for reflux symptom correlations is costly and invasive, patients are often prescribed acid suppression medications such as proton pump inhibitors (PPIs) to treat possible acid-related symptoms and only when patients do not improve symptomatically is additional testing with endoscopy and multichannel intraluminal impedance (pH-MII) testing pursued. Adult studies have demonstrated that the majority of patients with typical reflux symptoms such as chest pain, heartburn, and regurgitation do not have esophageal mucosal lesions visible at endoscopy.2,3 These patients have been traditionally categorized as having nonerosive reflux disease (NERD); this has emerged as the most common phenotype of GERD in adults.
Recent studies have suggested that this population of patients with typical reflux symptoms, no erosive esophagitis, and variable response to acid suppression is more heterogeneous than previously thought.3,4 The new Rome IV esophageal criteria now define a variety of nonerosive esophageal phenotypes based on the results of diagnostic testing including 24-hour pH-MII testing.5 This has been possible because clinicians can quantify the burden of both acidic and nonacidic reflux through the use of pH-MII testing, and can correlate patient-reported symptoms with reflux events.6 This allows for the categorization of symptomatic patients into 3 distinct phenotypes: (1) those with abnormal esophageal acid exposure (NERD), (2) those with normal esophageal acid exposure but a positive symptom association to acid or nonacid reflux (reflux hypersensitivity), and (3) those with normal esophageal acid exposure and a negative symptom association (functional heartburn).2,5 Appropriate categorization of patients into these subgroups has important therapeutic implications, because patients in each category may respond differently to medical and surgical interventions.5,7–9
Although >85%10 of children underdoing endoscopy do not have evidence of erosive esophagitis, little is known about the prevalence of nonerosive esophageal phenotypes in children and their response to acid suppression therapy. Therefore, the aims of this study were to characterize the prevalence of Rome IV nonerosive esophageal phenotypes in children using pH-MII testing, the rates of PPI responsiveness, and the frequency of microscopic esophagitis.
Methods
We conducted a retrospective review of all children ≥5 years of age who underwent esophagogastroduodenoscopy (EGD) and pH-MII testing for evaluation of typical gastroesophageal reflux symptoms (eg, heartburn, regurgitation, chest pain, or epigastric abdominal pain) between February 2004 and October 2016 at Boston Children’s Hospital. Children were included in the analysis if they: (1) had both an EGD and pH-MII testing, (2) did not have erosions present at the time of endoscopy, (3) reported symptoms during the pH-MII study, (4) had undergone a PPI trial for at least 8 weeks before diagnostic testing, and (5) were not on a PPI during the pH-MII testing. The age of patients in this study is similar to that used in prior pediatric functional GI disorder studies.11 Patients with documentation of at least partial improvement in the frequency and/or severity of symptoms after a PPI trial were classified as PPI responders. Those with no change in symptoms after a PPI trial were considered nonresponders. A total of 1269 pH-MII studies were reviewed for inclusion. Forty-five patients fulfilled the inclusion criteria and were included in this analysis.
All children underwent an EGD. During this procedure, biopsies were taken from a minimum of 2 esophageal heights. Patients with any erosive esophagitis were excluded. Patients were considered to have microscopic reflux esophagitis if there was distal esophageal inflammation such as basal zone hyperplasia or inflammatory cell infiltrate that did not meet the diagnostic criteria for eosinophilic esophagitis based on clinical history, symptoms, or histology.12
In all patients, a pH-MII study was completed within 6 months of the EGD.13 All patients stopped PPI therapy for a minimum of 48 hours before pH-MII testing. Patients underwent pH-MII recording for a minimum of 18 hours and ate at least 3 meals during this time. Meals were excluded from analysis. Each tracing was read manually in a blinded fashion by one of the investigators. Acid reflux episodes were defined as reflux episodes detected by at least 2 consecutive MII sensors and which were associated with a drop in pH to <4. Nonacid reflux episodes were defined as reflux events detected by at least 2 consecutive MII sensors that did not have any associated drop in pH to <4. Decreases in pH to <4 without any associated reflux on MII were defined as pH-only events.14 The pH portion of the pH-MII study was considered abnormal if the pH was <4 for >6% of the study.15 The MII portion of the study was considered abnormal if there were >72 reflux episodes.6,16
All patients reported typical reflux symptoms (heartburn, reflux, regurgitation, unspecified pain, chest pain, or abdominal pain) during the study. Symptoms reported within a 2-minute window before or after a reflux event were considered to be associated with that particular reflux event. Symptoms reported during mealtimes were excluded. We elected to use the symptom index (SI) as the measure of symptom association to define subgroups in our study. This is consistent with our standard clinical practice and similar to other published pediatric17–19 and adult7,20 studies. The SI was calculated for each patient by dividing the number of reported typical symptoms associated with reflux by the total number of reported symptom events and multiplying by 100. A positive SI was defined as a SI of ≥50% for any typical symptom. Although the primary classification for phenotypes in this study was based on the SI, we recognize that other institutions use different measures of symptom association and therefore the symptom sensitivity index (SSI) and the symptom association probability (SAP) were also calculated for each patient. The SAP was calculated by computing the statistical association between symptoms and reflux events using the Fisher exact test. A SAP was considered positive if it was ≥95%. The SSI was defined as the number of reflux events associated with symptoms divided by the total number of reflux events over 24 hours. SSI values ≥10% were considered positive.
Patients with a history of developmental delay; thoracic, esophageal, or gastric surgery; esophageal motility disorders; erosive esophagitis; or eosinophilic esophagitis were excluded.
Children were classified into nonerosive esophageal phenotypes based on Rome IV criteria.5 Children with abnormal esophageal acid exposure (pH <4 for >6% of the study) were classified as having true NERD. Those with normal acid exposure but a positive SI with acid reflux events were classified as having acid reflux hypersensitivity. Those with normal acid exposure and a positive SI with nonacid reflux events were classified as having nonacid reflux hypersensitivity. Children with normal esophageal acid exposure and a negative SI were classified into the functional heartburn group.
Statistical Analyses
Values are reported as mean ± standard deviation or as percentages. Comparisons between subgroups were performed using analysis of variance for continuous variables and χ2 testing or Fisher exact test for categorical variables. IBM SPSS Statistics Version 23 (Armonk, New York) was used to perform statistical analysis.
The study protocol was approved by the Institutional Review Board of the institution.
Results
Forty-five pediatric patients met the inclusion criteria and reported symptoms during pH-MII monitoring. The mean age was 11.8 ± 4.2 years. Fifty-three percent of patients were female. The mean time interval between the EGD and the pH-MII study was 2.3 ± 0.9 days. Reported symptoms during pH-MII monitoring included heartburn (47%), nonlocalized pain (49%), abdominal pain (22%), chest pain (7%), reflux (13%), and regurgitation (22%). The distribution of nonerosive esophageal phenotypes based on the SI is shown in Figure 1. The age and sex distributions were similar between phenotypes (P = .14 and .36, respectively). When the SAP, rather than the SI, was used to categorize patients, the distribution of Rome IV categories was identical (27% true NERD, 29% reflux hypersensitivity, and 44% functional heartburn). When using SSI for categorization, the subtype distribution was 27% true NERD, 20% reflux hypersensitivity, and 53% functional heartburn.
Figure 1.
Distribution of nonerosive esophageal phenotypes in 45 children.
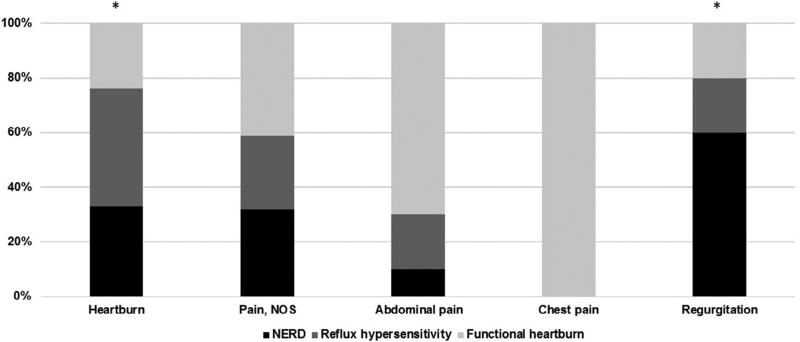
The distribution of phenotypes for each reported symptom is illustrated in Figure 2. Report of heartburn and report of regurgitation differed significantly between groups (P < .05). In general, children in the functional heartburn group were more likely to report nonspecific symptoms, such as abdominal pain and chest pain, although these differences did not attain statistical significance. Children with NERD were more likely to report regurgitation as a symptom. When children were stratified by age, older children (≥10 years) reported significantly more heartburn (P < .001) whereas younger children (<10 years) were more likely to report nonspecific pain (P = .047).
Figure 2.
Symptoms reported during pH impedance testing by phenotype. *P < .05. NOS, Not otherwise specified.
In total, there were 634 reported typical symptoms during pH-MII testing; 417 symptoms were associated with reflux events and 217 symptoms were not associated with any reflux. There were 230 reflux events associated with typical symptoms in the NERD subgroup, 111 events in those with reflux hypersensitivity, and 75 events in the children in the functional heartburn group. Distal events accounted for only 11% of all symptomatic reflux. Symptom perception for full column reflux did differ between groups with full column reflux triggering symptoms in the NERD group more than in the reflux hypersensitivity or functional heartburn groups (P = .023). There were no differences between groups for symptom perception from midesophagus or distal esophagus events.
Responsiveness to PPIs
The mean dose of PPIs trialed before evaluation was 0.8 ± 0.4 mg/kg per day. There was no significant difference in PPI response in children who received PPIs at a dose of <1 mg/ kg and those who receive a dose of ≥1 mg/kg (P = .73). Despite typical reflux symptoms, 42% of all subjects had no symptomatic improvement with empiric PPI therapy (Figure 3). Among subtypes, 58% of patients with NERD, 67% of patients with acid reflux hypersensitivity, 0% of patients with nonacid reflux hypersensitivity, and 55% of patients with functional heartburn had at least some symptomatic improvement with PPI use. There were no differences in PPI responsiveness between groups (P = .63).
Figure 3.
Differences in PPI responsiveness and microscopic esophagitis between phenotypes.
Endoscopy and Biopsies
Twenty percent of all children had evidence of microscopic reflux esophagitis on histology (Figure 3). Among subtypes, 17% of patients with NERD, 23% of patients with reflux hypersensitivity, and 20% with functional heartburn had microscopic esophagitis. There were no differences in microscopic esophagitis between subgroups (P = .93).
Impedance Testing
The NERD group had significantly more acid reflux events (P < .001), number of total reflux events (P = .004), number of pH-only events (P < .001), and the percentage of study time the pH was <4 (P < .001) than the two other groups. There were no differences in the number of nonacid events (P = .62), the percentage of proximal reflux events (.07), or the percentage of time that refluxate was in the esophagus (.07) between groups.
Discussion
We used Rome IV criteria and results from 24-hour pH-MII testing to classify children with symptomatic reflux but normal endoscopic evaluations into nonerosive esophageal phenotypes. This study reports the prevalence of these phenotypes in children using the new classification. The most important findings of the present study are that functional heartburn is the most common nonerosive esophageal phenotype in children and that microscopic esophagitis does not predict reflux phenotype. In addition, responsiveness to PPIs does not predict reflux phenotype.
Compared with the prevalence of reflux phenotypes reported in adult studies, we found that children are more likely to be categorized in the functional heartburn phenotype. Using the same classification system in 150 adults, Savarino et al3 found that 40% of patients had true NERD, 35% had reflux hypersensitivity, and 25% had functional heartburn. In another study of 221 patients referred for GERD testing, Cheng et al2 found 10% with erosive esophagitis, 35% with true NERD, 14% with reflux hypersensitivity, and 22% with functional heartburn and other functional disorders. There is no simple explanation for this discrepancy. However, it is possible that pediatric patients have more peripheral and central sensitization21,22 and that the expression of symptoms is different.
When we examine the subgroup of patients with reflux hypersensitivity, our cohort had a smaller proportion of patients with nonacid reflux-associated symptoms and fewer nonacid reflux events than has been previously reported in adults.4 This could be because our proportion of nonacid reflux events is smaller than typically seen in pediatrics or because nonacid reflux does not trigger typical symptoms in children. Although the literature reports that 45%–89% of pediatric reflux events are nonacidic,23 we found in this study that only 29% of reflux events were nonacidic. This small number of nonacid reflux episodes may explain the lack of symptom– reflux correlation simply because of the scarcity of events. Another possibility, that nonacid reflux events do not frequently trigger typical symptoms, would represent a novel finding; all prior pediatric symptom association studies, which were performed in children with atypical symptoms such as cough, suggest that nonacid reflux is more likely to trigger symptoms than acid reflux episodes.23–25
In addition to nonacid reflux, our prior studies and others have found that full column reflux events are more likely to trigger atypical symptoms than distal reflux in children.14,26 The present study shows for the first time that, in children, proximal reflux is also associated with typical symptoms of reflux. Although only 37% of total reflux events reached the proximal esophagus, these proximal events accounted for 58% of the symptomatic reflux in all groups. Distal reflux comprised only 11% of all symptomatic reflux episodes. Recent evidence in adults also shows that a proximal reflux event may be more likely to be perceived.27 This is a very interesting finding; traditional pH probe studies, which only reliably measure reflux in the distal esophagus, may fail to detect the extent to which reflux events contribute to typical symptoms. Therefore, this study not only describes the prevalence of nonerosive esophageal phenotypes in children, but also shows that although full column acid reflux seems to be a significant driver of symptoms in children, nonacid reflux and distal reflux are less important contributors.
Another important finding is that there is a difference in the distribution of symptoms depending on the age of the patient and this parallels a change in the frequency of reflux phenotypes. We found that functional disease was more common in younger children who experienced more general pain symptoms, whereas NERD was more common in older children who experienced more heartburn symptoms. Despite these age-related differences, we did not find any specific symptom profile that could reliably predict reflux phenotype. Although a single adult study by Savarino et al28 found more frequent reports of heartburn in patients with functional heartburn and epigastric pain in patients with true NERD, we and others have not found a consistent relationship between symptoms and reflux phenotype. In a study of 90 children 1–17 years of age with symptomatic GERD, Gupta et al29 found no differences in reports of regurgitation, heartburn, or abdominal pain in children with grossly normal endoscopic evaluations when compared with those with erosive esophagitis. In a study of 62 adult patients, Kandulski et al30 did not find any differences in reported heartburn, regurgitation, or dyspepsia between those with abnormal esophageal acid exposure, erosive esophagitis, and functional heartburn. Based on our study and these others in the literature, we conclude that clinical symptoms cannot be used as a reliable method to distinguish reflux phenotypes.
In addition to the inability to use symptoms to reliably predict subtypes, our study is the first pediatric study to report that PPI responsiveness also does not help to define phenotypes. Studies in adults have shown conflicting results about the usefulness of PPI responsiveness in identifying reflux subgroups. Bytzer et al31 analyzed data from adults in the DIAMOND study and found no significant differences in PPI responsiveness between patients with GERD, defined as either erosive esophagitis, abnormal esophageal pH, or positive symptom association and those with functional heartburn (69% vs 51%, respectively). However, other studies have found higher rates of PPI response in adults with abnormal esophageal acid exposure8 or those with a positive SI.9 Studies in children are limited. In a study of 20 children with grossly normal endoscopic evaluations, Lee et al32 found varying rates of responsiveness to an 8-week PPI trial: 35% of patients had symptom resolution, 50% had improved symptoms, and 15% had persistent symptoms. However, there was no phenotyping of patients using pH-MII so it is not possible to know if one phenotype had a superior PPI response.
As with symptom type and PPI responsiveness, we also found that microscopic esophagitis cannot be used to define reflux phenotypes; we found no differences in the rates of microscopic esophagitis in children with NERD, reflux hypersensitivity, and functional heartburn. We also found that there was no relationship between responsiveness to PPIs and the presence of microscopic esophagitis. These findings are important because, unlike the practice in adult gastroenterology, pediatric gastroenterologists routinely biopsy the esophagus to assess for microscopic esophagitis and our results suggest that these biopsies may not be helpful to differentiate patient subgroups or to predict PPI symptom response. Overall, the prevalence of microscopic esophagitis in our cohort (20%) is similar to that previously published in adult studies.33–35 However, adult studies have found differences in microscopic esophagitis between reflux phenotypes, with the highest rates of esophagitis in patients with NERD and the lowest rates in patients with functional heartburn.34 There is only a single pediatric study assessing the role of esophagitis in reflux phenotypes; Altaf et al36 found increased esophageal epithelial intercellular spaces in children with NERD compared with controls. However, in this study, NERD was defined using only clinical and endoscopic criteria, not impedance testing. Based on our results, endoscopic biopsies alone cannot be used to define GERD phenotypes.
Despite our novel findings, there are several limitations to our study. Because of our inclusion criteria and the fact that patients were referred for testing because of persistent symptoms, the proportion of nonresponsiveness to PPIs and the rate of functional diagnoses may be higher than expected. However, we feel this population is typical of what a pediatric gastroenterologist encounters in clinical practice (ie, patients who underwent a PPI trial but continued to have persistent symptoms). Second, this study was retrospective in nature, so the assessment of responsiveness to PPIs may be biased based on parent or physician recall. Furthermore, our sample size for this retrospective study is smaller than the sample sizes published in similar prospective adult studies on reflux phenotypes. This small number is related to our strict inclusion criteria including age. We believe that our review of >1200 studies done during a 12-year period and including a highly selected subset of these patients provides the largest possible sample of children for this retrospective analysis. Third, the measurement of symptom association is inherently dependent on patient report. This factor may be less reliable in younger children who are unable to verbalize symptoms appropriately or in those who experience symptoms so frequently that they cannot record all symptoms accurately.37 We restricted our inclusion criteria to children >5 years of age who did not have any significant developmental delays to ensure the most accurate reporting of symptoms. Given this, we would expect symptom reporting in our cohort to be similar to adults and currently patient-dependent symptom reporting is the gold standard method of performing pH-MII testing.
In conclusion, we document using the new Rome IV criteria that functional esophageal disorders are common in pediatrics. Because of the emerging literature documenting the risks associated with PPI use, we hope our results will encourage clinicians to correctly diagnose, using pH-MII testing, those patients with functional diagnoses in order to tailor therapies (PPIs vs neuromodulators) to reduce costs and morbidity of unnecessary or ineffective therapies.
Acknowledgments
Supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK097112 [to R.R.]), NIH (5T32DK007477-33 [to L.M.]), and the Cystic Fibrosis Foundation (to L.M.).
Glossary
- EGD
Esophagogastroduodenoscopy
- GERD
Gastroesophageal reflux disease
- NERD
Nonerosive reflux disease
- pH-MII
Multichannel intraluminal impedance
- PPIs
Proton pump inhibitors
- SAP
Symptom association probability
- SI
Symptom index
- SSI
Symptom sensitivity index
Footnotes
The authors declare no conflicts of interest.
References
- 1.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 2.Cheng F-KF, Albert DM, Maydonovitch CL, Wong RK, Moawad FJ. Categorization of patients with reflux symptoms referred for pH and impedance testing while off therapy. Clin Gastroenterol Hepatol. 2015;13:867–73. doi: 10.1016/j.cgh.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10:371–80. doi: 10.1038/nrgastro.2013.50. [DOI] [PubMed] [Google Scholar]
- 4.Savarino E, Zentilin P, Tutuian R, Pohl D, Casa DD, Frazzoni M, et al. The role of nonacid reflux in NERD: lessons learned from impedance-pH monitoring in 150 patients off therapy. Am J Gastroenterol. 2008;103:2685–93. doi: 10.1111/j.1572-0241.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 5.Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional esophageal disorders. Gastroenterology. 2016;150:1368–79. doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Hirano I, Richter JE. Practice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668–85. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 7.Limsrivilai J, Charatcharoenwitthaya P, Pausawasdi N, Leelakusolvong S. Imipramine for treatment of esophageal hypersensitivity and functional heartburn: a randomized placebo-controlled trial. Am J Gastroenterol. 2016;111:217–24. doi: 10.1038/ajg.2015.413. [DOI] [PubMed] [Google Scholar]
- 8.de Bortoli N, Martinucci I, Savarino E, Bellini M, Bredenoord AJ, Franchi R, et al. Proton pump inhibitor responders who are not confirmed as GERD patients with impedance and pH monitoring: who are they? Neurogastroenterol Motil. 2014;26:28–35. doi: 10.1111/nmo.12221. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Tan N, Zhang N, Xiong L, Peng S, Lin J, et al. Predictors of proton pump inhibitor failure in non-erosive reflux disease: a study with impedance-pH monitoring and high-resolution manometry. Neurogastroenterol Motil. 2016;28:674–9. doi: 10.1111/nmo.12763. [DOI] [PubMed] [Google Scholar]
- 10.Gilger MA, El-Serag HB, Gold BD, Dietrich CL, Tsou V, McDuffie A, et al. Prevalence of endoscopic findings of erosive esophagitis in children: a population-based study. J Pediatr Gastroenterol Nutr. 2008;47:141–6. doi: 10.1097/MPG.0b013e31815eeabe. [DOI] [PubMed] [Google Scholar]
- 11.Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. 2016;150:1456–68. e2. doi: 10.1053/j.gastro.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 13.Orenstein SR, Shalaby TM, Kelsey SF, Frankel E. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. Am J Gastroenterol. 2006;101:628–40. doi: 10.1111/j.1572-0241.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99:2452–8. doi: 10.1111/j.1572-0241.2004.40268.x. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph CD, Mazur LJ, Liptak GS, Baker RD, Boyle JT, Colletti RB, et al. J Pediatr Gastroenterol Nutr. 2001;32(Suppl 2):S1–31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 17.Salvatore S, Arrigo S, Luini C, Vandenplas Y. Esophageal impedance in children: symptom-based results. J Pediatr. 2010;157:949–54. e1–2. doi: 10.1016/j.jpeds.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Rosen R, Amirault J, Heinz N, Litman H, Khatwa U. The sensitivity of acoustic cough recording relative to intraesophageal pressure recording and patient report during reflux testing. Neurogastroenterol Motil. 2014;26:1635–41. doi: 10.1111/nmo.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilic D, Fröhlich T, Nöh F, Pappas A, Schmidt-Choudhury A, Köhler H, et al. Detection of gastroesophageal reflux in children using combined multichannel intraluminal impedance and pH measurement: data from the German Pediatric Impedance Group. J Pediatr. 2011;158:650–1. doi: 10.1016/j.jpeds.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Kohata Y, Fujiwara Y, Yamagami H, Tanigawa T, Shiba M, Watanabe K, et al. Usefulness of baseline impedance in patients with proton pump inhibitor-refractory nonerosive reflux disease. J Gastroenterol Hepatol. 2015;30(Suppl 1):36–40. doi: 10.1111/jgh.12743. [DOI] [PubMed] [Google Scholar]
- 21.Lawal A, Kern M, Sanjeevi A, Antonik S, Mepani R, Rittmann T, et al. Neurocognitive processing of esophageal central sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G787–94. doi: 10.1152/ajpgi.00421.2007. [DOI] [PubMed] [Google Scholar]
- 22.Herregods TVK, Bredenoord AJ, Smout AJPM. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202–13. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- 23.Mousa HM, Rosen R, Woodley FW, Orsi M, Armas D, Faure C, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011;52:129–39. doi: 10.1097/MPG.0b013e3181ffde67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen R, Amirault J, Johnston N, Haver K, Khatwa U, Rubinstein E, et al. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2014;49:1090–6. doi: 10.1002/ppul.22949. [DOI] [PubMed] [Google Scholar]
- 25.Loots CM, Benninga MA, Davidson GP, Omari TI. Addition of pH-impedance monitoring to standard pH monitoring increases the yield of symptom association analysis in infants and children with gastroesophageal reflux. J Pediatr. 2009;154:248–52. doi: 10.1016/j.jpeds.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Jadcherla SR, Gupta A, Fernandez S, Nelin LD, Castile R, Gest AL, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103:720–8. doi: 10.1111/j.1572-0241.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 27.van Hoeij FB, Weijenborg PW, van den Bergh Weerman MA, van den Wijngaard RMJGJ, Verheij J, Smout AJPM, et al. Mucosal integrity and sensitivity to acid in the proximal esophagus in patients with gastroesophageal reflux disease. Am J Physiol Gastrointest Liver Physiol. 2016;311:G117–22. doi: 10.1152/ajpgi.00134.2016. [DOI] [PubMed] [Google Scholar]
- 28.Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009;58:1185–91. doi: 10.1136/gut.2008.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta SK, Hassall E, Chiu Y-L, Amer F, Heyman MB. Presenting symptoms of nonerosive and erosive esophagitis in pediatric patients. Dig Dis Sci. 2006;51:858–63. doi: 10.1007/s10620-006-9095-3. [DOI] [PubMed] [Google Scholar]
- 30.Kandulski A, Jechorek D, Caro C, Weigt J, Wex T, Mönkemüller K, et al. Histomorphological differentiation of non-erosive reflux disease and functional heartburn in patients with PPI-refractory heartburn. Aliment Pharmacol Ther. 2013;38:643–51. doi: 10.1111/apt.12428. [DOI] [PubMed] [Google Scholar]
- 31.Bytzer P, Jones R, Vakil N, Junghard O, Lind T, Wernersson B, et al. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:1360–6. doi: 10.1016/j.cgh.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Kim MJ, Lee JS, Choe YH. The effects of three alternative treatment strategies after 8 weeks of proton pump inhibitor therapy for GERD in children. Arch Dis Child. 2011;96:9–13. doi: 10.1136/adc.2010.188565. [DOI] [PubMed] [Google Scholar]
- 33.Schneider NI, Plieschnegger W, Geppert M, Wigginghaus B, Hoess GM, Eherer A, et al. Validation study of the Esohisto Consensus Guidelines for the recognition of microscopic esophagitis (histoGERD Trial) Hum Pathol. 2014;45:994–1002. doi: 10.1016/j.humpath.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Savarino E, Zentilin P, Mastracci L, Dulbecco P, Marabotto E, Gemignani L, et al. Microscopic esophagitis distinguishes patients with non-erosive reflux disease from those with functional heartburn. J Gastroenterol. 2013;48:473–82. doi: 10.1007/s00535-012-0672-2. [DOI] [PubMed] [Google Scholar]
- 35.Thakkar K, Chen L, Tatevian N, Shulman RJ, McDuffie A, Tsou M, et al. Diagnostic yield of oesophagogastroduodenoscopy in children with abdominal pain. Aliment Pharmacol Ther. 2009;30:662–9. doi: 10.1111/j.1365-2036.2009.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altaf MA, Ciecierega T, Szabo S, Miranda A, Gorges C, Simpson P, et al. Comparison of light and electron microscopy in measurement of esophageal intercellular space in children. J Pediatr Gastroenterol Nutr. 2014;59:232–6. doi: 10.1097/MPG.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 37.Rosen R. Symptom association: an imperfect pairing. J Pediatr Gastroenterol Nutr. 2016;62:517–8. doi: 10.1097/MPG.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]