Abstract
Neutron capture therapy (NCT) is a targeted radiotherapy for cancer treatment. In this method, neutrons with a spectra/specific energy (depending on the type of agent used for NCT) are captured with an agent that has a high cross-section with these neutrons. There are some agents that have been proposed in NCT including 10B, 157Gd and 33S. Among these agents, only 10B is used in clinical trials. Application of 157Gd is limited to in-vivo and in-vitro research. In addition, 33S has been applied in the field of Monte Carlo simulation. In BNCT, the only two delivery agents which are presently applied in clinical trials are BPA and BSH, but other delivery systems are being developed for more effective treatment in NCT. Neutron sources used in NCT are fission reactors, accelerators, and 252Cf. Among these, fission reactors have the most application in NCT. So far, BNCT has been applied to treat various cancers including glioblastoma multiforme, malignant glioma, malignant meningioma, liver, head and neck, lung, colon, melanoma, thyroid, hepatic, gastrointestinal cancer, and extra-mammary Paget's disease. This paper aims to review physical, dosimetric and clinical aspects as well as delivery systems in NCT for various agents.
Keywords: NCT, BNCT, GdNCT, SNCT, Delivery system252Cf
1. Introduction
Neutron capture therapy (NCT) is a treatment method to selectively target malignant cells. In this method, neutrons with a spectra/specific energy are captured with an agent that has a high cross-section with those neutrons, and the neutron energy used depends on the type of the agent used. There are some agents that have been proposed in NCT including 10B, 157Gd and 33S and treatments with these agents are called BNCT, GdNCT and SNCT, respectively. In the following sections, each of these modalities are described.
1.1. BNCT
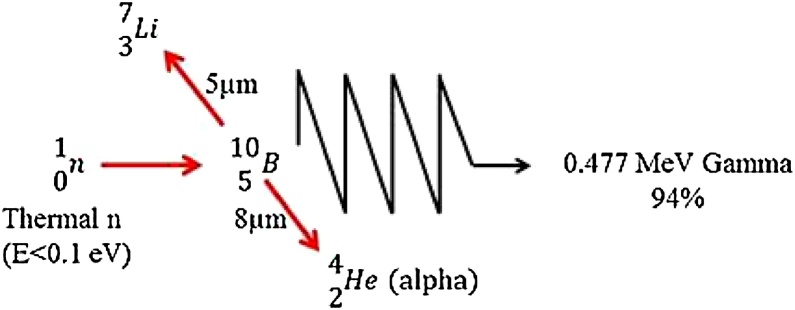
BNCT was first described in1935 by Taylor.1 It is a targeted radiotherapy based on the nuclear capture that occurs when 10B (non-radioactive) is irradiated with thermal neutrons (energies less than 0.5 eV). It is notable that 10B absorption cross-section follows a 1/ν law (ν is neutron velocity) very accurately in the energy range from 0 to 104 eV. Therefore, 10B-n capture reaction can occur even at energies higher than the thermal energies, although the thermal energies are more effective. Following the interaction, high linear energy transfer (LET) products (alpha particles and recoiling 7Li nuclei) are produced.[2], [3], [4], [5], [6], [7] The deposited energies of these heavy particles are shown below8:
These products deposit their energies in a range of 5–9 μm. This range corresponds to the diameter of a cell,9 and the harmful effects of these compounds with high LET are limited to boron comprising cells. Therefore, BNCT presents a method to selectively eradicate malignant cells and spare adjacent normal cells.6 Fig. 1 shows the nuclear reaction which is related to BNCT. For a successful BNCT, an adequate amount of 10B should be selectively accumulated in the tumor. Moreover, sufficient thermal neutrons should be available to create a large number of capture reactions.10 Also, it has been reported that when the efficiency of BNCT is limited with thermal neutrons, replacing light water with heavy water can increase the maximum therapeutic depth. As Blagojevic et al. showed, concentrations of heavy water in humans is not toxic up to 23%.[11], [12]Moreover, BNCT is clinically used for treatment of different tumors.[13], [14], [15], [16], [17], [18]
Fig. 1.
Neutron capture reaction with 10B.
1.2. GdNCT
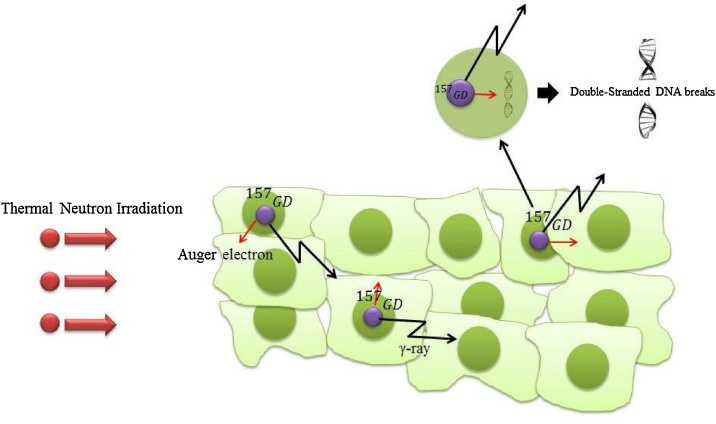
Besides 10B, 157Gd is used as an agent for NCT. Gadolinium element has seven stable isotopes including “152Gd (0.205%), 154Gd (2.23%) 155Gd (15.10%), 156Gd (20.60%), 157Gd (15.70%), 158Gd (24.50%), 160Gd (21.60%)”.19 Among these nuclides, 157Gd and 155Gd have the largest cross-sections to capture with thermal neutron, as their cross-sections of neutron capture are 255,000 and 60,800 barns, respectively.20 These are approximately 66 and 16 times greater than those of 10B, respectively. Furthermore, it is notable that cross sections of neutron capture for the other isotopes are insignificantly small for dose calculations.19 It is shown that the highest capture cross-section for gadolinium is at energies below 0.2 eV.21 GdNCT is not a fission reaction and is more complex than BNCT.22 The interaction products of the GdNCT are high energy gamma-rays, internal conversion (IC) electrons, X-rays, and auger electrons. The average energy of the gamma-rays and IC electrons are approximately 2.2 MeV and 45 eV, respectively, and their path lengths are several centimeters and several millimeters, respectively. In addition, the auger electrons have very low energy, as their path lengths in aqueous solutions are several nanometers.22 GdNCT is chiefly based on the action of internal conversion and auger electrons produced by 157Gd after neutron capture.23 In Fig. 2 the neutron capture reaction with 157Gd is illustrated.
Fig. 2.
Neutron capture reaction with 157Gd.
As this ionizing radiation is restricted to molecular dimensions, i.e. 5–40 nm, for inducting considerable DNA injury in malignant cells, it is necessary to position the 157Gd atoms within the DNA helix.22 It is notable that the success of GdNCT depends on the relative biological effectiveness (RBE) of gamma-rays, IC and auger electrons, as RBE directly relates to the placement of the 157Gd atoms with regard to the DNA of cancer cells.22 The auger electrons produced by the GdNCT reaction have a very high LET, due to their nanometer range. The auger emitters located adjacent to the DNA strands of malignant cells are able to induce a level of DNA injury which is 5–10 times greater than the high energy gamma-rays. Additionally, the RBE of the IC electrons can be considered intermediate between those of the auger electrons and the high energy gamma-rays.22 The following reaction formula shows capturing 157Gd and 155Gd with thermal neutrons19:
1.3. SNCT
In addition to 10B and 157Gd, a few nuclides capture higher energy neutrons at definite energies, because of resonances in the cross-section of NCT. A particular case among these nuclides is 33S that was introduced by Porras in 2008.24 This isotope is stable and there is a small proportion for it (about 0.75%) in natural sulfur.24 As there is sulfur in proteins related to tumor metabolism, it is a promising option for targeting tumor cell nuclei.25 In SNCT, alpha particle emission is the most probable decay pathway in the neutron capture reaction, as opposed to photon emission, which is dominant in all elements in the chart of nuclides.25 It has been proposed that in tumors with high concentration of 33S, the presence of this isotope can supplement the effect of 10B in BNCT if neutrons with energy of 13.5 keV are applied.26 In SNCT, the most significant resonance for NCT is 13.5 keV, because of the following characteristics: “(1) this energy is in the range of the energies which are utilized in epithermal BNCT; (2) it is a low energy for the background dose in the tissue; (3) both 33S and 30Si (the products of the reaction) are stable; (4) practically there is no gamma ray is emitted, therefore, all the energy produced is delivered locally”.25 In addition, at these energies, the cross-section of 33S (n, α) reaction is comparable to the cross-section of hydrogen elastic interaction, which corresponds to the main interaction of neutrons in water.24 It is notable that for tumor treatments which need a high dose both at a few centimeters and at the superficial depths, the simultaneous use of 10B and 33S is a possibility. Because the dose enhancement near the surface results from the presence of 33S, while at a few centimeters depth, it can mainly be caused by 10B.27 Porras showed using Monte Carlo simulations a neutron physical dose enhancement in a water medium in a high concentration of 33S when a neutron source of 13.5 keV was applied.24 Furthermore, this effect could be higher due to the high LET of alpha particles emitted which have a mean energy of 3.1 MeV. The following reaction formula shows capturing 33S with neutrons of 13.5 keV24:
2. Various delivery systems in NCT for targeted cancer therapy
The selective delivery of an adequate amount of NCT agents to the target cells within a tumor, low toxicity, accumulation and permanence in the tumor during neutron irradiations, and quick clearance from normal tissues and blood are the most important requirements for cancer treatment via NCT. Moreover, the tumor tissue heterogeneity is one of the most significant issues which lead to the heterogeneous uptake of the NCT therapy agents. Generally, within the tumor, the peripheral and necrotic regions have a higher uptake and dense areas have a lower uptake. Hence, proper cancer cell specific targeting and deliver strategies can eliminate these problem (Table 1).28
Table 1.
Summarized recent developments in nanotechnology based NCT agents’ delivery systems.
| No | Nanocarrier | NCT agent | Concentration | In-vitro/in-vivo model | Findings | Ref. |
|---|---|---|---|---|---|---|
| 1 | SWCNTs | C2B10 carborane cage |
23 and 50 mg/Ml | BALB/c mice bearing EMT6 tumor cells | The boron atoms are concentrated more in tumors cells than in blood and other organs | 54 |
| 2 | Boron nitride nanotubes | Boron nitride nanotubes | 10 µg/mL | T98G cells | Substantive and selective uptake of these nanocarriers by glioblastoma multiforme cells | 57 |
| 3 | Dendrimer | Isocyanato polyhedral borane | 1–75 µg/µL | Murine B16 melanoma |
Accumulation of bioconjugate in liver and spleen | 68 |
| 4 | Gold nanoparticle | B10C2 cage | 0.14–0.22 ppm | Osteosarcoma cells | Excellent biocompatibility | 74 |
| 5 | Chitosan | Gd-DTPA | 1200 µg/mouse | Mice bearing subcutaneous B16F10 melanomas |
92% retention of Gd-DTPA at the tumor site after 24 h | 78 |
| 6 | Liposome | Gd-DTPA | 6.8 ± 0.3 mg/mL | Tumor-bearing C57BL/6 mice | Prolonged blood circulation time and improved stability | 63 |
| 7 | Magnetic nanoparticles | 1-R-2-butyl-ortho-C2B10H10 | 35 mg/mL | BCAP-37 bearing BALB/c mice | High boron concentration of 51.4 μg/g tumor and magnetic field target ability | 73 |
Boric acid and its derivatives were used in 1950s and early 1960s as the BNCT delivery agents, but low tumor/brain ratios and poor tumor retention were attained and they were nonspecific.29 In the 1960s, two other boron compositions, one based on arylboronic acids, [(L)-4-dihydroxy-borylphenylalanine] named BPA30 and the other one based on polyhedral borane anion, sodium mercaptoundecahydro-closo-dodecaborate, named BSH31, were used as BNCT delivery agents. These components were classified as the second generation of BNCT delivery agents which had lower toxicity and longer tumor retention time than the related molecules. These two complexes in conjugation with fructose have been applied clinically in Argentina, the United States, Japan and Europe.3
Because of these delivery system's disadvantages and limitations, a third generation of delivery system has been developed based on targeting approaches. Conjugation of stable boron cluster to a tumor targeting moiety via hydrolytically stable linkage developed as the third-generation of boron delivery systems. To date, there are two main targeting strategies towards cancer cells and tissues: passive and active targeting. Passive targeting utilizes the enhanced permeability and retention (EPR) effect where the extravasation of nano-sized particles is facilitated. The EPR effect is due to the production of the vascular permeability factors like vascular endothelial growth factor (VEGF), nitric oxide (NO), bradykinin, peroxynitrite (ONOO–), prostaglandins and also defective vascular architecture.32 Active targeting is an alternative and complementary approach to passive targeting. This method is based on the conjugation of specific antibodies or ligands to the molecules expressed on cancer or the capillary endothelium cells. The vascular endothelial growth factor receptors and integrins are two main capillary endothelium targeting moieties which can be used to control delivery vectors biodistribution and to regress tumors.33
This class of delivery systems is divided into two main categories: (1) low molecular weight targeting system and (2) high molecular weight targeting system.
2.1. Low molecular weight agents
Different types of amino acids like aspartic acid, methionine, glycine, cysteine, alanine, tyrosine and non-naturally occurring amino acids have been used for BNCT.[34], [35], [36], [37] The main advantage of this delivery system is relatively high delivery efficacy which can deliver high concentration of boron to the tumor without affecting toxicity. Closo-B10H102− and closo-B12H122− are the polyhedral borane dianions and closo-C2B10H12 and nido-C2B9H12− are the icosahedral carboranes which have attracted attention due to their high boron content, hydrophobic character, negative charge, and chemical and hydrolytic stability.38
DNA-binding agents and biochemical precursors are the other types of low molecular delivery agents. Different analogues of purines, pyrimidines, nucleosides, and nucleotides such as the 3-(dihydroxypropyl-carboranyl-pentyl) thymidine derivative N5-2OH, plasmid DNA, and h-5-o-carboranyl-2V-deoxyuridine have been studied in cellular and animal models which have shown tumor selectivity and low toxicities.[39], [40]
Martin et al. for the first time demonstrated that the irradiation of plasmid DNA/Gd3+ mixture via Coster–Kronig electrons could induce DNA double-strand breaks (DSB). They also showed that addition of EDTA to the mixture markedly reduced the DSB due to the sequestering of the Gd3+ from DNA.41
Polyamines, groove binders, alkylating agents and intercalators are DNA-binding molecules which have been applied as boron-containing delivery agents. Derivatives of tribenzimidazoles, aziridines, arboranylpolyamines, Pt (II)-amine complexes, acridines, phenanthridines, and trimethoxyindoles are some examples of this class of delivery system. There is some controversy in this class of delivery agents, although some derivatives of these components have shown low toxicity.[40], [42], [43], [44]
Phthalocyanine, phenanthridines, and acridines derivatives are boron-containing fluorescent dyes which have this ability to interact with DNA to be quantified and detected with fluorescence microscopy. In this group, boron-containing porphyrins are more interesting due to their remarkable stability, low systemic toxicity and easy synthesis with high boron content.[45], [46], [47]
Sodium mercaptoundecahydro-dodecaborate (Na210B12H11SH:BSH) and borono-phenylalanine (10BPA) are two BNCT delivery agents currently applied in clinical trials but, due to their low tumor-to-normal brain tissue and tumor-to-blood 10B ratios, these components are not universally attractive.[48], [49]
In another study, Miura et al. compared boron uptake of BPA and BSH with carboranylporphyrin CuTCPH in mice bearing implanted EMT-6 mammary carcinoma. The results showed that CuTCPH has a longer retention time in comparison with BPA and BSH which is important in the timing of clinical BNCT treatment. Moreover, they showed that CuTCPH can deliver high concentrations of boron to tumor.50
2.2. High molecular weight delivery agents
Monoclonal antibodies (mAbs) and nanomaterials are major delivery agents in this class. Various studies have used mAb cetuximab (IMC-C225) and epidermal growth factor (EGF) to target mutant isoform of EGF receptor (EGFR) which are overexpressed in different tumors containing squamous cell carcinomas of the head and neck gliomas.51
2.2.1. Nanotechnology and nanomaterials
Nanotechnology is the engineering and science of particles that are on a nanoscale. These particles are approximately 1–100 nanometers (nm) in size. Nanomaterials of this size show novel characteristics. Nanotechnology and nanomaterials have attracted attention because of their unique physico-chemical and optical characteristics in addition to their nanometric size.[32], [52]Nanomaterials could be the best targeting vectors for different cancers due to their multifunctionality which could be conjugated with various cancer specific ligands.53 Moreover, there are different types of nanomaterials with different composition, size, shape, and physico-chemical properties which can be selected for different cancers and NCT delivery strategies.
2.2.2. Boron nitride and carbon nanotubes
Carbon nanotubes (CNTs) are interesting allotropes of carbon comprised of pure graphite (a hexagonal lattice of carbon) which is rolled as cylindrical hollow fiber. Their outstanding properties such as superb mechanical properties, nanoscaled size, and cylindrical arrangement of carbon atoms, low mass density, excellent conductivity, and high thermal stability have attracted attention. The flexibility and stability of CNTs are two important parameters in delivery applications which provide long circulation time and bioavailability. In this regard, Yinghuai et al. used nitrene cycloaddition to attach C2B10 carborane cage to the side wall of single wall carbon nanotubes (SWCNTs). Their biodistribution studies showed that concentration of boron atoms in tumor is more than blood and other organs suggesting that this nanocomplex can be a good nanocarrier for boron in BNCT of cancers.54
Toxicity and water solubility are the main challenges of carbon nanotube. Boron nitride and Carbon nanotubes (BNCNTs) are isosteres of CNTs because boron-nitride is isoelectronic with carbon and can be used in BNCT as a carrier. Studies have shown that BNCNTs can be functionalized with different biological moieties and have no toxicity against HEK293 cells.55 Various studies have used quinuclidine bases, glycodendrimers, polyethyleneimine (PEI) and poly-l-lysine (PLL) to make the complex water soluble. Folic acid and transferrin are two active targeting agents which conjugated with BNCNTs against human glioblastoma multiforme (GBM) T98G cells and human endothelia cells, respectively.[56], [57] These active targeting agents made BNNTs selective against target cells and assisted in the accumulation of BNCNTs into the tumors.
2.2.3. Liposome
Liposomes are versatile, small spherical and nontoxic nanocarriers composed of cholesterol and natural non-toxic phospholipids.58 Liposomes have attracted attention as the boron delivery carrier due to their biocompatibility, amphipathic nature and nanoscale size. The surface of these nanocarriers have the ability to be modified with various functionality and targeting agents, such as Polyethylene glycol (PEG), for increasing blood circulation time,59 mAb or their fragments, Epidermal Growth Factors (EGF), folic acid and transferrin for active cancer targeting.[59], [60], [61]
In 1992, Moore and his colleagues proposed that liposomes have the ability to overcome the low solubility problems of 5-(3-(1,2-decaboronyl) propyl)-6-methyl-thiouracil, DBTU-l. They encapsulated DBTU in liposome particles and confirmed the presence of boron using electron energy loss spectroscopy. The biodistribution study showed that liposomes facilitate the delivery of the insoluble DBTU-l and DBTU-2 to the amelanotic MM4l8 tumor and the highly melanised harding passey tumor, respectively.62
In their study, Le and Cui encapsulated Gd-DTPA into the liposome via freeze–thaw and increased blood circulation time with pegylation. They also limited the leakage of liposome via conjugation of Gd-DTPA with poly-l-lysine.63
In another study, Peters’ group has prepared different liposomal formulations of gadolinium-DTPA via lipid/film-extrusion method for NCT treatment of glioblastoma. Their results show that the nanocomplex has toxicity effects on F98 and LN229 cells following the radiation.64
2.2.4. Dendrimer
Dendrimers are nano-sized, highly branched, radially symmetric star-shaped macromolecules with well-defined, monodisperse and homogeneous structures.65 Core molecule, repeat units and reactive surface groups are three main parts of a typical dendrimer. Low toxicity and multiple types of reactive terminal groups make dendrimers ideal platforms for imaging and delivery applications. Several studies have applied targeting agents conjugated with dendrimers for BNCT. Terminal functional groups of dendrimers are ideal sites for attachment of boron components.66 Barth et al. attached water soluble isocyanato polyhedral borane [Na(CH3)3NB10H8NCO] to second- and fourth generation PAMAM dendrimers which have 12 and 48 reactive terminal amino groups, respectively.[67], [68] In other studies, EGF, folic acid and VEGF were used for targeting of dendrimers.[69], [70], [71]
In their study Kobayashi et al. prepared dual imaging and therapeutic avidin–dentrimer–(1B4M-Gd)254 (Av-G6Gd) for interperitoneal disseminated tumors which can be tracked with MRI. Their in-vitro and in-vivo (intraperitoneal) studies show that the human ovarian cell line, SHIN-3 cells, can uptake this nanocomplex effectively.72
2.2.5. Magnetic nanoparticles
Targeted delivery of a therapeutic agent to tumor location is the main challenge of cancer therapy. Apart from using targeting molecules, external physical manipulation, magnetic control of the therapeutic complex can also be very effective. Magnetic manipulation is a versatile tool to direct therapeutic agents to the tumor location. In this regard, appropriate cargos are attached to a magnetic nanoparticle and after administration into the patients circulatory system, an external high gradient magnetic field is applied to manipulate and direct the nanocomplex. Magnetic drug delivery can be used in NCT treatment of cancers.
Zhu et al. attached 1-R-2-butyl-ortho-C2B10H10 to starch covered iron oxide magnetic nanoparticle and examined its biodistribution. Their results show that in the presence of a magnetic field, the boron concentration in the tumor tissue is 51.4 µg/g of tumor, while the tumor/normal tissue ratio is 10:1. This study opened hope for combination of magnetic targeted BNCT with MRI modality.73
2.2.6. Metallic nanoparticles
Metallic nanoparticles are an important class of nanoparticles which have many applications due to their outstanding properties, but their application as NCT delivery agents is limited due to their intrinsic difficulties of attaining a sufficient amount of boron, toxicity, and poor water solubility of the resulting complex. In one study, Ciani et al. tried to address these limitations. They attached B10C2 cage to poly (ethylene oxide)-b-poly (caprolactone) diblock-copolymer (PEO-b-PCL). Next, this complex was conjugated to SH bearing carboranes functionalized gold nanoparticles. Their in-vitro results show that the nanocomplex has excellent biocompatibility and the ability to concentrate rational amount of boron atoms in the target cell.74 In another study, Mandal et al. developed multifunctional gold nanoparticle for boron delivery and florescence imaging. They covered GNPs with BPA and Fluorescein Isothiocyanate (FITC) functionalized oppositely charged polyelectrolytes via a layer by layer method and then attached folic acid to the outer layer of polyelectrolytes as the targeting agent. Their results show significant uptake of the nanoconjugate by folic acid over expressing cancer cells.75
2.2.7. Polymeric nanoparticles
Different types of polymeric nanoplatforms have been used as NCT delivery agents. Shogo Sumitani et al. used radical copolymerization of acetalpoly (ethyleneglycol)-block-poly (lactide)-methacrylate with 4-vinylbenzyl substituted closo-carborane to prepare polymer-based boron-containing nanoparticles. Their results show that the nanoconjugates were stable in PBS and 10% FBS with negligible leakage in a 50 h period and have a long circulation time.76
Chitosan is a natural linear polymer composed of D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) with β-(1–4) bonds. Various studies have used chitosan nanoparticles as the NCT delivery agent. Saha et al. developed Gd-DTPA-doped chitosan microparticles for NCT applications. Their in-vitro studies confirmed stable gamma-ray emission from this delivery agent.77 In another study, they improved Gd loading up to 45% via the emulsion-droplet coalescence technique and nearly 100% deacetylation of chitosan.78
Ichikawa et al. investigated the influence of Gd content, particle size and surface charge of Gd-loaded chitosan nanoparticles on their tumor-killing efficacy. Their results showed that the efficacy was related to the micrometric properties of the nanocomplex and smaller nanoparticles had higher tumor growth inhibition efficacy than larger ones because of the greater Gd retention in the tumor tissue.79
2.2.8. Sulfur containing components
Various studies have confirmed the efficacy of 33S either alone or in combination with boron in NCT cancer treatment.[24], [25] Different types of components can be used as sulfur delivery agents. MoS2 and WS2 nanoparticles are attractive candidates for high local concentration delivery of 33S to tumors. These nanostructures have this ability to concentrate about 7000 33S atoms in a three-layer octahedral of 5-nm size. More interestingly, WS2 nanoparticle is biocompatible and with size of 5 nm can be concentrated up to 7 mg/g in tumors.80 Protein based nanocarriers are other candidates for sulfur delivery. Excellent biodistribution properties and targeting ability make apoferritin an ideal carrier of 33S.81 In addition, sulfur containing proteins such as metallothioneins have the ability to concentrate 33S 10 times more than required for BNCT.82
3. Neutron sources for NCT
There are three neutron sources for NCT including fission reactor, accelerator, and californium-252 (252Cf).83
The most powerful neutron sources for NCT are nuclear reactors.22 In fission reactors, two methods are applicable to the design of epithermal neutron irradiation equipment. The first way is to use neutrons directly from the core as the source, which is the dominant way for conversion or modification of available reactors for NCT. The second way is related to using a fission converter; as it changes reactor's thermal neutrons to greater energy fission neutrons. Finally, to prepare epithermal neutrons, the beams resulting from fission are moderated and filtered.84
Another neutron source is radiofrequency quadrapole (RFQ) accelerator. The accelerators used as a neutron source in NCT range from electrostatic machines of low energy to cyclotrons of higher energy. In addition, there are much higher energy synchrotrons or linacs for this purpose.85 Recently, a cyclotron with 30 MeV proton energy was mounted at Kyoto University, which generates very high energy neutrons of up to 28 MeV.86 There is also an RFQ-DTL being installed at Ibaraki prefecture, near Tsukuba, intended to work with an 8 MeV proton beam, producing neutrons of up to 6 MeV. This source is considered as the most promising method for neutron generation.83 In other words, an accelerator would be considered as a beneficial neutron source for NCT in a hospital for following reasons: (1) in terms of public acceptance, accelerators are better than reactors; (2) it mainly involves less complexity with regards to accountability, licensing and disposal of nuclear fuel; (3) it has the ability to turn on and shut down; (4) the generated neutrons usually need less modification than those from a nuclear reactor (expect for cyclotrons of higher energy); (5) it could be cheaper and more compact than a comparable reactor.[83], [84] However, the accelerator generates neutron fluxes with low intensity in comparison with the nuclear reactors and this is a disadvantage for implementation. So, an increment in intensity would be essential if the accelerators are to be used for clinical NCT.[83], [84] Furthermore, the latter machines generate neutrons with very high energies and it should be moderated severely to use in NCT as a neutron source. A matter of concern related to these high-energy neutron is activation of surrounding materials.85 To date, most clinical irradiations have applied fission reactors as neutron sources, however, accelerators as a source of neutrons are under development and have been used less frequently.
In addition to fission reactors and accelerators, 252Cf is a neutron source that has been used in clinical trials as a brachytherapy source since the early 1970s. It is a man-made nuclide which emits at a neutron rate of 2.31 × 1012 neutrons/s per g.87 252Cf spontaneously emits a fission spectrum of neutrons with an average energy of 2.35 MeV and a yield of 3.76 neutrons per fission.88 The dose rate obtainable based on a very large (1.0 g) source of 252Cf is 4.1 RBE cGy/ min.89 Furthermore, gamma rays with a mean energy of 1 MeV are emitted.88 A way to diminish the total gamma contamination as well as to have an adequately high epithermal neutron flux is using various photon filters of different thickness.90 252Cf source has a very compact NCT facility and it also has better public acceptance than fission reactors due to its intrinsic criticality safety. However, due to the 2.6 year half-life of the isotope, it requires frequent substitution of the expensive 252Cf and also a large amount of 252Cf is needed (in the order of 1 g). In addition, neutron filtration and moderation would be required.83 Additionally, related to the dose rate of 252Cf, it is two to five times lower than those obtained by existing fission reactors and is a drawback of using 252Cf as a neutron source. However, radioisotope sources have the advantage of in-hospital installation.89 It should be noted that the use of 252Cf as an NCT source is mostly for statistical simulation studies rather than clinical trials.
4. Physical dosimetry of NCT
The total dose obtained from the sum of the dose compounds from gamma rays, protons, neutrons, and production of capture.10 It is notable that the capture agent determines the production type of capture. In addition, the dose received to the tumor volume is a compromise amongst the neutron fluence, the cross-section of the isotopes used in the various nuclear procedures, and the concentration of capturer agent in the tumor.27
In BNCT and GdNCT, the total dose includes four distinguished radiation components, each of them having various biological weighting factors. As a result, it is essential to quantify each of these compounds separately, so that significant biologically weighted doses can be calculated for various tumor and normal tissues. It should be noted that three out of four aforementioned components are common between BNCT and GdNCT. The three components are[19], [83]:
-
1)
The neutron dose: according to 1H (n,n) P reactions, fast and epithermal neutrons cause elastic neutron collisions with hydrogen in tissue.
-
2)
The gamma dose: the dose arising from gamma rays induced in the tissue itself, i.e. according to 1H (n.γ) 2H reactions, hydrogen in tissue absorbs thermal neutrons and emits gamma rays with 2.2 MeV energy. In addition, the gamma dose is because of gamma-rays accompanying the neutron beam.
-
3)
The proton dose from nitrogen-14 (14N) capture: given that 14N (n,p) 14C reaction, 14N element in tissue captures a thermal neutron and as a result, a proton is emitted. Dose is obtained from locally delivered energy from the recoiling 14C nucleus and the energetic proton.
The fourth dose component in BNCT is the dose arising from the 10B nuclear reactions: 10B captures a thermal neutron and, as a result, an alpha particle and recoiling 7Li ion are emitted, each of these particles has high LET with mean energy of about 2.31 MeV. As scheme 1 demonstrates, most of the time (93.7%), the recoiling 7Li ion is generated in an excited state and is de-excited by emitting a 0.477 MeV gamma photon. In the 6.3% reactions, the 7Li is transited to the stable state without gamma photon emission, in other words, the emitted gamma photons can be ignored from a dosimetry viewpoint because they are approximately two orders of amplitude less frequent and approximately half the energy of the 2.2 MeV gamma photons is due to the hydrogen capture, although they are mostly applied for 10B analysis purposes.[8], [83] Furthermore, the fourth dose component in GdNCT is natural gadolinium dose. 157Gd and 155Gd have the highest cross-sections to capture with thermal neutrons (cross-sections of neutron capture of 255,000 and 60,800 barns, respectively), so these two isotopes are considered as gadolinium dose (80% 57Gd and 20% 155Gd) and for the other isotopes, the nuclear reaction cross-sections are negligibly small for dose calculations.19
In SNCT, the total dose is obtained from the following dose compounds: sulfur, neutrons, primary and secondary gamma photons. Following the 33S (n,α) 30Si interaction, the emitted alpha particles have an average energy of 3.1 MeV with LET of 126 keV/μm and range of 15 μm.[24], [26], [27]
In addition to the items mentioned above, related to NCT dosimetry, there are several studies that have focused on methods of NCT dosimetry, for example the use of MOSFET, silicon, etc.[91], [92], [93]
5. Clinical studies of NCT for various cancers
To the best of our knowledge, the SNCT method has only been investigated in the field of statistical simulation. On the other hand, the GdNCT method has been evaluated in the field of in-vivo and in-vitro trials, so in this section, clinical studies related to BNCT methods are reviewed. Furthermore, there are numerous preclinical studies related to BNCT. For more details about these studies, please refer to them directly.[94], [95], [96], [97]
BNCT clinical trials have been carried out in the United States,98 Finland,99 Japan,100 Taiwan,101 Argentina,102 Netherland,103 Italy,104 Germany,105 Sweden106 and the Czech Republic.107 This treatment modality has been applied for the treatment of brain (glioblastoma multiforme, malignant glioma, and malignant meningioma), liver, head and neck, lung, colon, melanoma, thyroid, hepatic, gastrointestinal cancer, and extra-mammary Paget's disease.108
The first BNCT clinical trial was carried out on ten patients with terminal GBM brain tumor between the years 1951 and 1953. In this study, Na2B4O7 which was considered as a relatively nontoxic agent was applied as a BNCT drug. It is observed that all the ten patients died because of tumor recurrence without increase of their survival times.[109], [110] In addition to this study, three other studies (up to 1961) were conducted on patients with GBM tumor. Simple inorganic boron compounds were used in these studies. For many patients, drastic side effects like untreatable skin burns of the scalp and deep ulcerations were presented. The results of the four studies were similar to the consequences of conventional radiation therapy. Therefore, it was concluded that these drugs were not sufficiently tumor-specific and the thermal neutrons did not have deep penetration characteristics.22 The disappointments from the first pioneering BNCT studies in patients which were conducted from 1951 to 1961, resulted in the termination of BNCT clinical trials in the United States. Then all BNCT studies in the United States focused on the development and optimization of BNCT agents via in vivo and in vitro studies. Further searching for boron carriers resulted in the development of Na2[B12H11SH] or BSH.31 After the United States, in 1968 and in Japan, clinical trials were performed on patients with grade 3 and 4 GBM. All patients were administrated BSH and corticosteroids to manage adverse effects of the treatment. The results demonstrated considerable improvement with an average survival of 44 months and a median of 26 months.[111], [112], [113] In 1987 in Japan, Mishima et al.114 began to treat superficial melanoma utilizing BPA. Their study was a significant step towards the use of BNCT in treatment of other tumor kinds far from the central nervous system.[108], [114] As it was mentioned in the various delivery systems for NCT section, both BSH and BPA are still being used as carriers only in clinical trials for BNCT. Furthermore, many of these BNCT programs have been stopped due to high cost of nuclear reactors as well as their absence in the hospitals.108
Given the many clinical studies in the field of BNCT, a number of these studies are presented in Table 2. For more details about these studies, please refer to them directly.
Table 2.
Several clinical studies in the field of BNCT.
| Tumor type | Country | Treatment date | Carrier type | Ref. |
|---|---|---|---|---|
| GBM | Finland | 2008 | BPA | 115 |
| Head and neck | Finland | 2011 | BPA | 116 |
| Glioblastoma | Japan | 2009 | BPA and BSH | [117], [118] |
| Head and neck | Japan | 2014 | BPA | 119 |
| Malignant glioma | Japan | 2009 | BPA | [120], [121] |
| Malignant meningioma | Japan | 2012 | BPA | 122 |
| Head and neck | Taiwan | 2010–2011 | BPA | 123 |
| Hepatic | Japan | 2014 | BSH | 124 |
| Lung | Japan | 2012 | – | 125 |
| Extra-mammary Paget's disease | Japan | 2012 | – | 126 |
| GBM | USA | 1994–1999 | BPA | [86], [98], [127], [128] |
| GBM | Sweden | 2001–2003 | BPA | [106], [129], [130], [131] |
| GBM | Germany | 2004–2006 | BPA | 132 |
| GBM | Czech Republic | 2000–2002 | BSH | 133 |
6. Conclusion
NCT strives to improve the quality of life in patients by reducing the number of treatment fractionation sessions compared to standard radiotherapy and chemotherapy courses.
Among various agent types for NCT, only boron is used in clinical trials for treatment of various tumor types such glioblastoma multiforme, malignant glioma, malignant meningioma, melanoma, liver, head and neck, extra-mammary Paget's disease, etc.
With the recent advances in delivery systems, more effective treatment for neutron capture therapy has been provided, nevertheless in BNCT the only two delivery agents which are presently applied in clinical trials are BSH and BPA. Delivery systems based on nanotechnology can be considered as promising tools to improve the efficacy of NCT. Different kinds of nanoplatforms such as liposomes, dendrimers, boron nitride and carbon nanotubes, magnetic, metallic, and polymeric nanoparticles are being used in NCT as delivery agents.
The 157Gd is a dual purpose agent which can be used both for therapeutics and in imaging purposes. Additionally, 33S, as a new agent in NCT, can be effective due to having no background dose in soft tissue from interaction of neutrons with 33S.
It is notable that the use of accelerated-based neutron sources may increase the application of NCT for cancer treatment.
Funding
None declared.
Conflict of interests
None declared.
Acknowledgement
There is not a need for acknowledgement section in this study.
References
- 1.Taylor H. The disintegration of boron by neutrons. Proc Phys Soc. 1935;47:873–876. [Google Scholar]
- 2.Allen B. Epithermal neutron capture therapy: a new modality for the treatment of glioblastoma and melanoma metastatic to the brain. Med J Aust. 1995;153:296–298. doi: 10.5694/j.1326-5377.1990.tb136906.x. [DOI] [PubMed] [Google Scholar]
- 3.Barth R.F., Coderre J.A., Vicente M.G.H., Blue T.E. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 4.Farhood B., Ghorbani M. Effect of diameter of nanoparticles and capture cross-section library on macroscopic dose enhancement in boron neutron capture therapy. J Contemp Brachyther. 2014;6:377–385. doi: 10.5114/jcb.2014.48031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosroabadi M., Farhood B., Ghorbani M., Hamzian N., Moghaddam H.R., Davenport D. Tissue composition effect on dose distribution in neutron brachytherapy/neutron capture therapy. Rep Pract Oncol Radiother. 2016;21:8–16. doi: 10.1016/j.rpor.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckurts K.H., Wirtz K. Springer-Verlag; New York: 1964. Neutron physics; p. 14. [Google Scholar]
- 7.Capoulat M.E., Minsky D.M., Kreiner A.J. Computational assessment of deep-seated tumor treatment capability of the 9Be(d, n)10B reaction for accelerator-based Boron Neutron Capture Therapy (AB-BNCT) Phys Med. 2014;30:133–146. doi: 10.1016/j.ejmp.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Uusi-Simola J., Savolainen S., Kangasmäki A. Study of the relative dose-response of BANG-3® polymer gel dosimeters in epithermal neutron irradiation. Phys Med Biol. 2003;48:2895. doi: 10.1088/0031-9155/48/17/310. [DOI] [PubMed] [Google Scholar]
- 9.Cerullo N., Esposito J., Daquino G. Spectrum shaping assessment of accelerator-based fusion neutron sources to be used in BNCT treatment. Nucl Instrum Methods Phys Res B. 2004;213:641–645. [Google Scholar]
- 10.Khosroabadi M., Ghorbani M., Rahmani F., Knaup C. Neutron capture therapy: a comparison between dose enhancement of various agents, nanoparticles and chemotherapy drugs. Aust Phys Eng Sci Med. 2014;37:541–549. doi: 10.1007/s13246-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 11.Blagojevic N., Storr G., Allen B., Hatanaka H., Nakagawa H. Role of heavy water in boron neutron capture therapy. Top Dosimetry Treatm Plan Neutron Capture Therapy. 1994:125–134. [Google Scholar]
- 12.Wallace S., Mathur J., Allen B. The influence of heavy water on boron requirements for neutron capture therapy. Med phys. 1995;22:585–590. doi: 10.1118/1.597585. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda H., Honda C., Wadabayashi N. Pharmacokinetics of 10B-p-boronophenylalanine in tumours, skin and blood of melanoma patients: a study of boron neutron capture therapy for malignant melanoma. Melanoma Res. 1999;9:75–83. doi: 10.1097/00008390-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Diaz A.Z., Coderre J.A., Chanana A.D., Ma R. Boron neutron capture therapy for malignant gliomas. Ann Med. 2000;32:81–85. doi: 10.3109/07853890008995913. [DOI] [PubMed] [Google Scholar]
- 15.Kato I., Ono K., Sakurai Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004;61:1069–1073. doi: 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 16.Rosannanano S.B.A., Patriziachiari T.P., Francesca F.A. Efficacy of boron neutron capture therapy on liver metastases of colon adenocarcinoma: optical and ultrastructural study in the rat. Oncol Rep. 2004;11:149–153. [PubMed] [Google Scholar]
- 17.Dagrosa M.A., Crivello M., Perona M. First evaluation of the biologic effectiveness factors of boron neutron capture therapy (BNCT) in a human colon carcinoma cell line. Int J Radiat Oncol Biol Phys. 2011;79:262–268. doi: 10.1016/j.ijrobp.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H., Kirihata M. Neutron Capture Therapy. Springer; 2012. Boron compounds: new candidates for boron carriers in BNCT; pp. 99–116. [Google Scholar]
- 19.Abdullaeva G., Djuraeva G., Kim A. Evaluation of absorbed dose in Gadolinium neutron capture therapy. Open Phys. 2015;13:183–187. [Google Scholar]
- 20.Narmani A., Farhood B., Haghi-Aminjan H. Gadolinium nanoparticles as diagnostic and therapeutic agents: their delivery systems in magnetic resonance imaging and neutron capture therapy. J Drug Deliv Sci Technol. 2018;44:457–466. [Google Scholar]
- 21.Pennington E.M., Gajniak J.C. 1964. Compilation of ENDF/B data for magnesium, titanium, anadium, molybdenum and gadolinium. Available from: http://cds.cern.ch/record/1102998?ln=en [accessed 30.04.17] [Google Scholar]
- 22.Salt C., Lennox A.J., Takagaki M., Maguire J.A., Hosmane N.Sn. Boron and gadolinium neutron capture therapy. Russ Chem Bull. 2004;53:1871–1888. [Google Scholar]
- 23.Cerullo N., Bufalino D., Daquino G. Progress in the use of gadolinium for NCT. Appl Radiat Isot. 2009;67:157–160. doi: 10.1016/j.apradiso.2009.03.109. [DOI] [PubMed] [Google Scholar]
- 24.Porras I. Enhancement of neutron radiation dose by the addition of sulphur-33 atoms. Phys Med Biol. 2008;53:1–9. doi: 10.1088/0031-9155/53/7/L01. [DOI] [PubMed] [Google Scholar]
- 25.Porras I. Sulfur-33 nanoparticles: a Monte Carlo study of their potential as neutron capturers for enhancing boron neutron capture therapy of cancer. Appl Radiat Isot. 2011;69:1838–1841. doi: 10.1016/j.apradiso.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Porras I., Sabaté-Gilarte M., Praena J., Quesada J., Esquinas P. 33 S for Neutron Capture Therapy: nuclear data for Monte Carlo calculations. Nucl Data Sheets. 2014;120:246–249. [Google Scholar]
- 27.Praena J., Sabaté-Gilarte M., Porras I., Esquinas P., Quesada J., Mastinu P. 33 S as a cooperative capturer for BNCT. Appl Radiat Isot. 2014;88:203–205. doi: 10.1016/j.apradiso.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 28.Mishima Y. Springer Science & Business Media; 2013. Cancer neutron capture therapy. [Google Scholar]
- 29.Godwin J.T., Farr L.E., Sweet W.H., Robertson J.S. Pathological study of eight patients with glioblastoma multiforme treated by neutron capture therapy using boron 10. Cancer. 1955;8:601–615. doi: 10.1002/1097-0142(1955)8:3<601::aid-cncr2820080326>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Snyder H., Reedy A.J., Lennarz W.J. Synthesis of aromatic boronic acids. aldehydo boronic acids and a boronic acid analog of tyrosine1. J Am Chem Soc. 1958;80:835–838. [Google Scholar]
- 31.Soloway A., Hatanaka H., Davis M. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. J Med Chem. 1967;10:714–717. doi: 10.1021/jm00316a042. [DOI] [PubMed] [Google Scholar]
- 32.Samadian H., Hosseini-Nami S., Kamrava S.K., Ghaznavi H., Shakeri-Zadeh A. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J Cancer Res Clin Oncol. 2016;142:1–13. doi: 10.1007/s00432-016-2179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicha I. Strategies to enhance nanoparticle-endothelial interactions under flow. J Cell Biotechnol. 2016;1:191–208. [Google Scholar]
- 34.Srivastava R.R., Singhaus R.R., Kabalka G.W. 4-Dihydroxyborylphenyl analogues of 1-aminocyclobutanecarboxylic acids: potential boron neutron capture therapy agents. J Org Chem. 1999;64:8495–8500. [Google Scholar]
- 35.Díaz S., González A., de Riancho S.G., Rodríguez A. Boron complexes of S-trityl-L-cysteine and S-tritylglutathione. J Organ Chem. 2000;610:25–30. [Google Scholar]
- 36.Das B.C., Das S., Li G., Bao W., Kabalka G.W. Synthesis of a water soluble carborane containing amino acid as a potential therapeutic agent. Synlett. 2001;2001:1419–1420. [Google Scholar]
- 37.Kabalka G.W., Yao M.L. Synthesis of a novel boronated 1‐aminocyclobutanecarboxylic acid as a potential boron neutron capture therapy agent. Appl Organ Chem. 2003;17:398–402. [Google Scholar]
- 38.Hawthorne M.F., Lee M.W. A critical assessment of boron target compounds for boron neutron capture therapy. J Neuroncol. 2003;62:33–45. doi: 10.1007/BF02699932. [DOI] [PubMed] [Google Scholar]
- 39.Al-Madhoun A.S., Johnsamuel J., Barth R.F., Tjarks W., Eriksson S. Evaluation of human thymidine kinase 1 substrates as new candidates for boron neutron capture therapy. Cancer Res. 2004;64:6280–6286. doi: 10.1158/0008-5472.CAN-04-0197. [DOI] [PubMed] [Google Scholar]
- 40.Barth R.F., Yang W., Al-Madhoun A.S. Boron-containing nucleosides as potential delivery agents for neutron capture therapy of brain tumors. Cancer Res. 2004;64:6287–6295. doi: 10.1158/0008-5472.CAN-04-0437. [DOI] [PubMed] [Google Scholar]
- 41.Martin R.F., D’Cunha G., Pardee M., Allen B.J. Induction of double-strand breaks following neutron capture by DNA-bound 157Gd. Int J Radiat Biol. 1988;54:205–208. doi: 10.1080/09553008814551641. [DOI] [PubMed] [Google Scholar]
- 42.Cai J., Soloway A.H., Barth R.F. Boron-containing polyamines as DNA targeting agents for neutron capture therapy of brain tumors: Synthesis and biological evaluation. J Med Chem. 1997;40:3887–3896. doi: 10.1021/jm960787x. [DOI] [PubMed] [Google Scholar]
- 43.Woodhouse S.L., Rendina L.M. Synthesis and DNA-binding properties of dinuclear platinum (II)–amine complexes of 1, 7-dicarba-closo-dodecaborane (12) Chem Commun. 2001;23:2464–2465. doi: 10.1039/b108081d. [DOI] [PubMed] [Google Scholar]
- 44.Tietze L.F., Griesbach U., Bothe U., Nakamura H., Yamamoto Y. Novel carboranes with a DNA binding unit for the treatment of cancer by boron neutron capture therapy. ChemBioChem. 2002;3:219–225. doi: 10.1002/1439-7633(20020301)3:2/3<219::aid-cbic219>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Bregadze V.I., Sivaev I.B., Gabel D., Wöhrle D. Polyhedral boron derivatives of porphyrins and phthalocyanines. J Porphyr Phthalocyanines. 2001;5:767–781. [Google Scholar]
- 46.Vicente M. Porphyrin-based sensitizers in the detection and treatment of cancer: recent progress. Curr Med Chem Anticancer Agents. 2001;1:175–194. doi: 10.2174/1568011013354769. [DOI] [PubMed] [Google Scholar]
- 47.Evstigneeva R.P., Zaitsev A.V., Luzgina V.N., Ol'shevskaya V.A., Shtil A.A. Carboranylporphyrins for boron neutron capture therapy of cancer. Curr Med Chem Anticancer Agents. 2003;3:383–392. doi: 10.2174/1568011033482260. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda H., Hiratsuka J., Honda C. Boron neutron capture therapy of malignant melanoma using with special reference to evaluation of radiation dose and damage to the normal skin. Radiat Res. 1994;138:435–442. [PubMed] [Google Scholar]
- 49.Van Rij C.M., Wilhelm A.J., Sauerwein W.A., van Loenen A.C. Boron neutron capture therapy for glioblastoma multiforme. Pharm World Sci. 2005;27:92–95. doi: 10.1007/s11096-004-2850-7. [DOI] [PubMed] [Google Scholar]
- 50.Miura M., Morris G.M., Micca P.L. Boron neutron capture therapy of a murine mammary carcinoma using a lipophilic carboranyltetraphenylporphyrin 1. Radiat Res. 2001;155:603–610. doi: 10.1667/0033-7587(2001)155[0603:bnctoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Ang K.K., Berkey B.A., Tu X. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;26:7350–7356. [PubMed] [Google Scholar]
- 52.Afshar M.A.A., Moloudi K., Amirrashedi M.R., Ranjbar H.S. A brief review on polymer and protein based nanotheranostics. Int J Biol Pharm Allied Sci. 2015;5:112–128. [Google Scholar]
- 53.Khoshnevisan K., Daneshpour M., Barkhi M., Gholami M., Samadian H., Maleki H. The promising potentials of capped gold nanoparticles for drug delivery systems. J Drug Targ. 2017;12:1–8. doi: 10.1080/1061186X.2017.1387790. [DOI] [PubMed] [Google Scholar]
- 54.Yinghuai Z., Peng A.T., Carpenter K., Maguire J.A., Hosmane N.S., Takagaki M. Substituted carborane-appended water-soluble single-wall carbon nanotubes: new approach to boron neutron capture therapy drug delivery. J Am Chem Soc. 2005;127:9875–9880. doi: 10.1021/ja0517116. [DOI] [PubMed] [Google Scholar]
- 55.Chen X., Wu P., Rousseas M. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J Am Chem Soc. 2009;131:890–891. doi: 10.1021/ja807334b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciofani G., Raffa V., Menciassi A., Cuschieri A. Folate functionalized boron nitride nanotubes and their selective uptake by glioblastoma multiforme cells: implications for their use as boron carriers in clinical boron neutron capture therapy. Nanoscale Res Lett. 2008;4:113–121. doi: 10.1007/s11671-008-9210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciofani G., Del Turco S., Genchi G.G., D’Alessandro D., Basta G., Mattoli V. Transferrin-conjugated boron nitride nanotubes: protein grafting, characterization, and interaction with human endothelial cells. Int J Pharm. 2012;436:444–453. doi: 10.1016/j.ijpharm.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 58.Malam Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Wu G., Barth R.F., Yang W. Boron containing macromolecules and nanovehicles as delivery agents for Neutron Capture Therapy. Anticancer Agents Med Chem. 2006;6:167–184. doi: 10.2174/187152006776119153. [DOI] [PubMed] [Google Scholar]
- 60.Bohl Kullberg E., Bergstrand N., Carlsson J. Development of EGF-conjugated liposomes for targeted delivery of boronated DNA-binding agents. Bioconjug Chem. 2002;13:737–743. doi: 10.1021/bc0100713. [DOI] [PubMed] [Google Scholar]
- 61.Maruyama K., Ishida O., Kasaoka S. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT) J Control Release. 2004;98:195–207. doi: 10.1016/j.jconrel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Moore D.E., Chandler A.K., Corderoy-Buck S., Wilson J.G., Allen B.J. Progress in neutron capture therapy for cancer. Springer; 1992. Liposomes as carriers of boronated thiouracils for NCT of melanoma; pp. 451–454. [Google Scholar]
- 63.Le U.M., Cui Z. Long-circulating gadolinium-encapsulated liposomes for potential application in tumor neutron capture therapy. Int J Pharm. 2006;312:105–112. doi: 10.1016/j.ijpharm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Peters T., Grunewald C., Blaickner M. Cellular uptake and in vitro antitumor efficacy of composite liposomes for neutron capture therapy. Radiat Oncol. 2015;10:52. doi: 10.1186/s13014-015-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbasi E., Aval S.F., Akbarzadeh A. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9:1–10. doi: 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vivek K., Thakur N., Abhilash C., Nitan B., Neeraj B. Dendrimer: a review. World J Pharm Pharm Sci. 2016;5:526–536. [Google Scholar]
- 67.Barth R.F., Adams D.M., Soloway A.H., Alam F., Darby M.V. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: evaluation as a potential delivery system for neutron capture therapy. Bioconjug Chem. 1994;5:58–66. doi: 10.1021/bc00025a008. [DOI] [PubMed] [Google Scholar]
- 68.Liu L., Barth R., Adams D., Soloway A., Reisfeld R. Critical evaluation of bispecific antibodies as targeting agents for boron neutron capture therapy of brain tumors. Anticancer Res. 1995;16:2581–2587. [PubMed] [Google Scholar]
- 69.Capala J., Barth R.F., Bendayan M. Boronated epidermal growth factor as a potential targeting agent for boron neutron capture therapy of brain tumors. Bioconjug Chem. 1996;7:7–15. doi: 10.1021/bc950077q. [DOI] [PubMed] [Google Scholar]
- 70.Shukla S., Wu G., Chatterjee M. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug Chem. 2003;14:158–167. doi: 10.1021/bc025586o. [DOI] [PubMed] [Google Scholar]
- 71.Backer M.V., Gaynutdinov T.I., Patel V. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4:1423–1429. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 72.Kobayashi H., Kawamoto S., Saga T. Avidin-dendrimer-(1B4M-Gd) 254: a tumor-targeting therapeutic agent for gadolinium neutron capture therapy of intraperitoneal disseminated tumor which can be monitored by MRI. Bioconjug Chem. 2001;12:587–593. doi: 10.1021/bc010002o. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Y., Lin Y., Zhu Y.Z., Lu J., Maguire J.A., Hosmane N.S. Boron drug delivery via encapsulated magnetic nanocomposites: a new approach for BNCT in cancer treatment. J Nanomater. 2010:24. [Google Scholar]
- 74.Ciani L., Bortolussi S., Postuma I. Rational design of gold nanoparticles functionalized with carboranes for application in Boron Neutron Capture Therapy. Int J Pharm. 2013;458:340–346. doi: 10.1016/j.ijpharm.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Mandal S., Bakeine G.J., Krol S. Design, development and characterization of multi-functionalized gold nanoparticles for biodetection and targeted boron delivery in BNCT applications. Appl Radiati Isot. 2011;69:1692–1697. doi: 10.1016/j.apradiso.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Sumitani S., Oishi M., Nagasaki Y. Carborane confined nanoparticles for boron neutron capture therapy: improved stability, blood circulation time and tumor accumulation. React Funct Polym. 2011;71:684–693. [Google Scholar]
- 77.Saha T.K., Ichikawa H., Fukumori Y. Gadolinium diethylenetriaminopentaacetic acid-loaded chitosan microspheres for gadolinium neutron-capture therapy. Carbohydr Res. 2006;341:2835–2841. doi: 10.1016/j.carres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Tokumitsu H., Ichikawa H., Fukumori Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res. 1999;16:1830–1835. doi: 10.1023/a:1018995124527. [DOI] [PubMed] [Google Scholar]
- 79.Ichikawa H., Uneme T., Andoh T. Gadolinium-loaded chitosan nanoparticles for neutron-capture therapy: influence of micrometric properties of the nanoparticles on tumor-killing effect. Appl Radiat Isot. 2014;88:109–113. doi: 10.1016/j.apradiso.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Tenne R. Inorganic nanotubes and fullerene-like nanoparticles. J Mater Res. 2006;21:2726–2743. doi: 10.1038/nnano.2006.62. [DOI] [PubMed] [Google Scholar]
- 81.Hennequin B., Turyanska L., Ben T. Aqueous Near‐Infrared Fluorescent Composites Based on Apoferritin‐Encapsulated PbS Quantum Dots. Adv Mater. 2008;20:3592–3596. [Google Scholar]
- 82.Coyle P., Philcox J., Carey L., Rofe A. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.International Atomic Energy Agency . 2001. Current status of neutron capture therapy. TECDOC-1223. [Google Scholar]
- 84.Barth R.F., Vicente M.H., Harling O.K. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kreiner A.J., Bergueiro J., Cartelli D. Present status of accelerator-based BNCT. Rep Pract Oncol Radiother. 2016;21:95–101. doi: 10.1016/j.rpor.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka H., Sakurai Y., Suzuki M. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS) Appl Radiat Isot. 2011;69:1642–1645. doi: 10.1016/j.apradiso.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 87.Wierzbicki J., Maruyama Y., Porter A. Measurement of augmentation of 252Cf implant by 10B and 157Gd neutron capture. Med Phys. 1994;21:787–790. doi: 10.1118/1.597324. [DOI] [PubMed] [Google Scholar]
- 88.Beach J.L., Schroy C.B., Ashtari M., Harris M.R., Maruyama Y. Boron neutron capture enhancement of 252 Cf brachytherapy. Int J Radiat Oncol Biol Phys. 1990;18:1421–1427. doi: 10.1016/0360-3016(90)90317-d. [DOI] [PubMed] [Google Scholar]
- 89.Yanch J., Kim J., Wilson M. Design of a californium-based epithermal neutron beam for neutron capture therapy. Phys Med Biol. 1993;38:1145–1155. doi: 10.1088/0031-9155/38/8/013. [DOI] [PubMed] [Google Scholar]
- 90.Ghassoun J., Merzouki A., El Morabiti A., Jehouani A. On the 252 Cf primary and secondary gamma rays and epithermal neutron flux for BNCT. Nucl Instrum Methods Phys Res B. 2007;263:231–233. [Google Scholar]
- 91.Carolan M., Wallace S., Allen B.J. Cancer neutron capture therapy. Springer; 1996. Validation of Monte Carlo dose planning calculations by epithermal beam dose distribution measurements in phantoms; pp. 303–310. [Google Scholar]
- 92.Carolan M., Rosenfeld A., Mathur J., Allen B.J. Advances in neutron capture therapy. Elsevier; 1997. Characterisation and use of MOSFET gamma dosimeters and silicon PIN diode neutron dosimeters for epithermal neutron beam dosimetry; pp. 192–197. [Google Scholar]
- 93.Bradley P.D., Rosenfeld A.B., Allen B.J., Coderre J., Capala J. Performance of silicon microdosimetry detectors in boron neutron capture therapy. Radiat Res. 1999;151:235–243. [PubMed] [Google Scholar]
- 94.Parsons P.G., Allen B.J. In vitro uptake, incorporation and toxicity of chlorpromazine and thiouracil by human melanoma cells. Aust J Exp Biol Med Science. 1986;64:517–526. doi: 10.1038/icb.1986.56. [DOI] [PubMed] [Google Scholar]
- 95.Corderoy-Buck S., Allen B.J., Wilson J. Advances in radiopharmacology: proc six int symposium radiopharmacology. 1990. Investigation of boron conjugated thiouracil derivates for neutron capture therapy of melanoma. [Google Scholar]
- 96.Prashar J.K., Moore D.E., Wilson J.G., Allen B.J. Advances in neutron capture therapy. Springer; 1993. Synthesis of carboranylphenylalanine for potential use in neutron capture therapy; pp. 265–268. [Google Scholar]
- 97.Setiawan Y., Smith M.D., Moore D.E., Allen B.J. Cancer neutron capture therapy. Springer; 1996. Pharmacokinetics and effects of neutron capture on melanoma xenografts after administration of boronated low density lipoprotein; pp. 599–604. [Google Scholar]
- 98.Chanana A.D., Capala J., Chadha M. Boron neutron capture therapy for glioblastoma multiforme: interim results from the phase I/II dose-escalation studies. Neurosurgery. 1999;44:1182–1192. doi: 10.1097/00006123-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Joensuu H., Kankaanranta L., Seppälä T. Boron neutron capture therapy of brain tumors: clinical trials at the Finnish facility using boronophenylalanine. J Neuro-Oncol. 2003;62:123–134. doi: 10.1007/BF02699939. [DOI] [PubMed] [Google Scholar]
- 100.Ono K., Ueda S., Oda Y. Boron neutron capture therapy for malignant glioma at Kyoto University Reactor. Adv Neutron Capture Ther. 1997;1:39–45. [Google Scholar]
- 101.Liu Y.-W., Huang T., Jiang S., Liu H. Renovation of epithermal neutron beam for BNCT at THOR. Appl Radiat Isotop. 2004;61:1039–1043. doi: 10.1016/j.apradiso.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 102.González S., Bonomi M., Santa Cruz G. First BNCT treatment of a skin melanoma in Argentina: dosimetric analysis and clinical outcome. Appl Radiat Isotop. 2004;61:1101–1105. doi: 10.1016/j.apradiso.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 103.Sauerwein W., Zurlo A., Group EBNCT The EORTC boron neutron capture therapy (BNCT) group: achievements and future projects. Eur J Cancer. 2002;38:31–34. doi: 10.1016/s0959-8049(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 104.Pinelli T., Zonta A., Altieri S. Research and development in neutron capture therapy. 2002. TAOrMINA: from the first idea to the application to the human liver; pp. 1065–1072. [Google Scholar]
- 105.Wittig A., Hideghety K., Paquis P. Research and development in neutron capture therapy. 2002. Current clinical results of the EORTC-study 11961; pp. 1117–1122. [Google Scholar]
- 106.Capala J., Britta H., Sköld K. Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J Neurooncol. 2003;62:135–144. doi: 10.1007/BF02699940. [DOI] [PubMed] [Google Scholar]
- 107.Dbaly V., Tovarys F., Honova H. Contemporary state of neutron capture therapy in the Czech Republic (Part 2) Ceska a Slovenska Neurologie a Neurochirurgie. 2003;66:60–63. [Google Scholar]
- 108.Moss R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT) Appl Radiat Isot. 2014;88:2–11. doi: 10.1016/j.apradiso.2013.11.109. [DOI] [PubMed] [Google Scholar]
- 109.Farr L., Sweet W., Locksley H., Robertson J. Neutron capture therapy of gliomas using boron. Trans Am Neurol Assoc. 1953;13:110–113. [PubMed] [Google Scholar]
- 110.Farr L.E., Sweet W.H., Robertson J. Brookhaven National Lab; 1953. Neutron capture therapy with boron in the treatment of glioblastoma multifomre. [PubMed] [Google Scholar]
- 111.Hatanaka H. Boron neutron capture therapy for tumors. Int J Radiat Appl Instrum Part A. Appl Radiat Isot. 1986;37:79–80. [Google Scholar]
- 112.Hatanaka H., Sweet W., Sano K., Ellis F. The present status of boron-neutron capture therapy for tumors. Pure Appl Chem. 1991;63:373–374. [Google Scholar]
- 113.Hatanaka H., Sano K., Yasukochi H. Progress in neutron capture therapy for cancer. Springer; 1992. Clinical results of boron neutron capture therapy; pp. 561–568. [Google Scholar]
- 114.Mishima Y., Ichihashi M., Hatta S. First human clinical trial of melanoma neutron capture. Diagnosis and therapy. Strahlenther Onkol. 1989;165:251–254. [PubMed] [Google Scholar]
- 115.Kankaanranta L., Kiovunoro H., Seppala T. Proc. 13th Int. Cong. Neutron Capture Ther. 2008. Outcome of the first twelve patients with locally recurred inoperable head and neck cancer treated in the Finnish head and neck cancer BNCT trial; pp. 2–7. [Google Scholar]
- 116.Kankaanranta L., Seppälä T., Koivunoro H. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2012;82:67–75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 117.Kawabata S., Miyatake S.-I., Nonoguchi N. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot. 2009;67:15–18. doi: 10.1016/j.apradiso.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T., Nakai K., Kageji T. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol. 2009;91:80–84. doi: 10.1016/j.radonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 119.Fuwa N., Suzuki M., Sakurai Y. Treatment results of boron neutron capture therapy using intra-arterial administration of boron compounds for recurrent head and neck cancer. Br J Radiol. 2014;81:749–752. doi: 10.1259/bjr/65306248. [DOI] [PubMed] [Google Scholar]
- 120.Miyatake S.-I., Kawabata S., Nonoguchi N. Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro-Oncol. 2009;11:430–436. doi: 10.1215/15228517-2008-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miyatake S.-I., Kawabata S., Yokoyama K. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neuro-Oncol. 2009;91:199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 122.Kawabata S., Miyatake S.-I. Neutron capture therapy. Springer; 2012. Boron neutron capture therapy for malignant meningiomas; pp. 399–406. [Google Scholar]
- 123.Wang L., Wang S., Chu P. BNCT for locally recurrent head and neck cancer: preliminary clinical experience from a phase I/II trial at Tsing Hua open-pool reactor. Appl Radiat Isot. 2011;69:1803–1806. doi: 10.1016/j.apradiso.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 124.Yanagie H., Higashi S., Seguchi K. Pilot clinical study of boron neutron capture therapy for recurrent hepatic cancer involving the intra-arterial injection of a 10 BSH-containing WOW emulsion. Appl Radiat Isot. 2014;88:32–37. doi: 10.1016/j.apradiso.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki M., Suzuki O., Sakurai Y. Int Cancer Conf J. Springer; 2012. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT) pp. 235–238. [Google Scholar]
- 126.Makino E., Sasaoka S., Aihara T. 1013 the first clinical trial of boron neutron capture therapy using 10B-para-boronophenylalanine for treating extramammary Paget's disease. Eur J cancer. 2012;(48):244–245. [Google Scholar]
- 127.Chadha M., Capala J., Coderre J.A. Boron neutron-capture therapy (BNCT) for glioblastoma multiforme (GBM) using the epithermal neutron beam at the Brookhaven National Laboratory. Int J Radiat Oncol Biol Phys. 1998;40:829–834. doi: 10.1016/s0360-3016(97)00891-2. [DOI] [PubMed] [Google Scholar]
- 128.Diaz A.Z. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician's point of view. J Neuro-Oncol. 2003;62:101–109. doi: 10.1007/BF02699937. [DOI] [PubMed] [Google Scholar]
- 129.Henriksson R., Capala J., Michanek A. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA) Radiother Oncol. 2008;88:183–191. doi: 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 130.Sköld K., Diaz A., Giusti V., Pellettieri L., Hopewell J. Boron Neutron Capture Therapy for glioblastoma multiforme: advantage of prolonged infusion of BPA‐f. Acta Neurol. Scand. 2010;122:58–62. doi: 10.1111/j.1600-0404.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- 131.Sköld K., Gorlia T., Pellettieri L., Giusti V., H-Stenstam B., Hopewell J. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: an assessment of clinical potential. Br J Radiol. 2014;83:596–603. doi: 10.1259/bjr/56953620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wittig A., Sauerwein W., Moss R. Early phase II study on BNCT in metastatic malignant melanoma using the boron carrier BPA. EORTC Protocol. 2006;11011 [Google Scholar]
- 133.Burian J., Marek M., Rataj J. Int Cong Ser. Elsevier; 2004. Report on the first patient group of the phase I BNCT trial at the LVR-15 reactor; pp. 27–32. [Google Scholar]