Summary
Acinetobacter baumannii is a nosocomial, opportunistic pathogen that causes several serious conditions such as meningitis, septicemia, endocarditis and pneumonia. It can be found in the oral biofilm, which may be a reservoir for pneumonia and chronic obstructive pulmonary disease. Subgingival colonization by A. baumannii is associated with chronic and aggressive periodontitis as well as refractory periodontal disease. Porphyromonas gingivalis, a keystone periodontal pathogen localized to subgingival plaque, is also implicated in several chronic conditions including aspiration pneumonia. While both bacteria are found together in subgingival plaque and can cause multiple polymicrobial infections, nothing is known about the interactions between these two important human pathogens. In this study, we used RNA sequencing to understand the transcriptional response of both species as they adapt to heterotypic communities. Among the differentially regulated genes were those encoding a number of important virulence factors for both species including adhesion, biofilm formation, and protein secretion. Additionally, the presence of A. baumannii increased the abundance of P. gingivalis in model dual species communities Collectively these results suggest that both P. gingivalis and A. baumannii adapt to each other and have synergistic potential for increased pathogenicity. In identifying the mechanisms that promote pathogenicity and refractory disease, novel approaches to mitigate polymicrobial synergistic interactions may be developed to treat or prevent associated diseases.
Keywords: Porphyromonas gingivalis, Acinetobacter baumannii, RNA-seq, heterotypic communities
Introduction
Studies of the human microbiome have enhanced our understanding of polymicrobial communities and the interactions among organisms. Periodontal diseases arise from synergistic polymicrobial interactions between members of the oral community that result in dysbiosis and destructive inflammation1–4. The oral microbial community develops in a highly programmed process as early colonizers, predominantly Gram-positive facultative organisms such as the oral streptococci, adhere to mucosal and solid surfaces5,6, and then provide a substratum for attachment of later colonizers while also providing nutrient and physiological support. Porphyromonas gingivalis (Pg) is a Gram-negative late colonizer of oral biofilms. Pg has long been linked with periodontal diseases, but more recently has been associated with several severe conditions such as atherosclerosis, rheumatoid arthritis, respiratory infections and oral cancer7–11.
In the oral microbiome, in addition to classically defined periodontal pathogens, newly identified species and other medically relevant pathogens such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii (Ab) are being recognized12,13. Ab causes a variety of serious infections including pneumonia, urinary tract infections, endocarditis and skin and/or wound infections. While the role of Ab in periodontal disease is unknown and its interactions with other organisms within the oral microbial community are largely unstudied, it is often associated with anaerobic late colonizers. This is potentially due to antagonistic interactions with the early colonizer Streptococcus sanguinis that inhibits Ab growth14. The presence of Ab with classical periodontal pathogens such as Pg, Treponema denticola, Aggregatibacter actinomycetemcomitans, and Tannerella forsythia is associated with aggressive and chronic periodontitis15–18. Additionally, patients suffering from periodontitis are also more likely to be refractory to treatment if Ab is present19,20.
While there is a lack of evidence showing a causal relationship between periodontal and pulmonary diseases, oral plaque has been suggested as a reservoir for potential respiratory pathogens, and aspiration of these bacteria can lead to pneumonia21. Moreover, several studies have shown that the severity of periodontal disease is linked to adverse respiratory conditions. A study of elderly patients with periodontitis demonstrated that > 4 mm probing depth in at least 10 teeth was associated with an increased mortality due to pneumonia. Additionally, decreased periodontal ligament attachment and increased alveolar bone loss, two hallmark clinical manifestations of periodontitis, have been linked to reduced pulmonary function22–24.
Beyond periodontitis, specific periodontal pathogens may contribute to the etiology of respiratory diseases. Common periodontal pathogens Fusobacterium nucleatum and Bacteroides spp. (since re-classified as Pg and Prevotella intermedia) have been isolated from patients with aspiration pneumonia and lung abscesses25–27. Synergistic polymicrobial interactions involving oral bacteria have also been demonstrated for respiratory diseases. Mixed infections of Pg and T. denticola in a pneumonia mouse model caused more severe bronchopneumonia and increased mortality compared to monoinfections28. The bronchial clearance of Pg was delayed in the presence of T. denticola, and pro-inflammatory cytokines were increased in mixed infections. Polymicrobial infections of oral pathogens Pg, F. nucleatum or A. actinomycetemcomitans increased the invasion of P. aeruginosa into HEp-2 cells, stimulated greater cytokine production and elevated apoptotic cell death compared to infection with P. aeruginosa alone29. The commensal oral bacterial species Actinomyces naeslundii and Streptococcus gordonii did not affect P. aeruginosa invasion. Collectively these studies suggest that oral pathogens can either directly infect the lower respiratory system or synergistically influence respiratory pathogens and enhance disease.
As Pg and Ab can be located together in the oral biofilm, study of their polymicrobial communities and interspecies interactions may begin to illuminate their contributions to the etiology of both periodontal and respiratory diseases. Here, we have used RNA sequencing (RNA-Seq) to study the transcriptional response of Pg and Ab in a model community. The results provide novel insight into the physiological response and the potential synergistic interactions between Pg and Ab.
Materials and Methods
Bacterial strains and growth conditions
Porphyromonas gingivalis (Pg) ATCC 33277 was grown anaerobically in trypticase soy broth (TSB) supplemented with 1 mg/mL yeast extract, 5 ug/ml hemin and 1 ug/ml menadione. TSB broth was supplemented with 5% sheep blood and 5% agar for growth on solid media. Acinetobacter baumannii (Ab) AB0057 was grown aerobically in Brain Heart Infusion (BHI) broth or BHI agar plates.
Community Development
Polymicrobial communities for sequencing were generated as previously described30. 1x10^9 cells of Pg and Ab were pelleted by centrifugation and resuspended in phosphate buffered saline (PBS). The two species were then mixed, pelleted and held aerobically at 37C for 3 h. Control Pg and Ab cells alone were prepared under the same conditions.
RNA Purification, Sequencing and Analysis
Total RNA was isolated from the cells using the Qiagen RNAeasy kit (Qiagen) with DNase treatment. Ribosomal RNA depletion was performed using the Ribo-zero rRNA removal kit for bacteria (Illumina) and the libraries were constructed with the TruSeq mRNA library kit (Illumina). High throughput sequencing was performed on a HiSeq 2500v.4 (Illumina) at the University of Michigan Medical School Sequencing Core. Reads were mapped to the reference genomes from NCBI for A. baumannii AB005731 and P. gingivalis ATCC 3327732. Normalized read counts and p-values for differential expression were computed for four biological replicates. The p-values were corrected for multiple hypothesis testing using the q-value method33,34. Transcripts with an absolute log2 fold difference of greater than 0.5 and the q-value below 0.001 were considered statistically different. Data processing and analysis for differential expression was performed by the University of Michigan Bioinformatics core. All sequencing reads and analyzed data were deposited to the Gene Expression Omnibus (GEO, accession number GSE111038). Topological pathway analysis was performed using the Bioconductor software package which takes into account the position of the differentially expressed genes in the pathway map from the Kyoto Encyclopedia of Genes and Genomes (KEGG)35. Pathways were identified as significantly regulated when the false-discovery rate was <0.05.
Quantitative Reverse Transcriptase-PCR Validation
To validate the RNA sequencing results, expression of select genes was determined by qRT-PCR. Pg, Ab and PgAb communities were developed and RNA isolated as described above. RNA was isolated from three independent experiments and converted to cDNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). qRT-PCR was performed by StepOne Plus (Applied Biosystems) using the ΔΔCt method using 16s rRNA as an internal control. Primers used for qRT-PCR are described in Supplemental Table 1.
Heterotypic Community Development and Confocal Microscopy
Communities of Pg and Ab were generated essentially as described previously36. Ab cells (0.1 OD600) were stained with Texas Red in PBS for 30 min and coated on glass coverslips for 24 h aerobically at 37°C. P. gingivalis cells were labeled with FITC and reacted with Ab in PBS for 16 h aerobically 37°C. After washing, the coverslips were mounted to glass microscope slides and examined using a Leica SP8 confocal microscope using 488 nm (FITC) and 558 nm (Texas Red) lasers. XYZ stacks were digitally reconstructed and quantification of volume of each species was obtained using the Find Objects algorithm in the Volocity software. The images are representative of at least 3 experiments and the quantification is the average of 6 measurements from each biological replicate.
Results and Discussion
Transcripts from mixed communities of P. gingivalis (Pg) and A. baumannii (Ab) following three hours of aerobic co-incubation were analyzed to identify differentially expressed genes compared to both bacterial species in monoculture. Our previous analysis of the transcriptional response of Pg to S. gordonii (Sg) over-time revealed the most consistent transcriptional changes occurred between 2 and 6 hours of co-incubation37. Additionally, our incubation was conducted under aerobic conditions as Ab is a strict aerobe and Pg is an aerotolerant anaerobe with resistance to aerobic conditions for 5 hours38. Based on these previous finding, we selected a 3 hour incubation under aerobic conditions for our experiment. A total of 12 libraries, 4 biological replicates each from Pg and Ab alone and the dual species communities (PgAb) were prepared and sequenced using Illumina technology. The complete data sets are shown in Supplemental Table 2. Genes were determined to be differentially expressed in communities when transcripts with an absolute log2 fold difference of greater than 0.5 were identified compared to the mono-species controls and the q-value below 0.001 were considered statistically different. A comparison of gene expression levels between Pg and PgAb communities revealed 713 genes (34.1% of protein coding genes) were differentially expressed, and of those, 351 genes had increased transcript levels in mixed communities while 362 genes had reduced expression in the PgAb communities. Pathway analysis using Kyoto Encyclopedia of Genes and Genomes (KEGG) was attempted for the Pg vs PgAb data; however, no pathways were determined to be significant, likely due to over representation of genes encoding hypothetical proteins (discussed below). Changes in the Ab transcriptional program in the context of dual-species communities were also assessed. There were 1676 differentially expressed genes (44.1% of protein coding genes), with 847 of these up-regulated, and 829 down-regulated, in PgAb compared to Ab. Representative genes were selected for qRT-PCR validation of Ab genes and the results are shown in Supplemental Table 3. Pathway analysis identified 24 pathways of Ab that were significantly regulated in the mixed communities compared to single species communities (Table 1). The largest group, made up of 68 genes, related to purine metabolism. Many genes were also involved in peptidoglycan biosynthesis, propionate, pyruvate, and sulfur metabolism. This indicates that Ab is more physiologically active in the PgAb communities compared to Ab alone. As no exogenous nutrients were provided in the communities, these results suggest significant cross-feeding between Pg and Ab. While it is likely that incubation under aerobic conditions could influence the transcriptional landscape of Pg, it is likley that Pg, an aerotolerant organism at the leading edge of the subgingival community, will encounter higher oxygen environments when interacting with Ab, a strict aerobe.
Table 1.
Pathway analysis of Ab genes differentially expressed in PgAb communities
| Pathway Name | Genes UP | Genes DOWN | FDR1 |
|---|---|---|---|
| Aminoacyl-tRNA biosynthesis | 27 | 1 | 6.31E-06 |
| One carbon pool by folate | 11 | 1 | 0.000476599 |
| Fatty acid biosynthesis | 13 | 1 | 0.000569961 |
| Sulfur metabolism | 14 | 16 | 0.001322774 |
| Pyrimidine metabolism | 36 | 2 | 0.001330085 |
| Glycine, serine and threonine metabolism | 24 | 8 | 0.002933338 |
| Lysine biosynthesis | 12 | 4 | 0.006448065 |
| Purine metabolism | 47 | 21 | 0.007516597 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 21 | 2 | 0.008386424 |
| Peptidoglycan biosynthesis | 18 | 1 | 0.008877038 |
| Fructose and mannose metabolism | 8 | 2 | 0.011766396 |
| Cysteine and methionine metabolism | 21 | 8 | 0.011981639 |
| Methane metabolism | 15 | 4 | 0.013718309 |
| Vitamin B6 metabolism | 5 | 1 | 0.013718309 |
| Novobiocin biosynthesis | 4 | 0 | 0.025871218 |
| Arginine and proline metabolism | 12 | 5 | 0.025871218 |
| Propanoate metabolism | 27 | 10 | 0.030004433 |
| Pyruvate metabolism | 29 | 13 | 0.030004433 |
| Biotin metabolism | 10 | 3 | 0.030004433 |
| Amino sugar and nucleotide sugar metabolism | 14 | 5 | 0.035195636 |
| Oxidative phosphorylation | 35 | 4 | 0.04482427 |
| Folate biosynthesis | 11 | 8 | 0.04482427 |
| Tyrosine metabolism | 6 | 8 | 0.04482427 |
| Arginine biosynthesis | 11 | 9 | 0.047789592 |
Pathway analysis was performed using the Bioconductor package and pathways were considered significant with a false-discovery rate (FDR) < 0.05
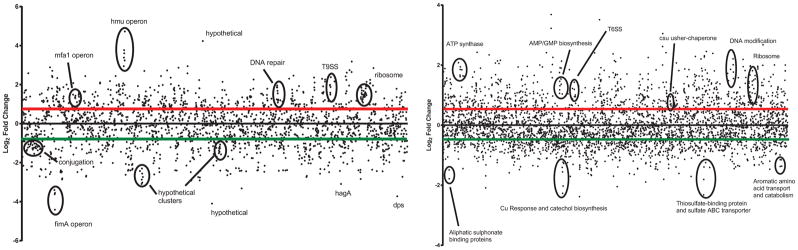
Figure 1 graphically represents the log2 fold change for every open reading frame from PgAb compared to Pg and Ab, respectively. For the PgAb vs Pg data set, a large portion of differentially expressed genes are annotated as hypothetical proteins (34.1%). Multiple large clusters of genes annotated as hypothetical proteins were differentially expressed and several were very highly regulated. Studies are ongoing to assess the function of these proteins. For the PgAb vs Ab data set, a number of functional clusters that were identified in the pathway analysis are highlighted. These include genes involved in sulfur metabolism which were negatively regulated by Ab in response to Pg. Multiple clusters of genes identified in purine metabolism are also highlighted. Genes of known functional and pathogenic significance were further analyzed and are described below.
Figure 1. Dot graph of DE genes and functional clusters.
Each dot represents the transcriptional response of every ORF numbered sequentially moving from left to right in PgAb vs Pg (left) and PgAb vs Ab (right). The red and green bars represent the significance cutoffs of 0.5 and −0.5 log2 fold change, respectively.
Phenylacetic acid catabolism
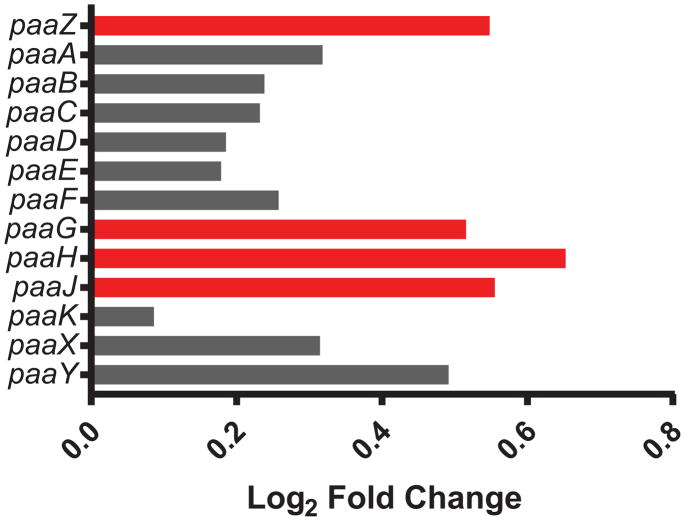
The Phenylacetic Acid Catabolism (PAA) pathway is encoded by the paa operon (AB57_1518 – AB57_1530) and is essential for the catabolism of several aromatic compounds such as phenylacetate, and yields acetyl- and succinyl-CoA, utilized by the TCA cycle39. The PAA pathway of Ab as well as other pathogens is associated with virulence and modulation of neutrophil chemotaxis40,41. PaaABCDE form a multi-protein complex that is most closely associated with virulence through production of toxic epoxides. Expression of the later genes in the paa pathway, paaZ, paaG, paaJ, and paaH were all significantly higher in the PgAb community compared to Ab alone (Figure 2), suggesting that Ab may produce more acetyl-CoA and succinyl-CoA in response to Pg. The increased production of both may feed into the TCA cycle for energy generation. Alternatively, increased succinyl-CoA production by the PAA pathway may be utilized by Pg. Similarly, Pg has been shown to induce succinate biosynthesis and secretion in T. denticola for cross-feeding42. While Pg lacks a PAA pathway, it does retain a paaK homolog (PGN_0228) which was up-regulated in communities with Ab. PaaK catalyzes the conversion of phenylacetate to phenylacetyl-CoA (PA-CoA), a terminal product for Pg. PA-CoA was shown to be the inducer of the PAA pathway in Pseudomonas putida43. Hence, Pg may synthesize PA-CoA which in turn stimulates the PAA pathway of Ab and lead to increased levels of acetyl-CoA and succinyl-CoA. Beyond the potential for metabolic cross-feeding, increased catabolism of phenylacetate may result in decreased neutrophil chemotaxis as phenylacetate from Ab is a neutrophil chemoattractant40. Such a process could link nutritional cross-feeding with polymicrobial synergy in PgAb communities.
Figure 2. Differential expression of genes within the phenylacetic acid catabolism pathway in the PgAb communities compared to Ab alone.
Results are expressed as log2 fold change in the PgAb community compared to Ab alone. Higher mRNA levels are represented as red bars and those with no significant change are shown as gray.
Expression of adhesins in PgAb communities
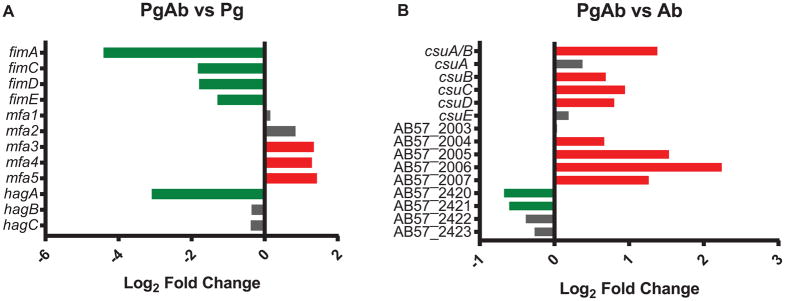
Colonization of the oral cavity and development of the polymicrobial biofilm involves attachment to both biotic and abiotic surfaces. Pg produces a number of adhesins such as the FimA and Mfa1 fimbriae along with the accessory proteins (FimB-FimE and Mfa2-Mfa5 respectively), and hemagglutinin A (HagA), HagB and HagC44. FimA and HagA were two of top 10 most negatively regulated Pg genes in the PgAb community. The expression of the long fimbriae major subunit FimA along with accessory genes PGN_0181 and PGN_0182 (the two fragments of FimB in 33277), FimC, FimD and FimE were all reduced in the PgAb communities compared to Pg alone (Figure 3). FimA fimbriae mediate attachment to diverse substrates such as other oral bacteria, the gingival epithelium and matrix proteins45,46. Expression of the hemagglutinin protein HagA was significantly decreased in the PgAb community compared to Pg alone, while expression of the structurally and functionally distinct HagB/C proteins was unchanged. HagA is a large protein composed of multiple repeating domains that each possess hemagglutinin activity, and is involved in attachment to endothelial and epithelial cells47.
Figure 3. Differential expression of adhesins in the PgAb communities.
Results are expressed as log2 fold change in the PgAb community compared to Pg (A) or Ab (B) alone. Higher mRNA levels are represented as red bars, lower levels are represented as green bars and those with no significant change are shown as gray.
The Mfa1 fimbriae accessory Mfa3-5 transcripts were higher in the PgAb community compared to Pg alone, while Mfa1 and Mfa2 were unchanged. Mfa1 is the structural fimbrial subunit while Mfa2 acts as an anchor and regulates filament length. Mfa3-5 are located along the fimbrial structure and have been shown to be co-expressed as an operon with Mfa1-248. Mfa3 is localized to the fimbrial tip and is essential for the Mfa4 and Mfa5 integration into the mature fimbriae49. Post-transcriptional regulation of Mfa3-5 may modulate the expression of the accessory proteins independently from mfa1. These findings are supported by our previous analysis of the transcriptional response of Pg to Streptococcus gordonii37. In both studies, detailed analysis of the reads mapped to the mfa operon reveal that mfa1 expression is detached from the downstream mfa2-5 genes. These differences may be due to inter-operon transcriptional start sites, differences in mRNA stability or transcriptional attenuation. Additional studies in our laboratory are exploring the role of Mfa2-5 in heterotypic communities. Collectively, these results indicate that P. gingivalis can dispense with the production of energetically costly adhesin proteins after community development is initiated, although Mfa3-5 may play specialized roles.
Ab encodes 4 distinct type 1 pili, 3 of which were differentially expressed in the polymicrobial community50. The first gene cluster comprising AB57_1744 through AB57_1747 was unchanged compared to Ab alone (Figure 3). This fimbrial cluster has been shown to be up-regulated in biofilm cells versus planktonic growth but a direct involvement in biofilm formation has not been established51. This cluster was also found to be down-regulated under iron limited conditions52. The type 1 pilus produced by the CsuA/BABCDE usher-chaperone assembly system is essential for initial biofilm development by attaching to abiotic surfaces and mediating early biofilm development51,53. These pili have additionally been shown to mediate attachment to alveolar epithelial cells and Candida albicans54. The major pilin subunit CsuA/B and the accessory CsuB,C,D transcripts were significantly up-regulated in the PgAb community versus Ab alone. A second usher-chaperone assembly system (AB57_2003 – AB57_2007) encodes type 1 pili, termed p-pili, that are also involved in biofilm and pellicle formation55. AB57_2007, encoding the fimbrial subunit, was increased in the PgAb community along with all other genes in the operon except AB57_2003. The putative type 3 filamentous fimbriae composed of FilABCDEF (AB57_0739 – AB57_0744) is up-regulated in biofilm pellicle compared to planktonic cells55. The genes encoding FilB, FilE and FilF were significantly decreased in response to Pg compared to untreated Ab. These data suggest that the CsuA/B pili and the p-pili are involved in heterotypic community maintenance while the FilA fimbriae may be dispensable following initial community development. In addition to polymicrobial community development, increased adhesion expression may be important for binding to oral epithelial cells and is the subject of future investigation.
Type VI secretion
Type VI secretion systems (T6SS) are analogous to bacteriophage contractile tail assemblies that mediate the injection of effectors into target cells56. T6SS effectors can be toxic to both bacteria and eukaryotic cells. The T6SS of Ab is encoded by a 20 gene cluster and has been shown to kill E. coli, suggesting this system may function for bacterial competition57. The T6SS genes are highly conserved among Ab isolates, and although not all Ab strains produce an operational T6SS58, AB0057 has been shown to produce a functional system59. Several of the T6SS machinery genes as well as T6SS effectors were differentially expressed in PgAb (Table 2) compared to Ab alone. TssB along with TssC assemble into cytoplasmic structures resembling bacteriophage sheathes that extend and contract60. Assembly of the TssB/C sheath is also dependent upon the gp25-like protein, TssE. TagX is a peptidoglycan hydrolase and is an essential enzyme in allowing transit of the T6SS machinery through the Ab peptidoglycan61. The T6SS leads to the export of two hallmark effector proteins, Hcp and VgrG60. Hcp forms hexameric rings and is homologous to the phage protein gpV. The presence of Hcp in the supernatant of T6SS encoding organisms is evidence for a functional secretion system. Hcp and VgrG are usually co-dependent for export. TssM, TssB, and Hcp have all been shown to be essential for Ab pathogenicity in a murine septicemia model58. The PgAb community showed increased levels of the tssB, tssC, and tssE along with tagX. AB0057 encodes three putative VgrG-like proteins one of which was differentially expressed, along with Hcp, in response to Pg. Additional genes within the operon were also up-regulated whose function within the T6SS are yet to be determined. In order to determine if the T6SS of AB0057 kills Pg, the PgAb community was incubated for three hours and Pg cells were then plated to determine CFU counts. There was no change in CFU when Pg was incubated alone or with AB0057 suggesting the T6SS is not lethal to Pg (data not shown). The ability to regulate the T6SS in the context of a complex polymicrobial community may alter the microbial community by favoring those resistant to T6SS-mediated killing such as Pg. In other Gram-negative bacteria, T6SS have been shown to be involved in metal acquisition such as iron62 as well as quorum sensing and biofilm formation63. In some bacteria, T6SS and effectors target eukaryotic cells and are required for virulence64.65. While the exact role the Ab T6SS plays in polymicrobial communities is unknown, the up-regulation may enhance Ab virulence by bactericidal-independent functions. Additionally, Pg induction of the Ab T6SS activity may lead to effector mediated killing of sensitive subgingival community members and alter the polymicrobial constituents.
Table 2.
Differential expression of Ab genes associated with the type 6 secretion system in PgAb communities
| Gene Name | Gene Name | log2 Fold Change1 | Product |
|---|---|---|---|
| AB57_1158 | 0.36272 | Rhs element Vgr protein, putative | |
| AB57_1442 | 0.18630 | putative VGR-related protein | |
| AB57_1479 | tssB | 1.4309 | type VI secretion protein, family |
| AB57_1480 | tssC | 1.19192 | type VI secretion protein, EvpB/family |
| AB57_1481 | Hcp | 0.79672 | type VI secretion system effector, Hcp1 family |
| AB57_1482 | tssE | 0.94882 | type VI secretion system lysozyme-related protein |
| AB57_1483 | tssF | 0.3391 | type VI secretion protein, family |
| AB57_1484 | tssG | −0.28688 | type VI secretion protein, family |
| AB57_1485 | −0.69577 | hypothetical protein | |
| AB57_1486 | tssM | 0.31656 | type VI secretion protein IcmF |
| AB57_1487 | tagF | 0.393 | type VI secretion-associated protein, family |
| AB57_1488 | tagN | 0.31515 | OmpA/MotB domain protein |
| AB57_1489 | PAAR Class I | −0.12307 | hypothetical protein |
| AB57_1490 | clpV | 0.45938 | type VI secretion ATPase, ClpV1 family |
| AB57_1491 | tssA | 1.00499 | type VI secretion-associated protein, ImpA family |
| AB57_1492 | tssK | 0.94866 | type VI secretion protein, family |
| AB57_1493 | tssL | 0.52583 | type IV / VI secretion system protein, DotU family |
| AB57_1494 | 0.72873 | hypothetical protein | |
| AB57_1495 | tagX | 0.53119 | D-alanyl-D-alanine carboxypeptidase family |
| AB57_3817 | 0.96823 | putative VGR-related protein |
Results are expressed as log2 fold change in PgAb compared to Ab alone. Higher mRNA levels are represented as red and statistically unchanged are in black.
Type IX Secretion
Pg produces a type 9 secretion system (T9SS) for the movement of proteins across the outer membrane66. Currently over 30 proteins that contain a conserved C-terminal domain (CTD), that is required for secretion, are transported through the T9SS. Genes encoding the core machinery of the T9SS are scattered across the Pg genome with the exception of the porKLMN operon. The transcript levels for the porKLMN operon were increased in the PgAb community (Table 3). Additional T9SS machinery genes sov, porU, porV, and porZ were also significantly up-regulated in response to Ab. The T9SS is regulated by the two-component system proteins PorX and PorY that functions through the transcriptional regulator SigP67. While neither the histidine kinase nor the response regulator were differentially expressed, transcript of SigP which has been shown to directly interact with the porKLMN promoter was increased68. A number of important virulence-associated proteins such as peptidylarginine deiminase (PPAD) and the gingipains are secreted through the T9SS66. Increased levels of the T9SS components may contribute to more significant virulence in the PgAb community compared to Pg. In addition to the core T9SS, 20 of the 30 T9SS secreted proteins also showed differential expression; 11 were up-regulated and 9 down-regulated (Table 3). PPAD and the arginine specific gingipain RgpB are two potential virulence factors that had the highest degree of up-regulation68–71. C5a citrullination by PPAD has been shown to reduce neutrophil chemotaxis72, suggesting a second potential mechanism of synergistic neutrophil manipulation by Pg and Ab in addition to phenylacetate metabolism and could be the focus of future studies.
Table 3.
Differential expression of Pg genes associated with the type 9 secretion system machinery, regulation and the C-terminal domain containing cargo proteins
| PGN Number | Product | Log2 Fold Change1 |
|---|---|---|
| T9SS Machinery | ||
|
| ||
| PGN_0022 | porU | 1.2229 |
| PGN_0023 | porV | 1.7245 |
| PGN_0274 | SigP | 0.8691 |
| PGN_0297 | conserved hypothetical protein | 0.2566 |
| PGN_0300 | conserved hypothetical protein | 0.0084 |
| PGN_0509 | porZ | 0.778 |
| PGN_0645 | porQ | 0.4576 |
| PGN_0778 | porT | 0.5775 |
| PGN_0832 | sov | 1.3021 |
| PGN_1019 | porX | −0.5066 |
| PGN_1235 | porS | 0.9197 |
| PGN_1236 | porR | 1.4053 |
| PGN_1296 | putative OmpA family protein | −0.4692 |
| PGN_1437 | conserved hypothetical protein | −0.4112 |
| PGN_1673 | porN | 1.9698 |
| PGN_1674 | porM | 1.9886 |
| PGN_1675 | porL | 1.5849 |
| PGN_1676 | porK | 1.4903 |
| PGN_1677 | porP | 1.8791 |
| PGN_1877 | porW | −0.1808 |
| PGN_2001 | porY | −0.2595 |
| T9SS Cargo Proteins | ||
|
| ||
| PGN_0022 | Por secretion system protein porU | 1.2229 |
| PGN_0123 | conserved hypothetical protein | −1.1650 |
| PGN_0152 | immunoreactive 61 kDa antigen | −2.0225 |
| PGN_0291 | accessory fimbrial protein, Mfa5 | 1.4341 |
| PGN_0335 | conserved hypothetical protein | 0.5009 |
| PGN_0509 | immunoreactive 84 kDa antigen | 0.7780 |
| PGN_0561 | trypsin like proteinase PrtT | 1.4692 |
| PGN_0654 | conserved hypothetical protein | −2.8665 |
| PGN_0657 | conserved hypothetical protein | −3.0214 |
| PGN_0659 | 35 kDa hemin binding protein | 0.9471 |
| PGN_0693 | conserved hypothetical protein | 0.5442 |
| PGN_0795 | conserved hypothetical protein | 0.5725 |
| PGN_0810 | conserved hypothetical protein | 2.3564 |
| PGN_0852 | immunoreactive 47 kDa antigen | 2.8683 |
| PGN_0898 | peptidylarginine deiminase PPAD | 1.0088 |
| PGN_0900 | thiol protease | 2.5795 |
| PGN_1115 | putative hemagglutinin | −2.6414 |
| PGN_1321 | conserved hypothetical protein | −2.1576 |
| PGN_1416 | probable lysyl endopeptidase precursor | −0.5973 |
| PGN_1466 | arginine-specific cysteine proteinase RgpB | 1.0239 |
| PGN_1476 | conserved hypothetical protein | 2.4632 |
| PGN_1556 | conserved hypothetical protein | −2.0303 |
| PGN_1611 | leucine-rich domain protein, InlJ | 0.0975 |
| PGN_1728 | lysine-specific cysteine proteinase Kgp | 0.0043 |
| PGN_1733 | hemagglutinin protein HagA | −3.0918 |
| PGN_1767 | immunoreactive 46 kDa antigen | −2.0675 |
| PGN_1770 | conserved hypothetical protein | 0.2688 |
| PGN_1790 | arginine-specific cysteine proteinase RgpA | −0.458 |
| PGN_2065 | putative Lys- and Rgp- gingipain domain protein | −2.7273 |
| PGN_2080 | conserved hypothetical protein | 1.6173 |
Results are expressed as log2 fold change in PgAb compared to Pg alone. Higher mRNA levels are represented as red, lower levels are represented as green, and statistically unchanged are in black.
Proteases
P. gingivalis is an asaccharolytic organism that relies on proteolysis to reduce proteins to peptides that serve as carbon and nitrogen sources44. A class of cysteine proteases, the gingipains, are important virulence factors73, and gingipains are specific for either arginine (RgpA/B) or lysine (Kgp). In addition to nutrient acquisition, gingipains are also involved in processing of surface proteins and degradation of host proteins. The gingipains degrade host immune proteins and can be released intracellularly and degrade host signaling proteins68,74,75. In our heterotypic communities, only RgpB was significantly up-regulated while RgpA and Kgp were not differentially expressed (Table 3). PrtT, a trypsin-like protease that is linked to virulence in a mouse abscess model76 was also up-regulated in the PgAb community.
Tetratricopeptide Repeat (TPR) Domain Proteins
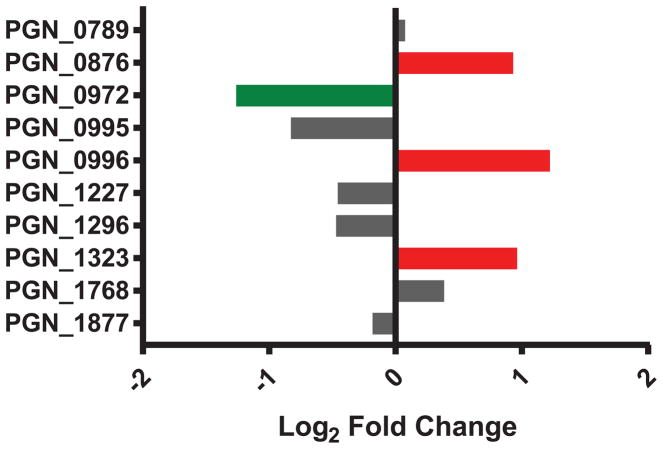
The TPR domain is a protein-protein binding motif that is often repeated within functionally diverse proteins. In Pg, genetic deletion of tprA (PGN_0876) was shown to reduce virulence in a mouse abscess infection model77. tprA was up-regulated in the heterotypic community compared to Pg alone (Figure 4). Among the other TPR proteins, PGN_0996 and PGN_1323 were also increased in response to Ab while PGN_0972 was reduced. In Ab, AB57_0888 is a TPR-domain containing lipoprotein that is annotated as a DNA uptake protein as part of the competence system, and this gene is increased in response to Pg. Collectively these data suggest that Pg may be more virulent in response to Ab, while Ab can up-regulate its DNA uptake system which provides an advantage in a competitive polymicrobial environment.
Figure 4. Differential expression of Pg TPR domain containing proteins in the PgAb communities.
Results are expressed as log2 fold change in the PgAb community compared to Pg alone. Higher mRNA levels are represented as red bars, lower expression is represented as green bars and those with no significant change are shown as gray.
A. baumannii capsule biosynthesis
Capsules are essential virulence factors in numerous bacterial species. Ab isolates produce at least 6 different capsule types with the enzymes involved in biosynthesis of sugar precursors and surface polysaccharide transferases being the most variable in gene arrangement78. Nearly every strain possesses a capsule encoded locus, including AB0057 used in this study (AB57_0090 - AB57_0116). Within this locus 10/27 genes were significantly up-regulated in the heterotypic community compared to Ab alone (Table 4), including the tyrosine kinase (AB57_0091) and the wza gene (AB57_0093) which have been shown to be essential for capsule production79. Genetic deletion of either gene results in increased sensitivity to human serum and decreased virulence in a murine subcutaneous infection model79. In addition to the capsule locus, AB57_1078 encodes a wzi homolog that in other bacteria has been shown to be essential for attachment of the capsule to the outer membrane80. These data suggest that Ab may increase capsule biosynthesis in the presence of Pg, which would make Ab more resistant to innate immune killing mechanisms and more virulent.
Table 4.
Differential expression of Ab genes associated with capsule biosynthesis and transport
| Gene ID | Product | log2 Fold Change1 |
|---|---|---|
| AB57_0090 | peptidyl-prolyl cis-trans isomerase Mip | 0.08133 |
| AB57_0091 | tyrosine-protein kinase ptk | 0.90987 |
| AB57_0092 | protein-tyrosine-phosphatase ptp | 0.71925 |
| AB57_0093 | polysaccharide export protein | 0.54358 |
| AB57_0094 | VI polysaccharide biosynthesis protein VipA/tviB | 1.35879 |
| AB57_0095 | VI polysaccharide biosynthesis protein VipB/tviC | 1.34966 |
| AB57_0096 | polysaccharide biosynthesis protein | 0.08478 |
| AB57_0097 | hypothetical protein | 0.54264 |
| AB57_0098 | hypothetical protein | −0.1949 |
| AB57_0099 | glycosyl transferase, group 1 | −0.35419 |
| AB57_0100 | hypothetical protein | −0.31633 |
| AB57_0101 | hypothetical protein | −0.47685 |
| AB57_0102 | putative glycosyl transferase family 1 | 0.32779 |
| AB57_0103 | glycosyl transferase, group 1 | −0.06922 |
| AB57_0104 | UDP-glucose 4-epimerase | 0.5243 |
| AB57_0105 | polyprenol phosphate:N-acetyl-hexosamine 1-phosphate transferase | 0.95193 |
| AB57_0106 | acetyltransferase | 0.3451 |
| AB57_0107 | nucleotide sugar epimerase/dehydratase | 0.46067 |
| AB57_0108 | UDP-glucose 4-epimerase | 0.7211 |
| AB57_0109 | hypothetical protein | −0.4545 |
| AB57_0110 | acyltransferase | −0.27185 |
| AB57_0111 | UTP-glucose-1-phosphate uridylyltransferase | −0.14983 |
| AB57_0112 | NDP-sugar dehydrogenase | −0.13951 |
| AB57_0113 | glucose-6-phosphate isomerase | 0.1811 |
| AB57_0114 | UDP-glucose 4-epimerase | 0.14976 |
| AB57_0115 | phosphomannomutase | 0.4157 |
| AB57_0116 | L-lactate permease | 0.67559 |
| AB57_1078 | hypothetical protein, wzi homolog | 0.99645 |
Results are expressed as log2 fold change in PgAb compared to Ab alone. Higher mRNA levels are represented as red, lower levels are represented as green, and statistically unchanged are in black.
Iron acquisition by Pg
Iron is essential for the growth and survival of bacteria and the human host as this critical metal is required as a cofactor for many essential enzymes involved in cellular and metabolic processes. In the host, iron is tightly sequestered by ferritin, lactoferrin and transferrin to protect against oxidative damage by the Fenton reaction. Acquisition of iron is an essential survival and virulence trait of all successful pathogens.
Pg growth is dependent upon iron acquisition in the form of hemin81,82. Interestingly the regulation of iron acquisition related genes within our heterotypic communities was variable (Table 5). The hmuYRSTUV operon encodes a hemin binding protein HmuY and the membrane bound receptor HmuR. The operon contains 4 of the 10 most positively regulated genes in the PgAb community. Consistent with increased need for iron acquisition, ferritin transcript levels were reduced indicating reduced need for iron storage. Additionally, PGN_1058 (bacterioferritin comigratory protein, bcp), which has previously been shown to be down-regulated during iron-limited conditions83 was significantly reduced in the PgAb community. In contrast, mRNA for the TonB-linked receptor, Tlr, and HtrABCD which comprise an ABC transport system involved in hemin acquisition84, were strongly reduced in the PgAb community. Additional hemin uptake systems encoded by the iht85 and hus86 operons were not differentially expressed in the PgAb communtities. HaeR/S is a two-component system (TCS) that regulates iron acquisition83. Both HaeR and HaeS showed reduced expression in the PgAb community, however only HaeR was determined to be significant due to a q-value that did not meet our strict significance cut-off for HaeS. HaeR was demonstrated to act as both an activator and repressor depending on promoter binding and hemin concentration. Interestingly HaeR has also been shown to regulate the hmu operon, htrA and bcp, suggesting that all differentially expressed genes in response to Ab may be through HaeR83, although transcript levels for TCS do not necessarily reflect activation status and information flow.
Table 5.
Differential expression of heme and iron acquisition genes in the PgAb communities compared to Pg alone
| PgAb vs Pg | ||
|---|---|---|
| Gene ID | Product | Log2 Fold Change1 |
| PGN_0553 | hmuV | 3.3866 |
| PGN_0554 | hmuU | 3.7695 |
| PGN_0555 | hmuT | 3.6057 |
| PGN_0556 | hmuS | 1.9810 |
| PGN_0557 | hmuR | 3.2401 |
| PGN_0558 | humY | 4.7244 |
| PGN_0604 | ferritin | −2.0719 |
| PGN_0659 | 35 kDa hemin binding protein | 0.9471 |
| PGN_0683 | tlr | −1.2954 |
| PGN_0684 | htrD | −1.3200 |
| PGN_0685 | htrC | −1.2234 |
| PGN_0686 | htrB | −1.2209 |
| PGN_0687 | htrA | −1.1410 |
| PGN_0704 | ihtA | 0.4271 |
| PGN_0705 | ihtB | 0.0173 |
| PGN_0706 | ihtC | 0.7127 |
| PGN_0707 | ihtD | 0.4343 |
| PGN_0708 | ihtE | 0.6481 |
| PGN_0752 | haeS | −1.7992 |
| PGN_0753 | haeR | −1.5937 |
| PGN_1058 | bcp | −1.7021 |
| PGN_1085 | feoB | 0.0982 |
| PGN_1087 | 1.3151 | |
| PGN_1335 | 1.7903 | |
| PGN_1336 | 1.1816 | |
| PGN_1503 | furR | 0.1741 |
| PGN_2037 | dps | −3.7272 |
| PGN_2090 | husB | −0.8785 |
| PGN_2091 | husA | −1.1772 |
Results are expressed as log2 fold change in PgAb compared to Pg alone. Higher mRNA levels are represented as red, lower levels are represented as green, and statistically unchanged are in black.
Dual-species community development
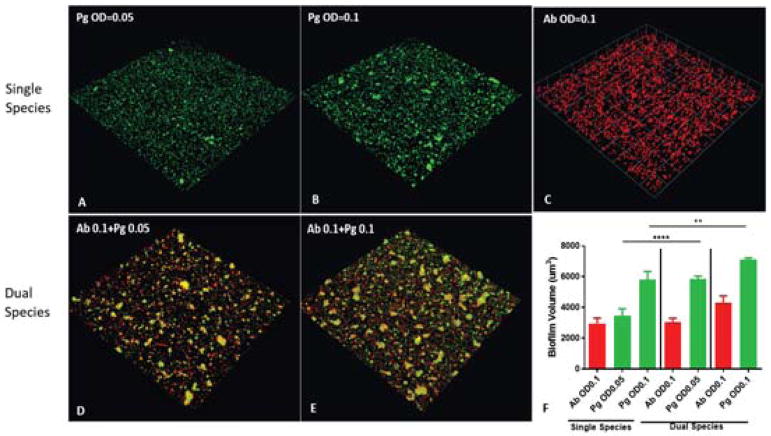
Both Pg and Ab are biofilm producing organisms that can be found as part of complex polymicrobial communities during infection. The RNA-Seq data revealed transcriptional responses in both Pg and Ab adhesins associated with biofilm formation in the mixed communities compared to mono-species biofilms. In a dual species community model we found the abundance of Pg was significantly increased in the presence of Ab compared to monotypic conditions (Figure 5). These data demonstrate that the transcriptional changes highlighted in the RNA-Seq communities can lead to increased heterotypic community development.
Figure 5. Ab enhances Pg community development.
(A–E) Pg (green) was incubated alone or reacted with a substratum of Ab (red) for 16 h at the cell densities indicated. A series of x-y sections were collected by confocal microscopy and digitally reconstructed into a three-dimensional image. Images are representative of 3 independent experiments. (F) Calculated biovolume of Pg and Ab in the images represented in (A–E) measured using the Volocity software. Results are means with standard deviation of the three experiments. **P<0.01, **** P<0.0001 compared between PgAb and Pg alone using ANOVA with Tukey’s multiple comparison test.
Comparison of heterotypic community responses
P. gingivalis thrives in a complex polymicrobial environment. Numerous studies have shown that Pg synergistically interacts with various members of the subgingival microbiome. One of the best characterized is the relationship between Pg and S. gordonii (Sg), including our recent analysis of the Pg transcriptional response to Sg by time-coursed RNA-Seq37. Comparing the transcriptional responses of Pg to Ab and Sg may provide insight into common responses to polymicrobial environments or highlight species-specific responses. One key difference between these two studies was the expression of fimbriae by Pg. PgSg communities resulted in significant increases in FimA and Mfa1 fimbrial expression over-time whereas in this study, the overall adhesive genes of Pg were down-regulated. This suggests that while Pg can directly interact with both Sg and Ab, Sg may provide prolonged stimuli for heterotypic community maintenance, whereas following initial community formation with Ab the fimbriae are no longer required. Another interesting comparison is the overall stress response of Pg to different community members. In this study we found no significant transcriptional changes in either the general stress response or the oxidative stress response of Pg, despite the presence of oxygen during community development. In contrast, Pg stress responses were down-regulated in the presence of Sg, highlighting their strong physiological compatibility. One notable exception was the oxidative stress response genes which were up-regulated in the presence of Sg. These differences may highlight that as a strict aerobe, Ab unlike Sg may provide a strong oxidative protection to Pg. One common response of Pg to both Ab and Sg is the up-regulation of the T9SS. This secretion system functions in the export of key virulence determinants such as PPAD and the gingipain proteases. While the community-associated function of these diverse effectors are largely uncharacterized, the increased secretion of potential community effectors in response to polymicrobial environments may result in community-wide alterations that are hallmarks of the dysbiosis associated with Pg infection.
Conclusion
Here we present a comprehensive transcriptional analysis of the key periodontal pathogen Pg and the opportunistic pathogen Ab as they sense and adapt to a heterotypic community. Model communities of Pg had greater numbers of bacteria in the presence of Ab compared to monotypic communities suggesting a synergistic relationship. Multiple adhesins were regulated by both Pg and Ab along with other biofilm associated factors. Pg and Ab showed synergistic metabolism in our model, and transcript levels of key virulence factors of both organisms were up-regulated in the heterotypic communities suggesting potential synergistic pathogenicity. The results of this study suggest that Pg and Ab may physically interact with each other during human infection to increase fitness due to cooperative metabolism and community development as well as promote immune dysregulation by manipulating neutrophil recruitment. In this study, we describe the transcriptional responses of Pg and Ab to a dual species community and provide key insights into the interaction of these two important human pathogens. While the scope of this study was limited to identifying and describing these interactions at the transcriptional level, additional studies are required to elucidate the underlying mechanisms and to functionally characterize the interactions described here, and to determine to extent to which the results from this model are applicable to an in vivo setting. In particular, study of the role of metabolic mutualism, differential protein secretion, and capsule biosynthesis may provide novel insights into synergistic pathogenicity.
Supplementary Material
Acknowledgments
Supported by NIH/NIDCR DE012505 (RL), DE023193 (RL), DE026939 (DM) and DE018276 (AW). We thank Ashley Best for helpful advice.
References
- 1.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Lamont RJ. Dancing with the Stars: How choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24(6):477–489. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacy A, McNally L, Darch SE, Brown SP, Whiteley M. The biogeography of polymicrobial infection. Nat Rev Microbiol. 2016;14(2):93–105. doi: 10.1038/nrmicro.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13(12):589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2(13):1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 7.Atanasova KR, Yilmaz O. Prelude to oral microbes and chronic diseases: past, present and future. Microbes Infect. 2015;17(7):473–483. doi: 10.1016/j.micinf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar PS. Oral microbiota and systemic disease. Anaerobe. 2013;24:90–93. doi: 10.1016/j.anaerobe.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. Am J Dent. 2013;26(5):249–254. [PubMed] [Google Scholar]
- 10.Okuda K, Kimizuka R, Abe S, Kato T, Ishihara K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J Periodontol. 2005;76(11 Suppl):2154–2160. doi: 10.1902/jop.2005.76.11-S.2154. [DOI] [PubMed] [Google Scholar]
- 11.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10(3):e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva-Boghossian CM, do Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56(9):899–906. doi: 10.1016/j.archoralbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Didilescu AC, Skaug N, Marica C, Didilescu C. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9(3):141–147. doi: 10.1007/s00784-005-0315-6. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Senba M, Ichinose A, Yamamoto T, Ariyoshi K, Matsumoto K. Bactericidal activity in filtrated supernatant of Streptococcus sanguinis against multidrug-resistant Pseudomonas aeruginosa. J Exp Med. 2009;219(2):79–84. doi: 10.1620/tjem.219.79. [DOI] [PubMed] [Google Scholar]
- 15.Ali RW, Velcescu C, Jivanescu MC, Lofthus B, Skaug N. Prevalence of 6 putative periodontal pathogens in subgingival plaque samples from Romanian adult periodontitis patients. J Clin Periodontol. 1996;23(2):133–139. doi: 10.1111/j.1600-051x.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 16.Slots J, Rams TE, Feik D, Taveras HD, Gillespie GM. Subgingival microflora of advanced periodontitis in the Dominican Republic. J Periodontol. 1991;62(9):543–547. doi: 10.1902/jop.1991.62.9.543. [DOI] [PubMed] [Google Scholar]
- 17.Souto R, Silva-Boghossian CM, Colombo AP. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45(2):495–501. doi: 10.1590/s1517-83822014000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards AM, Abu Kwaik Y, Lamont RJ. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol Oral Microbiol. 2015;30(1):2–15. doi: 10.1111/omi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo AP, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80(9):1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombo AP, Haffajee AD, Dewhirst FE, et al. Clinical and microbiological features of refractory periodontitis subjects. J Clin Periodontol. 1998;25(2):169–180. doi: 10.1111/j.1600-051x.1998.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 21.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70(7):793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 22.Hayes C, Sparrow D, Cohen M, Vokonas PS, Garcia RI. The association between alveolar bone loss and pulmonary function: the VA dental longitudinal study. Ann Periodontol. 1998;3(1):257–261. doi: 10.1902/annals.1998.3.1.257. [DOI] [PubMed] [Google Scholar]
- 23.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8(1):54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- 24.Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72(1):50–56. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202–207. doi: 10.1016/0002-9343(74)90598-1. [DOI] [PubMed] [Google Scholar]
- 26.Finegold SM, Strong CA, McTeague M, Marina M. The importance of black-pigmented gram-negative anaerobes in human infections. FEMS Immunol Med Microbiol. 1993;6(2–3):77–82. doi: 10.1111/j.1574-695X.1993.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson S, Laughon BE, Summer WR, Eckhaus MA, Bartlett JG, Jakab GJ. Characterization of the pulmonary inflammatory response to an anaerobic bacterial challenge. Am Rev Respir Dis. 1986;133(2):212–217. doi: 10.1164/arrd.1986.133.2.212. [DOI] [PubMed] [Google Scholar]
- 28.Kimizuka R, Kato T, Ishihara K, Okuda K. Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect. 2003;5(15):1357–1362. doi: 10.1016/j.micinf.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathogenesis. 2009;46(2):73–79. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Merritt J, Kreth J, Shi W, Qi F. LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component. Mol Microbiol. 2005;57(4):960–969. doi: 10.1111/j.1365-2958.2005.04733.x. [DOI] [PubMed] [Google Scholar]
- 31.Adams MD, Goglin K, Molyneaux N, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008;190(24):8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naito M, Hirakawa H, Yamashita A, et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008;15(4):215–225. doi: 10.1093/dnares/dsn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 35.Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12(2):115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuboniwa M, Tribble GD, James CE, et al. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol. 2006;60(1):121–139. doi: 10.1111/j.1365-2958.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- 37.Hendrickson EL, Beck DA, Miller DP, et al. Insights into dynamic polymicrobial synergy revealed by time-coursed RNA-Seq. Front Microbiol. 2017;8:261. doi: 10.3389/fmicb.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz PI, Slakeski N, Reynolds EC, Morona R, Rogers AH, Kolenbrander PE. Role of OxyR in the oral anaerobe Porphyromonas gingivalis. J Bacteriol. 2006;188(7):2454–2462. doi: 10.1128/JB.188.7.2454-2462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teufel R, Mascaraque V, Ismail W, et al. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci U S A. 2010;107(32):14390–14395. doi: 10.1073/pnas.1005399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhuiyan MS, Ellett F, Murray GL, et al. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2016;113(34):9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerqueira GM, Kostoulias X, Khoo C, et al. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis. 2014;210(1):46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 42.Grenier D. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis. Infect Immun. 1992;60(12):5298–5301. doi: 10.1128/iai.60.12.5298-5301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia B, Olivera ER, Minambres B, et al. Phenylacetyl-coenzyme A is the true inducer of the phenylacetic acid catabolism pathway in Pseudomonas putida. Appl Environ Micrbiol. 2000;66(10):4575–4578. doi: 10.1128/aem.66.10.4575-4578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immun. 2000;15(6):341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- 45.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62(4):1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishiyama S, Murakami Y, Nagata H, Shizukuishi S, Kawagishi I, Yoshimura F. Involvement of minor components associated with the FimA fimbriae of Porphyromonas gingivalis in adhesive functions. Microbiol. 2007;153(Pt 6):1916–1925. doi: 10.1099/mic.0.2006/005561-0. [DOI] [PubMed] [Google Scholar]
- 47.Belanger M, Kozarov E, Song H, Whitlock J, Progulske-Fox A. Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells. Anaerobe. 2012;18(1):128–134. doi: 10.1016/j.anaerobe.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa Y, Nagano K, Ikai R, et al. Localization and function of the accessory protein Mfa3 in Porphyromonas gingivalis Mfa1 fimbriae. Mol Oral Microbiol. 2013;28(6):467–480. doi: 10.1111/omi.12040. [DOI] [PubMed] [Google Scholar]
- 49.Ikai R, Hasegawa Y, Izumigawa M, et al. Mfa4, an accessory protein of Mfa1 Fimbriae, modulates fimbrial biogenesis, cell auto-aggregation, and biofilm formation in Porphyromonas gingivalis. PLOS One. 2015;10(10):e0139454. doi: 10.1371/journal.pone.0139454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT, Brown MH. Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics. 2014;15:1020. doi: 10.1186/1471-2164-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rumbo-Feal S, Gomez MJ, Gayoso C, et al. Whole transcriptome analysis of Acinetobacter baumannii assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLOS One. 2013;8(8):e72968. doi: 10.1371/journal.pone.0072968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics. 2011;12:126. doi: 10.1186/1471-2164-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiol. 2003;149(12):3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 54.Lee JC, Koerten H, van den Broek P, et al. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res Microbiol. 2006;157(4):360–366. doi: 10.1016/j.resmic.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Marti S, Nait Chabane Y, Alexandre S, et al. Growth of Acinetobacter baumannii in pellicle enhanced the expression of potential virulence factors. PLOS One. 2011;6(10):e26030. doi: 10.1371/journal.pone.0026030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pukatzki S, McAuley SB, Miyata ST. The type VI secretion system: translocation of effectors and effector-domains. Curr Opin Microbiol. 2009;12(1):11–17. doi: 10.1016/j.mib.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Sana TG, Lugo KA, Monack DM. T6SS: The bacterial “fight club” in the host gut. PLOS Pathogen. 2017;13(6):e1006325. doi: 10.1371/journal.ppat.1006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Repizo GD, Gagne S, Foucault-Grunenwald ML, et al. Differential role of the T6SS in Acinetobacter baumannii virulence. PLOS One. 2015;10(9):e0138265. doi: 10.1371/journal.pone.0138265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz FM, Santillana E, Spinola-Amilibia M, Torreira E, Culebras E, Romero A. Crystal structure of Hcp from Acinetobacter baumannii: A component of the Type VI secretion system. PLOS One. 2015;10(6):e0129691. doi: 10.1371/journal.pone.0129691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silverman JM, Brunet YR, Cascales E, Mougous JD. Structure and regulation of the type VI secretion system. Annu Rev Microbiol. 2012;66:453–472. doi: 10.1146/annurev-micro-121809-151619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber BS, Hennon SW, Wright MS, et al. Genetic dissection of the Type VI secretion system in Acinetobacter and identification of a novel peptidoglycan hydrolase, TagX, required for its biogenesis. mBio. 2016;7(5) doi: 10.1128/mBio.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen WJ, Kuo TY, Hsieh FC, et al. Involvement of type VI secretion system in secretion of iron chelator pyoverdine in Pseudomonas taiwanensis. Sci Rep. 2016;6:32950. doi: 10.1038/srep32950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallique M, Bouteiller M, Merieau A. The Type VI secretion system: A dynamic system for bacterial communication. Front Microbiol. 2017;8:1454. doi: 10.3389/fmicb.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burtnick MN, Brett PJ, Harding SV, et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun. 2011;79(4):1512–1525. doi: 10.1128/IAI.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma AT, Mekalanos JJ. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc Natl Acad Sci U S A. 2010;107(9):4365–4370. doi: 10.1073/pnas.0915156107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lasica AM, Ksiazek M, Madej M, Potempa J. The Type IX secretion system (T9SS): Highlights and recent insights into its structure and function. Front Cell Infect Microbiol. 2017;7:215. doi: 10.3389/fcimb.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadowaki T, Yukitake H, Naito M, et al. A two-component system regulates gene expression of the type IX secretion component proteins via an ECF sigma factor. Sci Rep. 2016;6:23288. doi: 10.1038/srep23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54(1):15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goulas T, Mizgalska D, Garcia-Ferrer I, et al. Structure and mechanism of a bacterial host-protein citrullinating virulence factor, Porphyromonas gingivalis peptidylarginine deiminase. Sci Rep. 2015;5:11969. doi: 10.1038/srep11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gully N, Bright R, Marino V, et al. Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis. PLOS One. 2014;9(6):e100838. doi: 10.1371/journal.pone.0100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Brien-Simpson NM, Paolini RA, Hoffmann B, Slakeski N, Dashper SG, Reynolds EC. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect Immun. 2001;69(12):7527–7534. doi: 10.1128/IAI.69.12.7527-7534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bielecka E, Scavenius C, Kantyka T, et al. Peptidyl arginine deiminase from Porphyromonas gingivalis abolishes anaphylatoxin C5a activity. J Biol Chem. 2014;289(47):32481–32487. doi: 10.1074/jbc.C114.617142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potempa J, Nguyen KA. Purification and characterization of gingipains. Curr Protoc Protein Sci. 2007;Chapter 21(Unit 21.20) doi: 10.1002/0471140864.ps2120s49. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Sztukowska M, Wang Q, et al. Noncanonical activation of beta-catenin by Porphyromonas gingivalis. Infect Immun. 2015;83(8):3195–3203. doi: 10.1128/IAI.00302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barth K, Genco CA. Microbial degradation of cellular kinases impairs innate immune signaling and paracrine TNFalpha responses. Sci Rep. 2016;6:34656. doi: 10.1038/srep34656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kesavalu L, Holt SC, Ebersole JL. Trypsin-like protease activity of Porphyromonas gingivalis as a potential virulence factor in a murine lesion model. Microb Pathogenesis. 1996;20(1):1–10. doi: 10.1006/mpat.1996.0001. [DOI] [PubMed] [Google Scholar]
- 77.Kondo Y, Ohara N, Sato K, et al. Tetratricopeptide repeat protein-associated proteins contribute to the virulence of Porphyromonas gingivalis. Infect Immun. 2008;78:2846–2856. doi: 10.1128/IAI.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenyon JJ, Hall RM. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLOS One. 2013;8(4):e62160. doi: 10.1371/journal.pone.0062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russo TA, Luke NR, Beanan JM, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010;78(9):3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, Chavez JD, Schweppe DK, et al. In vivo protein interaction network analysis reveals porin-localized antibiotic inactivation in Acinetobacter baumannii strain AB5075. Nat Commun. 2016;7:13414. doi: 10.1038/ncomms13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis JP. Metal uptake in host-pathogen interactions: role of iron in Porphyromonas gingivalis interactions with host organisms. Periodontol 2000. 2010;52(1):94–116. doi: 10.1111/j.1600-0757.2009.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smalley JW, Olczak T. Haem acquisition mechanisms of Porphyromonas gingivalis - strategies used in polymicrobial community in a haem-limited host environment. Mol Oral Microbiol. 2015 doi: 10.1111/omi.12149. [DOI] [PubMed] [Google Scholar]
- 83.Scott JC, Klein BA, Duran-Pinedo A, Hu L, Duncan MJ. A two-component system regulates hemin acquisition in Porphyromonas gingivalis. PLOS One. 2013;8(9):e73351. doi: 10.1371/journal.pone.0073351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olczak T, Simpson W, Liu X, Genco CA. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 2005;29(1):119–144. doi: 10.1016/j.femsre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Slakeski N, Dashper SG, Cook P, Poon C, Moore C, Reynolds EC. A Porphyromonas gingivalis genetic locus encoding a heme transport system. Oral Microbiol Immun. 2000;15(6):388–392. doi: 10.1034/j.1399-302x.2000.150609.x. [DOI] [PubMed] [Google Scholar]
- 86.Gao JL, Nguyen KA, Hunter N. Characterization of a hemophore-like protein from Porphyromonas gingivalis. J Biol Chem. 2010;285(51):40028–40038. doi: 10.1074/jbc.M110.163535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.