Abstract
Objectives
To evaluate the therapeutic effect of once-per-week low-intensity extracorporeal shock wave therapy (Li-ESWT) on underactive bladder (UAB) in the streptozotocin-induced diabetic rat model.
Materials and Methods
Thirty-six female Sprague-Dawley rats were assigned into 3 groups: normal control (NC), diabetes control (DMC), and diabetes underwent Li-ESWT (DM Li-ESWT). The two DM groups received intraperitoneal 60 mg/kg streptozotocin injection to induce diabetes mellitus. The Li-ESWT was applied toward the pelvis of the rats started 4 weeks after the streptozotocin administration and lasted for 4 weeks. The shock wave therapy was given once-per-week with energy flux density of 0.02 mJ/mm2 at 3 Hz for 400 pulses. All rats were subjected to conscious cystometry, leak point pressure, ex-vivo organ bath study, histology, immunofluorescence, and western blot analysis.
Results
Conscious cystometry revealed voiding dysfunction in DMC group, whereas DM Li-ESWT group showed significantly improved voiding function in reduced post-void residual urine and increased leak point pressure compared to DMC group. Ex-vivo organ bath study showed that Li-ESWT enhances muscle contractile activity (MCA) of bladder and urethra in electrical field stimulation (EFS) and drug stimulation. Histologically, Li-ESWT significantly restored bladder morphology in reducing intravesical lumen area and increasing muscle proportion of the bladder wall. Western blot analysis showed higher smooth muscle actin (SMA) expression of bladder wall in DM Li-ESWT compared to DMC group. Immunofluorescence showed decreased nerve-ending distribution, and destroyed and shortened nerve fibers in DMC group and recovery of neuronal integrity and innervation in DM Li-ESWT group.
Conclusions
In conclusion, Li-ESWT ameliorated underactive bladder and urinary incontinence in the diabetic UAB rat model. The improvement seems to be the results of restoration of bladder and urethra structure and function by Li-ESWT. Li-ESWT is non-invasive and may become a better alternative therapy for UAB. Further investigations are warranted.
Introduction
Diabetes mellitus is a highly prevalent metabolic disease and has various sequelae in multiple organs and systems. Diabetic bladder dysfunction (DBD) is one of the major sequelae, and its symptoms vary with the severity and progression of diabetes[1]. Patients with DBD express overactive bladder symptoms as increased daytime frequency and urgency in the early stage of diabetes, which subsequently transform to underactive bladder (UAB) in the late stage. UAB is the consequence of late-stage DBD with the symptoms of decreased bladder contractility and sensation, atonic bladder, increased post void residual urine, and recurrent urinary tract infection[2]. Furthermore, half of diabetic patients experience urinary incontinence after 10 years of diabetes progression[3].
Diabetes treatment focuses foremost on controlling the blood glucose level, and then on managing diabetes-related complications of affected target organs and systems. Muscarinic stimulants such as bethanechol have been used to improve bladder contraction in diabetic UAB; however, these drugs can cause serious side effects and sometimes produce unsatisfactory outcomes. Intermittent catheterization is usually recommended for UAB to drain away residual urine, reduce urinary tract infection, and prevent further kidney injury, but such treatments reduce the quality of life[4]. The pathomechanism of DBD with UAB has been investigated broadly. Polyuria and hyperglycemia play different roles in the pathogenesis. Polyuria plays an important role in the bladder wall thickness in DBD[5]. Moreover, hyperglycemia-induced oxidative stress products accumulate, cause damage to muscles, neurons, urothelium, and urethra[6], and result in the progression to UAB.
Several experimental treatment modalities have been proposed for enhancing the bladder function. Sasaki et al. used viral vectors to deliver the nerve growth factor (NGF) gene to the bladder wall, resulting in increased NGF levels and improved bladder function[7]. Gopinath et al. demonstrated the effect of smooth muscle transplantation into the diabetic bladder wall, which resulted in increased bladder contractile function and decreased post-voiding residual urine[8]. A previous study by our laboratory demonstrated a satisfactory outcome with adipose tissue-derived stem cell therapy in DBD with UAB, through inhibition of apoptosis and promotion of vascular integrity[9]. However, practical clinical modalities with fewer side effects are not available at the present.
Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been applied as a clinical treatment modality in many types of diseases, including myocardial infarction and heart failure[10], bone fracture and tendinopathy[11], chronic soft tissue wounds[12], and skin ischemia and skin graft[13]. Li-ESWT has also been applied to a variety of urological disorders. For example, a systemic review of the effects of Li-ESWT on erectile dysfunction revealed encouraging results: among 14 studies including 833 patients from 2005–2015, Li-ESWT was found to improve erectile function as measured by international index of erectile function (IIEF) and erectile hardness score (EHS)[14]. Other urological diseases such as prostatitis[15] and chronic pelvic pain syndrome[16] have also been shown to benefit from Li-ESWT. Regarding the application of Li-ESWT to DBD, a previous report from our laboratory demonstrated a positive effect in improving overactive bladder by defocused Li-ESWT administered three times per week[17]. To further evaluate (a) the therapeutic effects of Li-ESWT on UAB, which develops in later stage of diabetes, and (b) to explore whether reduced frequency can produce similar beneficial effects, we used a defocused Li-ESWT device applied once per week in streptozotocin-induced diabetic rats.
Materials and Methods
1. Animals and experimental design
In total, 36 female, 8-week-old Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA). The experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee of University of California San Francisco. The rats were divided into 3 groups: normal control (NC), diabetic mellitus control (DMC), and DM Li-ESWT. DMC and DM Li-ESWT rats received a single intraperitoneal injection of 60 mg/kg streptozotocin for diabetes induction. All rats were housed in a standard room with constant temperature and humidity, and a 12-hour light-dark cycle. The rats had access to tap water and standard rat chow ad libitum. Blood glucose and body weight were measured at 6 hours before STZ injection, 3 days after STZ injection, and then every week for the remainder of the study.
2. Low-intensity shock wave therapy
Li-ESWT was applied 4 weeks after diabetes induction and lasted for 4 weeks with once-per-week regime in DM Li-ESWT group applying following parameters: 0.02 mJ/mm2 energy flux density at 3 Hz for 400 pulses. Under isoflurane anesthesia and the prone position of the rats, Li-ESWT probe which contains a compact electromagnetic unit with a defocused shockwave source (LiteMed Inc., Taipei, Taiwan) was applied on the lower abdomen of the rats aiming toward the bladder and urethra. The sham procedures including the same anesthesia exposure and recovery were performed for the NC and DMC group for 4 weeks. After a one-week washout period, the rats were subjected to conscious cystometry and leak point pressure study, followed by sacrifice and tissue harvest for the ex-vivo organ bath study and molecular analyses.
3. Conscious cystometry
Twenty-four hours before conscious cystometry, the surgery for tube implantation was performed. Two polyethylene-90 (PE-90) tubes (Clay-Adam®) were placed in the intravesical and intraperitoneal regions respectively for pressure measuring. The next day, the rats were placed in the tunnel of the cystometry cage (Braintree Scientific®). The two implanted tubes were connected to pressure transducers (Utah Medical Products®) attached to a computer, and Labview 6.0 software (National Instruments®) was used to record the pressure continuously. Simultaneously, an electric scale was also connected to the computer to record the voided volume. Normal saline was infused into the bladder at a rate of 0.1 ml/min using an infusion pump. After 20 minutes to allow stabilization, an one-hour conscious cystometry was recorded.
4. Leak point pressure
Under urethane anesthesia, the rats were placed in the supine position and at the level of zero pressure. After incision of the lower abdomen and exposure of the bladder, the bladder was filled halfway with normal saline through the bladder catheter. The bladder capacity was obtained by adding the postvoid residual urine volume (obtained by intravesical catheter) to the voided volume. One half of the bladder volume was used to fill the bladder for the study. Using two cotton swabs, gradual pressure was applied to the bilateral sides of the bladder and then stopped immediately when urine leakage occurred from the urethra orifice. The pressure at which this leakage occurred was regarded as the leak point pressure. The procedure was repeated 5 times to calculate the average leak point pressure for each rat.
5. Ex-vivo organ bath study
The proximal 2/3 of urethra and bladder strips (1/3 bladder length) were prepared in Krebs solution. The length and weight of each specimen were measured. The ex-vivo organ bath procedures followed our published protocol[18]. The urethra and bladder tissues were positioned in the tissue bath system (Myobath Tissue Bath system II®). The chambers were loaded with Krebs solution maintained at 37°C and supplied continuously with 95% O2 and 5% CO2. The tissue samples were linked to force-displacement transducers. A pair of platinum electrodes was placed on the bilateral sides of the tissue, and electronic stimulators were connected to the electrodes. For urethra electrical field stimulation(EFS), square wave pulses were applied at 10–70 mA intensity and 0.2 ms duration with a 3-minute interlude in the urethral pulse study. For urethral fatiguing stimulation, repeated multi-pulse EFS were applied at maximal intensity 70 mA at 5 Hz square wave pulses for 5 minutes. For bladder EFS study, a train duration of 10 seconds and an inter-train interval of 90 seconds were used, and frequency response curves were recorded at 2, 5, 10, and 20 Hz. Tetrodotoxin, a neurotoxin that blocks nerve stimulation, was added into the chambers before each EFS to ensure the contractions are directly from muscle but not nerve mediated. The drug stimulation studies were performed with 40 mM caffeine in urethra and carbachol in bladder. Muscle contractile activity (MCA) was calculated by using the equation: MCA (N/cm2) = [force (g) × muscle length (cm) × 1.06]/[muscle weight (g) × 0.00981]. A Lab TRAX-4 data acquisition system (Myobath Tissue Bath system II®) was used for measuring the isometric tension.
6. Histology, immunofluorescence, and western blot analysis
Tissues were harvested immediately after the leak point pressure study. The rats in each group were divided into two groups for either the histological or organ bath study, which have different storage protocols. For the histologic analysis, the tissues were fixed, embedded and sliced as routine. Hematoxylin and eosin staining and Masson’s trichome staining were performed for histological evaluation. For immunofluorescence, primary antibodies were incubated with anti-s100 antibody and anti-SMA antibody (Abcam®). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen®). For western blot analysis, the procedures for western blot analysis followed our established protocols[19]. The primary antibodies were incubated with beta-actin antibody and anti-SMA antibody. Image analysis was performed with a ChemiImager 4000® to calculate the density of each protein band.
7. Image and statistical analyses
For histological image analysis, 5 separate fields from each slide were recorded with a Retiga Q image digital camera and ACT-1 software (Nikon Instruments Inc®). Image quantification was estimated by Image-Pro Plus (Media Cybernetics®). Numerical data were analyzed with Prism 5 (Graph Pad Software®) and expressed as mean ± standard error of the mean. To test for significant differences, multiple groups were analyzed by t-test and one-way analysis of variance. The Turkey-Kramer test was applied for post-hoc comparisons. P<0.05 was considered to be significant for the comparison of the two groups.
Results
1. Biological characteristics of the animals
Weekly body weight measurements revealed significantly higher body weights for NC compared with DMC and DM Li-ESWT rats (NC: 293.2 ± 4.8 vs. DMC: 227.2 ± 6.8 vs. DM Li-ESWT: 231.1 ± 4.0 g, P<0.05, at the 10th week) [Figure 1]. The difference in body weight between the groups became apparent two weeks after DM induction. DMC and DM Li-ESWT rats had significantly higher blood glucose levels compared with NC rats (NC: 115.3 ± 3.0 vs. DMC: 492.9 ± 15.0 vs. DM Li-ESWT: 499.0 ± 18.7 mg/dl, P<0.05, at the 10th week). There were no differences in body weight and blood glucose between DMC and DM Li-ESWT rats.
Figure 1.

2. Li-ESWT improves function of underactive bladder in diabetic rats
Conscious cystometry revealed significantly different urinary patterns between NC and DM rats. Representative results for a 30-minute recording interval for each group are shown in Figure 2. The cystometric graph shows the regular and stable urinary patterns in NC rats; weak, irregular and unstable urination in DMC rats; and improved bladder function in DM Li-ESWT group. The DM Li-ESWT group showed improved voiding function, reflected in: shorter micturition interval, higher urinary frequency, lower voided volume, and higher maximal detrusor pressure without abdominal-strained urination. Parameters in cystometry showed the deterioration of bladder function in DMC compared with NC rats [Table 1]. After Li-ESWT, DM Li-ESWT rats had significantly improved voiding function, reflected in: decreased post-voided residual urine (DMC: 0.91 ± 0.23 vs. DM Li-ESWT: 0.30 ± 0.07 ml, P<0.05) and increased leak point pressure (DMC: 31.3 ± 2.0 vs. DM Li-ESWT: 38.2 ± 1.8 cmH2O, P<0.05) compared with DMC rats. Furthermore, there were other parameters in DM Li-ESWT group that presented improving trends but did not reach statistical significance, including decreased void volume, decreased micturition interval, and increased maximal detrusor pressure. Taken together, these parameters show deterioration of the voiding function to UAB as a consequence of diabetes, and its amelioration by Li-ESWT.
Figure 2.
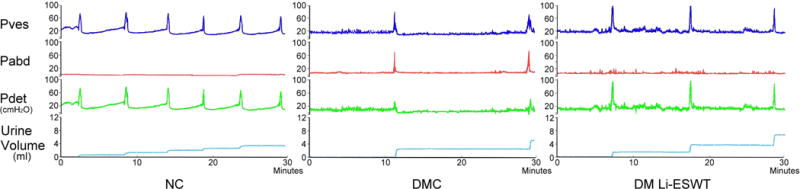
Table 1.
Parameters of conscious cystometry. DM Li-ESWT rats had significantly improved voiding function, reflected in: decreased post-voided residual urine, decreased bladder compliance, and increased leak point pressure compared with DMC rats.
| Parameters of conscious cystometry | ||||
|---|---|---|---|---|
|
| ||||
| Parameter\Group | Normal Control | DM Control | DM Li-ESWT | P value*** |
| Post-void residual urine (ml) | 0.15 ± 0.01 | 0.91 ± 0.23* | 0.30 ± 0.07** | <0.01, 0.74, 0.01 |
| Voided volume (ml) | 0.64 ± 0.05 | 2.05 ± 0.27* | 1.75 ± 0.06 | <0.01, <0.01, 0.40 |
| Micturition interval (second) | 411.3 ± 42.4 | 927.1 ± 119.5* | 792.2 ± 52.0 | <0.01, <0.01, 0.46 |
| Maximal detrusor pressure (cmH2O) | 71.3 ± 4.0 | 59.8 ± 6.8 | 76.5 ± 3.7 | 0.26, 0.74, 0.06 |
| Leak point pressure (cmH2O) | 42.9 ± 1.6 | 31.3 ± 2.0* | 38.2 ± 1.8** | <0.01, 0.19, 0.03 |
There were other parameters in DM Li-ESWT group which presented improving trends but did not reach statistical significance, including decreased void volume, decreased micturition interval, and increased maximal detrusor pressure.
: P<0.05 versus NC group;
: P<0.05 versus DMC group,
: P values are expressed in order as NC vs DMC, NC vs DM Li-ESWT, and DMC vs DM Li-ESWT.
Data are expressed as mean ± standard error of mean.
3. Li-ESWT enhances detrusor contractility
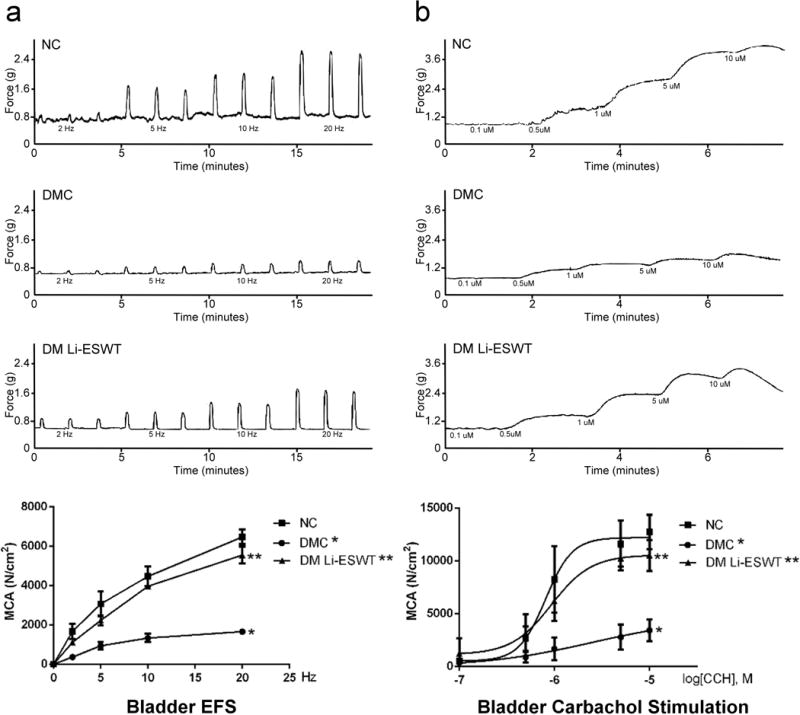
In ex-vivo organ bath studies for the urethra [Figure 3], DMC group showed significantly impaired MCA by EFS compared with NC and DM Li-ESWT group in the urethra study. DMC group had a significantly lower percentage of MCA in the fatigue stimulation test compared with NC and DM Li-ESWT groups (NC: 62.3 ± 8.1 vs. DMC: 38.1 ± 5.4 vs. DM Li-ESWT: 56.5 ± 1.9 %, P<0.05). DMC group had significantly impaired urethral MCA stimulated by caffeine compared with NC and DM Li-ESWT groups (NC: 4732 ± 487 vs. DMC: 1277 ± 83 vs. DM Li-ESWT: 3222 ± 694 N/cm2, P<0.05). In the bladder study [Figure 4], DMC group showed significantly impaired MCA stimulated by EFS compared with NC and DM-Li-ESWT groups. DMC group presented significantly lower carbachol-induced bladder MCA compared with NC and DM Li-ESWT groups. These organ bath studies demonstrate that Li-ESWT restores the muscle contractile functions of the bladder and urethra impaired by diabetes.
Figure 3.

Figure 4.
4. Li-ESWT activates bladder muscle regeneration
Bladder wall remodeling was found in DM and DM Li-ESWT groups [Figure 5]. DMC rats exhibited the pathological changes of UAB in the late-stage DBD, including chronic inflammation, tissue edema, and muscle atrophy of the bladder wall, which are characteristics of diabetic UAB. After Li-ESWT, DM Li-ESWT group exhibited the decrease in bladder size, leukocyte infiltration, tissue edema, wall thickness, and intravesical lumen areas compared with DMC group. Focally magnified images of the bladder wall showed that DMC rats had scattered and shrunken bladder muscle, whereas DM Li-ESWT rats had increased muscle volume, muscle bundle size and muscle proportion. The parameters of the bladder wall were deteriorated in DMC and partially reversed in DM Li-ESWT. DM Li-ESWT group had significantly smaller intravesical lumen areas (DMC: 7.5 ± 0.7 vs. DM Li-ESWT: 3.3 ± 0.8 mm2, P<0.05) and higher proportion of muscle content (DMC: 23.3 ± 1.1 vs. DM Li-ESWT: 28.3 ± 1.1 %, P<0.05). Furthermore, several additional histological findings in DM Li-ESWT group showed the improving trends but not in statistical significance, including lesser bladder cross-sectional area, bladder diameter, and bladder wall. Western blot analysis revealed significantly reduced relative SMA expression of the bladder wall in DMC rats compared with NC and DM Li-ESWT rats (NC: 100 vs. DMC: 68.7 ± 4.4 vs. DM Li-ESWT: 82.2 ± 4.2 %, P<0.05). Both the histological and western blot findings revealed restoration of the muscle content after Li-ESWT.
Figure 5.

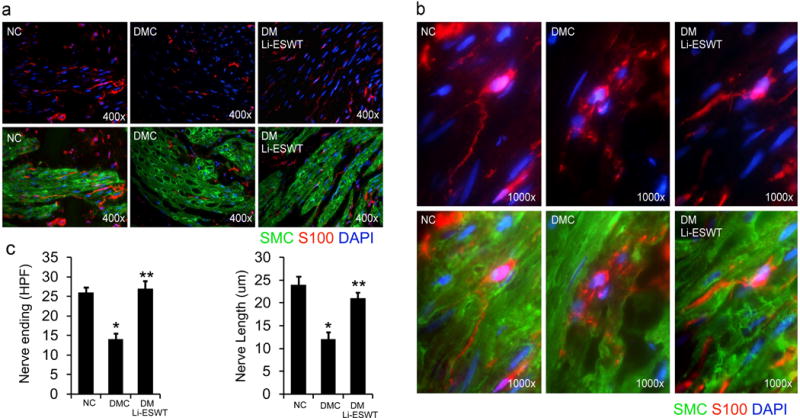
5. Li-ESWT enhances bladder nerve regeneration
Immunofluorescence microscopy with anti-S100 antibody staining under 400× magnification showed decreased nerve ending distribution and shortened nerve lengths in the bladder walls in DMC rats compared with NC and DM Li-ESWT rats [Figure 6]. Imaging at 1000× magnification showed clear differences in the morphology of the nerve fibers among groups: long and intact nerve fibers in NC group; atrophied and fragmented nerve fibers in DMC group; and regenerated nerve fibers in DM LI-ESWT group. Quantification of the high-power images revealed significantly decreased nerve-ending distribution (NC: 26.1 ± 1.2 vs. DMC: 13.9 ± 1.3 vs. DM Li-ESWT: 26.9 ± 1.9, P<0.05) and significantly shortened nerve length (NC: 23.8 ± 1.7 vs. DMC: 12.2 ± 1.5 vs. DM Li-ESWT: 20.9 ± 1.3 μm, P<0.05) in DMC animals compared with NC and DM Li-ESWT animals. These data demonstrated that Li-ESWT promotes bladder nerve regeneration and innervation.
Figure 6.
Discussion
UAB in the late stage of DBD is an intractable condition without efficacious treatment options[20, 21]. The major purposes of this study were to evaluate the therapeutic effect of Li-ESWT on UAB rat model and to examine the feasibility of a reduced frequency, once-a-week Li-ESWT. Both of these domains showed promising results in acutely diabetic rats. The current study revealed that Li-ESWT improved bladder wall composition, activated bladder muscle regeneration, enhanced bladder and urethra muscle contractile function, improved bladder innervation and promoted urethra continence. There are several strengths in the current study. First, the triad of conscious cystometry, leak point pressure and ex-vivo organ bath studies provided the details of the functional changes in diabetes and the effects of Li-ESWT on the bladder and urethra. Second, the study confirms the previous report of beneficial effect of the Li-ESWT on diabetic bladder. Third, the positive effects in functional studies were further supported by results of immunohistology and western blot analysis.
On the contrary, there are a number of weakness and the limitations of this study. First, we conducted once-per-week ESWT at a single energy level. Although we observed positive effects from this treatment regime, we cannot conclude that this is the optimal energy dosage for the once-a-week therapy. Elevating the energy level may increase the therapeutic benefit of ESWT, but also increase the risk of potential injury to the bladder. Further safety studies for Li-ESWT of the bladder should be conducted. Second, different UAB models may respond differently to Li-ESWT. Studies related to the effect of Li-ESWT on UAB are still sparse. Chuang et al. reported that Li-ESWT improved the voiding function and increased the bladder contractile amplitude for myogenic detrusor underactivity in a cryoinjury UAB model[22]. The current UAB model is diabetes-induced. Many factors may influence the induction of UAB, such as the degrees of the hyperglycemia and the durations of the diabetes. Further studies of Li-ESWT on UAB in different animal models are needed. Third, we did not use markers to label stem cells in this experiment. The therapeutic mechanism of ESWT related to stem cells has been previously investigated, including by our group[23, 24]. In the present study, the potential effect of once-per-week ESWT on bladder stem cells cannot be determined. All these weaknesses require further investigation.
In the present study, several parameters in conscious cystometry were improved after Li-ESWT. Lowering of the post void residual urine is one of the most meaningful changes. UAB in the late stage of DBD causes high post-void residual urine and results in serious complications such as urinary tract infection, bladder stone formation, reflux hydronephrosis, and chronic renal insufficiency[20, 21]. Much effort has been directed toward reducing the residual urine; however, few therapeutic modalities have been developed and the efficacies are still unsatisfactory. In the present study, we found that Li-ESWT reduces post-void residual urine by enhancing muscle contractile function, restoring bladder wall composition, promoting nerve regeneration, and ameliorating the coordination of bladder and urethra function. In the domains of the urinary incontinence, our study revealed the elevation of the leak point pressure and the increase of the urethra muscle contractile function in the organ bath studies, which provided the evidences for the therapeutic effect for the urinary incontinence by Li-ESWT. Urinary incontinence is a major complication of UAB in the late-stage DBD. A large cohort study demonstrated that diabetes causes a threefold higher risk of incontinence in patients who had diabetes for 5 years or more[3]. The pathophysiology of the urinary incontinence includes diabetic neuropathy, microvascular damage, muscular dysfunction, and discoordination of the bladder and urethra[25]. The therapeutic mechanism of Li-ESWT involves angiogenesis, nerve innervation, tissue regeneration[26] and muscle function restoration[26, 27]. Li-ESWT stimulates the regeneration of skeletal muscle and enhances tissue repair with the higher expression of the myonuclear content of regenerating muscle fibers[26]. Via the activation of protein kinase RNA-like endoplasmic reticulum kinase (PERK) and the transcription factor 4 (ATF4) pathway, Li-ESWT stimulates urethral muscle-derived stem cells (uMDSC), promotes myogenesis and ameliorates urinary incontinence[27]. Further investigations for the therapeutic effects and the mechanisms of Li-ESWT on incontinence are necessary.
Bladder remodeling comprises the morphological changes of the bladder wall due to polyuria and hyperglycemic damage related to diabetes. The degree of alteration of each component of the bladder varies with the severity and duration of diabetes[28]. Diabetes-related insulin resistance, metabolic syndrome, oxidative stress, chronic inflammation and chronic ischemia also play important roles in the progression of bladder remodeling[29]. In the present study, bladder enlargement, increased intravesical lumen area, increased bladder cross-sectional area, increased bladder diameter, increased bladder wall thickness, and decreased bladder smooth muscle content were all found in the DMC group. Li-ESWT partially reversed the morphological changes of the bladder, maintained the structure of the bladder, restored muscle volume, activated muscle regeneration, and decelerated the progression of DBD. Several studies regarding to diabetic bladder remodeling have been performed and yielded inconsistent results[5, 17, 30]. These differences for the changes of the bladder wall, smooth muscle thickness and contents among studies are likely due to dynamic changes of DBD and UAB associated with different severities and durations of diabetes in the different studies.
Regarding the weekly frequency of the regime for Li-ESWT, we reduced the frequency of ESWT to once per week. The main purpose of this reduction was to have the better patient compliance which had been well established to highly correlate with convenience of the medical treatment[31]. A review study of the association between dose regimen and medication compliance found that the daily prescribed dosage number is inversely correlated with compliance[32]. The lower dosing frequencies, the better compliance. Among the clinical applications of ESWT, most protocols use frequencies of two or three times per week, which is not only time- and cost-consuming for patients, but also reduces compliance[14, 33]. Few studies performed Li-ESWT once per week and yielded promising outcomes[34, 35]. ESWT is a dosage-dependent therapy[36]. We tried to balance the therapeutic effect and the weekly frequency of Li-ESWT to maintain both efficacy and compliance in patients. Here, we confirmed that reduced frequency, once-per-week, Li-ESWT has a satisfactory therapeutic outcome in the UAB rat model. This provides the potentiality for the intractable UAB treatment. Future studies should be investigated deeply to help translating the research finding into the possible clinical application for UAB.
Several mechanisms of ESWT have been proposed, including tissue regeneration, nerve re-innervation[37–39], angiogenesis[40], antiinflammation[41], and stem cell activation and recruitment[23, 24]. Vascular endothelial growth factor (VEGF) plays an important role for angiogenesis effect of Li-ESWT. Combining with the effects from angiogenesis and antiinflammation, the studies reveal Li-ESWT improves the skin flap tissue survival and promotes wound healing by enhancing neovascularization with increasing expression of VEGF and endothelial nitric oxide synthase (eNOS) and suppressing the inflammatory response by limiting leukocyte infiltration[40, 41]. The enhancement of nerve re-innervation by ESWT is one of the key mechanisms of the therapeutic effect of ESWT on UAB. In the present study, immunofluorescence microscopic observations with anti-S100 antibody staining (a marker for Schwann cells of the peripheral nervous system which has been used broadly for the identification of nerve innervation and nerve morphology[42]) revealed decreased nerve distribution and destroyed nerve structure caused by diabetes, which were found to be improved by Li-ESWT. Li-ESWT aids in re-innervation and therefore restoration of the nerve function in the bladder. The studies regarding to Li-ESWT for the nerve regeneration demonstrated that Li-ESWT promotes nerve regeneration with increasing neuronal nitric oxide synthase (nNOS)-positive neurons, resulting in the amelioration of the erectile dysfunction[38]. PERK and ATF4 pathway had been demonstrated to enhance brain-derived neurotrophic factor expression in Schwann cells and promote nerve regeneration[39]. In addition, stem cell activation and recruitment also play an important role in the mechanism of Li-ESWT. Li-ESWT stimulates the recruitment of endogenous progenitor cells[23], activates Schwann cells[24], induces the secretion and proliferation of bone marrow-derived mesenchymal stromal cells[43] and stimulates the expression of NGF and VEGF[17], resulting in angiogenesis, nerve regeneration and tissue restoration.
In conclusion, once-per-week Li-ESWT ameliorated bladder dysfunction and urinary incontinence in the diabetic UAB rat model. Li-ESWT improved bladder wall composition, activated bladder muscle regeneration, enhanced bladder and urethra muscle contractile function, increased bladder nerve innervation and promoted urethra continence. Once-per-week Li-ESWT seems to have a positive effect on diabetes induced UAB. Further investigations are warranted.
Acknowledgments
Research reported in this publication was supported by NIDDK of the National Institutes of Health under award number R56DK105097 and 1R01DK105097-01A1. Opinions, interpretations, conclusions and recommendations are those of the author and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- DBD
diabetic bladder dysfunction
- UAB
underactive bladder
- DM
diabetes mellitus
- Li-ESWT
low-intensity extracorporeal shock wave therapy
- NC
normal control
- DMC
diabetes mellitus control
- SMA
smooth muscle actin
- MCA
muscle contractile activity
- EFS
electrical field stimulation
Footnotes
DR. HSUN SHUAN WANG (Orcid ID : 0000-0003-1063-9089)
DR. BOHAN WANG (Orcid ID : 0000-0003-3561-9367)
DR. YAJUN RUAN (Orcid ID : 0000-0001-7767-7309)
Conflicts of Interest
Tom F. Lue is a consultant for Pfizer, Eli Lilly and Boston Scientific and chief medical officer and stock holder for AWCT, Inc.
References
- 1.Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006;176:380–6. doi: 10.1016/S0022-5347(06)00582-9. [DOI] [PubMed] [Google Scholar]
- 2.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, Chacko S. Diabetic bladder dysfunction: current translational knowledge. J Urol. 2009;182:S18–26. doi: 10.1016/j.juro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lifford KL, Curhan GC, Hu FB, Barbieri RL, Grodstein F. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53:1851–7. doi: 10.1111/j.1532-5415.2005.53565.x. [DOI] [PubMed] [Google Scholar]
- 4.Chapple CR, Osman NI, Birder L, et al. The underactive bladder: a new clinical concept? Eur Urol. 2015;68:351–3. doi: 10.1016/j.eururo.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Xiao N, Wang Z, Huang Y, Daneshgari F, Liu G. Roles of polyuria and hyperglycemia in bladder dysfunction in diabetes. J Urol. 2013;189:1130–6. doi: 10.1016/j.juro.2012.08.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology. 2004;64:1062–7. doi: 10.1016/j.urology.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki K, Chancellor MB, Goins WF, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–30. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- 8.Gopinath C, Ponsaerts P, Fransen E, Boeykens N, Pauwels P, Wyndaele JJ. Smooth muscle cell transplantation improves bladder contractile function in streptozocin-induced diabetic rats. Cytotherapy. 2013;15:869–78. doi: 10.1016/j.jcyt.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Qiu X, Shindel AW, et al. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev. 2012;21:1391–400. doi: 10.1089/scd.2011.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myojo M, Ando J, Uehara M, Daimon M, Watanabe M, Komuro I. Feasibility of Extracorporeal Shock Wave Myocardial Revascularization Therapy for Post-Acute Myocardial Infarction Patients and Refractory Angina Pectoris Patients. Int Heart J. 2017;58:185–90. doi: 10.1536/ihj.16-289. [DOI] [PubMed] [Google Scholar]
- 11.Furia JP, Rompe JD, Maffulli N, Cacchio A, Schmitz C. Radial Extracorporeal Shock Wave Therapy Is Effective and Safe in Chronic Distal Biceps Tendinopathy. Clin J Sport Med. 2017;27:430–7. doi: 10.1097/JSM.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 12.Porso M, Loreti S, Nusca SM, et al. Defocused Shock Wave Therapy for Chronic Soft Tissue Wounds in the Lower Limbs: A Pilot Study. Ultrasound Med Biol. 2017;43:362–9. doi: 10.1016/j.ultrasmedbio.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Mittermayr R, Hartinger J, Antonic V, et al. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg. 2011;253:1024–32. doi: 10.1097/SLA.0b013e3182121d6e. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Lin G, Reed-Maldonado A, Wang C, Lee YC, Lue TF. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol. 2017;71:223–33. doi: 10.1016/j.eururo.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 15.Fojecki GL, Tiessen S, Osther PJ. Extracorporeal shock wave therapy (ESWT) in urology: a systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J Urol. 2017;35:1–9. doi: 10.1007/s00345-016-1834-2. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann R, Cumpanas A, Miclea F, Janetschek G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: a randomised, double-blind, placebo-controlled study. Eur Urol. 2009;56:418–24. doi: 10.1016/j.eururo.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Xu L, Zhao Y, Wang M, Jin X, Zhang H. Endogenous Stem Cells Were Recruited by Defocused Low-Energy Shock Wave in Treating Diabetic Bladder Dysfunction. Stem Cell Rev. 2017;13:287–98. doi: 10.1007/s12015-016-9705-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee YC, Lin G, Wang G, et al. Impaired contractility of the circular striated urethral sphincter muscle may contribute to stress urinary incontinence in female zucker fatty rats. Neurourol Urodyn. 2017;36:1503–10. doi: 10.1002/nau.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin G, Li H, Zhang X, et al. Novel therapeutic approach for neurogenic erectile dysfunction: effect of neurotrophic tyrosine kinase receptor type 1 monoclonal antibody. Eur Urol. 2015;67:716–26. doi: 10.1016/j.eururo.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 20.van Koeveringe GA, Rademakers KL, Birder LA, et al. Detrusor underactivity: Pathophysiological considerations, models and proposals for future research. ICI-RS 2013. Neurourol Urodyn. 2014;33:591–6. doi: 10.1002/nau.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389–98. doi: 10.1016/j.eururo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Chuang YC, Tyagi P, Wang HJ, Huang CC, Lin CC, Chancellor MB. Urodynamic and molecular characteristics of detrusor underactivity in a rat cryoinjury model and effects of low energy shock wave therapy. Neurourol Urodyn. 2017:1–8. doi: 10.1002/nau.23381. [DOI] [PubMed] [Google Scholar]
- 23.Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med. 2017;14:493–501. doi: 10.1016/j.jsxm.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med. 2016;13:22–32. doi: 10.1016/j.jsxm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Izci Y, Topsever P, Filiz TM, Cinar ND, Uludag C, Lagro-Janssen T. The association between diabetes mellitus and urinary incontinence in adult women. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:947–52. doi: 10.1007/s00192-009-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zissler A, Steinbacher P, Zimmermann R, et al. Extracorporeal Shock Wave Therapy Accelerates Regeneration After Acute Skeletal Muscle Injury. Am J Sports Med. 2017;45:676–84. doi: 10.1177/0363546516668622. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Zhou J, Banie L, et al. Low-intensity extracorporeal shock wave therapy promotes myogenesis through PERK/ATF4 pathway. Neurourol Urodyn. 2017:1–9. doi: 10.1002/nau.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Daneshgari F. Temporal diabetes- and diuresis-induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R837–43. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 29.Uzun H, Ogullar S, Sahin SB, et al. Increased bladder wall thickness in diabetic and nondiabetic women with overactive bladder. Int Neurourol J. 2013;17:67–72. doi: 10.5213/inj.2013.17.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendig DM, Ets HK, Moreland RS. Effect of type II diabetes on male rat bladder contractility. Am J Physiol Renal Physiol. 2016;310:F909–22. doi: 10.1152/ajprenal.00511.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 32.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 33.Behr-Roussel D, Giuliano F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl Androl Urol. 2016;5:977–9. doi: 10.21037/tau.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Kim JY, Choi CM, et al. The Dose-Related Effects of Extracorporeal Shock Wave Therapy for Knee Osteoarthritis. Ann Rehabil Med. 2015;39:616–23. doi: 10.5535/arm.2015.39.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y, Lee JK, Lee BY, Kee HS, Jung KI, Yoon SR. Effectiveness of Lower Energy Density Extracorporeal Shock Wave Therapy in the Early Stage of Avascular Necrosis of the Femoral Head. Ann Rehabil Med. 2016;40:871–7. doi: 10.5535/arm.2016.40.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller A, Akin-Olugbade Y, Deveci S, et al. The impact of shock wave therapy at varied energy and dose levels on functional and structural changes in erectile tissue. Eur Urol. 2008;53:635–42. doi: 10.1016/j.eururo.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 37.Ohtori S, Inoue G, Mannoji C, et al. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci Lett. 2001;315:57–60. doi: 10.1016/s0304-3940(01)02320-5. [DOI] [PubMed] [Google Scholar]
- 38.Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–46. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Ning H, Reed-Maldonado AB, et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi D, Kawakami K, Ito K, et al. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: a critical role of endothelial nitric oxide synthase. Wound Repair Regen. 2012;20:887–95. doi: 10.1111/j.1524-475X.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuo YR, Wu WS, Hsieh YL, et al. Extracorporeal shock wave enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J Surg Res. 2007;143:385–92. doi: 10.1016/j.jss.2006.12.552. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Martinez T, Perez-Pinera P, Diaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633–8. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Wang J, Wang M, et al. Activation of bone marrow-derived mesenchymal stromal cells-a new mechanism of defocused low-energy shock wave in regenerative medicine. Cytotherapy. 2013;15:1449–57. doi: 10.1016/j.jcyt.2013.08.012. [DOI] [PubMed] [Google Scholar]


