Abstract
Although at first glance inflammation and social behavior may appear unrelated, research points to an important role for inflammation in shaping social processes. This review summarizes findings in this field, specifically highlighting work that provides support for the idea that inflammation can lead to 1) increases in sensitivity to negative, threatening social experiences and 2) increases in sensitivity to positive, socially rewarding experiences. These diverging sensitivities in response to inflammation may depend on context and be adaptive for recuperation and recovery from illness. This review also discusses the implications of these findings for health and future research, including implications for depression, loneliness, and inflammatory disorders.
Keywords: inflammation, social behavior, health
When asked about what they associate with inflammation, the average person may mention a cut or bruise, an acute illness, or a wide variety of chronic medical disorders. Whatever the answer, it seems unlikely that social behavior would be towards the top of the list of topics associated with inflammatory activity. Inflammation does indeed play a critical role in protecting against infection and injury, and chronic inflammation characterizes a wide host of diseases.1–3 Although inflammation and social behavior are not commonly thought of as related entities, recent research points to an important role for inflammation in shaping social behavior, or behaviors aimed at other conspecifics. In this paper, we will review the existing literature in both humans and non-human animals on the impact of inflammation on social behavior.
Sickness behavior
Pro-inflammatory cytokines, which are cell signaling molecules, aid in coordinating an inflammatory response to illness, injury, or infection. Importantly, pro-inflammatory cytokines also signal the brain to orchestrate a constellation of symptoms collectively called sickness behavior.4 These symptoms—including fatigue, increased pain, anhedonia, and loss of appetite—are thought to be part of an adaptive, motivational response to conserve energy and aid in recovery.4 Sickness behavior can be elicited by exposing animals to pro-inflammatory cytokines such as interleukin (IL)-1 or lippolysacccaride (LPS or endotoxin).5 Similarly, endotoxin administration has been used to model sickness behavior in humans.6
One of the key changes often mentioned in this collection of symptoms is disruptions in social behavior. Namely, social withdrawal or loss of interest in social activities is frequently cited as a principal component of inflammation-induced sickness behavior.5, 7 These changes in social behavior as a result of pro-inflammatory cytokine activity provided the first clues that inflammation may play an important role in shaping social behavior.
In order for the immune system to impact social behavior as part of sickness behavior, pro-inflammatory cytokines involved in peripheral inflammation would need to impact the central nervous system. Indeed, work in non-human animals has found increases in pro-inflammatory cytokines in the brain post-LPS administration in the periphery.8 Multiple pathways have been suggested to explain how peripheral immune activation could lead to a coordinated response by the brain to induce sickness behavior. These include activation of the vagus nerve, transport through the blood-brain barrier, and diffusion outside the blood-brain barrier in areas such as the choroid plexus.9
Although limited, there is some converging evidence in humans that intravenous endotoxin administration is also related to changes in inflammation in the central nervous system. Indeed, a study using positron emission tomography (PET) found that endotoxin led to increases in microglial activation,10 suggesting that LPS can activate a neuroinflammatory response. Furthermore, another study reported increases in the pro-inflammatory cytokine IL-6 in human cerebrospinal fluid following endotoxin administration.11 Together, these findings suggest that endotoxin administration and subsequent immune activation may end up impacting social behavior via central nervous system mechanisms.
Effects of inflammation on social experience
As noted above, a commonly listed component of sickness behavior is social withdrawal. Indeed, work in non-human animals indicates that heightened inflammatory activity leads to reduced social exploration,12 and data from humans showing that inflammation increases feelings of social disconnection support this.13 However, responses to inflammation appear to be context-dependent.14 For example, factors such as sleepiness can impact the effect of inflammation on sensitivity to reward.15 Similarly, in the social context, alterations towards this more negative or disconnected social state are not a definitive outcome of inflammation.16, 17 Work in both humans and non-human animals has provided evidence that in some cases, heightened inflammation may lead to an increased desire to affiliate.18, 19 Interestingly, the effects of inflammation on social behavior may depend on many factors, and whether to socially withdraw or connect may depend on which behavior would be more beneficial for survival. One factor that may drive this differentiation between socially withdrawing or socially affiliating behavior may be the familiarity of the other individual that the inflamed, sick individual is faced with.16 It may be evolutionarily advantageous to avoid socially threatening (or potentially threatening) individuals when one is sick and vulnerable. However, identifying a potential ally or source of care when one is sick is also evolutionarily advantageous, which could drive inflamed individuals to seek out familiar others. Below, we review the evidence for alterations in both negative and positive social experience as a consequence of inflammation, as well as a brief discussion of the effects on social cognition and a potentially unique role for interferon-gamma (IFN-γ), which has been referred to as an anti-viral cytokine.20 Understanding the effects of inflammation on social behavior is of vital importance, as social relationships are crucial for survival and well-being,21 and the effects of inflammation on social experience has historically been understudied, particularly in humans.
Effects of inflammation on negative social experience
It is reasonable to expect that inflammation may lead to heightened sensitivity to negative social experiences, as individuals who are experiencing sickness, and thus are in a vulnerable state, may need to more easily identify threats to their well-being. This increased sensitivity to negative social experiences may manifest behaviorally as a reduced interest in interacting with others or social withdrawal. In fact, work in non-human animals lends support to this idea. Rats injected with pro-inflammatory cytokines show reductions in their interest to explore novel juvenile rats, becoming more inhibited or more withdrawn in their social behavior.12, 22, 23 Indeed, this kind of reduction in social exploration has become so deeply entrenched in the animal model of sickness behavior that reviews of the state of the field have noted that “sickness behavior is frequently assessed as social exploration/investigation.”7 Thus, experimental work in non-human animals supports the idea that inflammation can lead to social withdrawal behavior.
Although work in animals is crucial in understanding the effects of inflammation on social processes, particularly social withdrawal, building on these studies in humans is essential in order to understand the effects on more subjective social experiences. For example, it is difficult or impossible to ascertain whether non-human animals feel socially disconnected or lonely after heightened inflammation, and thus, these complementary questions are necessary to examine in parallel human studies. In the first study exploring this in humans, Eisenberger and colleagues13 exposed subjects to an acute inflammatory challenge (endotoxin) or placebo and asked them to self-report on feelings of social disconnection (e.g., “I feel disconnected from others”). Building on the social withdrawal findings in the animal literature, results from this study showed that being exposed to endotoxin led to increased feelings of social disconnection. A later study, using a larger sample and a more comprehensive measure of social disconnection, replicated these findings that endotoxin leads to increases in feelings of social disconnection.24 Interestingly, these effects persist after controlling for sickness symptoms, which suggest that the effects of inflammation on feelings of social disconnection are not simply due to subjects feeling more sick (e.g., more fatigue) and instead represent a separate consequence of inflammation. Finally, another study in humans found that endotoxin leads to increased social anhedonia (“I want to be alone”).25 These studies in humans complement and extend the work in non-human animals, signifying that inflammation can lead to increases in subjective negative social experience such as feelings of social disconnection.
In addition to asking subjects to self-report on their socially relevant feelings, a complementary but distinct assessment is to examine their neural sensitivity to various social tasks. Changes in neural sensitivity may indicate neurocognitive factors that underlie changes in social behavior. For example, increased sensitivity to negative social experience (e.g., sensitivity to socially threatening experiences) may aid in explaining why inflammation increases feelings of social disconnection in humans and social withdrawal behavior in non-human animals. One type of negative social experience that predicts feelings of disconnection or loneliness is social rejection.26 In one study examining the effect of inflammation on neural sensitivity to social rejection,27 participants were given endotoxin or placebo and then completed a neuroimaging task at the height of the inflammatory response. While in the scanner, all participants were systematically excluded during an online ball-tossing game (Cyberball). Although there were no group differences in neural responses to rejection, among those in the endotoxin group, greater increases in inflammation were associated with greater activity in the dorsal anterior cingulate cortex (dACC) and the anterior insula (AI), which are often associated with pain-related activity.28, 29 Specifically, increases in the pro-inflammatory cytokine IL-6 were related to greater activity in the dACC and AI. Thus, it appears that increases in inflammation heighten neural sensitivity to the negative social experience of social rejection.
Another type of negative social experience is receiving negative feedback from others. Similar to social rejection, this type of social evaluation may present socially threatening information that may be particularly pertinent for vulnerable, sick individuals. It is perhaps unsurprising then that another study found that experimentally increasing inflammation led to heightened neural sensitivity to receiving negative social feedback.30 Subjects in this study who received endotoxin (vs. placebo) showed increases in the dACC and the amygdala, a neural region also involved in responses to threat,31 when receiving negative social feedback. This finding parallels the above results, suggesting that heightened inflammation can lead to increases in neural sensitivity to socially threatening information.
One question in response to these findings is whether these effects are specific to socially threatening experiences or whether they extend to all threatening experiences. In order to explore the social specificity of this effect, one study examined whether receiving endotoxin (vs. placebo) led to increases in neural sensitivity to social vs. non-social threatening stimuli.32 Subjects were shown four types of images: socially threatening stimuli (fear faces), non-socially threatening stimuli (guns), socially non-threatening stimuli (happy faces), and non-social, non-threatening stimuli (household items). Interestingly, endotoxin led to increases in threat-related neural activity—in the amygdala—in response to the socially threatening images (vs. all other types of stimuli). Furthermore, greater amygdala activity in response to socially threatening images (vs. non-socially threatening images) was associated with greater increases in self-reported feelings of social disconnection for participants who received endotoxin. Indeed, it appears that the effect of inflammation on threat was specific to social stimuli.
Overall, it appears that inflammation increases social withdrawal, feelings of social disconnection and anhedonia, as well as neural sensitivity to various kinds of negative social experience, including rejection, feedback, and threatening stimuli. This tendency for inflammation to heighten sensitivity to socially threatening information and increase social withdrawal behavior could potentially be adaptive. Avoiding others could aid in preventing exposure to threats to well-being for the vulnerable, infected individual, as well as prevent spread of infection in social networks.33
Effects of inflammation on positive social experience
Given the literature reviewed above, it would be easy to conclude that inflammation invariably leads to social withdrawal. However, some more work has found that, in some contexts, inflammation can actually lead to increases in social approach and sensitivity to social reward. Seeing as sick individuals are in a vulnerable state, it would be beneficial for them to be able to identify sources of help and care to aid in the recovery process (i.e., identifying and affiliating with close others). Indeed, some findings from studies with non-human animals showing increases in social behavior support this idea. Rhesus monkeys exposed to LPS (vs. saline) spent more time affiliating with their cage-mates, including being in close proximity to and clinging to this familiar other.19 Similarly, while rats exposed to LPS (vs. saline) decreased their active social behavior, they spent more time passively huddling with their cage-mates.34 This kind of increase in social behavior in response to inflammation has also been found in the context of pair bonding. Female prairie voles exposed to LPS (vs. saline) were faster to establish preferences for a male partner, suggesting that in some cases heightened inflammation may facilitate pair-bonding.35 These findings suggest that inflammation can lead to increases in social behavior, depending on the context, which may serve an adaptive purpose.17 Identifying and affiliating with others may provide sick individuals with resources to facilitate recovery from illness.
Although work in humans is limited in this area, some early findings parallel these findings from animal studies. In the first human study to directly test the effect of inflammation on sensitivity to close others, participants randomly received endotoxin or placebo. Then, at the peak of the inflammatory response, participants self-reported on their desire to be around their close others (“I feel like being around this person right now”) and completed a neuroimaging task.18 During the imaging portion, participants viewed images of their close others as well as sex-, race-, age-, and expression-matched strangers. In line with the behavioral findings in animals, participants reported a greater desire to be around their close others. Furthermore, participants who received endotoxin (vs. placebo) showed greater ventral striatum (VS) activity in response to viewing images of their close others (vs. strangers). The VS is a reward-related region, which has been shown to correlate with feelings of social connection.36 Interestingly, increases in the pro-inflammatory cytokine IL-6 in response to endotoxin were positively correlated with increases in VS activity. Together, these findings suggest that inflammation can lead to increased reward sensitivity to close others, as well as increases in desire to approach close others.
In another study examining neural sensitivity to reward, participants received positive feedback from an anonymous evaluator after being exposed to endotoxin or placebo.30 Similar to the findings in the previous study, participants who received endotoxin (vs. placebo) showed greater VS activity in response to receiving positive feedback. Endotoxin participants also showed greater activity in the ventromedial prefrontal cortex, another reward-related region.37 Although the evaluator in this study was not a close other, it is plausible the positive feedback alerted endotoxin participants that this person may be a potential ally. The enhanced reward-related activity in response to heightened inflammation may be adaptive in that it may aid vulnerable, sick individuals in identifying who may be able to provide assistance and care.
Although these studies suggest that inflammation can lead to increases in neural sensitivity to reward, it is important to point out that these are specifically in response to positive social experiences. Other work has pointed to reductions in reward-related activity in other domains in response to inflammation. For example, endotoxin (vs. placebo) can lead to reductions in VS activity in response to monetary reward.38 This distinction in direction of effects on reward sensitivity highlights the need to study social (vs. non-social) effects of inflammation.
Overall, it is important to note that inflammation can lead to increases in sensitivity to positive social stimuli, including affiliative behavior in non-human animals and increases in desire to affiliate in humans, as well as increases in neural sensitivity to socially rewarding experiences. This increased neural sensitivity and affiliative drive towards close others may be adaptive, in that it may allow the sick, vulnerable individual to receive care and support from a close other who can aid in recovery.
Other social effects of inflammation: social cognition
Another area of social experience that may be altered by inflammation and is vital to social functioning is social cognition or the ability to think about the minds (e.g., thoughts, feelings) of others. Indeed, alterations in inflammatory processes are implicated in the etiology of psychiatric disorders marked by social cognitive deficits such as schizophrenia and autism.39 For example, individuals with autism and schizophrenia often have difficulties accurately interpreting the emotional states of others and also have poor social relationships in general, including experiencing social isolation.40–42 Lending support to the notion that inflammation may drive social cognitive changes, one study in humans found that endotoxin (vs. placebo) led to declines in social cognitive performance.43 In this study, participants at the peak of inflammatory response completed the Reading the Mind in the Eyes (RME) test, which assesses ability to accurately identify others’ emotional states.44 Endotoxin participants (vs. placebo) decreased in their performance on this task, suggesting that inflammation may lead to impairments in social cognitive performance, and these effects remained after controlling for factors such as sickness behavior and mental confusion.
It is worth noting that another study using the same task but a lower dose of endotoxin and a smaller sample size did not find such differences in performance between the endotoxin and placebo groups.45 However, participants in this study completed the RME test in a neuroimaging environment, and the neural results revealed that endotoxin (vs. placebo) participants showed increased activity in neural regions related to social cognition. The authors suggest that this increased activity may be due to endotoxin-exposed individuals working harder to engage in a normal level of social cognitive processing, engaging in a compensatory strategy due to their compromised social cognitive ability. This enhanced activity in social cognition regions, coupled with the diverging findings on actual performance on social cognitive tasks, suggest that inflammation may play an important role in shaping social cognition but that future research is needed to more fully flesh out these effects.
A potentially unique role for interferon gamma (IFN-γ)?
Pro-inflammatory cytokines such as IL-1 and tumor necrosis factor (TNF)-α play a key role in inflammation-induced sickness behavior.7, 9 Another molecule, IFN-γ, which has been labeled as an anti-viral cytokine,20 has recently begun to emerge as another interesting contributor to the effect of the immune system on social behavior. Unlike primary pro-inflammatory cytokines—which are produced mainly by myeloid lineage, innate immune cells such as monocytes—IFN-γ is primarily produced by activated T cells and facilitates Th1 adaptive immune responses against intracellular pathogens such as viruses.46 IFN-γ can also indirectly promote inflammation by stimulating the antimicrobial activity of monocytes.46 Given both its association with and distinction from pro-inflammatory cytokines, it is perhaps unsurprising that the effect of IFN-γ on social behavior appears to be complicated.
One clue that IFN-γ was an interesting immune marker to examine in the context of social behavior comes from the noted relationships between IFN-γ and psychiatric disorders, which are often marked by alterations in social functioning. Unlike pro-inflammatory cytokines, where many disorders (e.g., major depressive disorder, bipolar disorder, schizophrenia) are associated with elevations in these markers,47 psychiatric disorders have been associated with both increased and decreased IFN-γ.48 For example, individuals with schizophrenia may display decreased IFN-γ49 but individuals with autism may display increased levels.50 Because of this inconsistency, it is unclear whether increases or decreases in IFN-γ are beneficial or detrimental.48
Recently, animal models using IFN-γ knockout mice are beginning to shed some light on potential reasons for this divergence. In one study, IFN-γ deficiency led to enhanced performance on cognitive tasks.51 In contrast, another study using IFN-γ knockout mice found that lacking IFN-γ led to deficits in the social domain.20 As such, it may be that alterations in this molecule may differentially impact the social vs. non-social domains, and of course, these relationships may also be altered by interactions with other biopsychosocial factors. Further research is needed, in both animals and humans, to determine whether increases in IFN-γ are related to social benefits (whereas decreases may be related to cognitive benefits) and in which contexts.
Implications for health
As outlined in this review, inflammation plays a role in shaping social behavior, including increasing sensitivity to both negative and positive social experience. These findings emphasize the importance of studying the effect of inflammation on a variety of social vs. non-social experiences in order to better understand the contexts and moderating factors of the influence of inflammation on social experience. Developing a better understanding of the effects of inflammation on social processes is particularly important when trying to extend this work into the implications of these findings for health.
Many psychiatric illnesses, including depression, have been associated with alterations in inflammatory processes.47 It has been proposed that heightened inflammation may play a role in the etiology of depression, at least in a subset of patients with depression.52, 53 Indeed, pro-inflammatory cytokines are elevated in individuals with depression.47 Furthermore, experimental studies using endotoxin in humans have found that experimentally increasing inflammation can lead to increases in depressed mood in otherwise healthy subjects.13, 24, 54 Given that depression is marked by “social risk factors, social impairments, and poor social functioning”,55 understanding the interactions between inflammatory and social processes could be a key factor in more fully understanding the links between inflammation and depression.
Loneliness, or feelings of social disconnection, is one such social factor. Although loneliness and depression are separable and distinct (i.e., not all individuals who are lonely are depressed and not all individuals with depression feel lonely),56–58 loneliness is very relevant to depression. Loneliness increases the risk for depression,59 and characteristics of depression include reduced social contact and social isolation.60, 61 Furthermore, as discussed above, experimentally inducing inflammation using endotoxin can lead to increases in both feelings of social disconnection and depressed mood.13, 24, 25, 54 In fact, one study found that increases in feelings of social disconnection mediated the relationship between inflammation and depressed mood.13 Given these findings, it has been suggested that loneliness may play a key role in inflammatory subtypes of depression.16 More broadly, these relationships suggest that further elucidating the links between social disconnection and inflammation may have implications for better understanding and treating depression.
In addition to its relationship with depression, loneliness and social isolation are constructs worth study in their own regard, particularly given their impact on health. Loneliness is associated with many physical and mental disorders,56 and perhaps most compellingly, the effect of social isolation on mortality is on par with risk factors such as smoking or obesity.62, 63 Given the noteworthy impact of loneliness on health outcomes, further understanding of the relationships between inflammation and social processes may also lead to breakthroughs in the treatment of loneliness, which may ultimately lead to health benefits. One potential avenue of future research is to test the effect of anti-inflammatory medications on loneliness. As discussed above, inflammation leads to increases in feelings of social disconnection, increases in sensitivity to social threat, and increases in sensitivity to social reward. Interestingly, lonely individuals also exhibit higher levels of inflammatory biology, as well as increased sensitivity to social threat64–66 and social reward.67 Given this overlap in the psychological states in both high levels of inflammation and high-lonely individuals, it is possible that dampening this inflammatory response through pharmacological intervention may lead to reductions in loneliness and improvements in health. Relatedly, researchers have conducted trials using anti-inflammatory drugs to treat depressive symptoms, and a recent meta-analysis found that this may be an effective treatment for decreasing these symptoms.68 However, these trials have not examined the effects of these drugs on loneliness or examined loneliness in the context of depressed individuals. As such, this remains an open, promising area of research.
Finally, another implication of the relationships between social processes and inflammation on health is in populations with chronic inflammatory disorders. Individuals with these disorders (e.g., rheumatoid arthritis, lupus) may experience alterations in social experiences for numerous reasons, including facets of the disease (e.g., pain, disability) that may alter social relationships. The findings discussed in this review would also suggest that the underlying inflammatory processes of the disease may also be directly impacting patients’ sensitivity to social experiences. Under chronic conditions, it is possible that some patients develop hypersensitivity to socially threatening experiences, potentially exacerbating tendencies to socially withdraw, as well as hypersensitivity to socially rewarding experiences with close others, potentially putting undue strain and expectations on those relationships. Furthermore, given the unprecedented growth in the percentage of the worldwide population over age 65 and that many diseases of aging involve inflammation (e.g., cardiovascular disease, Alzheimer’s disease), understanding the effects of inflammation on social processes could have broad public health implications.69–71 It is also possible that the findings in this paper, based on the effects of acute inflammation, may play out differently in a chronic inflammatory context. Indeed, this is an understudied area of research which may have wide-ranging implications for patients with inflammatory disorders and an aging population.
Conclusion
Overall, it appears that inflammation is a powerful organizer of social behavior. These changes in neural sensitivity and behavior as a result of inflammation—including increases in sensitivity to both negative and positive social experience, dependent on context—are likely adaptive, allowing the individual to allocate resources for rest and recovery and to identify close others who may help with this process. These relationships between inflammation and social processes have various health implications, including for individuals with depression, lonely individuals, and those with chronic inflammatory disorders, emphasizing the need to further study and understand these complex relationships.
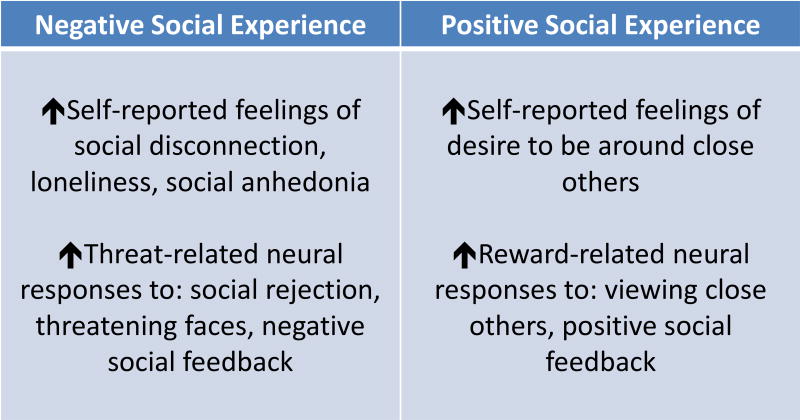
Figure 1.
Figure summarizing the effects of experimental inflammation on negative and positive social experiences in humans. These divergent effects (increases in sensitivity to negative social experience and increases in sensitivity to positive social experience) may depend on context. For example, individuals with heightened inflammation, in their vulnerable state, may choose to avoid potentially threatening individuals but also may choose to affiliate with familiar or close others as they may be potential sources of care and comfort.
Acknowledgments
This research was funded, in part, by an R01 from NIMH to N.I.E. (5R01MH091352). We acknowledge the additional support provided to M.M. by a Cousins Center for Psychoneuroimmunology Post-Doctoral Fellowship.
Footnotes
Competing Interests
The authors declare on conflicts of interest.
References
- 1.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 2.Marx J. Inflammation and cancer: the link grows stronger: research into a long-suspected association between chronic inflammation and cancer reveals how the immune system may be abetting tumors. Science. 2004;306:966–969. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, et al. Markers of inflammation and cardiovascular disease. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelley KW, et al. Cytokine-induced sickness behavior. Brain, behavior, and immunity. 2003;17:112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 6.Schedlowski M, Engler H, Grigoleit J-S. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain, behavior, and immunity. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 7.McCusker RH, Kelley KW. Immune–neural connections: how the immune system’s response to infectious agents influences behavior. Journal of Experimental Biology. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakizaki Y, et al. Temporal profiles of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: estimation by push-pull perfusion. Endocrine journal. 1999;46:487. doi: 10.1507/endocrj.46.487. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandiego CM, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences. 2015;112:12468–12473. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler H, et al. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Molecular Psychiatry. 2017 doi: 10.1038/mp.2016.264. [DOI] [PubMed] [Google Scholar]
- 12.Bluthe R, et al. Synergy between tumor necrosis factor α and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberger NI, et al. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, behavior, and immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin MR, Eisenberger NI. Context-Dependent Effects of Inflammation: Reduced Reward Responding is Not an Invariant Outcome of Sickness. Neuropsychopharmacology. 2017;42:785. doi: 10.1038/npp.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasselin J, et al. Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacology. 2017;42:801. doi: 10.1038/npp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberger NI, et al. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology. 2017;42:242. doi: 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hennessy MB, Deak T, Schiml PA. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain, behavior, and immunity. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inagaki TK, et al. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain, behavior, and immunity. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain, behavior, and immunity. 2007;21:807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filiano AJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological bulletin. 1995;117:497. [PubMed] [Google Scholar]
- 22.Bluthe R-M, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain research. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 23.Marvel FA, et al. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain, behavior, and immunity. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Moieni M, et al. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannestad J, et al. Citalopram reduces endotoxin-induced fatigue. Brain, behavior, and immunity. 2011;25:256–259. doi: 10.1016/j.bbi.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boivin M, Hymel S, Bukowski WM. The roles of social withdrawal, peer rejection, and victimization by peers in predicting loneliness and depressed mood in childhood. Development and Psychopathology. 1995;7:765–785. [Google Scholar]
- 27.Eisenberger NI, et al. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 29.Rainville P, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 30.Muscatell KA, et al. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain, behavior, and immunity. 2016;57:21–29. doi: 10.1016/j.bbi.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki TK, et al. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole SW. The complexity of dynamic host networks. Complex systems science in biomedicine. 2006:605–629. [Google Scholar]
- 34.Yee JR, Prendergast BJ. Sex-specific social regulation of inflammatory responses and sickness behaviors. Brain, behavior, and immunity. 2010;24:942–951. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilbo SD, et al. Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiology & behavior. 1999;68:151–156. doi: 10.1016/s0031-9384(99)00154-7. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki TK, Eisenberger NI. Shared neural mechanisms underlying social warmth and physical warmth. Psychological Science. 2013;24:2272–2280. doi: 10.1177/0956797613492773. [DOI] [PubMed] [Google Scholar]
- 37.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenberger NI, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation. Pediatr Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brüne M, Brüne-Cohrs U. Theory of mind—evolution, ontogeny, brain mechanisms and psychopathology. Neuroscience & Biobehavioral Reviews. 2006;30:437–455. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Magiati I, Tay XW, Howlin P. Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: a systematic review of longitudinal follow-up studies in adulthood. Clinical Psychology Review. 2014;34:73–86. doi: 10.1016/j.cpr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Millier A, et al. Humanistic burden in schizophrenia: a literature review. Journal of psychiatric research. 2014;54:85–93. doi: 10.1016/j.jpsychires.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Moieni M, et al. Inflammation impairs social cognitive processing: a randomized controlled trial of endotoxin. Brain, behavior, and immunity. 2015;48:132–138. doi: 10.1016/j.bbi.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron-Cohen S, et al. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:241–251. [PubMed] [Google Scholar]
- 45.Kullmann JS, et al. Experimental human endotoxemia enhances brain activity during social cognition. Social cognitive and affective neuroscience. 2013;9:786–793. doi: 10.1093/scan/nst049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy K, Weaver C. Janeway's immunobiology. Garland Science 2016 [Google Scholar]
- 47.Najjar S, et al. Neuroinflammation and psychiatric illness. Journal of neuroinflammation. 2013;10:816. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monteiro S, et al. Brain interference: Revisiting the role of IFNγ in the central nervous system. Progress in neurobiology. 2017;156:149–163. doi: 10.1016/j.pneurobio.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Arolt V, et al. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Molecular Psychiatry. 2000;5:150. doi: 10.1038/sj.mp.4000650. [DOI] [PubMed] [Google Scholar]
- 50.Li X, et al. Elevated immune response in the brain of autistic patients. Journal of neuroimmunology. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monteiro S, et al. Absence of IFNγ promotes hippocampal plasticity and enhances cognitive performance. Translational psychiatry. 2016;6:e707. doi: 10.1038/tp.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller AH, Raison CL. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA psychiatry. 2015;72:527–528. doi: 10.1001/jamapsychiatry.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichenberg A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 55.Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neuroscience & Biobehavioral Reviews. 2016;69:313–332. doi: 10.1016/j.neubiorev.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Cacioppo S, et al. Loneliness: Clinical import and interventions. Perspectives on Psychological Science. 2015;10:238–249. doi: 10.1177/1745691615570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perissinotto CM, Cenzer IS, Covinsky KE. Loneliness in older persons: a predictor of functional decline and death. Archives of internal medicine. 2012;172:1078–1084. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stek ML, et al. Is depression in old age fatal only when people feel lonely? American journal of psychiatry. 2005;162:178–180. doi: 10.1176/appi.ajp.162.1.178. [DOI] [PubMed] [Google Scholar]
- 59.Cacioppo JT, et al. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychology and aging. 2006;21:140. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- 60.Rottenberg J, Gotlib IH. Socioemotional functioning in depression. Mood disorders: A handbook of science and practice. 2004:61–77. [Google Scholar]
- 61.Wade TD, Kendler KS. The relationship between social support and major depression: cross-sectional, longitudinal, and genetic perspectives. The Journal of nervous and mental disease. 2000;188:251–258. doi: 10.1097/00005053-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Holt-Lunstad J, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspectives on Psychological Science. 2015;10:227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 63.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS medicine. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in cognitive sciences. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cacioppo S, et al. Loneliness and implicit attention to social threat: A high-performance electrical neuroimaging study. Cognitive neuroscience. 2016;7:138–159. doi: 10.1080/17588928.2015.1070136. [DOI] [PubMed] [Google Scholar]
- 66.Qualter P, et al. Loneliness across the life span. Perspectives on Psychological Science. 2015;10:250–264. doi: 10.1177/1745691615568999. [DOI] [PubMed] [Google Scholar]
- 67.Inagaki TK, et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Social cognitive and affective neuroscience. 2015;11:1096–1101. doi: 10.1093/scan/nsv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Köhler O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 69.Libby P. Inflammation and cardiovascular disease mechanisms–. The American journal of clinical nutrition. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 70.Population Reference Bureau. World Population Aging: Clocks Illustrate Growth in Population Under Age 5 and Over Age 65 2011 [Google Scholar]
- 71.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. The international journal of biochemistry & cell biology. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]