Abstract
Studies of xenotransplantation from swine have identified porcine viruses as potential barriers to clinical trials. The biology of these viruses has not been extensively investigated in the in vivo xeno-environment. Enhancement of viral gene expression by viral and cellular factors acting in trans has been demonstrated for certain viruses, including bidirectional interactions between human herpesviruses and endogenous (HERV) and exogenous (HIV) retroviruses. Both porcine cytomegalovirus (PCMV) and porcine endogenous retrovirus (PERV) infections have been identified in xenografts from swine. PERV receptors exist on human cells with productive infection in vitro in permissive human target cell lines. PCMV is largely species-specific with infection restricted to the xenograft in pig-to-baboon transplants. It is unknown whether coinfection by PCMV affects the replication of PERV within xenograft tissues which might have implications for the risk of retroviral infection in the human host. Methods: We studied a series of 11 pig-to-baboon kidney xenografts from PERV-positive miniature swine in the presence or absence of PCMV infection. PERV replication was not altered in the presence of PCMV coinfection (p=0.70). The absence of variation with coinfection was confirmed when PERV quantitation was expressed relative to simultaneous cellular GAPDH levels with or without PCMV coinfection (p=0.59). PCMV coinfection does not alter replication of PERV in life-supporting renal xenotransplantation in vivo in baboons.
Introduction
The development of clinical xenotransplantation using organs from swine has been limited by immunologic, metabolic and infectious barriers. Among infectious challenges, both porcine cytomegalovirus (PCMV) and porcine endogenous retrovirus (PERV) infections have been identified in xenografts from swine1–4. PERV has no apparent adverse impact on the porcine host and there are no data to suggest a direct interaction between PERV and PCMV on the virulence or replication of either virus. However, interactions between exogenous human retrovirus (human immunodeficiency virus, HIV) and herpesviruses such as human cytomegalovirus (HCMV) have been demonstrated in vitro5. Increased replication of HIV was observed with HCMV coinfection and was initially attributed to the effects of HIV on the status of the host immune system.6–9 This supposition is consistent with clinical observations that deployment of highly active antiretroviral therapies (HAART) led to a reduction in the incidence of opportunistic infections in AIDS including invasive CMV disease while nonresponding individuals remained at risk for CMV infections due to the impact of AIDS on CMV-specific CD8+ and CD4+ T lymphocytes in control of CMV replication6. However, interest emerged in cofactors that might enhance HIV replication or predispose individuals to progression to AIDS, possibly via activation of HIV-infected T cells by HCMV. Studies of human CMV-HIV coinfections of H9 cells demonstrated enhanced productive CMV and HIV-1 infections5. Thus, the interactions between HIV and CMV are bidirectional and occur at the cellular level as well as due to immunosuppressive effects.5 Given concerns regarding the potential infection of human recipients of porcine xenografts, we hypothesized that a similar interaction might exist between PERV and PCMV.
PCMV is largely species-specific with infection restricted to the xenograft in pig-to-baboon transplants10–12. PCMV infection causes endothelial activation in cultured cells in vitro and in pig vessels in vivo with increased expression of ICAM-1, vascular thrombosis, disseminated intravascular coagulation and neutropenia; as a result, PCMV infection increases graft rejection and reduces the survival of porcine xenografts in baboon recipients11,13–17. PCMV is susceptible to antiviral agents in vitro including ganciclovir18,19. In contrast, PERV has no clinical manifestations in swine, and baboon cells lack functional PERV-receptors preventing productive infection in xenograft recipients20–24. PERV receptors have been identified on human cells and productive infection demonstrated in vitro in certain permissive human target cells25–31. PERV is susceptible to antiretroviral agents in vitro32,33. Any enhancement of retroviral replication due to herpesviral coinfection might alter strategies for infectious disease surveillance or immunosuppressive regimens in xenograft recipients. We examined a series of xenografts derived from baboon recipients of porcine GalT-KO renal xenotransplants in vivo to determine whether evidence exists of an interaction between PERV and PCMV within xenograft kidneys.
Materials and Methods
Animals
Pig kidneys used for life-supporting function were obtained from GalT-KO miniature swine and implanted in recipient baboons using tolerance induction protocols14,34–38. Male or female recipient baboons (Papio anubis) were purchased from Mannheimer Foundation (Homestead, FL). Xenogeneic organs were obtained from GalT-KO miniature swine. Frozen biopsy samples from 11 functioning porcine xeno-kidney grafts were derived from pig-to-baboon xenotransplants at 13.7 days (6 animals, mean p<.05) for PCMV-infected grafts and mean of 53.2 days (5 animals) for PCMV uninfected grafts. Samples were analyzed for the presence of PCMV and PERV, using real-time PCR. Excised GalT-KO kidney tissue samples were frozen for viral analysis. Details of the preparation of these animals have been published previously14. All animals were cared for per guidelines of the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Immunosuppression
All recipients received a tolerance induction regimen that included transient T cell and B cell depletion37,38. Ten recipients received Rituximab (20 mg/kg IV) prior to transplantation38. All recipients received maintenance therapy with 110 mg/kg/day IV mycophenolate mofetil (Roche, Nutley, NJ) through an Omniflow 4000 Plus infusion pump (Abbott Laboratories, North Chicago, IL) continuously throughout the experiment but tapered after day 30; humanized anti-human CD154 mAb (ABI, Novartis, Basel) was administered at 20–25 mg/kg/day IV every 2–4 days, and methylprednisolone 2 mg/kg IV, starting on day 0 and tapered continuously thereafter. One CMV-negative recipient received 100 rads WBI and LoCD2 (4 mg/kg/day IV) (rat anti-primate CD2b, Immerge, BioTherapeutics, Charlestown, MA) followed by horse anti-thymocyte globulin (50 mg/kg/day IV) (ATG, Pharmacia/Upjohn, Peapack, NJ) in place of Rituximab prior to transplantation37,38. All recipients received a 14-day course of intravenous ganciclovir at 5–10 mg/day as prophylaxis against baboon CMV (BCMV) infection19.
Total RNA collection
Total RNA was collected using a two-step method comprised of an initial purification using RNA STAT-60 (AMS Biotechnology, Milton Park, Abingdon, UK) followed by RNeasy Mini Kit (Qiagen, Germantown, MD). Transplanted kidneys were harvested and approximately one half cm3 aliquots were frozen in liquid nitrogen and stored at −80°C. Individually, frozen tissue was transferred to frozen mortar and pestle positioned in a liquid nitrogen bath. The tissue was cryominced and ground into a course powder, transferred to a frozen pre-weighed tube and weighed. Sufficient RNA STAT-60 was added to a bring the concentration of ground tissue to RNA STAT-60 to 100mg tissue per ml RNA STAT-60. RNA extraction continued using the manufacturer’s Total RNA Protocol. Purification continued using the RNeasy Mini Kit per manufacturer’s protocol including on-membrane DNase. RNA yield quantified using Nano-Drop ND-1000 (Nano Drop Technologies, Wilmington, DE). RNA was stored at −80°C.
Reverse Transcription Reaction
RT reaction was carried out using the Omniscript RT Kit (Qiagen, Germantown, MD) using the manufacturer’s protocol. 1992 ng of Total RNA was run in each 12 ul reaction with a dilution of 1:625. Each RNA sample was run with, and without RT enzyme. A cocktail of 3 reverse primers (PERV pol, MHC, GAPDH) were added to a final concentration of 333 nM for each primer.
Quantitative Real-Time PCR
Target DNA sequences were quantified by real-time PCR using a Stratagene Mx3005P (Agilent Technologies, Cedar Creek, Texas). Sequence-specific primers and TaqMan probe were generated for each gene target (Primer Express software, Applied Biosystems, Foster City, CA)(Table 1). Each 25uL PCR reaction included target DNA, 800nM primers (InVitrogen-Life Technologies, Grand Island, NY) 200nM TaqMan probe (Applied Biosystems-Life Technologies, Grand Island, NY), 20 nM Rox reference and 1× Brilliant III Ultra Fast Master Mix (Agilent Technologies, Cedar Creek, Texas). The PCR cycling conditions were as follows: 1 cycle at 95°C for 5 min followed by 50 cycles of denaturation at 95°C for 10 seconds, and annealing-extension at 60°C for 30 seconds with data collection following each extension. Serial dilutions of gel-extracted amplicon cloned into Invitrogen TOPO plasmid served as quantifying standards. Target DNA is detected with a linear dynamic range of 10 to 106 copies. For quantification of PCMV DNA, 300 ng of xenograft pig kidney DNA was run in triplicate. Primers and probes specific for PCMV DNA polymerase gene have been shown to have no cross-reactivity with PLHV-112,39.
Table 1.
PCR Primers and Probe Sequences
| PCMV forward primer | 5′-GTTCTGGGATTCCGAGGTTG-3′ |
| PCMV reverse primer | 5′-ACTTCGTCGCAGCTCATCTGA-3’ |
| PCMV probe | 5′-FAM-CAGGGCGGCGGTCGAGCTC-TAMRA-3′ |
| PERV pol forward primer | 5’ AGC TCC GGG AGG CCT ACT C 3’ |
| PERV pol reverse primer | 5’ ACA GCC GTT GGT GTG GTC A 3’ |
| PERV pol probe | 5’ FAM-CCA CCG TGC AGG AAA CCT CGA GAC T-TAMRA 3’ |
| pMHC-I forward primer | 5’ GCC CTG GGC TTC TAC CCT AA 3’ |
| pMHC-I reverse primer | 5’ TCT CAG GGT GAG TGG CTC 3’ |
| pMHC-I probe | 5’ FAM- CCA GGA CCA GAG CCA GGA CAT GGA GCT CGT T-TAMRA 3’ |
| pGAPDH forward primer | 5’ TCA ACG ACC ACT TCG TCA AGC 3’ |
| pGAPDH reverse primer | 5’ GGA TGG AAA CTG GAA GTC AGG AGA 3’ |
| pGAPDH probe | 5’ FAM-TCT CTC CTC CTC GCG TGC TCT TGC T-TAMRA 3’ |
PERV pol quantitation
10uL of a 1:625 dilution of the reverse transcription (RT) reaction was amplified in a 50 cycle PERV polymerase quantitative TaqMan PCR in triplicate using a Stratagene MX300P real-time thermocycler (Agilent Technologies). 10uL of a 1:25 dilution of the “No RT enzyme” control RT reaction was similarly treated. PCR conditions included PERV pol forward and reverse primers at 800nM final concentration and PERV pol probe at 200 nM final concentration. Brilliant III Ultra Fast master mix (600880 Agilent Technologies) was used supplemented to 20 nM with ROX reporter dye (600880 Agilent Technologies) and 0.04 Units/uL UNG nuclease (N8080096, Life Technologies). Cycling conditions included 1 cycle of 10 minutes at 50°C followed by one cycle of 10 minutes at 95°C and 50 cycles of 10 seconds at 95°C followed by 30 seconds at 60°C with data collected at the end of each cycle. Assay sensitivity is at least 10 copies/ul. Absolute copies of PERV pol, and of porcine MHC-I and porcine GAPDH nucleic acids were measured per nanogram of input cDNA40.
Statistics
Data sets were tested for normality using the Wilcoxin/S Test for Normality and all were found to be not significantly different from a normal population, accordingly the Student’s T-Test was used when comparing PERV pol expression in PCMV positive and PCMV negative xenokidneys.
Results
Xenografts were classified as negative for PCMV (6 animals) if no amplification was observed. Xenografts classified as positive for PCMV amplified with superimposable amplification plots in triplicate typically with low cycle thresholds as measured at the end of each amplification cycle indicative of viral reactivation. The quantitative detection limit for this assay was 7 copies and values are expressed as positive or negative.
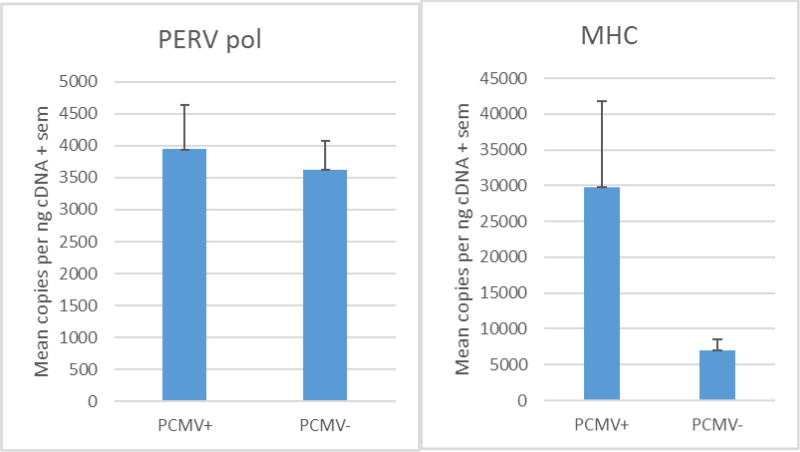
Nucleic acids were successfully amplified from all reactions containing reverse transcriptase. PERV assay sensitivity was at least 10 copies/ul. PERV expression was not altered in the presence (3944 +/− 690 copies per nanogram input DNA) or absence (3617 +/− 460 copies) of PCMV coinfection (p=0.70) (Figure 1A). To control for variability in reaction efficiency, ratios of PERV pol to MHC and PERV pol to GAPDH were examined. Control values for GAPDH DNA in kidney tissues did not vary with (2,482,230 +/− 888,506 or without (1,932,384 +/−665,051 PCMV infection (p=0.63). Thus, in this system using porcine renal tissue biopsies, GAPDH was demonstrated to be a useful internal control. The absence of variation in PERV replication with PCMV coinfection was confirmed when PERV quantitation was expressed as a ratio to GAPDH with or without PCMV coinfection (p=0.59). By contrast, porcine MHC-I RNA (cDNA) tended to increase with PCMV infection of xenograft tissue (more than three-fold, Figure 1B) and therefore was not a useful internal control for PERV quantitation (Figure 1B) (p=0.12).
Figure 1.
A. Absolute values of PERV nucleic acids were measured per nanogram of input cDNA in porcine renal xenograft biopsies. PERV expression was not altered in the presence or absence of PCMV coinfection (p=0.70). The absence of variation with coinfection was confirmed when PERV quantitation was expressed as a ratio to GAPDH with or without PCMV coinfection (p=0.59). Control values for GAPDH DNA did not vary with PCMV infection (p=0.723). B. MHC RNA (cDNA) tended to increase with PCMV infection of xenograft tissue and therefore was not a useful internal control for PERV quantitation (p=0.12).
Discussion
Inbred miniature swine studied as xenograft donors all express PERV A, B and C (and AC recombinants) in vitro and in vivo. While PCMV has diverse effects in vitro and in vivo, these studies demonstrated the absence of enhancement of PERV expression during co-infection by PCMV in vivo. The mechanisms underlying the permissive effect of CMV infection on HIV replication remain to be elucidated. Limitations of the study might include a modest number of samples tested which was necessitated by the desire to use animals undergoing uniform immunosuppression. Prior studies of herpesvirus-retrovirus interactions have been performed with high titer viral infections in vitro with an increased likelihood that cellular coinfection exists. However, given the diverse systemic effects of CMV infection, it is possible that the effects of viral interactions would be observed even if cellular coinfection did not occur within the xenograft. Such interactions were not observed in vivo. Despite these observations, as PERV receptors are defective in nonhuman primates, clinical infectious risk cannot be readily assessed in this model. Interestingly, the selection of internal controls for amplification reactions was critical given that MHC expression was increased by over three-fold in the animals with PCMV infection and could not be used as an internal reaction control for these studies15. This may reflect either specific or nonspecific stimulation of MHC transcription by infection. CMV infection in vivo tends to provoke allograft rejection as well as xenograft rejection, possibly via T-cell priming and endothelial activation11,13–15,41–46. Similar variation was not observed for GAPDH amplification and therefore the ratio of PERV-pol expression to GAPDH ratio serves as a useful control for input cellular nucleic acids.
Enhancement of viral gene expression by viral and cellular factors acting in trans has been demonstrated in various systems including bidirectional interactions between herpesviruses and endogenous (HERV) and exogenous (HIV, HTLV-1, Visna) retroviruses47–52. Human endogenous retroviral (HERV-W) elements, including elements lacking regulatory LTRs, are expressed in cell-specific patterns which can be modulated by environmental influences including placental development, as well as with herpes simplex virus 1 (HSV-1) or influenza A/WSN/33 viruses. LTR-directed transcription of the human endogenous retrovirus K can be induced by HSV-1 infection immediate early protein, ICP0 (KWUN) and by EBV infection53,54. LTR-directed transcription of the HERV-W is induced by HSV-1 infection. HSV-1 Us11 protein is involved in post-transcriptional trans-regulation of retroviral glycoprotein expression in HIV and HTLV-1.
In vitro, like CMV, Herpes simplex virus type 1 functions as a trans-activator of HIV55–59. Epstein-Barr virus gene product BMLF-1 functions as trans-activators of promotors derived from simian virus 40, adenovirus, and herpes simplex virus48,51,59–61. Of interest, HIV-related gene products or cellular factors induced by HIV-1 infection also causes trans-activation of CMV expression in vitro. Studies of the cellular trans-activation of viral replication have been performed in permissive target cell lines (H9) and may not extrapolate to in vivo conditions. Like human CMV infection, PCMV has been shown to increase the risk for xenograft rejection and graft loss and has diverse effects on intracellular processes and host immunity14,15,62. However, in the present study in vivo, PERV, an endogenous retrovirus, does not have a similar bi-directional relationship with PCMV in porcine xenografts in immunosuppressed baboons. In this study, a uniform immunosuppressive regimen was used for xenotransplantation. As we and others have demonstrated, the specific immunosuppressive regimen (as well as graft rejection or inflammation) is important relative to the activation of replication of porcine herpesviruses (PCMV and porcine lymphotropic herpesviruses, PLHV)11,12,14,21,39,63. Data on the impacts of specific immunosuppressive regimens or the organ transplanted on PERV replication are incomplete and merit study21. Preclinical studies in nonhuman primates provides a valuable model for infectious and immunological effects of xenotransplantation.
Acknowledgments
This research was supported by the Project 1 and Core B of the NIH/NIAID 2P01AI45897 and the MGH Swine Facility grant C06 RR020135-01.
References
- 1.Denner J, Tonjes RR, Takeuchi Y, Fishman J, Scobie L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 5: recipient monitoring and response plan for preventing disease transmission. Xenotransplantation. 2016;23:53–9. doi: 10.1111/xen.12227. [DOI] [PubMed] [Google Scholar]
- 2.Fishman J, Patience C. Xenotransplantation: Infectious Risk Revisited. Am J Transplantation. 2004;4:1383–90. doi: 10.1111/j.1600-6143.2004.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19:72–81. doi: 10.1111/j.1399-3089.2012.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierson RN, 3rd, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–80. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnik PR, Kosloff BR, Hirsch MS. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. The Journal of infectious diseases. 1988;157:508–14. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- 6.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur A, Daniel MD, Hempel D, Lee-Parritz D, Hirsch MS, Johnson RP. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J Virol. 1996;70:7725–33. doi: 10.1128/jvi.70.11.7725-7733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur A, Hale CL, Noren B, Kassis N, Simon MA, Johnson RP. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J Virol. 2002;76:3646–58. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiala M, Cone LA, Chang CM, Mocarski ES. Cytomegalovirus viremia increases with progressive immune deficiency in patients infected with HTLV-III. AIDS Res. 1986;2:175–81. doi: 10.1089/aid.1.1986.2.175. [DOI] [PubMed] [Google Scholar]
- 10.Mueller NJ, Barth RN, Yamamoto S, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002;76:4734–40. doi: 10.1128/JVI.76.10.4734-4740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller NJ, Kuwaki K, Dor FJ, et al. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78:1449–53. doi: 10.1097/01.tp.0000141361.68446.1f. [DOI] [PubMed] [Google Scholar]
- 12.Mueller NJ, Livingston C, Knosalla C, et al. Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in pig-to-baboon xenotransplantation. The Journal of infectious diseases. 2004;189:1628–33. doi: 10.1086/383351. [DOI] [PubMed] [Google Scholar]
- 13.Gollackner B, Mueller NJ, Houser S, et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75:1841–7. doi: 10.1097/01.TP.0000065806.90840.C1. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Tasaki M, Sekijima M, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98:411–8. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminski H, Fishman JA. The Cell Biology of Cytomegalovirus: Implications for Transplantation. Am J Transplant. 2016;16:2254–69. doi: 10.1111/ajt.13791. [DOI] [PubMed] [Google Scholar]
- 16.Knight DA, Waldman WJ, Sedmak DD. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation. 1999;68:1814–8. doi: 10.1097/00007890-199912150-00030. [DOI] [PubMed] [Google Scholar]
- 17.Sedmak DD, Knight DA, Vook NC, Waldman JW. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation. 1994;58:1379–85. [PubMed] [Google Scholar]
- 18.Fryer JF, Griffiths PD, Fishman JA, Emery VC, Clark DA. Quantitation of porcine cytomegalovirus in pig tissues by PCR. Journal of clinical microbiology. 2001;39:1155–6. doi: 10.1128/JCM.39.3.1155-1156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller NJ, Sulling K, Gollackner B, et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. American Journal of Transplantation. 2003;3:1057–64. doi: 10.1034/j.1600-6143.2003.00192.x. [DOI] [PubMed] [Google Scholar]
- 20.Akiyoshi DE, Denaro M, Zhu H, Greenstein JL, Banerjee P, Fishman JA. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 1998;72:4503–7. doi: 10.1128/jvi.72.5.4503-4507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa NC, Wilkinson RA, Griesemer A, et al. Absence of replication of porcine endogenous retrovirus and porcine lymphotropic herpesvirus type 1 with prolonged pig cell microchimerism after pig-to-baboon xenotransplantation. J Virol. 2008;82:12441–8. doi: 10.1128/JVI.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon AR, Templin C, Schroder C, et al. No evidence for productive PERV infection of baboon cells in in vivo infection model. Annals of transplantation. 2003;8:24–34. [PubMed] [Google Scholar]
- 23.Martin U, Steinhoff G, Kiessig V, et al. Porcine endogenous retrovirus (PERV) was not transmitted from transplanted porcine endothelial cells to baboons in vivo. Transplant International. 1998;11:247–51. doi: 10.1007/s001470050136. [DOI] [PubMed] [Google Scholar]
- 24.Martin U, Tacke SJ, Simon AR, et al. Absence of PERV specific humoral immune response in baboons after transplantation of porcine cells or organs. Transplant International. 2002;15:361–8. doi: 10.1007/s00147-002-0428-7. [DOI] [PubMed] [Google Scholar]
- 25.Ericsson TA, Takeuchi Y, Templin C, et al. Identification of receptors for pig endogenous retrovirus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6759–64. doi: 10.1073/pnas.1138025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martina Y, Marcucci KT, Cherqui S, et al. Mice transgenic for a human porcine endogenous retrovirus receptor are susceptible to productive viral infection. J Virol. 2006;80:3135–46. doi: 10.1128/JVI.80.7.3135-3146.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonjes RR, Czauderna F, Fischer N, et al. Molecularly cloned porcine endogenous retroviruses replicate on human cells. Transplant Proc. 2000;32:1158–61. doi: 10.1016/s0041-1345(00)01165-9. [DOI] [PubMed] [Google Scholar]
- 28.Specke V, Tacke SJ, Boller K, Schwendemann J, Denner J. Porcine endogenous retroviruses: in vitro host range and attempts to establish small animal models. Journal of General Virology. 2001;82:837–44. doi: 10.1099/0022-1317-82-4-837. [DOI] [PubMed] [Google Scholar]
- 29.Czauderna F, Fischer N, Boller K, Kurth R, Tonjes RR. Establishment and characterization of molecular clones of porcine endogenous retroviruses replicating on human cells. J Virol. 2000;74:4028–38. doi: 10.1128/jvi.74.9.4028-4038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denner J, Specke V, Schwendemann J, Tacke SJ. Porcine endogenous retroviruses (PERVs): adaptation to human cells and attempts to infect small animals and non-human primates. Annals of Transplantation. 2001;6:25–33. [PubMed] [Google Scholar]
- 31.Argaw T, Figueroa M, Salomon DR, Wilson CA. Identification of residues outside of the receptor binding domain that influence the infectivity and tropism of porcine endogenous retrovirus. J Virol. 2008;82:7483–91. doi: 10.1128/JVI.00295-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelm M, Fishman JA, Pontikis R, Aubertin AM, Wilhelm FX. Susceptibility of recombinant porcine endogenous retrovirus reverse transcriptase to nucleoside and non-nucleoside inhibitors. Cell Mol Life Sci. 2002;59:2184–90. doi: 10.1007/s000180200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denner J. Can Antiretroviral Drugs Be Used to Treat Porcine Endogenous Retrovirus (PERV) Infection after Xenotransplantation? Viruses. 2017;9 doi: 10.3390/v9080213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol. 2000;164:3079–86. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Shimizu A, Ierino FL, et al. Thymic transplantation in miniature swine. I. Development and function of the "thymokidney". Transplantation. 1999;68:1684–92. doi: 10.1097/00007890-199912150-00011. [DOI] [PubMed] [Google Scholar]
- 36.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 38.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller NJ, Barth RN, Yamamoto S, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002;76:4734–40. doi: 10.1128/JVI.76.10.4734-4740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–4. 6, 8–9. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- 41.van den Elsen PJ. Expression regulation of major histocompatibility complex class I and class II encoding genes. Front Immunol. 2011;2:48. doi: 10.3389/fimmu.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedmak DD, Guglielmo AM, Knight DA, Birmingham DJ, Huang EH, Waldman WJ. Cytomegalovirus inhibits major histocompatibility class II expression on infected endothelial cells. The American journal of pathology. 1994;144:683–92. [PMC free article] [PubMed] [Google Scholar]
- 43.Waldman WJ, Knight DA, VanBuskirk A, Adams PW, Orosz CG, Sedmak DD. Endothelial HLA class II induction mediated by allogeneic T cells activated by cytomegalovirus-infected cultured endothelial cells. Transplant Proc. 1993;25:1439–40. [PubMed] [Google Scholar]
- 44.van Dorp WT, van Wieringen PA, Marselis-Jonges E, et al. Cytomegalovirus directly enhances MHC class I and intercellular adhesion molecule-1 expression on cultured proximal tubular epithelial cells. Transplantation. 1993;55:1367–71. doi: 10.1097/00007890-199306000-00029. [DOI] [PubMed] [Google Scholar]
- 45.Craigen JL, Grundy JE. Cytomegalovirus induced up-regulation of LFA-3 (CD58) and ICAM-1 (CD54) is a direct viral effect that is not prevented by ganciclovir or foscarnet treatment. Transplantation. 1996;62:1102–8. doi: 10.1097/00007890-199610270-00014. [DOI] [PubMed] [Google Scholar]
- 46.Dzabic M, Rahbar A, Yaiw KC, et al. Intragraft cytomegalovirus protein expression is associated with reduced renal allograft survival. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:969–76. doi: 10.1093/cid/cir619. [DOI] [PubMed] [Google Scholar]
- 47.Sodroski J, Rosen C, Wong-Staal F, et al. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985;227:171–3. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- 48.Hess JL, Clements JE, Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985;229:482–5. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- 49.Sodroski J, Patarca R, Rosen C, Wong-Staal F, Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985;229:74–7. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- 50.Arya SK, Guo C, Josephs SF, Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III) Science. 1985;229:69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- 51.Gendelman HE, Phelps W, Feigenbaum L, et al. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986;83:9759–63. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–3. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 53.Sutkowski N, Chen G, Calderon G, Huber BT. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J Virol. 2004;78:7852–60. doi: 10.1128/JVI.78.14.7852-7860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–89. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- 55.Kwun HJ, Han HJ, Lee WJ, Kim HS, Jang KL. Transactivation of the human endogenous retrovirus K long terminal repeat by herpes simplex virus type 1 immediate early protein 0. Virus Res. 2002;86:93–100. doi: 10.1016/s0168-1702(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 56.Lee WJ, Kwun HJ, Kim HS, Jang KL. Activation of the human endogenous retrovirus W long terminal repeat by herpes simplex virus type 1 immediate early protein 1. Mol Cells. 2003;15:75–80. [PubMed] [Google Scholar]
- 57.Diaz JJ, Dodon MD, Schaerer-Uthurralt N, et al. Post-transcriptional transactivation of human retroviral envelope glycoprotein expression by herpes simplex virus Us11 protein. Nature. 1996;379:273–7. doi: 10.1038/379273a0. [DOI] [PubMed] [Google Scholar]
- 58.Mosca JD, Bednarik DP, Raj NB, et al. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987;84:7408–12. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosca JD, Bednarik DP, Raj NB, et al. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987;325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 60.Wong KM, Levine AJ. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986;60:149–56. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieberman PM, O'Hare P, Hayward GS, Hayward SD. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986;60:140–8. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856–79. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 63.Dor FJ, Doucette KE, Mueller NJ, et al. Posttransplant lymphoproliferative disease after allogeneic transplantation of the spleen in miniature swine. Transplantation. 2004;78:286–91. doi: 10.1097/01.tp.0000128342.64240.cf. [DOI] [PubMed] [Google Scholar]