Abstract
The loss of miR-200 family, through DNA methylation, results in cancer cells undergoing an epithelial to mesenchymal transition (EMT), and metastasis. In this study, we established that the transcriptional repressor Kaiso directly binds methylated regions of the miR-200 family, and this is reversed with 5-aza treatment. sh-Kaiso PC-3 cells display increased miR-200-a/b/c, miR-141, and miR-429 expression, with miR-200c demonstrating the most significant increase. Interestingly, overexpression of EGFR or treatment with EGF decreases miR-200c expression and this is reversed after treatment with EGFR specific kinase inhibitor PD153035. However, EGF did not have a significant effect on miR-200c in sh-Kaiso DU-145 or PC-3 cell lines, suggesting Kaiso silences miR-200c through the activation of EGFR signaling. Overexpression of Kaiso in LNCaP cells results in decreased expression of miR-200-a/b/c, miR-141, and miR-429, along with increased expression of ZEB1, p-EGFR and total EGFR levels. Overexpression of miR200c in PC-3 cells results in decreased expression of EGFR, ZEB1, ERK1/2 and Kaiso. Additionally, sh-Kaiso PC-3 demonstrates reduced in vivo tumor formation and metastasis. Thus, our data suggests that EGFR signaling regulates the silencing of miR-200 family through Kaiso binding to methylated regions in the promoter.
Keywords: DNA methylation, Kaiso, Epithelial to Mesenchymal Transition, Prostate Cancer, Metastasis
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignancy in men, with African American men experiencing a rate 60% higher than white patients [1]. At the time of diagnosis, approximately 50% of men have clinically advanced disease. Acquisition of invasive properties of the disease requires epithelial cells to lose their intracellular contacts or epithelial phenotype and acquire a mesenchymal phenotype [2–6] This process is termed epithelial-mesenchymal transition (EMT), and is characterized by the down-regulation of the epithelial marker E-cadherin, which modulates cell-cell junctions. The miR-200 family has been shown to be master regulators of both epithelial to mesenchymal (EMT) and mesenchymal to epithelial [7]) transitions [8–10], where decreased expression of one or more of these miRNAs, depending on the tumor type, support dissemination from the primary tumor and subsequent metastasis.
Multiple reports demonstrate that individual miRNAs in the 200 family are regulated by growth factor signaling [11]. In particular, miR-200b is regulated by PDGF signaling in prostate cancer [12]. Furthermore, miR-200c targets epidermal growth factor receptor (EGFR) and transforming growth factor beta (TGFβ) receptor signaling to drive tumor cells toward mesenchymal state, through a double feedback loop with the two transcriptional regulators of E-Cadherin, ZEB1 and ZEB2. This intercellular feedback look promotes an extracellular autocrine signaling loop that supports the maintenance of the mesenchymal phenotype required for metastasis [10]. The loss of expression of miRNA-200c has been shown in other cancer types (breast, ovarian and renal cancers) to correlate with EMT and ultimately promotion of cancer metastasis[8–10]. Interestingly at the miRNA level, these miRNAs are often silenced by DNA promoter methylation.
Recently, we have demonstrated that Kaiso, a POZ transcriptional repressor, binds to methylated regions in numerous genes including E-cadherin, and is a critical regulator of EMT in both prostate cancer, breast cancer [13, 14] and recently in pancreatic cancer [15]. Furthermore, increased Kaiso expression and cytoplasmic to nuclear localization is influenced by activation of EGFR[16]. In support of a feedback mechanism, recent reports have demonstrated that miR-200c directly influences EGFR and TGFβ signaling cascades to promote mesenchymal to epithelial transition, MET [17]. We have reported that Kaiso binds to the methylated regions in the miR-31 promoter, resulting in silencing of miR-31 expression and increased migratory and invasive capability [14]. However, whether Kaiso has a role in the silencing of the miR-200 family has not been determined.
In this report, we demonstrate that Kaiso binds miR-200 a/b/c, miR-141, and miR-429 through direct binding to methylated regions in the promoter, and this silencing of Kaiso expression causes re-expression of all miR-200, with the most significant increases in miR-200c. Furthermore, EGFR overexpression in DU-145 cells or treatment with EGF in both DU-145 and PC-3 showed decreases in miR-200c expression in a dosage dependent manner, and this does not occur in sh-Kaiso DU-145 or PC-3 cells, suggesting that EGFR signaling induces Kaiso to silence miR-200c expression. Overexpression of Kaiso in LNCaP cells results in decreased expression of miR-200 a/b/c, miR-141, and miR-429 and increased expression of ZEB1, p-EGFR and total EGFR levels. Overexpression of miR-200c in PC-3 cells results in decreased expression of EGFR, p-EGFR, ZEB1, and Kaiso expression. Additionally, sh-Kaiso PC-3 demonstrates reduced in vivo tumor formation and metastasis in vivo capability. Our data suggest that EGFR-Kaiso signaling axis is a critical regulator of the miR-200-ZEB1 feedback loop promoting EMT and metastasis in prostate cancer.
MATERIALS AND METHODS
Cell Culture
PREC Non-malignant CA prostate epithelial cells (PrEC) were obtained from Clonetics Lonza (Switzerland) and maintained in Prostate Epithelial Cell Growth Medium (Clonetics) cell were grown in Prostate Epithelial Cell Basal Medium. RC-77N, RC-170N, RC-165N; RC-92a, and RC-77T were cultured in keratinocyte serum-free medium (KGM, Life Technologies, Carlsbad, CA) supplemented with bovine pituitary extract, recombinant epidermal growth factor, and 1% penicillin-streptomycin-neomycin were maintained in KGM medium. LNCaP and C42B cells were grown in high glucose (4.5 gm/l) Dulbecco’s modified Eagles medium. This medium was supplemented with fetal bovine serum (FBS; 10%), penicillin-streptomycin (10,00 IU/ml and 10,00 ug/ml), sodium pyruvate (100mM), non-essential amino acids (1×), and L-glutamine (200 mM) (37° C, 90% humidity, 5% CO2 and 95% air). WT-DU-145 transduced sublines were passaged in G418 (1000mg/ml) until sub-passage for experimental testing. PC-3, cells where grown in T-media. This media was supplemented with fetal bovine serum (10%) and penicillin-streptomycin (10, 00 IU/ml and 10.00 μg/ml) (37° C, 90% humidity, 5% CO2 and 95% air).
Antibodies
Abcam, Anti-Kaiso antibody (ab12723), Anti -EGFR antibody (ab52894), Anti-EGFR (phospho Y1068) (ab32430), AB clonal, dilution:1:1000, Anti-Zeb1 antibody (A5600); Cell Signaling Anti-ERK1/2 antibody (4695T), Anti-Twist-antibody (46702), Anti-Snail-antibody (3879); Sigma, Anti-Zeb2 antibody (SAB2102760), Anti-B-Actin-antibody (A5441) All antibodies for immunoblots were used at dilution:1:1000. For immunofluorescence, For Immunofluorescence assays: Abcam, Anti-Kaiso antibody (ab12723) and Anti-E-cadherin (610181) were used at dilution:1:100.
Treatment with 5-aza-2-deoxycytidine
PC-3 cells were cultured to about 60% confluence and then treated with 5 uM of 5-aza- 2-deoxycytidine for 72 hours. The culture media supplemented with 5-aza-2-deoxycytidine was replaced every 24 hours. On Day 3, cells were harvested, and lysate was prepared for chromatin immunoprecipitation assay (ChIP) or qRT-PCR analysis.
Treatment with EGFR and EGF Inhibitor (PD153035)
DU-145 cells, EGFR overexpressing DU-145 cell, and PC-3 wild type cells were treated with increasing concentrations of EGF (10 ng and 50 ng) for 72 hours. These cells were also treated with an EGFR specific kinase inhibitor, PD153035 (500 nM) for 72 hours. After 72 hours, cells were harvested, and RNA lysate was prepared for qRT-PCR analysis.
Stably Transfected Cell Lines
LNCaP cells that express lower levels of Kaiso as compared with PC-3 cells were stably transfected to over-express Kaiso using Lipofectamine 2000 according to the manufacturer’s protocol (Invitrogen) with the ZBTB33 expression driven by a CMV promoter and an RFP reporter, pLenti-GIII-CMV-RFP-2A-Puro vector (Cat.LV361231 ABM) and pLenti-III-CMV-RFP-2A-Puro-Blank Control Lentiviral Vector (Cat.LV591 ABM). RNA lysate from previous sh-Kaiso PC-3 cells was used to measure the expression of microRNA-200a, b and c. Controls used were lysates from normal PC-3 cells and PC-3 cells transfected with Scramble nucleotide sequence.
Immunofluorescence Microscopy
Cells were grown to approximately 80% confluence on 4-well chambered slides and fixed with warm 3.7% formaldehyde for 10 min at room temperature. Cells were permeabilized after rinsing thoroughly in 0.2% Triton X-100 for 5 min and rinsed 3-4 times with PBS. Cells were incubated with approximately 200 uL of FX Signal enhancer (Invitrogen) for 30 min in a humid environment and at room temperature. Cells were stained after rinsing thoroughly with anti-Kaiso antibody for 1 hour at room temperature, protected from light. Cells were washed thoroughly and stained with secondary antibody, Alexa 594 anti-mouse (Invitrogen) for 30 min and washed thoroughly. Nuclear staining was achieved by adding Vectashield mounting medium with DAPI and cell staining was observed under DSU confocal microscope (Olympus, New York) using Metamorph Imaging Software (Molecular devices, LLC, Sunnyvale, CA) to capture cell images.
Immunoblotting
Total cell extracts were boiled for 5 min in Laemmli sample buffer, resolved in SDS polyacrylamide gels, and electrophoretically transferred to PVDF membrane (Millipore, Billerica, MA). For Western blot analysis, filters were incubated in Tris-buffered saline (TBS) solution containing 0.1% Tween 20 and 5% nonfat milk. Filters were then incubated overnight in the cold room with the appropriate antibodies diluted in a TBS also containing 0.1% Tween 20 and 5% nonfat milk. After extensive washing in TBST solution, filters were incubated with horseradish peroxidase–conjugated anti-mouse (GE Healthcare). The filters were washed as above and developed using chemiluminescence detection system.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from each cell type using RNAzol (Molecular Research Center, Inc.) according to the manufacturer’s protocol. RNA (10 ng) was reverse transcribed using TaqMan microRNA reverse transcription kits (Life Technologies). Relative expression of microRNAs was quantified with the TaqMan Universal PCR Master Mix, No AmpErase UNG, using the One Step Real-Time PCR system (Life Technologies). Thermal cycling conditions included enzyme activation for 10 min at 95°C, 40 cycles at 95°C for 15 sec, and 60°C for 60 sec. MicroRNAs tested were miR-200a/b and c, miR-141 and miR-429 in cell lines including Kaiso knockout PC-3 cells (sh-Kaiso) negative control with Scramble nucleotide sequence (sh-scr), PC-3 cells treated with 5-aza, WT PC-3 cells, PC-3 and DU145 cells treated with EGF/PD153035, PC-3 cells overexpressing miR200c and a panel of prostate cancer parental cell lines. The analyses for each were performed in triplicate and RNU48 microRNA was used as endogenous control. Messenger RNA (mRNA)- Reverse transcription for RNA was accomplished using RNA reverse transcription kits (Life Technologies) according to the manufacturer’s protocol. SYBR Green ROX qPCR Mastermix (Qiagen Sciences) was used to amplify the cDNA product using the Step One qRT-PCR (Applied Biosystems) instrument at 95°C for 10 min for enzyme activation, 95°C for 15 sec, 60°C for 60 sec at 40 cycles. Genes tested for were Kaiso, Zeb 1/2 in cell lines including WT LNCaP cells, WT PC-3 cells and PC-3 cells overexpressing miR200c and Scramble control. The fold change was calculated using the 2-ΔCt value method. Analyses were performed in triplicate.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed with the use of ChIP-IT Kits (Milipore) to determine Kaiso binding to the methylated promoter. In brief, 5-aza treated and untreated PC-3 cells were fixed in 1% formaldehyde at room temperature for 10 minutes and 1:10 volume of 10× Glycine was added to quench the fixation reaction for 5 min at room temperature. Cells were pelleted and lysed using SDS lysis buffer supplemented with protease cocktail inhibitor. 300-400 uL aliquots of the lysates were sonicated on wet ice to shear DNA at optimized conditions to an average size between 200 and 1500 bp. 10 uL of each sonicated cell lysate was saved for input DNA control. Sheared crosslinked chromatin was incubated in dilution buffer containing protease inhibitor cocktail II and protein G agarose beads. Kaiso antibody (Abcam, Boston, MA) was used for chromatin immunoprecipitation with Anti-RNA Polymerase II and Normal Mouse IgG as positive and negative control, respectively. 1 ng each of ChIP and input DNA were used for real-time PCR analysis. Primers were designed to amplify a 217 bp fragment of the methylated CpG islands of the miR-200c/141 promoter region: 5’-GGGTAGGGGAAGGTGGCTCAGAG-3’ and 5’-GGATCCTGGGCCTGAAGCTGC-3’.300 bp fragment of the methylated CpG islands of miR-200a/b/429 promoter region: 5’-GCAGAGGTGGAGAGGCGAG-3’ and 5’-CCTGGCACAGGAAGTCAGTT-3’ were used [18]. Quantitative real-time PCR was conducted to amplify the genes using the Step One qRT-PCR (Applied Biosystems) instrument at 95°C for 10 min for enzyme activation, 95°C for 15 secs, 60°C for 60 secs at 30 cycles. The analyses were performed in triplicate and input was used as endogenous control.
Animal Studies
Athymic nude mice were injected subcutaneously with 3 × 106 Kaiso Scramble control (sh-src) cells and Sh-Kaiso PC-3 cells. After the baseline tumor measurement, subsequent tumor measurements were done to determine tumor proliferation and growth in these mice. Athymic nude mice were intracardially inoculated with 1 × 106 RFP-tagged sh-scr and sh-Kaiso cells and subsequently imaged for tumor formation and metastasis weekly, over a period of 30 days.
Statistical Analysis
All quantitative experiments were performed at least two times and each individual data point in triplicates. Statistical analysis was performed using Microsoft Excel or GraphPad Prism software. Microsoft Excel was used to determine standard deviation and statistical significance was determined using, one-tailed T-test. The P-value for statistical significance was 0.05.
RESULTS
Previously we reported that Kaiso induces EMT and increased cell migration through silencing of both E-cadherin and miR-31 in aggressive prostate and breast cancer cell lines, through direct binding to methylated regions in the E-cadherin [15, 16] or miR-31 promoter [14]. However multiple reports have highlighted that members of the microRNA-200 family are silenced through hypermethylation of their promoter to induce EMT in multiple cancer types [11, 19]. Therefore, to determine if Kaiso directly interacts with the miR-200 family genes, we designed primer sequences for each of these sites and the location of each is shown in (Figure 1A). ChIP assays were performed for each site using ChIP-grade Kaiso 6F8 antibody. The ChIP assay utilizing primers in the −246 to −169 region showed Kaiso binding to the miR-200c/141 promoter and the −324 to +123 region showed Kaiso binding to miR-200a/200b/429 promoter, and this binding was abrogated in cells treated with 500nM 5-aza, highlighting that repressor activity of Kaiso (Figure 1B).
Figure 1. Kaiso binds to CpG sites directly regulating miR-200a, miR-200b, miR-200c, miR-141 and miR-429 expression.
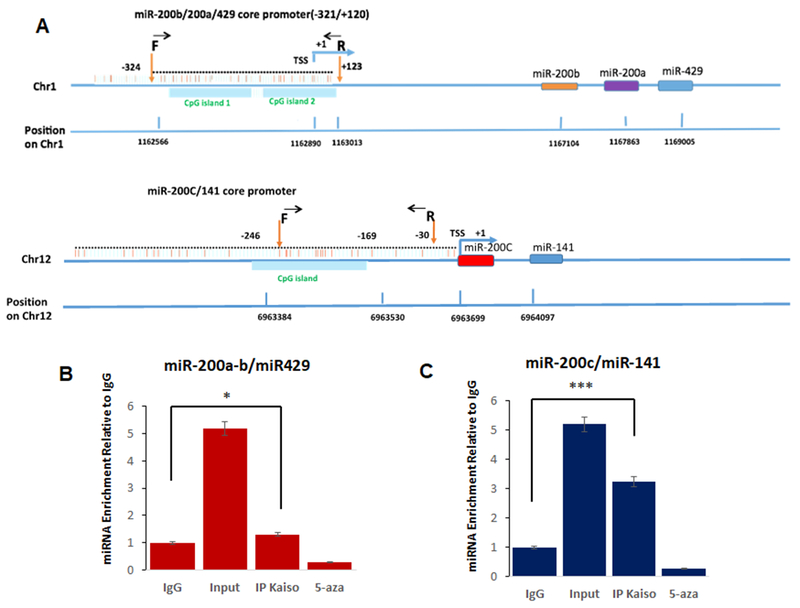
(A) in silico analysis of Kaiso binding sites on the promoters of each miRNA in 200 family. (B) Chromatin immunoprecipitation products were analyzed by qRT-PCR specific miR-200a, miR-200b, miR-429 core promoter primers (−321 to +120) to amplify the methylated region in PC3 cells in the presence or absence of 5-aza. (C) Chromatin immunoprecipitation products were analyzed by qRT-PCR miR-200c and miR-141 core promoter primers (246 to −169) to amplify the methylated region in PC3 cells in the presence or absence of 5-aza Mouse IgG as a negative control. Input DNA served as a positive control. All data presented are the means of three independent experiments, performed in triplicate ± S. E. ***P < 0.001 is significant
To determine if Kaiso has a functional role on the miR-200 family, we utilized previously published, sh-Kaiso PC-3 cell line model compared to sh-scr control cells [14, 16]. All of the miR-200 family, miR-200a, miR-200b, miR-429, miR-200c and miR-141 were highly expressed as determined by qRT-PCR in PC-3 sh-Kaiso cells compared to PC3-scr control cell, similar to PC-3 cells treated with 500nM 5-aza-2′-deoxycytidine (5-aza), a demethylation agent (Figure 2). Since miR-200c demonstrated the highest level of increases in sh-Kaiso compared to sh-scr cells, we therefore focused on the miR-200c Kaiso relationship for the reminder of the studies.
Figure 2. Kaiso expression is correlated with expression of classical EMT markers.
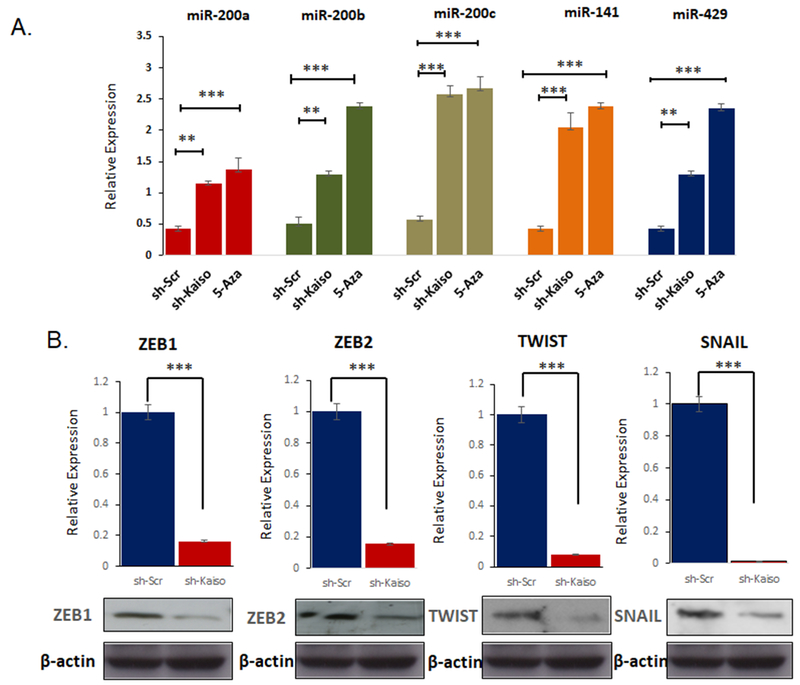
A. Relative expression of the miR-200 family expression were measured using qRT-PCR in sh-Kaiso compared to untreated scr-control cells and 5 aza treated sh-scr control cells. B. ZEB1, SNAIL and TWIST were measured by qRT-PCR and immunoblot in sh-Kaiso PC-3 cells compared to sh-scr control. *P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant.
Since a number of reports have indicated that multiple members of the miR-200 family, are responsible for regulation of ZEB1 and ZEB2 [20–22], we next sought to determine if Kaiso would influence the expression of these genes, as well as other known master regulators of EMT TWIST, and SNAIL. ZEB1 and ZEB2 showed decreased expression at both the RNA levels by qRT-PCR, and decreased protein levels, using immunoblot (Figure 2B). We also observed decreases in TWIST and SNAIL expression in this sh-Kaiso cells as compared to sh-Scr cells as well. Thus, it appears that Kaiso has a functional role in regulating the miR-200c ZEB1 signaling axis.
Of the miR-200 family, miR-200c has not been widely investigated in prostate cancer. Therefore, we first determined the expression in a panel of normal to highly metastatic prostate cell lines, which includes non-malignant PREC, RC-77N, RC-170N, RC-165N; malignant cell lines RC-92a, RC-77T, LNCaP; and metastatic cell lines DU-145, DU-145-EGFR, PC-3 and C42B cells. . miR-200c is expressed in normal and non-metastatic prostate cancer cell lines, and expression is lost in metastatic prostate cancer cell lines. Interestingly, we observed that miR-200c is significantly lower in DU-145 cells that overexpress EGFR [23, 24] compared to parental cell lines (Figure 3A). This is interesting, since we have previously reported that this cell line has higher levels of Kaiso [16]. To explore this relationship further, we treated DU-145, DU-145 EGFR, and PC-3 cells with increasing concentrations of EGF, as well as an EGFR specific kinase inhibitor, PD153035. miR-200c levels were significantly decreased after both 10ng/mL and 50ng/mL of EGF, which was completely reversed after treatment with PD153035 where we observed a 2-fold increase in miR-200c expression (Figure 3B). Since we demonstrated above that Kaiso regulates miR-200c expression, we then treated our sh-Kaiso PC-3 and DU-145 cell lines with 10ng/mL of EGF. Interestingly, we did not observe any changes in miR-200c expression in sh-Kaiso cells suggesting that EGFR induced decreased in miR-200c is dependent on Kaiso (Figure 3C). To confirm EGFR-Kaiso-miR-200c relationship, we overexpressed Kaiso in non-metastatic LNCaP cells. LNCaP Kaiso overexpressing cells (LNCaP-O) demonstrated increased ZEB1 expression along with increased in EGFR and p-EGFR expression (Figure 3D). Furthermore, RT-PCR results demonstrated that LNCaP-O cells showed a decrease in miR-200a, miR-200b, miR-429, miR-200c and miR-141 expression compared to LNCaP Scr- control cells (Figure 3E).
Figure 3. Kaiso regulates miR-200c through EGFR activity.
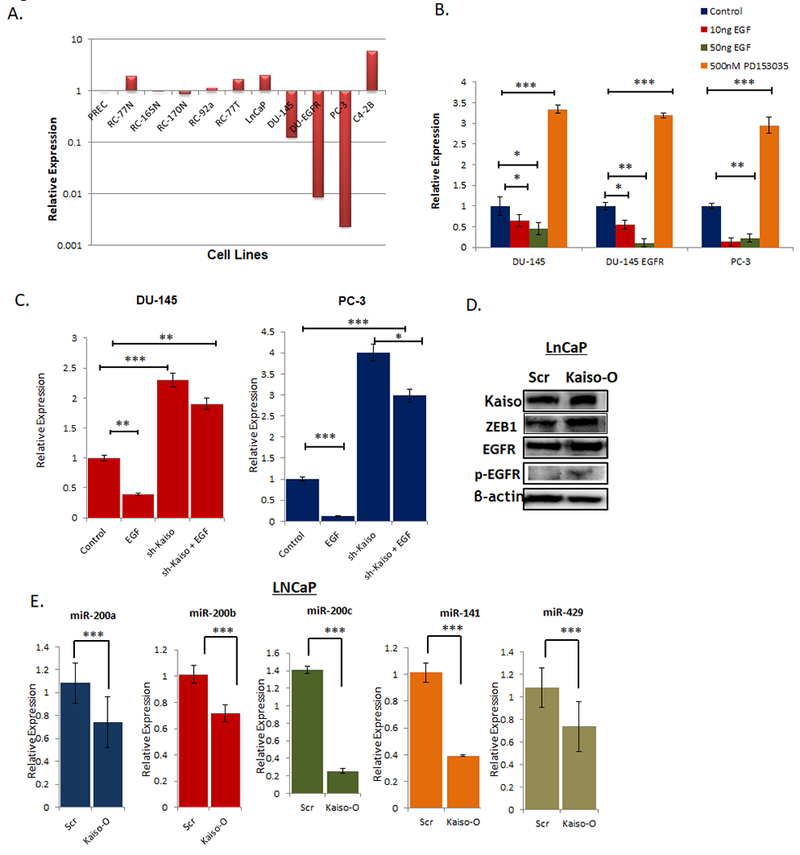
A. Relative expression levels of miR-200c expression levels were measured by qRT-PCR in a panel of prostate cancer cells lines shows that miR-200c is significantly lower in DU-145 cells that overexpress EGFR compared to parental cell lines. B. miR-200c expression was measured by qRT-PCR in DU-145, DU-145 EGFR, and PC-3 cells were treated with 10ng/mL and 50ng/mL of EGF, as well as an EGFR specific kinase inhibitor, PD153035. C. miR-200c expression was measured by in sh-Kaiso DU-145 and PC-3 cells in the presence or absence of 10ng/mL EGF. D. Stable LNCaP Kaiso overexpression (LNCaP-O) and LNCaP SCR-control cell were measured for Kaiso, EGFR, p-EGFR, and ZEB1 by immunoblot with β-actin as loading control. E. miR-200a, miR-200b, miR-429, miR-200c and miR-141 were measured using RT-PCR in LNCaP Kaiso overexpressing and LNCaP scr-control cells. All data presented are the means of three independent experiments, performed in triplicate ± S. E * P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant.
Next, we wanted to investigate if there is a feed forward miR-200c/Kaiso loop. To test this, stably transfected clones re-expressing miR-200c in PC-3 cells were used. Here, we verified the expression of two distinct clones to PC-3 wild type, negative control cells with Scramble nucleotide sequence (PC-3 scr) and non-metastatic LNCaP cells (Figure 4A). Next, we measured the expression of Kaiso, ZEB1 and ZEB2 by qRT-PCR. These genes were significantly decreased in the PC-3 cells over-expressing miR-200c as compared to PC-3 control, scr-control cells and non-metastatic LNCaP cells (Figures 4B). We also probed these cells for Kaiso, ZEB1, EGFR and p-EGFR expressions by immunoblot. All four protein expressions were significantly decreased in both PC-3 cells over-expressing miR-200c comparable to scr-control cells and LNCaP (Figure 3C). Utilizing the same model, we probed for Kaiso localization by immunofluorescence. Interestingly, we observed increased E-cadherin expression (Supplemental Figure 1) and decreased Kaiso expression in the miR-200c clone compared to scr–control clone (Figure 4).
Figure 4. miR-200c Re-expression Reverses EMT.
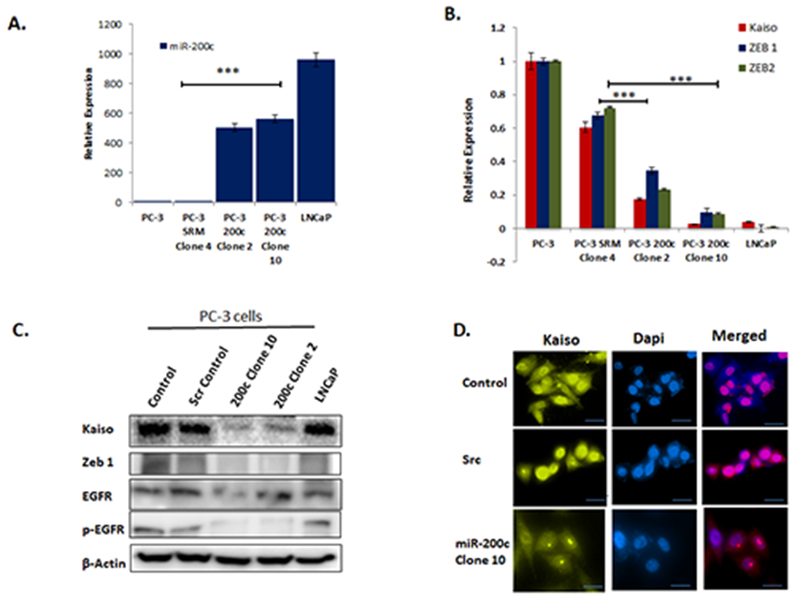
A. Two stably transfected clones 4 and 10 in PC-3 cells were measured for qRT-PCR for miR-200c expression compared to cells transfected with SCR-control and LNCaP cells. B. PC-3 miR-200c clone 10 was probed for Kaiso, Zeb 1 and Zeb 2 expression by qRT-PCR and compared to SCR-control and LNCaP cells. C. Kaiso, EGFR, p-EGFR, and ZEB1 were measured by immunoblot in miR-200 clone PC-3 cells, SCR-control and LNCaP cells. Immunofluorescence using Anti-Kaiso antibody (yellow) expression in miR-200c PC-3 clone compared to PC-3 wild type and scramble control cells. Blue color =DAPI nuclear stain. All data presented are the means of three independent experiments, performed in triplicate ± S. E * P < 0.05 is significant. **P < 0.01 is significant. ***P < 0.001 is significant
Since EGFR is known to activate MAPK-ERK signaling pathway we used PC-3 sh-Kaiso cells to determine ERK activity. We observed robust decreases in ERK activity in sh-Kaiso cells compared to scr-control by immunoblot (Supplemental Figure 2A). To confirm MAPK-ERK signaling has role in Kaiso expression, we used 500nM PD98059, MAPK inhibitor, in the presence or absence of 10ng/ml EGF. PD98059 resulted in a decrease in Kaiso protein expression and blocked the ability of EGF to increase Kaiso expression (Supplemental Figure 2B. To confirm the role of miR-200c, we further observed that 200c clone 10 PC-3 cells display decreased p-ERK and total ERK expression, compared to control, scr, and LNCaP cells (Supplemental Figure 2C).
To determine if the Kaiso-miR-200c-ZEB1 signaling axis as a role in in vivo, we inoculated the sh-Kaiso and sh-scr cells in the subcutaneous in athymic nude mice. Using a xenograft model, we showed a significant increase in tumor size in athymic nude mouse inoculated subcutaneously with the PC-3 Scramble control than in athymic nude mouse inoculated with the sh-Kaiso PC-3 cells (Figures 5A & B). Excised tumors after five weeks from both xenograft groups maintained their initial Kaiso and Zeb1 expression levels as determined by IHC (Figures 5C and D). Interestingly, microRNA-200a, miR-200b and miR-200c expression levels were preserved in the sh-Kaiso xenograft tumor with miR-200c demonstrating the most significant levels (Figure 5E). Finally, we sought to determine if Kaiso promotes tumor metastasis. Therefore, we intra-cardiac inoculated athymic mice with RFP-tagged sh-scr and sh-Kaiso cells Intriguingly, scr- PC-3 control cells formed metastatic lesions by days 7, while the mice with sh-Kaiso cells showed no tumor formation and metastasis by days 18 and 30 (Figure 6A). Furthermore, we did not observe any evidence of bone metastasis by X-ray or IHC of intra tibial in sh-Kaiso cells (Figure 6B). Thus, it appears that there is a feed forward Kaiso-miR-200c signaling axis that promotes epithelial to mesenchymal transition and metastasis in prostate cancers. A diagram predicting this relationship is depicted in (Figure 6C).
Figure 5. Kaiso depleted PC-3 cells exhibit decreased in vivo tumor growth.
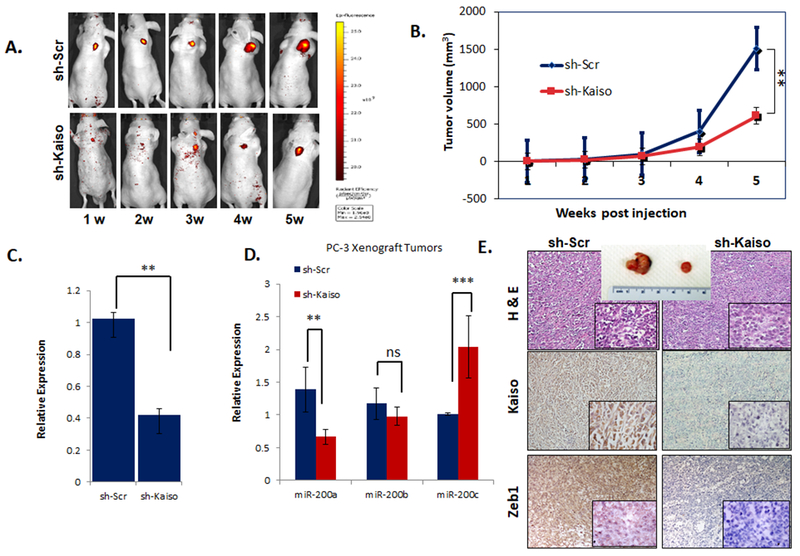
A. sh-Kaiso PC-3 RFP cells were inoculated subcutaneously in (n=7) a thymic nude mice and measure for RFP expression over a 6 week. B. Tumor volume was measured using C. miR-200 a, b, and c were measured by qRT-PCR in tumors excised from the in vivo experiment. D. H&E analysis and Immunohistochemical staining for Kaiso and ZEB1 was performed on tumor excised from in vivo experiment.
Figure 6. Kaiso Depleted PC-3 RFP cells show decreased in vivo metastasis.
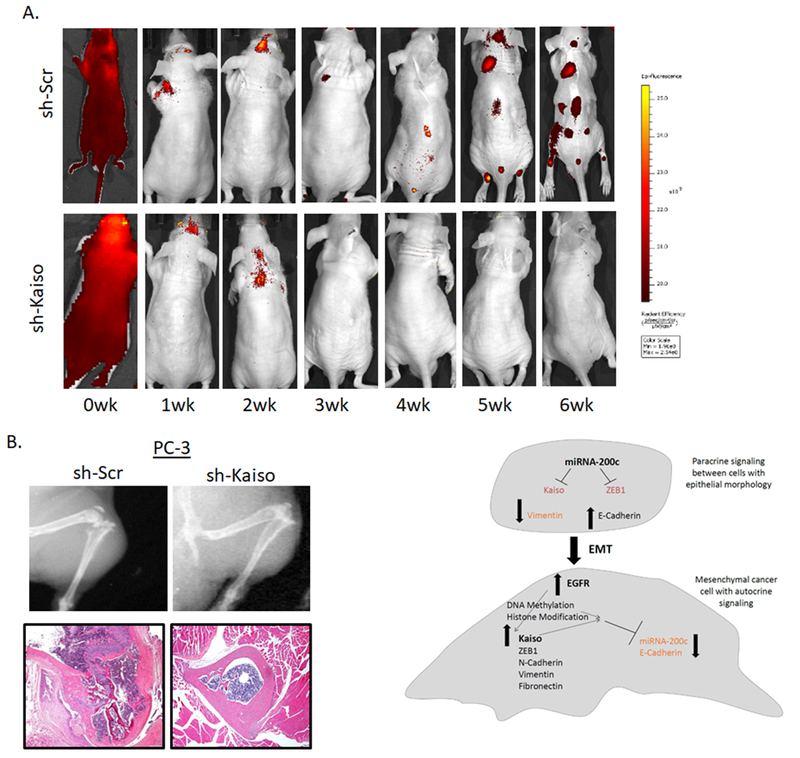
A. RFP sh-scr and sh-Kaiso cells in mice (n=7) were inoculated through intra cardiac injection and tumor formation/metastasis was measured by RFP expression over 30 days. B. X-Ray Image is representative of the tibia from mice after 30 days, and H&E image is representative of tumors excised from bone tibia After 30 days, C. Schematic of EGFR-Kaiso-miR-200 regulation in epithelial and mesenchymal prostate cancer cells.
DISCUSSION
There is mounting evidence that Kaiso is involved in the pathogenesis of multiple tumor types. While some reports have suggested that Kaiso is a tumor suppressor, other reports have suggested that Kaiso is proto-oncogene. There are even fewer reports on the molecular mechanism of Kaiso in either of these situations. Previously, we reported that Kaiso is elevated in late stage prostate cancer, including lymph node and bone metastasis[16], and has a functional role in promoting cell migration and invasion of prostate cancer cell at least in part through methylation dependent silencing of miR-31 [14]. However, a role of Kaiso in additional miRNA’s silenced by DNA hypermethylation is poorly understood. In the current study we demonstrate that Kaiso similarly has a direct regulatory role in the expression of the microRNA-200 family, which has been shown to highly influence EMT in aggressive tumors through the direct regulation of a number of genes, such as ZEB1 and ZEB1 [7, 11, 25, 26].
The miR-200 family consists of 5 miRNAs that are closely related in sequence, but are predicted to comprise two distinct classes in terms of their targets, with miR-200a and miR-141 sharing identical seed regions and miR-200b, miR-200c, and miR-429 constituting a separate targeting class [27]. Our initial findings through ChIP binding assays confirm that Kaiso directly binds to methylated regions in chr 1 , which is responsible for the expression of miR-200a, miR-200b, and miR-429, and chr 12 which is responsible for miR-200c/141 expression. Although Kaiso appears to have a role in other miR-200 family member, miR-200c is the strongest interaction, and upon depletion of Kaiso, miR-200c demonstrated the most significant increases of all miRNAs compared to Scramble control. EMT inducing platelet-derived growth factor-D (PDGF-D) has been demonstrated to induce EMT through repression of miR-200b expression [12, 26]. However, little is known about the regulation of miR-200c. Interestingly, we observed that over expression of EGFR or treatment with EGF in DU-145 cells results in significant decreases in miR-200c expression, and this repression was reversed in both DU-145 and PC-3 cell lines treated with EGFR specific inhibitor PD153035. These results are in agreement with our previous findings that both DU-145 PC-3 cell line exposed to EGF exhibit increased Kaiso expression and cytoplasmic to nuclear localization to induce cell migration [16] . Furthermore, EGF could not repress miR-200c expression in sh-Kaiso DU-145 or sh-Kaiso PC-3, highlighting that Kaiso has a direct role in regulating miR-200c expression. Although the TGF-β -SMAD signaling has been demonstrated to regulate Kaiso in breast cancer models [28], it is likely that TGF-β and EGFR signaling act cooperativity to regulate Kaiso expression. Although we cannot rule out differences in prostate and breast cancer specific signaling cascades. However, since, there are a large number of reports in multiple tumor types other than prostate cancer, that highlight the role of miR-200c in cell migration and metastasis, our findings suggest a role of this regulatory mechanism in these cancers as well.
LNCaP cells have been well documented to express high levels of miR-200c and lack DNA methylation [29] and low levels of Kaiso expression [16]. Therefore, we utilized this cell line to stably overexpress Kaiso. Interestingly, LNCaP-O cells demonstrated a significant decrease in all miR-200 family members. While many reports have demonstrated that decreases in miR-200c expression result in increased ZEB1 expression [26], our data suggest that Kaiso is an intermediate regulator of ZEB1 through miR-200c expression. Congruent with EGF stimulation decreasing miR-200c through Kaiso, we further observed that EGF induced increases in Kaiso expression is dependent on MAPK activity. In support of this we observed that sh-Kaiso PC-3 cells display robust decreases in EGFR, p-ERK expression and ZEB1 expression. Several reports have demonstrated that miR-200c directly regulates EGFR expression in other tumor types, and that miR-200c overexpression enhances sensitivity to EGFR inhibitor gefitinib [30]. The fact that Kaiso overexpressing LNCaP cells demonstrate increases in EGFR expression levels and phosphorylated EGFR, and miR-200c overexpressing PC-3 cells demonstrate decreased expression of these protein compared to control cells, suggest that Kaiso-miR-200c regulatory axis is regulated by EGFR signaling in a feed forward loop that drives EMT and tumor progression. This is plausible since a similar report as our findings demonstrates that ectopic expression of miR-200c results in decreased EGFR expression, and bioinformatics analysis suggest that miR-200c directly regulates EGFR multiple breast cancer cells [31].
To validate our in vitro mechanistic studies, we preformed two in vivo assays to determine the effect of Kaiso in tumor growth and metastasis. sh-Kaiso PC-3 cells inoculated subcutaneously in mice. sh-Kaiso cells demonstrate a delay in tumor formation compared to sh-scr control cells. Furthermore, to confirm the role of Kaiso in cell migration and metastasis, we subsequent performed intracardiac injection of sh-Kaiso PC-3 cells. Interestingly, we did not observe any metastases in these mice compared to sh-scr control. These finding are similar to studies in ovarian cancer have shown that microRNA-200c regulates the E-cadherin transcriptional repressors, Kaiso and Zeb1 [10]. Our findings suggest a link to the EGF receptor-signaling cascade of events, which regulates Kaiso expression and localization [32]. Our focus in this study is due to the immense need for detailed studies on epigenetic mechanisms involved in the regulation of tumor suppressor microRNAs, miR-200 family. This is important because studies have shown that epigenetic silencing mechanisms are reversible and has great potential for cancer therapy [33]. further highlights that this signaling axis can be exploited for therapy.
In conclusion, our results have showed that downregulation of miR-200c is at least in part regulated with EGFR-Kaiso signaling axis. Overexpression of miR-200c decreased Kaiso and ZEB1 expression and decreased invasion and EMT of prostate cancer cells. Therefore, our study provided functional evidence that supports a role of Kaiso in EMT and the progression of prostate tumors.
Supplementary Material
Supplemental Figure 1. Re-expression of miR-200c causes MET in PC-3 cells. IF staining for E-cadherin (Red) in PC-3 miR-200c clone 10 compared to PC-3 wild type and scramble control cells. Blue color =DAPI nuclear stain.
Supplemental Figure 2. Kaiso expression is influenced by MAPK activity. A. PC-3 cells were treated with 10ng/mL EGF or MAPK specific kinase inhibitor PD 98059 and probed with Anti-Kaiso antibody. B. sh-Kaiso PC-3 cells were probed with Anti-ERK and p-ERK antibody. PC-3 miR-200c clone 2 and clone 10 were probed with Anti-ERK and p-ERK antibody. Images are representative of three individual experiments. β-actin was used as loading control in each experiment.
Supplemental Table 1. ChIP primers for miR-200a, miR-200b, miR-200c, miR-141 and miR-429
Acknowledgments
This work was supported by grants U54MD007585, (NIH/RCMI) [CY], U54CA118623-01 (NIH/NCI) [CY], (NIH/NCI) 1 R21 CA188799-01 [CY]; and a Department of Defense Grant, PC120913, W81XWH-10-1-0543. Statistical analysis was performed by the University Alabama at Birmingham and Tuskegee University Statistical Cores. We also thank members of the Yates laboratory and the Davis laboratory for their technical assistance, comments and discussions.
Footnotes
Competing interests
The authors have no conflicting financial interests.
REFERENCES
- [1].Crawford ED, Understanding the epidemiology, natural history, and key pathways involved in prostate cancer, Urology, 73 (2009) S4–10. [DOI] [PubMed] [Google Scholar]
- [2].Bockhorn M, Jain RK, Munn LL, Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed?, The Lancet Oncology, 8 (2007) 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures.pdf, Nat Clin Pract Oncol, 4 (2007) 699–710. [DOI] [PubMed] [Google Scholar]
- [4].Fidler IJ, The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited.pdf, Nat Rev Cancer, 3 (2003) 453–458. [DOI] [PubMed] [Google Scholar]
- [5].Lee JM, Dedhar S, Kalluri R, Thompson EW, The epithelial-mesenchymal transition: new insights in signaling, development, and disease, J Cell Biol, 172 (2006) 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tse JC, Kalluri R, Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment.pdf, J Cell Biochem, 101 (2007) 816–829. [DOI] [PubMed] [Google Scholar]
- [7].Neves R, Scheel C, Weinhold S, Honisch E, Iwaniuk KM, Trompeter H, Niederacher D, Wemet P, Santourlidis S, Uhrberg M, Role of DNA methylation in miR-200c,141 cluster silencing in invasive breast cancer cells.pdf, BMC Research Notes, 3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ, The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat Cell Biol, 10 (2008) 593–601. [DOI] [PubMed] [Google Scholar]
- [9].Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, Nguyen LT, Hijiya N, Uchida T, Sato F, Mimata H, Seto M, Moriyama M, Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c, J Pathol, 216 (2008) 418–427. [DOI] [PubMed] [Google Scholar]
- [10].Park SM, Gaur AB, Lengyel E, Peter ME, The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2, Genes Dev, 22 (2008) 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, Stampfer MR, Futscher BW, Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells, PLoS One, 5 (2010) e8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH, miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells, Stem Cells, 27 (2009) 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jones J, Wang H, Karanam B, Theodore S, Dean-Colomb W, Welch DR, Grizzle W, Yates C, Nuclear localization of Kaiso promotes the poorly differentiated phenotype and EMT in infiltrating ductal carcinomas, Clin Exp Metastasis, 31 (2014) 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang H, Liu W, Black S, Turner O, Daniel JM, Dean-Colomb W, He QP, Davis M, Yates C, Kaiso, a transcriptional repressor, promotes cell migration and invasion of prostate cancer cells through regulation of miR-31 expression.pdf, Oncotarget, 7 (2015) 5677–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jones J, Mukherjee A, Karanam B, Davis M, Jaynes J, Reams RR, Dean-Colomb W, Yates C, African Americans with pancreatic ductal adenocarcinoma exhibit gender differences in Kaiso expression, Cancer Lett, 380 (2016) 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jones J, Wang H, Zhou J, Hardy S, Turner T, Austin D, He Q, Wells A, Grizzle WE, Yates C, Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells, Am J Pathol, 181 (2012) 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perdigao-Henriques R, Petrocca F, Altschuler G, Thomas MP, Le MT, Tan SM, Hide W, Lieberman J, miR-200 promotes the mesenchymal to epithelial transition by suppressing multiple members of the Zeb2 and Snail1 transcriptional repressor complexes, Oncogene, 35 (2016) 158–172. [DOI] [PubMed] [Google Scholar]
- [18].Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ, A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition, Cancer Res, 68 (2008) 7846–7854. [DOI] [PubMed] [Google Scholar]
- [19].Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M, Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis, Oncogene, 31 (2012) 2062–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jurmeister S, Baumann M, Balwierz A, Keklikoglou I, Ward A, Uhlmann S, Zhang JD, Wiemann S, Sahin O, MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F, Mol Cell Biol, 32 (2012) 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li X, Roslan S, Johnstone CN, Wright JA, Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ, Gregory PA, Khew-Goodall Y, MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways, Oncogene, 33 (2014) 4077–4088. [DOI] [PubMed] [Google Scholar]
- [22].Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR, miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor, Proc Natl Acad Sci U S A, 107 (2010) 20828–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Turner T, Chen P, Goodly LJ, Wells A, EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells, Clin Exp Metastasis, 14 (1996) 409–418. [DOI] [PubMed] [Google Scholar]
- [24].Xie H, Turner T, Wang MH, Singh RK, Siegal GP, Wells A, In vitro invasiveness of DU-145 human prostate carcinoma cells is modulated by EGF receptor-mediated signals, Clin Exp Metastasis, 13 (1995) 407–419. [DOI] [PubMed] [Google Scholar]
- [25].Taube JH, Malouf GG, Lu E, Sphyris N, Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, Herschkowitz JI, Rosen JM, Issa JP, Calin GA, Chang JT, Mani SA, Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties, Sci Rep, 3 (2013) 2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Williams LV, Veliceasa D, Vinokour E, Volpert OV, miR-200b inhibits prostate cancer EMT, growth and metastasis, PLoS One, 8 (2013) e83991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bracken CP, Li X, Wright JA, Lawrence DM, Pillman KA, Salmanidis M, Anderson MA, Dredge BK, Gregory PA, Tsykin A, Neilsen C, Thomson DW, Bert AG, Leerberg JM, Yap AS, Jensen KB, Khew-Goodall Y, Goodall GJ, Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion, EMBO J, 33 (2014) 2040–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bassey-Archibong BI, Kwiecien JM, Milosavljevic SB, Hallett RM, Rayner LG, Erb MJ, Crawford-Brown CJ, Stephenson KB, Bedard PA, Hassell JA, Daniel JM, Kaiso depletion attenuates transforming growth factor-beta signaling and metastatic activity of triple-negative breast cancer cells, Oncogenesis, 5 (2016) e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lynch SM, O’Neill KM, McKenna MM, Walsh CP, McKenna DJ, Regulation of miR-200c and miR-141 by Methylation in Prostate Cancer, Prostate, 76 (2016) 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou G, Zhang F, Guo Y, Huang J, Xie Y, Yue S, Chen M, Jiang H, Li M, miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1, Biomed Pharmacother, 85 (2017) 113–119. [DOI] [PubMed] [Google Scholar]
- [31].Koo T, Cho BJ, Kim DH, Park JM, Choi EJ, Kim HH, Lee DJ, Kim IA, MicroRNA-200c increases radiosensitivity of human cancer cells with activated EGFR-associated signaling, Oncotarget, 8 (2017) 65457–65468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, Christgen M, Lehmann U, Daniel J, van Diest PJ, Derksen PW, Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer, PLoS One, 7 (2012) e37864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li LC, Carroll PR, Dahiya R, Epigenetic changes in prostate cancer: implication for diagnosis and treatment, J Natl Cancer Inst, 97 (2005) 103–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Re-expression of miR-200c causes MET in PC-3 cells. IF staining for E-cadherin (Red) in PC-3 miR-200c clone 10 compared to PC-3 wild type and scramble control cells. Blue color =DAPI nuclear stain.
Supplemental Figure 2. Kaiso expression is influenced by MAPK activity. A. PC-3 cells were treated with 10ng/mL EGF or MAPK specific kinase inhibitor PD 98059 and probed with Anti-Kaiso antibody. B. sh-Kaiso PC-3 cells were probed with Anti-ERK and p-ERK antibody. PC-3 miR-200c clone 2 and clone 10 were probed with Anti-ERK and p-ERK antibody. Images are representative of three individual experiments. β-actin was used as loading control in each experiment.
Supplemental Table 1. ChIP primers for miR-200a, miR-200b, miR-200c, miR-141 and miR-429


