Abstract
Introduction:
Goal-directed hemostatic resuscitation based on thrombelastography (TEG) has a survival benefit compared to conventional coagulation assays such as INR, aPTT, fibrinogen level, and platelet count. While TEG-based transfusion thresholds for patients at risk for massive transfusion (MT) have been defined using rapid TEG (rTEG), cutoffs have not been defined for TEG using other activators such as kaolin. The purpose of this study was to develop thresholds for blood product transfusion using citrated kaolin TEG (CK-TEG) in patients at risk for MT.
Methods:
CK-TEG was assessed in trauma activation patients at two level-one trauma centers admitted between 2010–2017. ROC curve analyses were performed to test the predictive performance of CK-TEG measurements in patients requiring MT, defined as >10 units of RBC or death within the first 6 hours. The Youden Index defined optimal thresholds for CK-TEG-based resuscitation.
Results:
Of the 825 trauma activations, 671 (81.3%) were male, 419 (50.8%) suffered a blunt injury, and 62 (7.5%) received a MT. Patients who had a MT were more severely injured, had signs of more pronounced shock, and more abnormal coagulation assays. CK-TEG R-Time was longer (4.9vs4.4 min, p=0.0084), Angle was lower (66.2vs70.3 degrees, p<0.0001), MA was lower in MT (57vs65.5 mm, p<0.0001) and LY30 was greater (1.8vs1.2 percent, p=0.0012) in patients with MT compared to non-MT. To predict MT, R-Time yielded an area under the ROC curve (AUROC)=0.6002, and a cut point of>4.45 min. Angle had an AUROC=0.6931, and a cut point of<67 degrees. CMA had an AUROC=0.7425, and a cut point of<60mm. LY30 had an AUROC=0.623 with a cut point of>4.55%.
Conclusion:
We have identified CK-TEG thresholds that can guide MT in trauma. We propose plasma transfusion for R-time > 4.45 min, fibrinogen products for an angle <67 degrees, platelet transfusion for MA < 60mm, and antifibrinolytics for LY30 >4.55%.
Level of Evidence:
Therapeutic study, level V
Keywords: Massive transfusion, TEG, citrated kaolin TEG, coagulopathy, resuscitation
Introduction
Exsanguination from uncontrolled hemorrhage is the leading cause of preventable death following trauma, accounting for up to 40% of deaths, half of which occur within the first 2 hours (1) (2). An endogenous trauma-induced coagulopathy (TIC) contributes to the majority of hemorrhagic deaths and is a multifocal process attributed to reduced thrombin generation, fibrinogen depletion, platelet dysfunction, systemic hyperfibrinolysis, and fibrinolysis shutdown (3–8).
The implementation of massive transfusion protocols (MTPs) improve survival in the treatment of patients in hemorrhagic shock (2, 9–12) (13). Significant resources have been directed towards identifying the ideal ratio of blood products in resuscitation (2) (9, 11). While ratio based resuscitation may be appropriate in the initial stages of managing the critically injured, subsequent blood component akaolinth other activatiors such as ional coagulation assaysrdministration has traditionally been guided by conventional coagulation assays such as the international normalized ratio (INR) of the prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen level, and platelet count(10). Through the use thrombelastography (TEG), a viscoelastic assay that provides comprehensive assessment of clot formation and clot degradation, our group have shown that a goal-directed MTP guided by TEG improves survival compared with MTP guided by conventional coagulation assays. Furthermore, this improved survival is accomplished with the transfusion of fewer plasma and platelet units during the early phases of resuscitation of the injured patient (10).
We have previously used rapid TEG (rTEG) to define optimal transfusion thresholds that are used to guide hemostatic resuscitation for patients at risk for massive transfusion (MT). These defined thresholds include transfusion of plasma for an activated clotting time (ACT) <128 seconds, transfusion of cryoprecipitate for an angle <65 degrees, transfusion of platelets for a maximum amplitude (MA) <55 mm, and administration of antifibrinolytics for a LY30 >5% (9). However, these thresholds are not directly translatable for use in TEG with other activators such as kaolin alone (citrated kaolin TEG) (14) which is used by some centers to guide hemostatic resuscitation. The type of activator differentiates rTEG from other activated TEGs (citrated kaolin TEG). Rapid TEG is activated with tissue factor and kaolin while citrated kaolin TEG (CK-TEG) is activated only with kaolin (14). While activated TEG (with either tissue factor or kaolin) enhances clotting and speeds the time to completion of the assay, there are potential mechanistic limitations to using rTEG. The use of tissue factor in rTEG may overwhelm endogenous clot activation and mask native circulating factors that promote clot formation following trauma (5). Indeed, it is likely that TEG initiated by kaolin may (as a result of a more subtle activation) prove superior at evaluating hypercoagulable patients, while rTEG may be better to evaluate hypocoagulable patients. Consequently, our objective was to identify coagulation abnormalities, identified as a need for massive transfusion (a surrogate for significant coagulopathy), for each CK-TEG parameter of clot formation and breakdown and use these parameters to define thresholds to guide hemostatic resuscitation for patients at risk for massive transfusion.
Methods
Study Design
This is an analysis of prospectively collected data from adult (age≥18 years) trauma activation patients who met criteria for the highest-level trauma activation (Supplemental 1) at the Denver Health Medical Center (DHMC) and Zuckerberg San Francisco General Hospital (ZSFGH) between 2010–2017. Both centers are ACS verified and state certified Level 1 trauma centers. Exclusion criteria were: unsalvageable injuries (defined by patients in asystole at emergency department arrival), isolated gunshot wounds to the head, pregnancy, incarcerated patients, transferred from another hospital, documented chronic liver disease, or a known coagulation disorder.
Samples were obtained under protocols approved by the institutional review board for each institution. The studies contributing to this database were approved by the Colorado Multiple Institution Review Board under a waiver of consent for Denver Health. Informed consent was obtained for all patients at ZSFGH under a protocol approved by the Committee on Human Research at University of California, San Francisco. Trained research professional assistants (PRAs) performed all viscoelastic assays within one-hour postinjury. Clinicians were blinded to research data.
Massive transfusion activation was under physician discretion. The transfusion of products other than RBCs during this period was guided by rapid thrombelastography (rTEG) criteria, as previously described at DHMC(10, 15). For this protocol 4 units of RBC units and 2 plasma units were delivered to the patient’s bedside. These first units were administered according to the treating clinicians’ criteria while awaiting results of rTEG (10). A non-protocolized, transfusion based on clinical evaluation and conventional coagulation assays at ZSFGH as previously described(16). However, if patients at ZSFGH was resuscitated in the trauma operating room, a ROTEM-based MTP was initiated that transfused plasma for an EXTEM CT >80 sec, cryoprecipitate or fibrinogen products for a FIBTEM MCF <8mm, platelets for an EXTEM MCF < 45 mm AND a FIBTEM MCF >10mm and tranexamic acid for an EXTEM ML >10%. The primary outcome of this study was massive transfusion (MT), defined as >10 units of RBCs or death in first 6 hours from injury based on findings previously published by our group (17) in order to account for survivor bias. This endpoint was used to define thresholds for blood component therapy in massively transfused patients.
Citrated Kaolin TEG (CK-TEG)
Citrated kaolin TEG (CK-TEG) was performed on whole blood collected in citrated vacuum tubes to prevent clotting prior to assays. CK-TEG assays yield the following parameters to assess the dynamic process of clot formation and breakdown in this study: time to clot initiation (reaction time [R-time, min]), dynamics of clot formation (alpha angle [α, degrees]), clot strength (maximum amplitude [MA, mm]) and fibrinolysis (lysis 30 minutes after MA is achieved [LY30, %]) run according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics Cooperation, Niles, IL).
Prolonged clot initiation (CK-TEG R-time) is an indication for plasma transfusion. Abnormal dynamics of clot formation (CK-TEG angle) is an indication for fibrinogen products (cryoprecipitate). Low clot strength (CK-TEG MA) is an indication for platelet transfusion. Increased fibrinolysis (CK-TEG LY30) is an indication for antifibrinolytics (9, 15, 18–20).
Conventional coagulation assays (CCA)
Samples were collected during trauma activations upon arrival to the ED in tubes containing 3.2% citrate and 4 mL of heparin (19 units/mL). Values for conventional coagulation assays (INR and aPTT) were determined by the clinical laboratory at Denver Health Medical Center and Zuckerberg San Francisco General Hospital by standard protocol.
Statistical Analysis
GraphPad Prism version 7.0a (GraphPad Software, Inc; La Jolla, CA) and Excel version 12.2.5 (Microsoft Corporation; Redmond, WA) were used for statistical analysis. For non-normally distributed variables, data were expressed as median and interquartile range (IQR) using a two-tailed Mann-Whitney test. Area under the receiver operating characteristics (AUROC) curve analysis was performed for each CK-TEG measurement to assess its predictive performance for massive transfusion. For each of the CK-TEG measurement, we selected the thresholds with the strongest differentiation of the outcome (MT) as identified by the maximum Youden Index (J = sensitivity + specificity −1). The maximum Youden’s Index is the value closest to 1 (21). The sensitivity, specificity, positive, and negative predictive values were also determined for CK-TEG variables, INR, and aPTT.
Results
There were 825 patients who had CK-TEG performed on presentation. The demographic characteristics of these patients are summarized in Table 1. Of the patients included, 671 (81.3%) were male, 419 (50.8%) patients suffered a blunt injury, and 62 (7.5%) received a MT. Patients who had a MT were more severely injured, had signs of more pronounced shock, had more abnormal conventional coagulation assay values, and more abnormal CK-TEG parameters compared to those that did not require MT (Table 2 and Table 3). Of the 763 that did not receive a MT, 191 (25%) received 1 or more units of RBC with a median of 3 units (IQR 2–5 units). Furthermore, the characteristics of MT patients between centers is shown in Table 4. Patients included from the Denver database had increased signs of shock (lower systolic blood pressure, elevated lactate, increased base deficit) as well as some more abnormal coagulation studies (increased LY30 and longer INR) when compared to the San Francisco database.
Table 1–
Demographics and Characteristics of Patients
| Variable | |
|---|---|
| Age (years) (n=825) | 33 (25–48) |
| ISS (n=762) | 10 (2–25) |
| ED SBP (mmHg) (n=816) | 129 (107–148) |
| ED Heart Rate (BPM) (n=815) | 97 (80–114) |
| ED GCS (n=820) | 14 (9–15) |
| ED Temp (Celsius) (n=587) | 36.6 (36.2–36.9) |
| Lactate (mg/dL) (n=405) | 3.3 (2.4–5.5) |
| Base Deficit (n=352) | 4.4 (0.7–8.2) |
| Hgb (g/dL) (n=814) | 14 (12.6–15.2) |
| Platelet Count (1,000/mL) (n=813) | 263 (214–311) |
| INR (n=797) | 1.1 (1.0–1.2) |
| PTT (seconds) (n=795) | 27.7 (25–30.6) |
| Fibrinogen (mg/dL) (n=467) | 225 (172–287) |
| D-Dimer (ng/mL) (n=478) | 1.29 (0.35–5.22) |
| R-Time (min) (n=825) | 4.5 (3.6–5.45) |
| Angle (degrees) (n=825) | 70.1 (66.2–73.3) |
| MA (mm) (n=825) | 65.1 (61.3–68.7) |
| LY30 (%)(n=825) | 1.2 (0.3–2.5) |
| RBC Units (first 6 hrs) (n=825) | 0 (0–1) |
| Plasma Units (first 6 hrs) (n=825) | 0 (0–1) |
| Platelet Units (first 6 hrs) (n=825) | 0 (0–0) |
| Cryo Units (first 6 hrs) (n=825) | 0 (0–0) |
Data are presented as median and interquartile range. ISS=injury severity score, ED=emergency department, SBP=systolic blood pressure, GCS=Glasgow coma scale, Hgb=hemoglobin, INR=international normalized ratio, PTT=partial thromboplastin time, R-Time=reaction time, MA=maximum amplitude, LY30=fibrinolysis 30 minutes after achieving MA.
Table 2–
Demographic and Characteristics of Patients by Massive Transfusion Status
| Variable | No Massive Transfusion | Massive Transfusion | p-value |
|---|---|---|---|
| Age (years) | 33 (25–48) | 35 (25–52) | 0.5081 |
| ISS | 10 (2–22) | 36 (29–50) | <0.0001 |
| ED SBP (mmHg) | 131 (110–149) | 86 (70–120) | <0.0001 |
| ED Heart Rate (BPM) | 96 (80–112) | 111(88–137) | 0.0009 |
| ED GCS | 15 (10–15) | 8 (3–14) | <0.0001 |
| ED Temp (Celsius) | 36.6 (36.2–36.9) | 36.2 (35–36.7) | 0.0057 |
| Lactate (mg/dL) | 3.2 (2.3–4.7) | 8.7 (5.9–12.7) | <0.0001 |
| Base Deficit | 4 (0.4–7.6) | 12 (7–16) | <0.0001 |
| Hgb (g/dL) | 14.1 (12.7–15.3) | 11.5 (10–13.1) | <0.0001 |
| Platelet Count (1,000/mL) | 268 (220–314) | 183 (130–237) | <0.0001 |
| INR | 1.1 (1–1.2) | 1.4 (1.3–1.8) | <0.0001 |
| PTT (seconds) | 27.5 (24.9–30.1) | 37.5 (30.1–54.1) | <0.0001 |
| Fibrinogen (mg/dL) | 229 (178–292) | 168 (136–224) | <0.0001 |
| D-Dimer (ng/mL) | 0.94 (0.31–4.04) | 6.7 (3.87–9.27) | <0.0001 |
| RBC Units (first 6 hrs) | 0 (0–1) | 16 (11–32) | <0.0001 |
| Plasma Units (first 6 hrs) | 0 (0–0) | 10 (4–14) | <0.0001 |
| Platelet Units (first 6 hrs) | 0 (0–0) | 2 (0–3) | <0.0001 |
| Cryo Units (first 6 hrs) | 0 (0–0) | 0 (0–2) | <0.0001 |
Data are presented as median and interquartile range. MT is defined as >10 units of RBCs or death in first 6 hours from injury. ISS=injury severity score, ED=emergency department, SBP=systolic blood pressure, GCS=Glasgow coma scale, Hgb=hemoglobin, INR=international normalized ratio, PTT=partial thromboplastin. Comparison made using a using a two-tailed Mann-Whitney test with significance set at p<0.05.
Table 3–
Citrated Kaolin Thrombelastography (CK-TEG) Parameters by Massive Transfusion Status
| CK-TEG Parameter | No Massive Transfusion | Massive Transfusion | p-value |
|---|---|---|---|
| R-Time (min) | 4.4 (3.6–5.4) | 4.9 (4.3–6.1) | 0.0084 |
| Alpha angle (degrees) | 70.3 (66.7–73.5) | 66.2 (59.8–69.9) | <0.0001 |
| MA (mm) | 65.45 (61.6–68.9) | 57 (48–65.5) | <0.0001 |
| LY30 (%) | 1.2 (0.3–2.4) | 1.8 (0.3–19.7) | 0.0012 |
Data are presented as median and interquartile range. MT is defined as >10 units of RBCs or death in first 6 hours from injury. R-Time=reaction time, MA=maximum amplitude, LY30=fibrinolysis 30 minutes after achieving MA.
Table 4–
Demographic and Characteristics of Massive Transfusion Patients at Two Centers that Participated in Study
| Variable | Denver (n=41) | San Francisco (n=21) | p-value |
|---|---|---|---|
| Age (years) | 34 (25–50) | 38 (24–63) | 0.5723 |
| ISS | 34 (28–48) | 38 (28–55) | 0.5955 |
| ED SBP (mmHg) | 82 (58–96) | 105 (91–131) | 0.0016 |
| ED Heart Rate (BPM) | 116 (81–139) | 109 (87–134) | 0.8162 |
| ED GCS | 7 (3–15) | 8 (3–14) | 0.9414 |
| ED Temp (Celsius) | 36.2 (35–38.8) | 35.6 (35.4–37.0) | 0.9656 |
| Lactate (mg/dL) | 9.8 (6.5–13.2) | 6.0 (4.5–7.3) | 0.0234 |
| Base Deficit | 15 (11.1–19.4) | 6.6 (3.5–8.4) | <0.0001 |
| Hgb (g/dL) | 11 (9.3–12.3) | 13 (11.0–13.8) | 0.0048 |
| Platelet Count (1,000/mL) | 170 (114–208) | 226 (196–296) | 0.0003 |
| INR | 1.54 (1.31–2.01) | 1.3 (1.2–1.5) | 0.0226 |
| PTT (seconds) | 43.5 (30.2–59.8) | 35.8 (29.4–50.3) | 0.1788 |
| Fibrinogen (mg/dL) | 154 (129–197) | 173 (155–249) | 0.1347 |
| D-Dimer (ng/mL) | 7.68 (3.72–20.01) | 6.66 (4–7.42) | 0.3388 |
| R-Time (min) | 5 (4.4–6.25) | 4.7 (3.6–5.85) | 0.1857 |
| Angle (degrees) | 64 (58–69) | 70 (62–71) | 0.0922 |
| MA (mm) | 57 (38–65) | 61 (52–67) | 0.0759 |
| LY30 (%) | 2.8 (1.2–40.7) | 0.9 (0–7.3) | 0.0312 |
Data are presented as median and interquartile range. MT is defined as >10 units of RBCs or death in first 6 hours from injury. R-Time=reaction time, MA=maximum amplitude, LY30=fibrinolysis 30 minutes after achieving MA. Comparison made using a using a two-tailed Mann-Whitney test with significance set at p<0.05.
The AUROC for CK-TEG parameters are shown in Table 5. Of all CK-TEG parameters that measured dynamics of clot formation, CK-TEG MA was the best predictor of MT (AUROC = 0.74 95% CI 0.67 to 0.82, p<0.0001).
Table 5–
Area under the receiver-operating characteristics curve (AUROC) for all CK-TEG Parameters
| AUROC | 95% Confidence Interval | p value | |
|---|---|---|---|
| Clot Initiation | |||
| CK R-Time | 0.6002 | 0.5261 to 0.6743 | 0.0086 |
| Dynamics of Clot Formation | |||
| CK Alpha angle | 0.6931 | 0.6202 to 0.766 | <0.0001 |
| Clot Strength | |||
| CK MA | 0.7425 | 0.6663 to 0.8186 | <0.0001 |
| Fibrinolysis | |||
| CK LY30 | 0.623 | 0.5354 to 0.7105 | 0.0013 |
Table illustrates the AUROC with 95% confidence intervals of CK-TEG parameters for each aspect of clot formation and breakdown (clot initiation, dynamics of clot formation, clot strength, and fibrinolysis or clot breakdown). Clot initiation, dynamics of clot formation, clot strength, and fibrinolysis are commonly only measured with a single value that is subsequently used clinically so only the most commonly used parameter is reported for each aspect of clot formation and breakdown.
The optimal thresholds for the CK-TEG-guided MT based on the Youden Index are shown in Table 6 and Figure 1. Predictive capacity of each CK-TEG thresholds measured is shown in Table 6.
Table 6–
Predictive Performance of CK-TEG Variables for Massive Transfusion
| CK-TEG Variables | Sensitivity | Specificity |
|---|---|---|
| R-Time > 4.45 min | 72.58% | 50.2% |
| Angle < 67 degrees | 58.06% | 74.97% |
| MA < 60 mm | 61.29% | 83.99% |
| LY30 > 4.55% | 38.71% | 90.29% |
Figure 1– Proposed Citrated Kaolin TEG-guided resuscitation thresholds for patients receiving a massive transfusion.
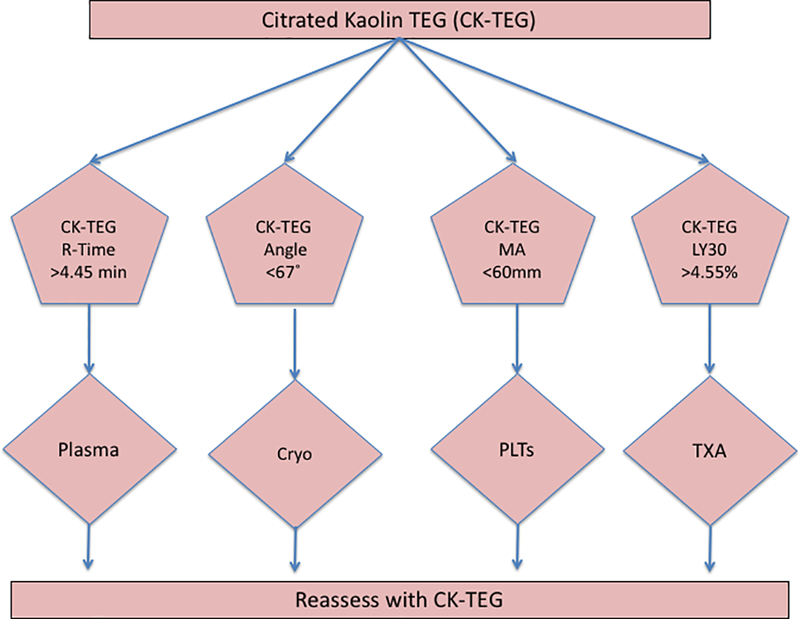
A schematic representation of the proposed thresholds in a CK-TEG-guided MTP for injured patients. These thresholds represent proposed points at which administration of specific blood products should be initiated in the bleeding patient. Cryo=cryoprecipitate, PLTs=platelets, and TXA=tranexamic acid
Discussion
In this study, we determined the degree of discrimination offered by CK-TEG parameters for MT. While most centers that use TEG employ rTEG for MT, others continue to use CK-TEG. Based on the CK-TEG parameters evaluated, thresholds were identified that could be used to guide hemostatic resuscitation for injured patients that receive a MT. Based on these, we propose plasma transfusion for R-Time >4.45 min, fibrinogen products for an angle <67 degrees, platelet transfusion for MA <60 mm, and antifibrinolytics for LY30 >4.55% (Figure 1).
The CK-TEG is an assay that is activated with the use of kaolin, which stimulates coagulation through the intrinsic or contact pathway. Various methods for restoring and maintaining normal hemostasis have been described including ratio-based transfusion or goal-directed therapy with viscoelastic based transfusions. Goal-directed therapy is used to optimize a normal hemostatic competence until this hemostasis is obtained and has been shown to improve survival(22). When using different TEG assays, measurements cannot be generalized, as activators (e.g. tissue factor, kaolin, or endogenous contact to surface of assay cup) differ between these tests(23).
One study has shown that the results of conventional coagulation tests correlate moderately with rTEG parameters, and these rTEG parameters correlate strongly with the most aspects of the respective kaolin TEG parameters(24). Furthermore, rTEG and CK-TEG show comparable predictive capacity in patients that receive a blood transfusion(24). In this study, we found the CK-TEG parameters that yielded the greatest AUROC curves were the angle (AUROC=0.6931) and MA (AUROC=0.7425). These parameters have routinely been shown to produce the greatest AUROC in studies evaluating rotational thromboelastometry (ROTEM) and rTEG (9) (24, 25), indicating that clot strength and dynamics of clot strength have the best predictive capacity for MT. Clot initiation (R-time), however, had poor predictability of MT (AUROC=0.6002). This is consistent with previous studies that have shown that measures of clot initiation do not have a strong correlation with transfusions(26). A poor correlation between rTEG and CK-TEG measures of clot initiation further supports the notion that clot initiation is an inconsistent predictor of MT(24). Other factors besides just coagulation factors may also influence clot initiation and include the effects of fibrinolysis and genetic variability(27, 28). Finally, as proteomic analysis of individual patients becomes more accessible, changes in plasma proteins in response to injury may also show to be directly influential toward clot initiation and subsequent laboratory tests used to identify abnormalities in this aspect of blood clotting(29).
The CK-TEG parameter for fibrinolysis, LY30, also yielded a suboptimal AUROC curve of 0.6024. This finding is consistent with evaluation of other viscoelastic assays that had suboptimal AUROC curves for markers of fibrinolysis(9) (30). Extent of fibrinolysis and early mortality exhibit a quadratic relationship which may be responsible for the diminished ability of viscoelastic measurements of fibrinolysis to predict massive transfusion(31). The exact nature of this quadratic relationship is difficult to discern. The quadratic relationship refers to a U-shape in which mortality is increased on either of the extreme. We found the same complexity in assessing FFP:RBC ratios in previous studies(17).
The value of TEG is in identifying specific mechanistic perturbations of coagulation and therefore guiding specific blood component resuscitation. Our goal was to determine CK-TEG thresholds that predict MT (a surrogate for significant coagulopathy) for the purpose of use in goal-directed resuscitation. The thresholds illustrated in Figure 1 reflect proposed points at which administration of specific blood products should be initiated for CK-TEG. It is important to consider the type of viscoelastic assay that is used for the treatment of the bleeding injured patient. TEG is used at our institution and more commonly in the United States, while ROTEM is prominently used in European centers (14). Not only are there different commercially available viscoelastic assay (ROTEM and TEG), but there are different activators for these assays(14). Similarly, more specialized viscoelastic assays can look specifically at fibrinogen function or platelet function. For example, the FIBTEM assay (ROTEM) and the Functional Fibrinogen assay (TEG) can address the fibrinogen contribution to cloth strength while TEG Platelet Mapping (TEG-PM) and ROTEM Platelet can address the function of platelets in the presence of various platelet agonists (4, 8, 32, 33). While the exact use of these specialized assays is still under debate, we have previously described that adenosine diphosphate (ADP) receptor inhibition did not add predictive value in predicting mortality, massive transfusion, or platelet transfusion with TEG-PM(33).
Some consider the rTEG (activated with kaolin and tissue factor) to be more physiologically relevant to trauma, and these combined activators provide results sooner. However, CK-TEG is also used for the treatment of injured patients to guide blood product transfusion and may be better at identifying hypercoaguability as a large multicenter study using tissue factor to activate viscoelastic assays showed that 10% of trauma patients had a hypercoaguable state within 24 hours of admission (33). The use of tissue factor may subsequently mask endogenous circulating factors that create a hypercoaguable state (5, 34). As we have shown that resuscitation based on rTEG improves survival compared to conventional coagulation assays, it may not matter which viscoelastic assay (rTEG, CK-TEG, or ROTEM) is used to guide hemostatic resuscitation in injured patients.
There were some important differences seen between the two centers included in the study (Table 4). The systolic blood pressure was lower, lactate and base deficit were increased, and INR and LY30 were longer in the Denver massively transfused patients compared to San Francisco. The increased shock could explain the enhanced INR and longer LY30(35, 36). However, the injury severity, and a majority of the coagulation parameters (fibrinogen, PTT, R-Time, Angle, and MA) were not different between groups.
There are several limitations notable to this study. These data reflect a single time point of a dynamics process and does not take into account the temporal changes of the coagulation process through the acute phase of resuscitation. It should be noted that rTEG resuscitation was only practiced at one of the centers which may introduce some variability. Some of these patients received a transfusion based on goal-directed resuscitation based on rTEG, and as such, these CK-TEG thresholds are predictive of a massive transfusion that is already partially guided by viscoelastic testing (rTEG). We acknowledge that these measurements are analyzed for MT as an endpoint and not individual components and should be used cautiously for continued guidance of blood product transfusion. However, at this time, these thresholds provide the best guidelines for specific components and can act as a frame work for the refinement of goal-directed CK-TEG based resuscitation. Furthermore, these data do not evaluate the effects of intervention of blood products on treating patients that received a MT and the study is not designed to define mortality related outcomes. Finally, as our focus was on MT, we did not evaluate other clotting related outcomes such as macrovascular and microvascular thromboembolic events or multiple organ failure (MOF).
In conclusion, the proposed thresholds provide an important addition to the evolution of viscoelastic-based resuscitation strategies. Our goal was to identify the best predictor of significant coagulopathy, using MT as a surrogate, for each aspect of the dynamic process of clot formation and breakdown using CK-TEG, a commonly used viscoelastic assay. These initial observations may serve as a building block for a multicenter trial to test the proposed algorithm, refine these recommendations, and compare viscoelastic assays. Refinement of the CK-TEG-based resuscitation strategies should include optimizing the respective clinical interventions for each given CK-TEG parameter to determine if targeting these thresholds will reduce mortality. While TEG can define which aspect of clot formation and breakdown is abnormal, assays such as proteomic or metabolomic analysis of injured patients may help better define the specific abnormality within clot initiation, dynamics of clot formation, clot strength, or fibrinolysis that accounts for the deranged TEG parameter.
Supplementary Material
Acknowledgments
Disclosure: Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart Lung and Blood Institute UM1-HL120877, the Department of Defense USAMRAA, W81XWH-12–2-0028, and W911QY-15-C-0044, in addition to the National Institute of Environmental Health Sciences K01ES026834. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood institute, or the Department of Defense. Additional research support provided by Haemonetics with shared intellectual property.
Footnotes
Author Contributions: G.R.S and J.J.S implemented the study, interpreted data, drafted and critically revised the manuscript. G.R.N interpreted data, drafted and critically revised the manuscript. L.Z.K. interpreted data, drafted and critically revised the manuscript. A.S.C. interpreted data, drafted and critically revised the manuscript. E.E.M, M.J.C, R.A.C, C.C.S, A.B, and A.S. are principal investigators, were responsible for study conception and design, implementation of study, completion of study, interpretation of data, manuscript drafting, and critical revision.
References
- 1.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fries D, Martini WZ. Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth. 2010;105(2):116–21. [DOI] [PubMed] [Google Scholar]
- 4.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214(5):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13(10):1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma Acute Care Surg. 2011;71(5 Suppl 1):S427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie SA, Kornblith LZ, Howard BM, Conroy AS, Kunitake RC, Nelson MF, Hendrickson CM, Calfee CS, Callcut RA, Cohen MJ. Characterization of distinct coagulopathic phenotypes in injury: Pathway-specific drivers and implications for individualized treatment. J Trauma Acute Care Surg. 2017;82(6):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82(1):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma Acute Care Surg. 2011;71(2 Suppl 3):S318–28. [DOI] [PubMed] [Google Scholar]
- 13.Meyer DE, Vincent LE, Fox EE, O’Keeffe T, Inaba K, Bulger E, Holcomb JB, Cotton BA. Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg. 2017;83(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli S. TEG(R) and ROTEM(R) in trauma: similar test but different results? World J Emerg Surg. 2012;7 Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36(7):723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutcher ME, Howard BM, Sperry JL, Hubbard AE, Decker AL, Cuschieri J, Minei JP, Moore EE, Brownstein BH, Maier RV, et al. Evolving beyond the vicious triad: Differential mediation of traumatic coagulopathy by injury, shock, and resuscitation. J Trauma Acute Care Surg. 2015;78(3):516–23. [DOI] [PubMed] [Google Scholar]
- 17.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma Acute Care Surg. 2008;65(2):261–70; discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 18.Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, Falk V, Mohr FW. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140(5):1117–24 e2. [DOI] [PubMed] [Google Scholar]
- 19.Haas T, Fries D, Velik-Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106(5):1360–5, table of contents. [DOI] [PubMed] [Google Scholar]
- 20.Schochl H, Maegele M, Solomon C, Gorlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - A systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraçoğlu AYA, Tetik S. The role of viscoelastic tests in trauma: “TEG and ROTEM”. J Pharmacol Med Chem. 2017;1(1):1–5. [Google Scholar]
- 24.Jeger V, Willi S, Liu T, Yeh DD, De Moya M, Zimmermann H, Exadaktylos AK. The Rapid TEG alpha-Angle may be a sensitive predictor of transfusion in moderately injured blunt trauma patients. ScientificWorldJournal. 2012;2012:821794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore HB, Moore EE, Chapman MP, Huebner BR, Einersen PM, Oushy S, Silliman CC, Banerjee A, Sauaia A. Viscoelastic Tissue Plasminogen Activator Challenge Predicts Massive Transfusion in 15 Minutes. J Am Coll Surg. 2017;225(1):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20(4):548–53. [DOI] [PubMed] [Google Scholar]
- 27.Tang W, Schwienbacher C, Lopez LM, Ben-Shlomo Y, Oudot-Mellakh T, Johnson AD, Samani NJ, Basu S, Gogele M, Davies G, et al. Genetic associations for activated partial thromboplastin time and prothrombin time, their gene expression profiles, and risk of coronary artery disease. Am J Hum Genet. 2012;91(1):152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee VH, Conners JJ, Cutting S, Song SY, Bernstein RA, Prabhakaran S. Elevated international normalized ratio as a manifestation of post-thrombolytic coagulopathy in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(8):2139–44. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee A, Silliman CC, Moore EE, Dzieciatskowa M, Kelher M, Sauaia A, Jones K, Chapman MP, Gonzalez E, Moore HB, et al. Systemic Hyperfibrinolysis after Trauma: A Pilot study of Targeted Proteomic Analysis of Superposed Mechanisms in Patient Plasma. J Trauma Acute Care Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaletel-Kragelj L, Bozikov J, Forum for Public Health Collaboration in South Eastern E Methods and tools in public health a handbook for teachers, researchers and health professionals. Lage: Jacobs; 2010. [Google Scholar]
- 31.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, Silliman CC. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stettler GR, Moore EE, Moore HB, Nunns GR, Huebner BR, Einersen P, Ghasabyan A, Silliman CC, Banerjee A, Sauaia A. Platelet adenosine diphosphate receptor inhibition provides no advantage in predicting need for platelet transfusion or massive transfusion. Surg. 2017;162(6):1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller MC, Balvers K, Binnekade JM, Curry N, Stanworth S, Gaarder C, Kolstadbraaten KM, Rourke C, Brohi K, Goslings JC, et al. Thromboelastometry and organ failure in trauma patients: a prospective cohort study. Crit Care. 2014;18(6):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore HB, Moore EE, Morton AP, Gonzalez E, Fragoso M, Chapman MP, Dzieciatkowska M, Hansen KC, Banerjee A, Sauaia A, et al. Shock-induced systemic hyperfibrinolysis is attenuated by plasma-first resuscitation. J Trauma Acute Care Surg. 2015;79(6):897–903; discussion −4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, Sauaia A, Cotton BA. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J Am Coll Surg. 2016;222(4):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


