Abstract
The cyclic nucleotide cGMP is an intracellular second messenger with important roles in neuronal functions and animals’ behaviors. The phosphodiesterases (PDEs) are a family of enzymes that hydrolyze the second messengers cGMP and cAMP. Inhibition of phosphodiesterase 9 (PDE9), a main isoform of PDEs hydrolyzing cGMP, has been shown to improve learning and memory as well as cognitive function in rodents. However, the role of PDE9 in regulating neuronal structure and function in vivo remains unclear. Here we used in vivo two-photon microscopy to investigate the effect of a selective PDE9 inhibitor PF-04449613 on the activity and plasticity of dendritic spines of layer V pyramidal neurons in the mouse primary motor cortex. We found that administration of PF-04449613 increased calcium activity of dendrites and dendritic spines of layer V pyramidal neurons in mice under resting and running conditions. Chronic treatment of PF-04449613 over weeks increased dendritic spine formation and elimination under basal conditions. Furthermore, PF-04449613 treatment over 1-7 days increased the formation and survival of new spines as well as performance improvement after rotarod motor training. Taken together, our studies suggest that elevating the level of cGMP with the PDE9 inhibitor PF-04449613 increases synaptic calcium activity and learning-dependent synaptic plasticity, thereby contributing to performance improvement after learning.
Keywords: Phosohodiesterase 9 inhibitor, Synaptic calcium activity, Dendritic calcium activity, Synaptic plasticity, Rotarod training
Introduction
Phosphodiesterases (PDEs) are a superfamily of enzymes that hydrolyze the second messengers cGMP and cAMP. In mammals, there are ~100 isoforms of PDEs, which are subcategorized into 11 families (PDE1-11) (Beavo, 1995; Bender et al., 2006; Liu et al., 2008; Wunder et al., 2005). PDEs have different substrate preferences: PDE4, PDE7 and PDE8 hydrolyze cAMP, whereas PDE5, PDE6 and PDE9 are cGMP-specific. PDE1, PDE2, PDE3, PDE10 and PDE11 can hydrolyze both cAMP and cGMP (Mehats et al., 2002). Of all PDEs, PDE9 has the highest affinity for cGMP (Fisher et al., 1998).
The cyclic nucleotide cGMP is one of the key intracellular second messengers in the N-methyl-D-aspartate (NMDA) signaling transduction pathway that regulates synaptic plasticity (Serulle et al., 2007; Son et al., 1998). In postsynaptic neurons, NMDA receptor activation increases calcium and activates calcium/calmodulin-dependent neuronal NO synthases, which lead to the generation of cGMP via NO-sensitive soluble guanylyl cyclase (East et al., 1990; Garthwaite, 1991). cGMP-dependent protein kinase II (cGKII), which phosphorylates GluA1, is important for promoting long-term synaptic potentiation (LTP) (Kim et al., 2015; Lu et al., 1999; Serulle et al., 2007). Consistently, inhibition of PDE9 has been shown to increase cGMP in the brain tissue and cerebrospinal fluid (CSF) (Hutson et al., 2011; Kleiman et al., 2012; van der Staay et al., 2008) and facilitate LTP in hippocampal culture (Hutson et al., 2011) and slices (Kroker et al., 2014; Kroker et al., 2012; van der Staay et al., 2008).
Given the fact that the NMDA-NO-cGMP-PKG pathway is critically involved in learning and memory (Arancio et al., 1995; Bernabeu et al., 1997; Taqatqeh et al., 2009) and that patients with Alzheimer’s disease (AD) and Huntington’s disease (HD) and Schizophrenia show reduced levels of cGMP in their CSF (Beckmann et al., 2002; Bonkale et al., 1995; Gattaz et al., 1983; Hesse et al., 2017; Saavedra et al., 2013), blocking the activity of PDEs to increase cGMP levels has been suggested to treat cognitive and memory deficits in various diseases (Domek-Lopacinska et al., 2010; Duinen et al., 2015; Fusco et al., 2015). Indeed, several studies have shown that selective PDE9 inhibitors significantly improve cognitive and learning performance in rodents under various behavioral paradigms (social recognition (van der Staay et al., 2008), passive avoidance (van der Staay et al., 2008), T-maze (van der Staay et al., 2008) and object place memory (Kroker et al., 2014) in mice for PDE9 inhibitor BAY 73-6691; Y-maze (Hutson et al., 2011), social recognition (Hutson et al., 2011) in mice; and novel object recognition (Hutson et al., 2011), reversed amphetamine-induced deficit in auditory gating (Kleiman et al., 2012) in rats for PF-04447943).
Despite the beneficial effects of PDE9 inhibitors on learning and behavioral improvements (Hutson et al., 2011; Kleiman et al., 2012; Kroker et al., 2014; van der Staay et al., 2008), how PDE9 inhibitors affect the processes of learning and memory in vivo remains unclear. In the present work, we used two-photon microscopy to examine the effect of inhibiting PDE9 on synaptic activity and plasticity in vivo with a potent and selective inhibitor PF-04449613 (6-[(1R)-1-(3-phenoxyazetidin-1-yl)ethyl] -1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo [3,4-d] pyrimidin-4-one) (Claffey et al., 2012b; Kleiman et al., 2012; Lee et al., 2015). We found that PF-04449613 treatment increased dendritic and spine calcium activity of layer V pyramidal neurons under resting and running conditions in the mouse primary motor cortex. Furthermore, treatment of PF-04449613 increased the degree of dendritic spine formation and survival, as well as performance improvement after motor training. These findings suggest that PDE9 inhibitors facilitate learning and memory by increasing synaptic plasticity and activity.
Materials and methods
Animals
Mice expressing YFP predominantly in cortical layer V pyramidal cells were purchased from the Jackson Laboratory (YFP-H line) and housed in the animal facility in the Peking University Shenzhen Graduate School (PKUSZ). Mice were maintained at 22 ± 2 °C with a 12-hour light/dark cycle (lights on at 8 a.m., lights off at 8 p.m.). Food and water were available ad libitum. Transgenic mice expressing GCaMP6 in layer V pyramidal neurons (GCaMP6S line1 mice) were generated in the Gan lab and housed in the Skirball Animal Facility at New York University School of Medicine. Four- to five-week-old male and female mice were used in the present study. All experimental protocols were conducted in accordance with the institutional guidelines.
Drugs
PF-04449613 (6-[(1R)-1-(3-phenoxyazetidin-1-yl)ethyl]-1-(tetrahydro-2H-pyran -4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) was provided by Pfizer and also purchased from Sigma-Aldrich (CAS Number: 1236858-52-8). PF-04449613 is a selective and potent inhibitor of PDE9, which has been shown to significantly increase the cerebral cGMP level within 30 min from 1 mg/kg to 32 mg/kg in a dose-dependent manner (Kleiman et al., 2012). The drug was dissolved in vehicle 5/5/90 (5% DMSO/5% Cremophor/90% Injection Saline).
Surgical preparations for imaging awake, head-restrained mice
Ca2+ imaging was carried out in awake, head-restrained mice through a thinned-skull window according to previous studies (Cichon et al., 2015; Yang et al., 2013) (Fig. 1A). Surgical preparations were done 24 h before imaging. Briefly, mice were deeply anesthetized with ketamine (100 μg/g) and xylazine (10 μg/g). The mouse’s head was shaved and followed by a scalp incision; and the skull surface was exposed, cleaned, and applied with a thin layer of cyanoacrylate-based glue. The imaging area (~1 mm in diameter) in the forelimb motor cortex was identified based on stereotactic coordinates (1.3 mm anterior to bregma, 1.2 mm lateral from midline). A head holder composed of two parallel light metal bars was attached to the mouse’s skull anterior and posterior to the motor cortex. The head holder helped restrain the animal’s head and reduced motion-induced artefact during imaging. The bars were mounted to the skull outside the imaging region with dental acrylic cement, and the imaging area was covered by 1% agar or silicone elastomer (World Precision Instruments). After the surgery, the mice were returned to their own cages to recover before next-day imaging.
Figure 1. Subcutaneous administration of PF-04449613 increased spine calcium activity in motor cortex.

(A) Experimental paradigm for spine and dendritic calcium imaging under resting and running conditions. (B) Images and fluorescent traces of representative spines of layer V pyramidal neurons expressing GCaMP6S during 1 min resting and 1 min running period for vehicle and PF-04449613 treated group. Yellow circles indicate spines. Gray traces and blue traces on the right panels represent before and after either vehicle or PF-04449613 administration respectively. (C-J) Changes of calcium transients were quantified as changes of average amplitude, frequency, duration and integrated activity. Data of before and after either vehicle or PF-04449613 application were compared during resting (C-F) and running period (G-J). Data are presented as mean value ± S.E.M.. n.s. P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired nonparametric test. Scale bar, 10 μm.
Two-photon Ca2+ imaging of spines and dendrites of L5 neurons expressing GCaMP6S
Transgenic mice expressing GCaMP6 in layer V pyramidal neurons (GCaMP6S line 1) were used for Ca2+ imaging of dendritic and dendritic spine activity. 24 h after surgical preparation, mice were head-restrained to a metal base and the skull of the imaged area was thinned using a high-speed drill under a dissecting microscope. The skull of interest was immersed in artificial cerebrospinal fluid (ACSF) during drilling and carefully polished with a microsurgical blade to ~20 μm in thickness. Next, head-retrained mice were placed on the top of a custom-built free-floating treadmill and habituated about 10 min. GCaMP6-positive spines and dendrites at the depth of 0-50 μm below the pial surface were imaged for detecting Ca2+ activities. Mice were recorded over 2 trials of resting and 2 trials of treadmill running before and about 30 minutes after subcutaneous administration of either drug or vehicle. Each trial lasted over 2 minutes. The treadmill was set at a slow speed about 1 m/min.
Ca2+ images were acquired at 2 Hz using an Olympus two-photon microscope (FV1000MPE) equipped with a Ti:Sapphire laser (MaiTai DeepSee, Spectra Physics) tuned to 935 nm. All imaging were performed using a 25× objective (numerical aperture 1.1) immersed in ACSF solution with a 3× digital zoom.
Transcranial two-photon imaging of dendritic spine dynamics
Transcranial dendritic spine imaging was performed according to previous studies (Grutzendler et al., 2002; Yang et al., 2009; Yang et al., 2010; Zuo et al., 2005) (Fig. 3A). In brief, mice were anesthetized with pentobarbital sodium (100 mg/kg, intraperitoneally). The periosteum was exposed by a midline incision of the scalp and then removed with a microsurgical blade. The forelimb region of motor cortex to be imaged was identified based on stereotactic coordinates (1.3 mm anterior to bregma, 1.2 mm lateral from midline) and marked with a fine marker. The head was then immobilized, and a high-speed micro-drill and microsurgical blade were used to thin a circular area of the skull over the forelimb region to ~20 μm in thickness. Image stacks of dendritic segments projecting to superficial cortical layers were obtained using an Olympus two-photon microscope (FV1000MPE) with the laser tuned to 920 nm and with a 60× objective immersed in ACSF solution. A 3× digital zoom was used to yield high-magnification images suitable for quantification of dendritic spines. For multiple imaging, the above procedure was repeated and the localization of the same region was facilitated by low-magnification image stacks at 1× digital zoom and with reference to vascular landmarks in light microscopic photographs of the thinned skull area.
Figure 3. Chronic PF-04449613 treatment increased dendritic spine remodeling.
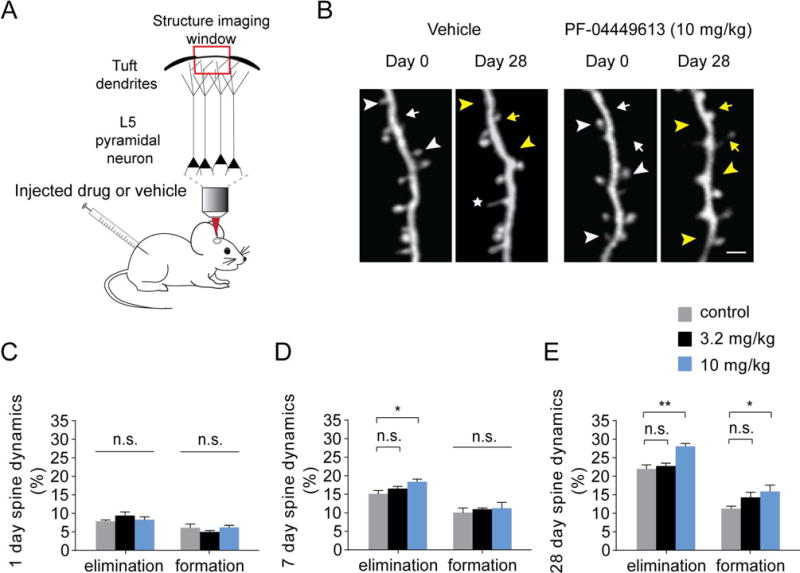
(A) Schematic of two-photon dendritic spine imaging of layer V pyramidal neurons expressing YFP. Animals were injected with PF-04449613 or vehicle for 1, 7 or 28 days. (B) Day 0 and day 28 imaging of dendritic spines of layer V pyramidal neurons in vehicle injected and 10 mg/kg PF-04449613 injected mice. Arrows and arrowheads indicate spine formation and elimination on day 28, respectively. Yellow arrows and arrowheads indicate newly formed spines and eliminated spines, respectively. An asterisk marks a filopodium. (C) PF-04449613 did not affect spine dynamics over 1 day (n = 5, 6, 7 mice for control, 3.2 mg/kg, 10 mg/kg respectively). (D) 7-day treatment of 10 mg/kg PF-04449613 increased spine elimination rate but did not change spine formation rate (n = 5, 6, 6 mice for control, 3.2 mg/kg, 10 mg/kg respectively). (E) 28-day treatment of 10 mg/kg PF-04449613 increased the rates of both spine elimination and formation (n = 6, 5, 5 mice for control, 3.2 mg/kg, 10 mg/kg respectively). Data are presented as mean value ± S.E.M.. n.s. P > 0.05, *P < 0.05, **P < 0.01 versus vehicle group according to ANOVA followed by the Dunnett’s test. Scale bar, 2 μm.
Rotarod training/testing procedure
The Rotarod training/testing procedure was performed as described before (Yang et al., 2009). A Rotarod system with six individual chambers (Taimeng Technology Corporation of Chengdu, China) was used in this study. Animals were placed on the motorized rod (30 mm in diameter) in the chamber. The rotation speed gradually increased from 0 to 100 r.p.m. over the course of 180 seconds. The time latency and rotation speed were recorded when the animal was unable to keep up with the increasing speed and fell. Each rotarod training session consisted of 20 trails and lasted less than 30 minutes.
Examine the effects of PF-04449613 on dendritic spine plasticity and motor learning
To test the effect of PF-04449613 on dendritic spine turnover under basal conditions, mice were anesthetized and image stacks of dendritic segments were obtained at about 5:00 p.m. (day 0). PF-04449613 (3.2 mg/kg and 10 mg/kg) or control vehicle (5/5/90: 5% DMSO/5% Cremophor/90% Injection Saline) were administered subcutaneously at a volume of 10 ml/kg at 6:00 p.m.; On the next day (day 1), PF-04449613 or vehicle were administered twice (9:00 a.m. and 6:00 p.m.), and image stacks were obtained at about 5:00 p.m.; PF-04449613 or vehicle were administered twice a day for an additional 6 or 27 days; and image stacks were obtained only on day 7 or 28.
To test the effect of PF-04449613 on motor learning-induced dendritic spine plasticity, mice were anesthetized and image stacks of dendritic segments were acquired in the morning on day 0. Mice were then trained on an accelerated rotarod for 20 trials at ~6:00 p.m. PF-04449613 (10 mg/kg) or control vehicle were administered immediately after the training. On the following days, PF-04449613 or vehicle were administered twice a day (9:00 a.m. and 6:00 p.m.) until the end of experiment. On day 1 and 7, image stacks were obtained after rotarod training/testing in the afternoon.
Analysis of Ca2+ imaging data
Ca2+ transients of spines and dendrites during resting and running periods, as indicated by changes of GCaMP6 fluorescence (F), were analyzed post hoc using Image J software (NIH). Visually identifiable spines and dendrites were selected as regions of interests (ROI) for quantification. A background value of vessels was first subtracted from GCaMP6 fluorescence values. All Ca2+ transients were calculated as ΔF/F0, where ΔF/F0 was (F-F0)/F0, and F0 was defined as the average of 10% minimum F fluorescence intensity over 1 min as described previously (Bai et al., 2017; Zhao et al., 2017). Because the frequencies of calcium transients in dendrites and spines were low (few times per minute), >10% of fluorescence traces did not contain calcium transients from active spines or calcium spikes from active dendrites. Each dendritic Ca2+ transient was defined as an event when changes of ΔF/F0 in dendritic shaft (average length >30 μm) were above 50% for GCaMP6S. As described previously (Chen et al., 2013; Cichon et al., 2015), for spine transient activity, back propagating action potential-related Ca2+ component was removed from spine Ca2+ transient signals by subtracting 70% of the dendritic shaft signal, measured as ΔF/F0 spine_specific = (F-F0)/F0 spine – 0.7 * (F-F0)/F0 dendrite. The threshold for a Ca2+ signal is >3 s.d. of baseline fluorescence noise and > 50% for GCaMP6S. The amplitude is the average of the peak ΔF/F0 of each Ca2+ transient over 1 min. The duration is the average of the duration of each Ca2+ transient over 1 min. The frequency is the number of Ca2+ transients per 1 min and the integrated activity is the sum of Ca2+ transients above threshold over 1 min.
Analysis of dendritic spine formation and elimination
The procedure for quantifying spine dynamics has been described in the earlier studies (Li et al., 2017; Yang et al., 2009). In brief, image stacks were analyzed post hoc using NIH ImageJ software. For each dendritic segment analyzed, filopodia were identified as long, thin protrusions with the ratio of head diameter to neck diameter <1.2:1 and ratio of length to neck diameter > 3:1. The remaining protrusions were classified as spines. Spines or filopodia were considered the same between views if their positions remained the same distance from relative adjacent landmarks. Spines were considered different if they were more than 0.7 μm away from their expected positions based on the first view.
Statistics
All data were presented as mean value ± standard error of the mean (S.E.M.). We used one-sample Kolmogorov-Smirnov test to determine the normality of data distribution. Mann-Whitney nonparametric tests were used to test for differences between groups whose distributions did not pass the tests for normality, and Student’s test was used between groups with normal distributions. Mann-Whitney nonparametric tests (two-tailed) were used to test the differences between PF-04449613 groups and vehicle groups in Ca2+ imaging results (Figs. 1 and 2). Significant differences of spine dynamics (percentage of added and eliminated spines) between groups were determined by the Student’s t test (groups treated with only 10 mg/kg PF-04449613 and vehicle, Fig. 4) or Dunnett’s test for multiple comparisons after one-way ANOVA (groups treated with 3.2 mg/kg and 10 mg/kg PF-04449613 and vehicle, Fig. 3). In the behavioral test, motor skill performance was quantified by the average running speed that mice mastered on rotarod (the speed that the animal was unable to keep up with the increasing speed and fell). Significant differences between behavioral tests were determined by the Student’s t test (Fig. 4). Significant levels were set at P ≤ 0.05. All statistical analyses were performed using IBM SPSS statistics or GraphPad Prism software.
Figure 2. PF-04449613 administration increased dendritic calcium activity in motor cortex.
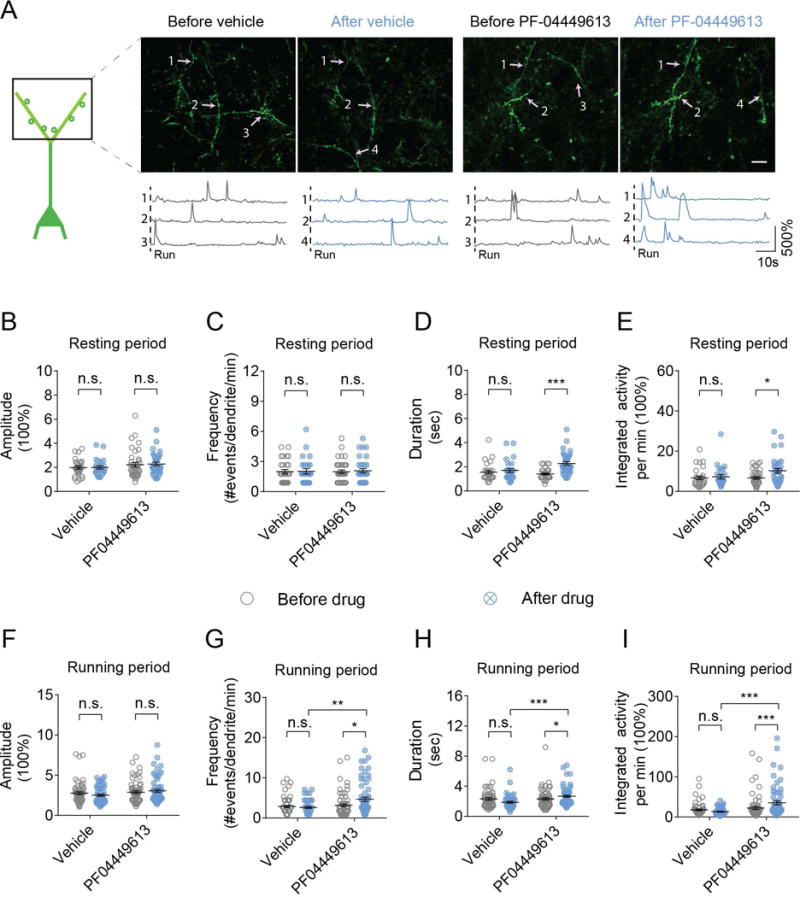
(A) Images and fluorescent traces of representative apical tuft dendrites of layer V pyramidal neurons expressing GCaMP6S during 1 min running period. Different dendrites active during running period are merged in presented images. Gray traces and blue traces represent before and after either vehicle or PF-04449613 administration respectively. Vertical dash lines represent the onset of treadmill running. (B-I) Changes of dendritic calcium transients were quantified as changes of average amplitude, frequency, duration and integrated activity. The average amplitude, frequency, duration and integrated activity of dendritic calcium transients were compared before and after either vehicle or PF-04449613 administration under resting state (B-E) and running state (F-I). Data are presented as mean value ± S.E.M.. n.s. P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired nonparametric test. Scale bar, 10 μm.
Figure 4. PF-04449613 administration increased spine turnover and performance improvement after motor learning.

(A) Repeated imaging of dendritic spines of layer V pyramidal neurons expressing YFP in vehicle and 10 mg/kg PF-04449613 injected mice after rotarod training. Yellow arrows and arrowheads indicate newly formed spines and eliminated spines after training, respectively. An asterisk marks a filopodium. 1-day (B) and 7-day (C) treatment of 10 mg/kg PF-04449613 increased the rates of both spine elimination and formation after rotarod training (n = 6 pairs of mice for vehicle and 10 mg/kg PF-04449613). (D) Effect of PF-04449613 administration on the survival rate of new spine formed on day 1 and persisted on day 7 after rotarod training (n = 6 pairs of mice for control and 10mg/kg PF-04449613). (E) 1-day and 7-day treatment of 10 mg/kg PF-04449613 improved rotarod skill performance (n = 10 and 11 mice for control and 10 mg/kg PF-04449613 respectively). Data are presented as mean value ± S.E.M.. *P < 0.05, **P < 0.01 versus vehicle group according to Student’s test. Scale bar, 2 μm.
Results
Acute subcutaneous administration of PF-04449613 increases dendritic and dendritic spine calcium activity of layer V pyramidal neurons
Previous studies have shown that PDE9 inhibitors facilitate long-term synaptic potentiation in hippocampal slices (Hutson et al., 2011; Kroker et al., 2012; van der Staay et al., 2008). To examine how PF-04449613 affects synaptic activity in vivo, we performed Ca2+ imaging of apical tuft dendrites of layer V pyramidal neurons in the primary motor cortex before and ~30 minutes after subcutaneous administration of either PF-04449613 or vehicle (Fig. 1A,B). In mice under quiet resting conditions, we found no significant difference in the amplitude, duration and integrated activity of spine Ca2+ transients before and ~30 minutes after vehicle treatment (Fig. 1C-F; amplitude: 2.28 ± 0.14 vs 2.20 ± 0.17, P = 0.267; duration: 1.78 ± 0.21 vs 1.64 ± 0.10, P = 0.443; integrated activity: 9.26 ± 1.20 vs 6.67 ± 0.60, P = 0.284; n = 68 and 72 spines before and after vehicle administration respectively). There was a slight but significant decrease in the frequency of spine Ca2+ transients ~30 minutes after vehicle administration (1.66 ± 0.08 vs 1.54 ± 0.12, P = 0.035). In contrast, the frequency, duration and integrated activity, but not amplitude, of spine Ca2+ transients showed significant increases ~30 minutes after administration of PF-04449613 at 10 mg/kg (Fig. 1C-F; amplitude: 2.56 ± 0.19 vs 3.07 ± 0.24, P = 0.085; frequency: 1.35 ± 0.07 vs 1.93 ± 0.13, P < 0.001; duration: 1.75 ± 0.16 vs 2.32 ± 0.20, P = 0.011; integrated activity: 9.68 ± 1.84 vs 17.22 ± 2.05, P < 0.001; n = 84 and 81 spines before and after drug application respectively).
In mice under treadmill running conditions, we observed a general reduction in the activity of spine Ca2+ transients ~30 minutes after vehicle administration (Fig. 1G-J; amplitude: 3.08 ± 0.19 vs 2.73 ± 0.20, P = 0.096; frequency: 1.90 ± 0.11 vs 1.63 ± 0.10, P = 0.033; duration: 2.70 ± 0.24 vs 1.54 ± 0.13, P < 0.001; integrated activity: 19.85 ± 2.45 vs 11.18 ± 1.92, P < 0.001; n = 79 spines before and after vehicle administration). The reduction in various parameters of spine Ca2+ transients was likely due to the fact that mice were running during the period of ~30 minutes after vehicle administration. In contrast, ~30 minutes after subcutaneous administration of PF-04449613 at 10 mg/kg, there were significant increases in the amplitude, duration and integrated activity of Ca2+ transients (Fig. 1G-J; amplitude: 2.97 ± 0.22 vs 3.75 ± 0.28, P = 0.009; frequency: 2.18 ± 0.16 vs 3.01 ± 0.28, P = 0.073; duration: 1.76 ± 0.14 vs 2.63 ± 0.24, P = 0.001; integrated activity: 15.77 ± 2.15 vs 40.16 ± 6.23, P < 0.001; n = 88 spines before and after drug application). Taken together, these results indicate that subcutaneous administration of PF-04449613 at 10 mg/kg leads to a significant increase in spine Ca2+ activities under both quiet resting and treadmill running conditions.
In addition to spine Ca2+ activity, we found that PF-04449613 at 10 mg/kg also increased the duration and integrated activity, but not the amplitude and frequency, of Ca2+ transients across long segments of apical dendrites of layer V pyramidal neurons under quiet resting conditions (Fig. 2A-E; duration:1.41 ± 0.07 vs 2.14 ± 0.20, P < 0.001; integrated activity: 6.67 ± 0.60 vs 10.24 ± 1.15, P = 0.017; amplitude: 2.27 ± 1.15 vs 2.21 ± 0.19, P > 0.05; frequency: 1.98 ± 0.17 vs 2.07 ± 0.20, P > 0.05; n = 38 and 35 dendrites before and after drug administration respectively). Furthermore, under treadmill running conditions, the frequency, duration and integrated activity, but not amplitude, of dendritic Ca2+ transients were increased ~30 minutes after PF-04449613 administration (Fig. 2F-I; frequency: 3.15 ± 0.36 vs 4.66 ± 0.52, P = 0.014; duration: 2.31 ± 0.15 vs 2.68 ± 0.52, P < 0.05; integrated activity: 22.21 ± 3.80 vs 35.26 ± 4.87, P < 0.001; amplitude: 2.89 ± 0.16 vs 3.05 ± 0.19, P > 0.05; n = 64 dendrites for both conditions). Taken together, these findings show that the PDE9 inhibitor PF-04449613 increases calcium activities of dendrites and dendritic spines under both resting and running conditions.
Chronic PF-04449613 treatment increases dendritic spine remodeling
To determine whether the PDE9 inhibitor, PF-04449613, affects not only calcium activity of dendritic spines but also their structural dynamics, we examined spine formation and elimination in the primary motor cortex over various time intervals (1-, 7- and 28-day) with or without subcutaneous administration of PF-04449613 at a dosage of either 3.2 mg/kg or 10 mg/kg (Fig. 3A). Dendritic spines on apical dendrites of layer V pyramidal neurons in 1-month-old mice were imaged with transcranial two-photon microscopy as described previously (Grutzendler et al., 2002; Yang et al., 2010). PF-0449613 or vehicle was administered twice a day over 1, 7 or 28 days. The same dendritic branches were repeatedly imaged after the drug treatment (Fig. 3B). We found that 1-day treatment of PF-04449613 at either low or high dosage did not affect spine elimination (7.8% ± 0.4%, 9.4 ± 1.1% and 8.3% ± 1.0% for vehicle, 3.2 mg/kg and 10 mg/kg PF-04449613, respectively. F (2,15) = 0.962, P = 0.404) nor spine formation (6.1% ± 1.1%, 5.0% ± 0.4% and 6.2% ± 0.8% for vehicle, 3.2mg/kg and 10 mg/kg PF-04449613, respectively. F (2,15) = 1.176, P = 0.336) (Fig. 3C). Over 7 days, only high-dose PF-04449613 treatment (10 mg/kg) increased spine elimination (15.7% ± 1.4%, 16.5% ± 0.7% and 18.4% ± 0.8% for vehicle, 3.2mg/kg and 10 mg/kg PF-04449613, respectively. F (2,14) = 4.906, P < 0.05), but had no significant effect on spine formation (10.1% ± 1.4%, 11.0% ± 0.4% and 11.2% ± 1.7% for vehicle, 3.2mg/kg and 10 mg/kg PF-04449613, respectively. F (2,14) = 0.248, P = 0.784) (Fig. 3D). PF-04449613 treatment over 28 days enhanced both spine elimination (22.0% ± 1.2%, 22.8% ± 0.9% and 28.1% ± 0.9% for vehicle, 3.2mg/kg and 10 mg/kg PF-04449613, respectively. F (2,13) = 12.463, P < 0.01) and formation (11.2% ± 0.8%, 14.3% ± 1.6% and 15.9% ± 2.0% for vehicle, 3.2mg/kg and 10 mg/kg PF-04449613, respectively. F (2,13) = 3.478, P < 0.05) (Fig. 3E). Further Dunnett’s test showed that spine dynamics over 28 days were affected following the treatment of PF-04449613 at 10 mg/kg (P < 0.01 for spine elimination and P < 0.05 for spine formation), but not 3.2 mg/kg (P = 0.780 for spine elimination and P = 0.200 for spine formation). Together, these results indicate that chronic inhibition of PDE9 with PF-04449613 at a dosage of 10 mg/kg increases dendritic spine formation and elimination in the mouse primary motor cortex.
PF-04449613 increases motor learning-induced spine formation and elimination
It has been shown that rotarod training leads to a significant increase in spine formation and elimination (Yang et al., 2009). To determine whether PF-04449613 has an effect on spine plasticity induced by rotarod training, we tracked dendritic spines in the forelimb region of the motor cortex before and one day after training (Fig. 4A). PF-04449613 at 10 mg/kg or control vehicle was administered twice over 1 day. We found that administration of PF-04449613 significantly increased spine formation (8.4% ± 0.4% and 14.3% ± 1.2% for vehicle and 10 mg/kg PF-04449613, respectively; P < 0.01) and elimination (7.1% ± 0.5% and 8.9% ± 0.5% for vehicle and 10 mg/kg PF-04449613, respectively; P < 0.05) over 1 day compared to the control group (Fig. 4B). The rates of spine formation (11.0% ± 1.5% and 15.2% ± 1.5% for vehicle and 10 mg/kg PF-04449613, respectively) and elimination (16.5% ± 1.5% and 19.7% ± 0.9% for vehicle and 10 mg/kg PF-04449613, respectively) over 7 days were also significantly higher after 7-day treatment with PF-04449613 as compared to the vehicle treatment (P < 0.05) (Fig. 4C). Furthermore, the percentage of new spine formed during the first day and persisted at day 7 was significantly higher after PF-04449613 treatment as compared to the vehicle-treated control group (1.0% ± 0.2% and 4.1% ± 0.9% for vehicle and 10 mg/kg PF-04449613, respectively. P < 0.01) (Fig. 4D). Together, these results indicate that inhibition of PDE9 activity with PF-04449613 increases motor learning-induced spine formation and elimination.
PF-04449613 improves motor skill learning
Our findings above show that PF-04449613 increases rotarod training-induced persistent new spines. Previous studies have shown that the extent of persistent new spines induced after motor training correlates with performance improvement (Hayashi-Takagi et al., 2015; Liston et al., 2013; Xu et al., 2009; Yang et al., 2009). Therefore, PF-04449613 may enhance performance improvement after rotarod training. To test this possibility, we trained mice on an accelerated rotarod task with administration of vehicle or PF-04449613 at 10 mg/kg over 1-7 days. We found that the inhibitor effectively led to an improvement in motor skill performance as quantified by the average running speed that mice mastered on rotarod after 1 day treatment (35.41 ± 1.78 and 41.85 ± 1.79 for vehicle and 10 mg/kg PF-04449613, respectively. P < 0.05) (Fig. 4E). Moreover, motor skill performance continued to be better after treatment with PF-04449613 for 7 days (33.66 ± 1.73 and 39.85 ± 2.19 for vehicle and 10 mg/kg PF-04449613, respectively. P < 0.05) (Fig. 4E). Consistent with previous studies on beneficial effects of PDE9 inhibitors, these findings indicate that PF-04449613 enhances performance improvement after motor learning.
Discussion
In the present work, we investigated the effect of a selective PDE9 inhibitor PF-04449613 on dendritic spine activity and plasticity of layer 5 pyramidal neurons as well as behavioral improvement after motor learning in the living mouse motor cortex. We found that PF-04449613 at the dose of 10 mg/kg increased the activity of spines and dendrites under both resting and running states. Remodeling of dendritic spines of layer V pyramidal neurons was also elevated under basal (no motor training) condition after 7 or 28 days treatment of PF-04449613. Importantly, PF-04449613 treatment over 1-7 days significantly increased rotarod training-induced new spines and performance improvement. Taken together, these findings suggest that the PDE9 inhibitor PF-04449613 facilitates learning and memory by promoting learning-related synaptic activity and plasticity.
PDE9 is widely distributed in brain regions including neocortex, hippocampus, basal forebrain, basal ganglia, thalamus, pons, olfactory bulb and cerebellum (Andreeva et al., 2001; Reyes-Irisarri et al., 2007; Van Staveren et al., 2003). It is predominantly expressed in neurons (Kleiman et al., 2012; van Staveren et al., 2002; Van Staveren et al., 2003). In vitro studies indicate that PDE9 inhibitors promote synaptic transmission (van der Staay et al., 2008), increase synapse formation (indicated by synapsin 1 expression) and phosphorylated GluR1 (Hutson et al., 2011), as well as facilitate long-term potentiation (LTP) in hippocampal neurons (Hutson et al., 2011; Kroker et al., 2014; Kroker et al., 2012; van der Staay et al., 2008). In addition to PDE9 inhibitors, other PDE inhibitors also affect LTP-related plasticity (Heckman et al., 2015; Puzzo et al., 2008; Reneerkens et al., 2009). Specific inhibitors of PDE1 (Snyder et al., 2016), PDE2 (Boess et al., 2004; Wang et al., 2017), PDE3 (Hiramatsu et al., 2010), PDE4 (Bruno et al., 2011; Ricciarelli et al., 2017), PDE5 (Prickaerts et al., 1997; Prickaerts et al., 2002b), PDE7 (Perez-Gonzalez et al., 2013), and PDE9 (Hutson et al., 2011; van der Staay et al., 2008) have been shown to enhance memory performance in rodent. For example, the selective PDE2 inhibitor BAY 60-7550 improves memory acquisition and consolidation in the object recognition task (Boess et al., 2004; Domek-Lopacinska et al., 2008; Rutten et al., 2007), and reverses the deficit in object recognition produced by acute tryptophan depletion (van Donkelaar et al., 2008). PDE4 inhibitors rolipram, L-454, 560, and PDE5 inhibitors sildenafil, vardenafil, tadalafil, zaprinast have also been shown to improve cognitive functions in various animal models (Reneerkens et al., 2009; Schmidt, 2010). PDE9 inhibitors BAY 73-6691 and PF-04447943 also improve learning and memory and enhance cognitive function in rodents (Hutson et al., 2011; Kroker et al., 2014; van der Staay et al., 2008). Consistent with the beneficial effects of various PDE inhibitors, we have found that PF-04449613 improves performance improvement after rotarod training. By determining spine formation and elimination after motor training, our results further suggest that PDE9 inhibitors facilitate motor behavioral improvement by promoting learning-induced synaptic plasticity.
PDE9 is highly specific for cGMP hydrolysis and shows the highest affinity for cGMP of all PDEs (Km = 0.07-0.17 μM) (Fisher et al., 1998; Wunder et al., 2005). The intracellular level of cGMP is regulated by the balance between synthesis by guanylyl cyclase and breakdown by PDEs (Diederen et al., 2007; Francis et al., 1999). Previous studies have shown that PF-04449613 is a selective and potent inhibitor of PDE9 (Claffey et al., 2012a; Kleiman et al., 2012; Lee et al., 2015). Subcutaneous administration of PF-04449613 from 1 mg/kg to 32 mg/kg significantly increases the cerebral cGMP level in a dose-dependent manner (Kleiman et al., 2012). The level of cGMP peaks around 30-60 min after subcutaneous administration and gradually returns to baseline level a few hours later (Kleiman et al., 2012). In our study of spine plasticity, PF04449613 was administered twice a day. Therefore, we expect that the elevation of cGMP levels due to PF04449613 administration would be maintained most of the time in the brain to modulate synaptic plasticity. Furthermore, we found that only high dosage and long-term PF04449613 application significantly affect synaptic structural plasticity, indicating chronic PF04449613-related synaptic changes may need persistent elevation of cGMP.
How the elevation of cGMP after PDE9 inhibitor administration affects synaptic plasticity remains to be determined. It has been shown that cGMP activates cGMP-dependent protein kinases (PKGs/cGKs), which phosphorylates synaptic proteins that modulate the synthesis and/or release of neurotransmitters (Arancio et al., 2001; Schmidt et al., 1993; Taqatqeh et al., 2009). Therefore, the increases in dendritic spine calcium and spine remodeling could be due to an increase in presynaptic glutamate release. Furthermore, higher synaptic and dendritic calcium activity after PDE9 inhibitor application could be due to cGKII-regulated calcium release from IP3Rs in postsynaptic neurons, which has been shown to play an important role in the induction of LTP, a synaptic model of learning and memory (Kim et al., 2015). It is thus possible that PF-04449613 causes an elevation of cellular cGMP and calcium activity in both pre- and postsynaptic sites, leading to enhanced synaptic plasticity and improved learning and memory through the NO/cGMP/PKG pathway. Consistently, the inhibition of cGMP synthesis impairs the performance in several learning and memory behavioral tasks (Bernabeu et al., 1997; Edwards et al., 2002; Izquierdo et al., 2000; Kendrick et al., 1997; Yamada et al., 1996), whereas administration of cGMP analog 8-bromo-cGMP improves learning and memory (Bernabeu et al., 1996; Prickaerts et al., 2002a).
While PDE inhibitors in preclinical animal studies have shown promising cognitive and memory improvement, the clinical phase data of PDE inhibitors show little or no cognitive performance improvement in AD patients as compared with the placebo group (Prickaerts et al., 2017; Schwam et al., 2014). The gap between the animal studies and the clinical studies remains to be investigated. Recent studies in an AD mouse model have shown that abnormal and prolonged dendritic calcium spikes occur on a subset of dendrites and cause depotentiation of synaptic strength (Bai et al., 2017). Because the PDE9 inhibitor PF-04449613 increases Ca2+ activities of dendrites and dendritic spines, PDE9 inhibition may be beneficial for wild type control mice, but not for AD mice. Short-term PDE inhibition or an optimal dose range of PDE9 inhibitors over long-term intake might be necessary for achieving beneficial cognitive and learning performance in AD patients. Further studies with relevant animal models of various diseases are needed to assess whether long-term decrease or increase of PDE9 activities could serve as a therapeutic target for treatment of age-related memory deficits.
Acknowledgments
This study was supported by the NIH grant R01 NS047325 to W.-B.G., and by funding from Peking University Shenzhen Graduate School, Pfizer, the National Natural Science Foundations of China (No. 81771428, 31700915 and 81100839), Shenzhen Science and Technology Innovation Funds (JCYJ20170306091732304, JCYJ20170306165303295, JCYJ20160428154351820, JCYJ20170807144417251 and JCYJ20170807144259233), and the Oversea Study Program of Guangzhou Elite Project.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
References
- Andreeva SG, Dikkes P, Epstein PM, Rosenberg PA. Expression of cGMP-specific phosphodiesterase 9A mRNA in the rat brain. J Neurosci. 2001;21:9068–9076. doi: 10.1523/JNEUROSCI.21-22-09068.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Antonova I, Gambaryan S, Lohmann SM, Wood JS, Lawrence DS, Hawkins RD. Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation. J Neurosci. 2001;21:143–149. doi: 10.1523/JNEUROSCI.21-01-00143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Bai Y, Li M, Zhou Y, Ma L, Qiao Q, Hu W, Li W, et al. Abnormal dendritic calcium activity and synaptic depotentiation occur early in a mouse model of Alzheimer’s disease. Mol Neurodegener. 2017;12:86. doi: 10.1186/s13024-017-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Gattaz WF. Multidimensional analysis of the concentrations of 17 substances in the CSF of schizophrenics and controls. J Neural Transm (Vienna) 2002;109:931–938. doi: 10.1007/s007020200076. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Schmitz P, Faillace MP, Izquierdo I, Medina JH. Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. Neuroreport. 1996;7:585–588. doi: 10.1097/00001756-199601310-00050. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Schroder N, Quevedo J, Cammarota M, Izquierdo I, Medina JH. Further evidence for the involvement of a hippocampal cGMP/cGMP-dependent protein kinase cascade in memory consolidation. Neuroreport. 1997;8:2221–2224. doi: 10.1097/00001756-199707070-00026. [DOI] [PubMed] [Google Scholar]
- Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, et al. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Winblad B, Ravid R, Cowburn RF. Reduced nitric oxide responsive soluble guanylyl cyclase activity in the superior temporal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1995;187:5–8. doi: 10.1016/0304-3940(95)11323-o. [DOI] [PubMed] [Google Scholar]
- Bruno O, Fedele E, Prickaerts J, Parker LA, Canepa E, Brullo C, Cavallero A, et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–2063. doi: 10.1111/j.1476-5381.2011.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey MM, Helal CJ, Verhoest PR, Kang Z, Fors KS, Jung S, Zhong J, et al. Application of structure-based drug design and parallel chemistry to identify selective, brain penetrant, in vivo active phosphodiesterase 9A inhibitors. J Med Chem. 2012a;55:9055–9068. doi: 10.1021/jm3009635. [DOI] [PubMed] [Google Scholar]
- Claffey MM, Helal CJ, Verhoest PR, Kang ZJ, Fors KS, Jung S, Zhong JY, et al. Application of Structure-Based Drug Design and Parallel Chemistry to Identify Selective, Brain Penetrant, In Vivo Active Phosphodiesterase 9A Inhibitors. J Med Chem. 2012b;55:9055–9068. doi: 10.1021/jm3009635. [DOI] [PubMed] [Google Scholar]
- Diederen RM, La Heij EC, Markerink-van Ittersum M, Kijlstra A, Hendrikse F, de Vente J. Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3′5′ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br J Ophthalmol. 2007;91:379–384. doi: 10.1136/bjo.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domek-Lopacinska K, Strosznajder JB. The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res. 2008;1216:68–77. doi: 10.1016/j.brainres.2008.02.108. [DOI] [PubMed] [Google Scholar]
- Domek-Lopacinska KU, Strosznajder JB. Cyclic GMP and nitric oxide synthase in aging and Alzheimer’s disease. Mol Neurobiol. 2010;41:129–137. doi: 10.1007/s12035-010-8104-x. [DOI] [PubMed] [Google Scholar]
- Duinen MV, Reneerkens OA, Lambrecht L, Sambeth A, Rutten BP, Os JV, Blokland A, et al. Treatment of Cognitive Impairment in Schizophrenia: Potential Value of Phosphodiesterase Inhibitors in Prefrontal Dysfunction. Curr Pharm Des. 2015;21:3813–3828. doi: 10.2174/1381612821666150605110941. [DOI] [PubMed] [Google Scholar]
- East SJ, Garthwaite J. Nanomolar N(G)-nitroarginine inhibits NMDA-induced cyclic GMP formation in rat cerebellum. Eur J Pharmacol. 1990;184:311–313. doi: 10.1016/0014-2999(90)90623-e. [DOI] [PubMed] [Google Scholar]
- Edwards TM, Rickard NS, Ng KT. Inhibition of guanylate cyclase and protein kinase G impairs retention for the passive avoidance task in the day-old chick. Neurobiol Learn Mem. 2002;77:313–326. doi: 10.1006/nlme.2001.4021. [DOI] [PubMed] [Google Scholar]
- Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- Fusco FR, Giampa C. Phosphodiesterases as therapeutic targets for Huntington’s disease. Curr Pharm Des. 2015;21:365–377. doi: 10.2174/1381612820666140826113957. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Cramer H, Beckmann H. Low CSF concentrations of cyclic GMP in schizophrenia. Br J Psychiatry. 1983;142:288–291. doi: 10.1192/bjp.142.3.288. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman PR, Wouters C, Prickaerts J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer’s disease: a translational overview. Curr Pharm Des. 2015;21:317–331. doi: 10.2174/1381612820666140826114601. [DOI] [PubMed] [Google Scholar]
- Hesse R, Lausser L, Gummert P, Schmid F, Wahler A, Schnack C, Kroker KS, et al. Reduced cGMP levels in CSF of AD patients correlate with severity of dementia and current depression. Alzheimers Res Ther. 2017;9:17. doi: 10.1186/s13195-017-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu M, Takiguchi O, Nishiyama A, Mori H. Cilostazol prevents amyloid beta peptide(25-35)-induced memory impairment and oxidative stress in mice. Br J Pharmacol. 2010;161:1899–1912. doi: 10.1111/j.1476-5381.2010.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson PH, Finger EN, Magliaro BC, Smith SM, Converso A, Sanderson PE, Mullins D, et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydr o-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology. 2011;61:665–676. doi: 10.1016/j.neuropharm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Vianna M, Barros DM, Mello e Souza T, Ardenghi P, Sant’Anna MK, Rodrigues C, et al. Short- and long-term memory are differentially affected by metabolic inhibitors given into hippocampus and entorhinal cortex. Neurobiol Learn Mem. 2000;73:141–149. doi: 10.1006/nlme.1999.3925. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guevara-Guzman R, Zorrilla J, Hinton MR, Broad KD, Mimmack M, Ohkura S. Formation of olfactory memories mediated by nitric oxide. Nature. 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- Kim S, Titcombe RF, Zhang H, Khatri L, Girma HK, Hofmann F, Arancio O, et al. Network compensation of cyclic GMP-dependent protein kinase II knockout in the hippocampus by Ca2+-permeable AMPA receptors. Proc Natl Acad Sci U S A. 2015;112:3122–3127. doi: 10.1073/pnas.1417498112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman RJ, Chapin DS, Christoffersen C, Freeman J, Fonseca KR, Geoghegan KF, Grimwood S, et al. Phosphodiesterase 9A regulates central cGMP and modulates responses to cholinergic and monoaminergic perturbation in vivo. J Pharmacol Exp Ther. 2012;341:396–409. doi: 10.1124/jpet.111.191353. [DOI] [PubMed] [Google Scholar]
- Kroker KS, Mathis C, Marti A, Cassel JC, Rosenbrock H, Dorner-Ciossek C. PDE9A inhibition rescues amyloid beta-induced deficits in synaptic plasticity and cognition. Neurobiol Aging. 2014;35:2072–2078. doi: 10.1016/j.neurobiolaging.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Kroker KS, Rast G, Giovannini R, Marti A, Dorner-Ciossek C, Rosenbrock H. Inhibition of acetylcholinesterase and phosphodiesterase-9A has differential effects on hippocampal early and late LTP. Neuropharmacology. 2012;62:1964–1974. doi: 10.1016/j.neuropharm.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Lee DI, Zhu GS, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–476. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Mansour MN, Dillman KS, Perez JR, Danley DE, Aeed PA, Simons SP, et al. Structural basis for the catalytic mechanism of human phosphodiesterase 9. Proc Natl Acad Sci U S A. 2008;105:13309–13314. doi: 10.1073/pnas.0708850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Pascual C, Antequera D, Bolos M, Redondo M, Perez DI, Perez-Grijalba V, et al. Phosphodiesterase 7 inhibitor reduced cognitive impairment and pathological hallmarks in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:2133–2145. doi: 10.1016/j.neurobiolaging.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, de Vente J, Honig W, Steinbusch HW, Blokland A. cGMP, but not cAMP, in rat hippocampus is involved in early stages of object memory consolidation. Eur J Pharmacol. 2002a;436:83–87. doi: 10.1016/s0014-2999(01)01614-4. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Heckman PRA, Blokland A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26:1033–1048. doi: 10.1080/13543784.2017.1364360. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Steinbusch HW, Smits JF, de Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337:125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van Staveren WC, Sik A, Markerink-van Ittersum M, Niewohner U, van der Staay FJ, Blokland A, et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002b;113:351–361. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Sapienza S, Arancio O, Palmeri A. Role of phosphodiesterase 5 in synaptic plasticity and memory. Neuropsychiatr Dis Treat. 2008;4:371–387. doi: 10.2147/ndt.s2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Markerink-Van Ittersum M, Mengod G, de Vente J. Expression of the cGMP-specific phosphodiesterases 2 and 9 in normal and Alzheimer’s disease human brains. Eur J Neurosci. 2007;25:3332–3338. doi: 10.1111/j.1460-9568.2007.05589.x. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Brullo C, Prickaerts J, Arancio O, Villa C, Rebosio C, Calcagno E, et al. Memory-enhancing effects of GEBR-32a, a new PDE4D inhibitor holding promise for the treatment of Alzheimer’s disease. Sci Rep. 2017;7:46320. doi: 10.1038/srep46320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A. Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors. Eur J Pharmacol. 2007;558:107–112. doi: 10.1016/j.ejphar.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Giralt A, Arumi H, Alberch J, Perez-Navarro E. Regulation of hippocampal cGMP levels as a candidate to treat cognitive deficits in Huntington’s disease. PLoS One. 2013;8:e73664. doi: 10.1371/journal.pone.0073664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ. Phosphodiesterase inhibitors as potential cognition enhancing agents. Curr Top Med Chem. 2010;10:222–230. doi: 10.2174/156802610790411009. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schwam EM, Nicholas T, Chew R, Billing CB, Davidson W, Ambrose D, Altstiel LD. A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:413–421. doi: 10.2174/1567205011666140505100858. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, et al. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Prickaerts J, Wadenberg ML, Zhang L, Zheng H, Yao W, Akkerman S, et al. Preclinical profile of ITI-214, an inhibitor of phosphodiesterase 1, for enhancement of memory performance in rats. Psychopharmacology (Berl) 2016;233:3113–3124. doi: 10.1007/s00213-016-4346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Lu YF, Zhuo M, Arancio O, Kandel ER, Hawkins RD. The specific role of cGMP in hippocampal LTP. Learn Mem. 1998;5:231–245. [PMC free article] [PubMed] [Google Scholar]
- Taqatqeh F, Mergia E, Neitz A, Eysel UT, Koesling D, Mittmann T. More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci. 2009;29:9344–9350. doi: 10.1523/JNEUROSCI.1902-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Barfacker L, Devry J, Erb C, Heckroth H, Karthaus D, et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology. 2008;55:908–918. doi: 10.1016/j.neuropharm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, Rutten K, Blokland A, Akkerman S, Steinbusch HW, Prickaerts J. Phosphodiesterase 2 and 5 inhibition attenuates the object memory deficit induced by acute tryptophan depletion. Eur J Pharmacol. 2008;600:98–104. doi: 10.1016/j.ejphar.2008.10.027. [DOI] [PubMed] [Google Scholar]
- van Staveren WC, Glick J, Markerink-van Ittersum M, Shimizu M, Beavo JA, Steinbusch HW, de Vente J. Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J Neurocytol. 2002;31:729–741. doi: 10.1023/a:1025704031210. [DOI] [PubMed] [Google Scholar]
- Van Staveren WC, Steinbusch HW, Markerink-Van Ittersum M, Repaske DR, Goy MF, Kotera J, Omori K, et al. mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol. 2003;467:566–580. doi: 10.1002/cne.10955. [DOI] [PubMed] [Google Scholar]
- Wang L, Xiaokaiti Y, Wang G, Xu X, Chen L, Huang X, Liu L, et al. Inhibition of PDE2 reverses beta amyloid induced memory impairment through regulation of PKA/PKG-dependent neuro-inflammatory and apoptotic pathways. Sci Rep. 2017;7:12044. doi: 10.1038/s41598-017-08070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol. 2005;68:1775–1781. doi: 10.1124/mol.105.017608. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Hiramatsu M, Noda Y, Mamiya T, Murai M, Kameyama T, Komori Y, et al. Role of nitric oxide and cyclic GMP in the dizocilpine-induced impairment of spontaneous alternation behavior in mice. Neuroscience. 1996;74:365–374. doi: 10.1016/0306-4522(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Chang PC, Gooden F, Gan WB. Transcranial two-photon imaging of synaptic structures in the cortex of awake head-restrained mice. Methods Mol Biol. 2013;1010:35–43. doi: 10.1007/978-1-62703-411-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RH, Hu WL, Tsai JL, Li W, Gan WBA. Microglia limit the expansion of beta-amyloid plaques in a mouse model of Alzheimer’s disease. Mol Neurodegener. 2017;12:47. doi: 10.1186/s13024-017-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]


