Abstract
Objective
A single bout of exercise followed by intake of carbohydrates leads to glycogen supercompensation in prior exercised muscle. Our objective was to illuminate molecular mechanisms underlying this phenomenon in skeletal muscle of man.
Methods
We studied the temporal regulation of glycogen supercompensation in human skeletal muscle during a 5 day recovery period following a single bout of exercise. Nine healthy men depleted (day 1), normalized (day 2) and supercompensated (day 5) muscle glycogen in one leg while the contralateral leg served as a resting control. Euglycemic hyperinsulinemic clamps in combination with leg balance technique allowed for investigating insulin-stimulated leg glucose uptake under these 3 experimental conditions. Cellular signaling in muscle biopsies was investigated by global proteomic analyses and immunoblotting. We strengthened the validity of proposed molecular effectors by follow-up studies in muscle of transgenic mice.
Results
Sustained activation of glycogen synthase (GS) and AMPK in combination with elevated expression of proteins determining glucose uptake capacity were evident in the prior exercised muscle. We hypothesize that these alterations offset the otherwise tight feedback inhibition of glycogen synthesis and glucose uptake by glycogen. In line with key roles of AMPK and GS seen in the human experiments we observed abrogated ability for glycogen supercompensation in muscle with inducible AMPK deletion and in muscle carrying a G6P-insensitive form of GS in muscle.
Conclusion
Our study demonstrates that both AMPK and GS are key regulators of glycogen supercompensation following a single bout of glycogen-depleting exercise in skeletal muscle of both man and mouse.
Keywords: AMP-activated protein kinase (AMPK), TBC1 domain family member 4 (TBC1D4), Glycogen synthase (GS), Glucose uptake, Exercise, Insulin action
Highlights
-
•
A single bout of exercise followed by carbohydrate intake leads to glycogen supersompensation in the prior exercised muscle.
-
•
Skeletal muscle AMPK and glycogen synthase remain activated beyound normalized muscle glycogen content.
-
•
Glycogen synthesis above resting levels is mediated independent of muscle insulin sensitivity.
1. Introduction
Glycogen is formed as a branched polymer of glucose serving as an essential energy depot in times of nutritional surplus that can be readily mobilized when energy is needed [1]. While liver glycogen directly contributes to glucose homeostasis by releasing glucose into the blood, skeletal muscle lacks this ability but accounts for ∼80% of glucose disposal under insulin-stimulated conditions [2]; in this way, muscle glucose uptake and storage becomes crucial for controlling blood glucose levels. The majority of glycogen is stored in skeletal muscle (a total of ∼400 g) and the “set-point” for glycogen concentration in human skeletal muscle appears to be tightly controlled at a level of ∼1.5 g/100 g muscle. This is mediated by an efficient feedback-regulation of glycogen synthase (GS) by muscle glycogen concentration [3]. Intriguingly, when a single bout of exercise is followed by intake of carbohydrates, the muscle cell favors glycogen synthesis far beyond resting levels (i.e. glycogen supercompensation) and muscle glycogen concentration can increase up to ∼4 g/100 g muscle. In 1966, Bergström and Hultman demonstrated that the ability to supercompensate muscle glycogen is restricted to the prior exercised muscle [4]. The authors concluded that a single bout of exercise enhances factors within the prior exercised muscle that were maintained for several days after exercise, elevating the “set-point” for muscle glycogen storage. It is noteworthy that the molecular nature of these exercise-induced “enhancing factors” that allow for glycogen synthesis far beyond normalized muscle glycogen concentration remains to be demonstrated.
The activity of glycogen synthase (GS), the key regulatory enzyme in glycogen synthesis, is controlled by the allosteric activator glucose-6-phosphate (G6P), and by covalent phosphorylation at inhibiting residues [5], [6]. Insulin enhances glycogen synthesis through increased glucose uptake and by promoting de-phosphorylation and thus activation of GS [1], [7]. Compared to insulin, exercise results in a more pronounced de-phosphorylation and hence higher activation of GS in skeletal muscle [8], [9] than during insulin stimulation. A synergy exists between allosteric and covalent regulation of GS such that GS affinity for the allosteric activator G6P is enhanced in the de-phosphorylated state [10], [11]. It remains an open question whether this molecular regulation of GS is involved in the phenomenon of glycogen supercompensation in skeletal muscle.
AMP activated protein kinase (AMPK) serves as a cellular energy regulator by monitoring cellular nucleotide status [12]. AMPK binds to the glycogen particle [13], [14], [15] and may also act as a cellular fuel sensor as muscle glycogen is a negative regulator of AMPK activation [16], [17]. Supporting this concept, prolonged AMPK activation in vivo by different pharmacological means (e.g. AICAR, PF739 and MK-8722) is accompanied by elevated muscle glycogen concentration [18], [19], [20]. Moreover, missense mutation (gain-of-function) of the AMPK γ3 or γ1 regulatory subunits is associated with elevated muscle glycogen content [21], [22], [23], [24], [25]. Thus, a tight coupling exists between muscle glycogen and AMPK. AMPK in human skeletal muscle exists in three different complexes (α2β2γ1, α1β2γ1 and α2β2γ3) and kinase activity increases in a trimer-specific manner during exercise [26], [27], [28], [29], and may stay elevated for several hours into exercise recovery [30]. Studies in rodents suggest that this AMPK activation is necessary for insulin sensitization of skeletal muscle in the period immediately following exercise [31], [32]. However, whether AMPK is involved in the regulation of glycogen supercompensation in skeletal muscle remains to be investigated.
Here, we have used an invasive study in man and advanced muscle proteomics to revisit the phenomenon of glycogen supercompensation in skeletal muscle. In the course of 5 days both in vivo physiological measurements and classical biochemical as well as proteomic analyses in muscle biopsies revealed temporal regulation of insulin sensitivity for muscle glucose uptake, intracellular signaling, capacity for glucose uptake as well as glucose/fatty acid oxidation.
2. Methods
2.1. One-legged glycogen supercompensation regime in man
The study was approved by the regional ethics committee in Denmark (Journal number: H-4-2013-071) and performed in accordance with the Declarations of Helsinki II. Each subject gave written informed consent before participating. Nine healthy male subjects (age, 26 ± 3 years; BMI, 23.5 ± 1.7 kg/m2; VO2 peak, 45 ± 4 ml O2/min/kg; data presented as means ± SD) underwent a glycogen supercompensation regime. The study design is illustrated in Figure 1 and a detailed description of preclinical investigation of the subjects and the experimental design is provided in Supplemental I. The subjects were familiarized to the one-legged knee extensor model at light intensity prior to the testing. The working leg was chosen randomly in order to avoid possible confounding effects of leg dominance. On a separate day, the peak workload (PWL) of the knee extensors during one-legged extensor exercise was determined as previously described [33]. On day 1, the subjects performed one-legged knee extensor exercise until exhaustion. This exercise protocol consisted of one-legged knee extensor exercise for 1 h at 80% of PWL interspersed by 5 min bouts at 90% of PWL every 10 min. This was followed by interval exercise until exhaustion containing 4 min bouts starting at 100% PWL followed by 1 min at 50% of PWL. When the subjects were unable to maintain kicking frequency during these intervals, the exercise intensity was lowered by 10% and finished when the subjects were unable to finish 4 min at 60% of PWL.
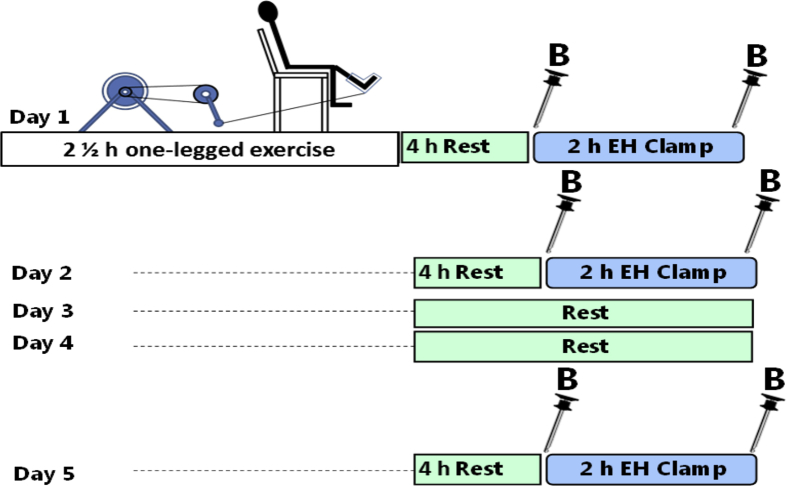
Figure 1.
Study design for one-legged glycogen supercompensation in man. Nine lean and healthy male subjects underwent a one-legged glycogen supercompensation regime. Muscle glycogen content was reduced (day 1), normalized (day 2), and supercompensated (day 5) in one leg while remaining unchanged in the resting control leg. On average the subjects exercised for 2 ½ h. Througout the 5 days the subjects consumed a eucaloric carbohydrate-rich diet. Muscle insulin action was evaluated under these three different situations by euglycemic hyperinsulinemic clamps (EH Clamps) and femoral catheterization in combination with measurement of femoral leg blood flow enabled to calculate insulin-stimulated leg glucose uptake. Muscle biopsies were obtained from both legs before and after insulin infusion. EH Clamp: Euglycemic hyperinsulinemic clamp. B: Biopsy from vastus lateralis muscle.
After 4 h of rest, a 120 min euglycemic hyperinsulinemic- (∼100 μU/ml) clamp was performed. This procedure was repeated in the same subjects in the rested state (without prior exercise) at day 2 and day 5 in order to investigate insulin-stimulated glucose uptake during the glycogen supercompensation regime. Catheters were inserted in the femoral veins of both legs and in a heated hand vein for sampling of arterialized venous blood in order to measure insulin-stimulated glucose uptake across the legs using A-V balance technique combined with ultrasound Doppler measurements of femoral arterial blood flow. Muscle biopsies from m. vastus lateralis were obtained before (basal state) and after 120 min of insulin infusion (insulin-stimulated state) from both legs at day 1, day 2, and day 5. Throughout the 5 day supercompensation regime, the subjects consumed a eucaloric diet composed of 80 E% carbohydrates, 10 E% fat and 10 E% protein.
2.2. Proteomic analyses of human muscle biopsies
Muscle biopsies for proteomic analyses were processed and analyzed as previously described [34]. A detailed description can be found in Supplemental I. For MS data repository and the complete data set, see Supplemental III.
2.3. Muscle processing and western blotting
Homogenates from human muscle biopsies were prepared from freeze-dried muscle, dissected free of visible fat, blood and connective tissue. Homogenates from mouse muscle were prepared from wet muscle. Muscle tissue was subsequently homogenized as described in detail in Supplemental I. Muscle homogenates and lysates were prepared in sample buffer. Except for GLUT1 and GLUT4 measurements, all samples were heated for 5 min at 96 °C. Equal amounts of sample were loaded on self-cast gels (7–15%) and separated by SDS-PAGE. Gels were transferred to polyvinylidene fluoride membranes using semidry blotting. Membranes were blocked in 2% skim milk or BSA and subsequently probed overnight at 4 °C with primary antibody (see Supplemental I for full list of primary antibodies). Membranes were incubated with secondary antibody conjugated with horseradish peroxidase (HRP) for 45 min at room temperature and protein abundance of phosphorylated proteins and total expression of proteins was visualized with chemiluminescence (Millipore) and a digital imaging system (ChemiDoc MP System; Bio-Rad, Copenhagen, DK). Sample loading and protein transfer were ensured by use of Coomassie-staining.
2.4. Muscle glycogen content
Muscle glycogen content was measured by a fluorometric method as glycosyl units after acid hydrolysis on 150 μg homogenates for human samples and 10–15 mg w.w. muscle for mouse samples [35]. Glycogen content in human muscle homogenates was determined as average from basal and insulin-stimulated samples as no effect of insulin was observed.
2.5. Enzyme activity of GS and AMPK
Enzyme activity for GS and AMPK was assayed in muscle homogenate and lysate, respectively, as specified in Supplemental I.
2.6. Animal studies
Animal experiments were performed on male mice in compliance with the European Union convention for protection of vertebra animals used for scientific purposes (Council of Europe 123, Strasbourg, France, 1985) and were approved by the Danish Animal Experimental Inspectorate. Muscle-specific inducible AMPK α1/α2 double KO mice (AMPK α imdKO) were generated by use of the Cre/loxP system as described in detail in Supplemental I. GYS1 KI mice (R582A/R582A) carrying a G6P-insensitive form of GS in muscle were generated as previously described [36]. A detailed description of genetic mouse models and procedures can be found in Supplemental I.
2.7. In vivo glycogen synthesis and in vivo glucose uptake in mice
Animals were restricted from access to chow 2 h prior to the experiments. Mice performed treadmill exercise for 1½ h at 60% (10% incline) of maximal running capacity and received oral gavage containing either saline (10 μl/g body wt) (Saline experiment) or glucose (10 μl/g body wt, 0.2 g/ml glucose) in combination with [3H]-2-deoxyglucose (2.2 MBq/ml; Perkin Elmer, USA) and [14C]-glucose (0.74 MBq/ml; Perkin Elmer, USA). Blood samples from the tail vein were obtained at 0, 20, 40, and 60 min after oral gavage and blood glucose concentration was monitored by glucometer (Contour XT; Bayer, Leverkusen, DE). Concurrently, blood plasma was obtained for determination of 3H and 14C radioactivity. In vivo glycogen synthesis rate was determined as [14C]-glucose incorporation into glycogen. Glycogen fraction was purified by overnight precipitation of muscle homogenates (10–20 mg muscle boiled in 1 M NaOH for 30 min) in ice cold (−20 °C) 96% ethanol in the presence of glycogen (1.14 mg, Sigma, G-8876, DK). After overnight precipitation, samples were centrifuged (15 min at 3000 g) and washed 3 times in cold ethanol (−20 °C); the pellet was suspended in water and counted for 14C radioactivity by liquid scintillation counting. In vivo glucose uptake was determined as trapped 2-[3H]-2-deoxyglucose-6-phosphate related to specific activity in the blood and the time of exposure as described in details in Supplemental I.
2.8. Glycogen supercompensation in mouse muscle
Mice were restrained from access to chow 2 h prior to the experiment. The exercise protocol consisted of 1½ h treadmill exercise (10% incline) at 60% of individual maximal running capacity. Glucose gavage (2 g/kg body wt) was given immediately after exercise and after 1 h of rest. Five hours after termination of exercise, the mice were killed by cervical dislocation, and tissues were quickly removed and frozen in liquid nitrogen. Glucose gavage solution was obtained by dissolving glucose in saline (0.2 g/ml) and was administered relative to mouse body weight (10 μl/g body wt).
2.9. Statistical analysis
Data are presented as means ± SEM. For the human glycogen supercompensation regime and in vitro mice studies data are analyzed by two-way ANOVA with repeated measurements. In vivo experiments in mice were analyzed by two-way ANOVA. Student-Newman-Keuls test was used for post hoc testing. A statistical significant difference was accepted for p ≤ 0.05
3. Results
3.1. Muscle insulin sensitivity and glucose uptake capacity remain enhanced in recovery from a single bout of glycogen-depleting exercise
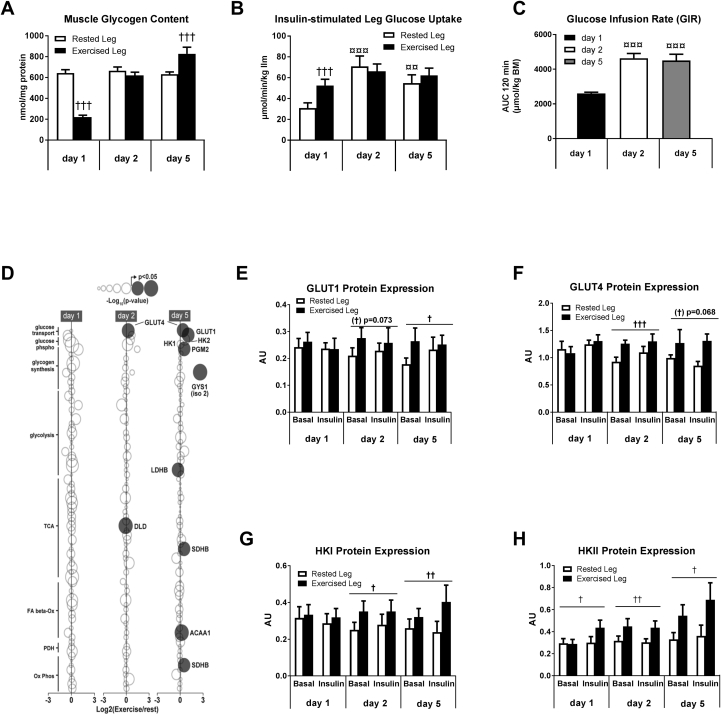
To investigate the molecular regulation of glycogen supercompensation, nine male subjects underwent a one-legged glycogen supercompensation regime. Glycogen content was reduced in muscle of one leg by one-legged exercise until exhaustion (Exercised Leg) (day 1) (p < 0.001), while the contralateral leg (Rested Leg) served as a resting control with unchanged glycogen content (p = 0.33) (Figure 2A). Intake of a carbohydrate-rich, eucaloric diet (80 E% CHO) in the following days normalized (day 2) and supercompensated (p < 0.001) (day 5) glycogen content in muscle of the prior exercised leg while remaining unchanged in muscle from the rested leg (Figure 2A). Euglycemic-hyperinsulinemic clamps in combination with catheterization of the femoral veins enabled the measurement of insulin-action on glucose uptake in the glycogen-depleted muscle in recovery from exercise (day 1) and under resting conditions with normalized (day 2) and supercompensated muscle glycogen content (day 5), respectively. These measurements revealed enhanced insulin-stimulated glucose uptake across the prior exercised leg compared to the rested control leg (day 1) (p < 0.001) (Figure 2B). This mechanism ensures glucose availability for initial glycogen resynthesis in the prior exercised muscle. Surprisingly, insulin-stimulated glucose uptake in the rested leg was higher on day 2 and day 5 compared to day 1. This was associated with higher insulin sensitivity at the whole body level (GIR) at day 2 and 5 compared to day 1 (Figure 2C). Plasma FFA levels were markedly enhanced in recovery from exercise and RER values indicated increased reliance on fat as energy substrate at day 1 compared to day 2 and 5 (Fig S1,S2). Availability of FA has been indicated as negative regulator of muscle insulin sensitivity and we speculate that this may be associated with the lowered insulin sensitivity at a whole body level on day 1. However, as insulin-stimulated glucose uptake was similar in both legs on day 2 and 5 (p = 0.35 and p = 0.15, respectively), enhanced insulin sensitivity itself seems not to drive glycogen synthesis beyond resting levels (i.e. glycogen supercompensation).
Figure 2.
Muscle glycogen supercompensation in human skeletal muscle involves coordinated upregulation in glucose uptake capacity. Muscle glycogen was depleted in one leg (day 1), normalized (day 2), and supercompensated (day 5) while remained unchanged in the rested control leg (A). A 120 min euglycemic hyperinsulinemic clamp was performed to investigate insulin action on glucose uptake 4 h post exercise (day 1) and under resting conditions on day 2 and 5. Insertion of femoral leg catheters in combination with measurements of leg blood flow allowed for calculation of insulin-stimulated glucose uptake across the prior exercised and rested leg (B). Whole body insulin sensitivity is given as glucose infusion rate (GIR) during the clamp and presented as AUC for 120 min (μmol/kg body mass) (C). Muscle biopsies were obtained before (basal state) and under insulin-stimulated conditions (following 120 min insulin infusion). Proteomic analyses was performed on basal biopsies obtained at day 1, 2, and 5 (n = 5). The exercised leg is presented relative to the rested control leg as log2 ratio. Circle size indicates the magnitude of increase/decrease and significant p-value is illustrated by black circles (D). Basal and insulin-stimulated muscle lysates were investigated for protein expression of GLUT1, GLUT4, HKI, and HKII by use of immunoblotting (E–H). Data are expressed as means ± SEM (n = 8–9). Western blotting data are given as arbitrary units (AU). †p ≤ 0.05, ††p ≤ 0.01 and †††p ≤ 0.001 for effect of leg. ¤¤p ≤ 0.01 and ¤¤¤p ≤ 0.001 for significant different from day 1. Main effect is indicated by horizontal line.
We next investigated basal skeletal muscle biopsies for key metabolic proteins by use of proteomic analyses in the rested and exercised leg on day 1, 2 and 5 (n = 5) (Figure 2D and Supplemental Table S1). These data showed a marked upregulation of proteins involved in glucose transport (GLUT1 and GLUT4) (p < 0.05) and a tendency for upregulation of proteins involved in glucose phosphorylation (HKI and HKII) (p-values in the range of 0.07–0.19) while proteins involved in fat oxidation and glycolysis largely remained unchanged during the supercompensation period (Figure 2D and Supplemental Table S1). A single peptide from glycogen synthase 1 isoform 2 (GYS1-2) was increased at day 5 (p = 0.02); however, canonical GYS-1 was not upregulated, and the maximal activity of GS was not altered (Supplemental Figs. S3,S4). These observations by proteomics were strengthened by immunoblotting, revealing a coordinated increase in the protein expression of HKI, HKII, GLUT1, and GLUT4 (Figure 2E–H and Fig. S8). Overall, these alterations induced by a single bout of exercise became apparent the day following exercise (day 2) and remained throughout the whole glycogen supercompensation regime.
Collectively, these data demonstrate that a single bout of exercise induces a robust increase in glucose uptake capacity at the level of glucose transport and phosphorylation. We interpret the temporal order of these cellular alterations as a mechanism to antagonize the otherwise negative feedback that glycogen normally exerts on insulin-stimulated muscle glucose uptake [37], [38].
3.2. Muscle AMPK activity is elevated for 5 days following a single bout of exercise
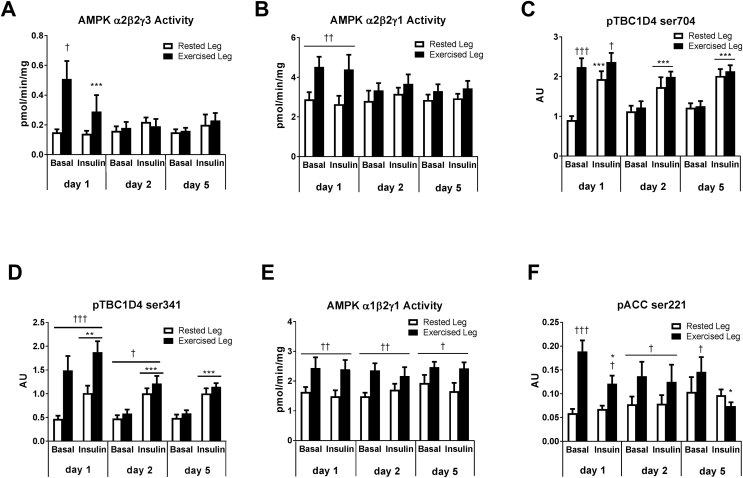
Given that AMPK contains a glycogen-binding domain and serves as a cellular fuel-sensor, we evaluated regulation of muscle AMPK throughout the human glycogen supercompensation regime. A temporal and heterotrimeric-specific regulation of AMPK activity was evident in muscle (Figure 3A,B and E). Thus, AMPK α2-containing complexes were highly activated in recovery from exercise (day 1) (p < 0.05) and reversed to resting levels the subsequent days (day 2 and 5) (Figure 3A–B). This regulation coincided with enhanced insulin-stimulated muscle glucose uptake (Figure 2B) and elevated phosphorylation at Ser704 and Ser341 of the AMPK downstream target TBC1D4 (day 1) (p < 0.001) (Figure 3C–D). In accordance with previous studies demonstrating that the proximal insulin-signaling cascade in human muscle remains unaffected by prior exercise [33], [39], [40], [41], we observed that Akt phosphorylation at Ser308 and Thr473 increased similarly in both legs in response to insulin (data not shown). Given the link between AMPK and insulin sensitivity in rodent muscle [31], [32], [42], these findings support that enhanced α2-AMPK signaling via distal insulin signaling molecule – TBC1D4 – mediates enhanced insulin sensitivity also in muscle of man.
Figure 3.
AMPK trimer-specific activation in human skeletal muscle during a glycogen supercompensation regime. Muscle lysate was investigated for AMPK activity of α2β2γ3 (A), α2β2γ1 (B), and α1β2γ1 (E) by immunoprecipitation followed by kinase activity assay. TBC1D4 phosphorylation at ser704 (C) and ser341 (D) as well as ACC phosphorylation at ser221 (F) was investigated in muscle lysate by use of immunoblotting with site-specific antibodies. Data are expressed as means ± SEM (n = 8–9). ∗p ≤ 0.05, ∗∗p ≤ 0.01 and ∗∗∗p ≤ 0.001 for effect of insulin infusion/time. †p ≤ 0.05, ††p ≤ 0.01 and †††p ≤ 0.001 for effect of leg. Main effect is indicated by horizontal line.
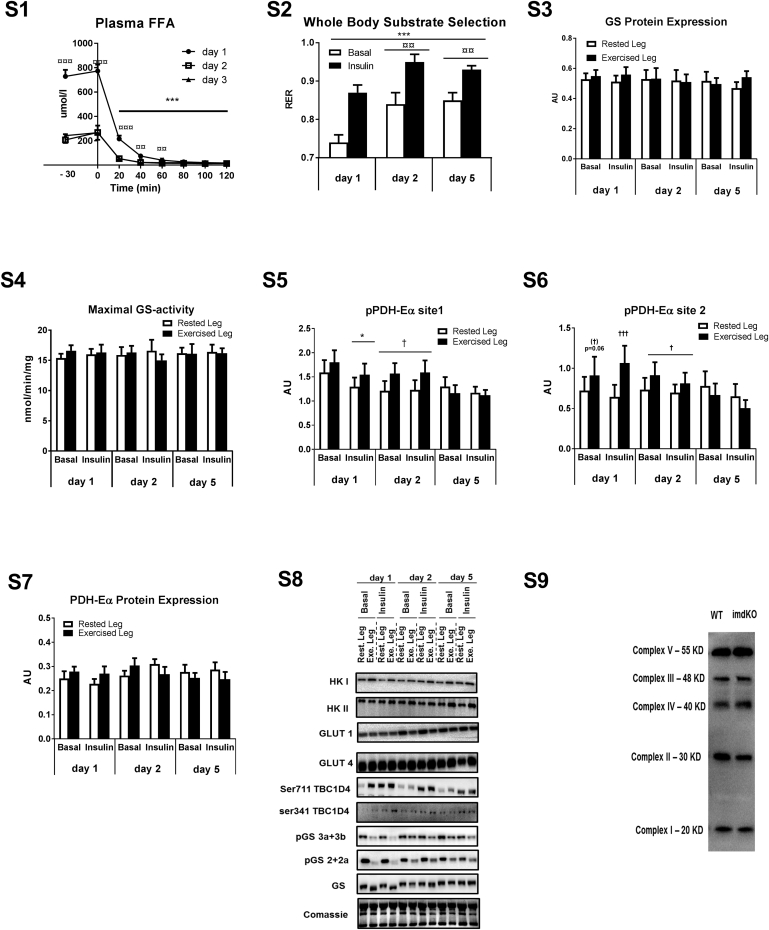
A selective increase in activity of the AMPK α1-containing complex was present throughout the 5 days supercompensation regime in muscle of the exercised leg only (p < 0.05) (Figure 3E). This unexpected observation was supported by increased phosphorylation under basal conditions of the well-established AMPK target acetyl-CoA carboxylase 2 (ACC2) (p < 0.05) (Figure 3F). Given that AMPK promotes fatty acid oxidation by phosphorylation of ACC2 in the resting state [43], these observations suggest substrate selection towards fat oxidation in the prior exercised muscle. Notably, judged by proteomic analyses, this fuel switch was largely independent of alterations in expression of proteins involved in fatty acid β-oxidation, glycolysis and glucose oxidation (Supplemental Table S1). However, we observed that exercise induced phosphorylation (hence deactivation) of the glucose oxidation-controlling protein complex pyruvate dehydrogenase (PDH-Eα) on day 1 (Figs. S5–7). Notably, elevated PDH phosphorylation was still present (p < 0.05) when glycogen had reversed to resting levels in the prior exercised muscle on day 2 (Figs. S5–7).
Collectively, these observations suggest that glycogen supercompensation involves AMPK-dependent reduced glucose oxidation in expense of fat oxidation as a cellular mechanism for sustained glycogen synthesis and hence glycogen supercompensation.
3.3. Acute deletion of AMPK activity in skeletal muscle abrogates the ability for glycogen supercompensation following exercise
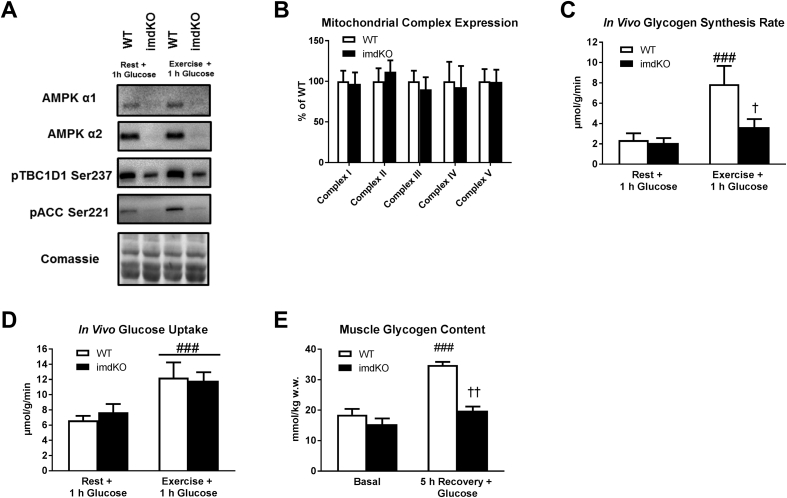
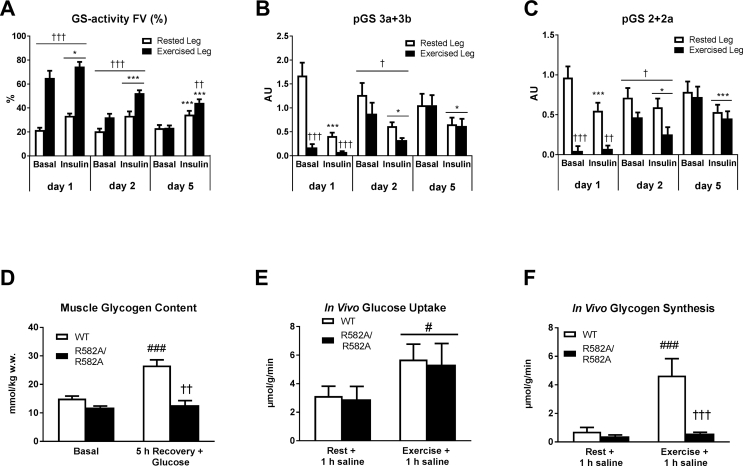
In order to illuminate a necessary role of AMPK in muscle glycogen supercompensation, we generated a muscle-specific inducible AMPK catalytic isoform knock out mouse model (AMPK α1/α2 imdKO). Three weeks after induction of gene deletion, these mice show marked deletion of both AMPK α1 and α2 protein expression (Figure 4A). In line with previous AMPK deficient models, muscle of these mice shows markedly lower phosphorylation of the AMPK downstream targets TBC1D1 and ACC (Figure 4A). Importantly, and in contrast to previous AMPK deficient models, these mice do not suffer from a mitochondrial phenotype (Figure 4B and Fig. S9). This is highly relevant because 1) mitochondrial dysfunction may affect substrate utilization and glucose partitioning toward glycolysis, and 2) muscle of all previous models used to link AMPK to glycogen storage display mitochondrial dysfunction [44], [45]. Intriguingly, muscle glycogen synthesis in vivo was markedly reduced in the AMPK imdKO mice (p < 0.05) when glucose was administered orally following exercise (Figure 4C) even though muscle glucose uptake in vivo was similar between genotypes (Figure 4D). As a consequence of the reduced glycogen synthesis following exercise, the ability to supercompensate muscle glycogen was abrogated in mice with acutely induced deletion of AMPK catalytic activity (p < 0.001) (Figure 4E).
Figure 4.
Acute muscle-specific deletion impairs glycogen synthesis following exercise and abrogates the ability for glycogen supercompensation following exercise. A novel mouse model with acute genetic deletion of AMPK catalytic activity in skeletal muscle was generated. Deletion of the catalytic subunits AMPK α1 and α2 and reduced signaling of the downstream targets TBC1D1 and ACC was verified by immunoblotting (A). Protein expression of mitochondrial complex proteins (Complex I-V) in quadriceps muscle was investigated by use of specific antibodies (B). In vivo glycogen synthesis rate (C), glucose uptake (D) and muscle glycogen concentration (E) were assessed in quadriceps muscle from WT mice and mice with acute inducible deletion of AMPK catalytic activity (imdKO). Glycogen synthesis rate and glucose uptake was investigated in vivo in response to oral glucose gavage (2 g/kg body wt) in the rested state (Rest + 1 h Glucose) and exercised state (Exercise + 1 h Glucose). Muscle glycogen content in quadriceps muscle was measured in the basal state and 5 h post exercise with glucose gavage (2 g/kg body wt) given immediately after 1½ h exercise and again following 1 h recovery. ###p ≤ 0.001 for different from corresponding resting value. †p ≤ 0.005 and ††p ≤ 0.01 for effect of genotype. Data are presented as means ± SEM (n = 4–8).
In combination with our observations in human muscle, these findings suggest that sequential activation of AMPK trimeric complexes in recovery from exercise enhances insulin sensitivity to stimulate glucose uptake and may promote fat oxidation so that intracellular glucose is directed towards glycogen synthesis even when muscle glycogen has returned to normal levels. Thus, AMPK is essential for glycogen supercompensation.
3.4. Glycogen synthase remains in an activated state beyond normalized muscle glycogen content
Next, we examined whether glycogen supercompensation in human skeletal muscle was associated with alterations in proteins involved in the pathway of glycogen synthesis. The formation of new glycogen particles is initiated by the self-glucosylated enzyme glycogenin. The observation of unchanged expression of glycogenin-1 (p ≥ 0.60) and other key proteins in glycogen synthesis suggest that glycogen supercompensation is associated with increased particle size of existing glycogen granules rather than formation of additional glycogen particles (Supplemental Table S1).
GS promotes the elongation of glucose chains within the glycogen particle and is considered as rate-controlling enzyme for glycogen synthesis [1]. Protein expression of GS remained similar between the exercised and rested muscle (Fig. S3). In contrast, fractional enzyme activity of GS was markedly elevated in the prior exercised muscle (day 1) (p < 0.001) and remained elevated the following days (p < 0.01) even though muscle glycogen content was normalized to resting levels or supercompensated (Figure 5A). In accordance, phosphorylation of key inhibitory residues on GS was markedly reduced by exercise (day 1) (p < 0.001) and remained lower the day after exercise (day 2) (p < 0.05) (Figure 5B–C). Lowered phosphorylation of GS enhances the sensitivity for allosteric activation by intracellular G6P [11]. Thus the maintained dephosphorylation by exercise presents a mechanism for sensitizing GS for allosteric activation by G6P beyond normalized glycogen content. By using knockin mice (KI) (R582A/R582A) carrying a G6P-insensitive form of GS in muscle [36], we verified that allosteric activation of GS by G6P indeed is essential for glycogen supercompensation following exercise (Figure 5D). It has previously been reported that G6P tends to accumulate in muscle of GS KI (R582A/R582A) compared to WT controls [36]. As G6P accumulation exerts negative feedback on glucose uptake, we cannot fully exclude that the absence of glycogen supercompensation in these mice partly arises from inhibited glucose uptake due to accumulation of G6P. However, in an additional experiment with saline injection, in which accumulation of G6P would not be expected, we could demonstrate that in vivo muscle glucose uptake was similar between genotypes (Figure 5E). Under these experimental conditions prior exercise increased in vivo glycogen synthesis rate in WT mice but not in GS KI (R582A/R582A) mice (Figure 5F), demonstrating that muscle glycogen synthesis following exercise is fully dependent on allosteric regulation of GS. Because GS is rate-limiting for glycogen synthesis from intracellular glucose, the above described alterations on GS regulation in man will indeed favor glycogen storage in the prior exercised muscle.
Figure 5.
Muscle glycogen supercompensation in man reveals sustained activation of glycogen synthase (GS) far beyond normalized muscle glycogen content. Enzyme-activity of GS was determined in muscle homogenates as fractional activity (activity in the presence of 0.17 mM G6P relative to 8 mM G6P) (A). GS phosphorylation at the key regulatory sites 3a+3 b and 2+2a was measured by immunoblotting (B–C). Glycogen content in quadriceps muscle from mice expressing a G6P-insensitive form of GS in muscle (R582A/R582A) was measured under basal conditions (Basal) and 5 h following exercise where glucose gavage (2 g/kg body wt) was given immediately and 1 h post exercise (D). In vivo glucose uptake (E) and in vivo glycogen synthesis (F) in quadriceps muscle from WT and R582A/R582A mice was determined in the rested state (Rest + 1 h saline) and prior exercised state (Exercise + 1 h saline) by oral gavage of saline containing [14C]- Glucose and [3H]-2-deoxyglucose. Data are expressed as means ± SEM (n = 8–9 for A–C and n = 6–8 for D–E). ∗p ≤ 0.01 and ∗∗∗p ≤ 0.001 for effect of insulin infusion/time. (†) p ≤ 0.1, †p ≤ 0.05, ††p ≤ 0.01 and †††p ≤ 0.001 for effect of leg in human experiment and for effect of genotype in mice experiment. #p ≤ 0.05 and ###p ≤ for significantly different from corresponding resting value. Main effect is indicated by horizontal line.
4. Discussion
Based on our observations, we propose the following model of molecular mechanisms induced by exercise enhancing glycogen storage in human skeletal muscle: The cellular fuel-sensor AMPK α2 is activated by a single bout of glycogen-depleting exercise and remains activated into the immediate recovery period. This provides an insulin sensitizing effect on insulin-stimulated glucose uptake in the prior exercised muscle and secures adequate glucose supply for glycogen re-synthesis. Moreover, we suggest that the prolonged activation of AMPK α1 following exercise promotes glucose sparing for glycogen synthesis in expense of fat oxidation. These alterations lead to high cellular glucose availability in the exercised muscle, and the final partitioning towards glycogen is secured as the rate limiting enzyme - GS - remains activated and insulin responsive even when glycogen content is normalized or supercompensated. Collectively, these cellular alterations provide a potential mechanism to offset the otherwise tight inhibitory effect that glycogen exerts on glucose uptake and glycogen synthesis [37], [46], hereby allowing for muscle glycogen supercompensation.
The supercompensated glycogen level reported by Bergström and Hultman was ∼35% higher compared to the one observed in the current study (890 vs. 660 μmol/g dw muscle). It has previously been demonstrated that the timing of carbohydrates following exercise is essential for obtaining maximal glycogen synthesis [47]. In order to determine insulin sensitivity at a time-point where glucose uptake and leg blood flow were normalized, the current study involved a period of 6 h (4 h rest post exercise and 2 h euglycemic hyperinsulinemic clamp) before carbohydrates were provided to the subjects on day 1. Although not fully clear from their paper, we find it unlikely that intake of carbohydrate in the Bergström and Hultman study should have been delayed by 6 h. This difference in study design may offer some explanations to the difference in maximal obtainable glycogen levels between these two studies.
Proteomic analyses in combination with immunoblotting revealed that a single exercise bout resulted in a marked upregulation in proteins involved in muscle glucose uptake (GLUT1, GLUT4, HKI, and HKII) in the prior exercised muscle, while expression of key metabolic proteins in glycogen synthesis, glycolysis and fatty acid β oxidation remained unchanged. Indeed, glucose uptake in the prior exercised leg in the supercompensated state was similar to the resting leg within normal glycogen content. We hypothesize that increased expression of proteins involved in glucose uptake may be necessary to maintain glucose uptake when muscle glycogen is supercompensated. Supporting this concept, increased protein expression of GLUT4 and hexokinase in rat and human muscle has been reported ∼3–16 h following a single exercise bout [48], [49], [50]. High glycogen is a known negative regulator of glucose uptake and glycogen synthesis in both basal and insulin-stimulated conditions [46]. However, this phenomenon is offset in the conditions during glycogen supercompensation as evident from the equal basal as well as the similar increase in insulin-stimulated glucose uptake on day 2 and 5 in the prior exercised compared to rested muscles. We propose that the elevated expression of proteins determining glucose uptake capacity (GLUT1, GLUT4, HKI, and HKII) provide a mechanism to antagonize the otherwise negative feedback glycogen exerts on insulin-stimulated glucose uptake. This may be essential during hyperglycemic conditions repeatedly experienced during the carbohydrate loading as part of the glycogen supercompensation regime. In line, hyperglycemia together with hyperinsulinemia achieved by prolonged infusion (8 h) forces glucose uptake even when muscle glycogen levels are normal [51]. Under these conditions GS regulation becomes crucial for the partitioning of glucose towards glycogen storage. The activity of GS was markedly elevated following exercise and remained elevated even at day 2 and 5. At day 2, this coincided with sustained dephosphorylation of GS. These modulations enhance the affinity for the substrate UDP-glucose and the affinity for G6P binding (i.e. potential allosteric activation of GS) and thus secure optimal conditions for GS function [10], [11]. Intriguingly, whereas the basal GS activity was similar in muscle of the two legs on day 5, insulin's ability to activate GS was on average 38% higher in the prior exercised muscle. These modulations signify that the covalent regulation of the G6P-binding affinity is a potential important regulatory mechanism for glycogen supercompensation. Our observation in mouse muscle expressing the G6P-insensitive GS mutant indeed supports such a view. The functional outcome of these alterations is a condition allowing prior exercised muscle to maintain a high rate of glycogen synthesis, even in the face of normalized or elevated glycogen content, ultimately leading to glycogen supercompensation.
The maintained activation of all AMPK-complexes in recovery from exercise (day 1) coincided with increased insulin-stimulated glucose uptake in the prior exercised muscle as well as increased regulatory phosphorylation of TBC1D4. We interpret this signaling convergence of AMPK and TBC1D4 on day 1 as a physiological mechanism that provides glucose uptake for the highly prioritized glycogen synthesis in the early recovery period from exercise. This interpretation is supported by genetic evidence in mouse muscle where the insulin sensitizing effect of prior AMPK activation by AICAR and exercise is dependent on intact AMPK α2 activity and correlated to TBC1D4 phosphorylation [31], [32]. In line, in human muscle, we observed that the disappearance of the insulin-sensitizing effect of prior exercise coincided with the loss of elevated AMPK α2 activity on day 2 and 5. Thus, these observations provide a plausible mechanism for the immediate return of glycogen to resting level but they do not provide a mechanism for glycogen synthesis beyond that point.
Notably, insulin-stimulated glucose uptake in the rested leg was found to be lower at day 1 compared to day 2 and 5. This observations coincides with elevated plasma FA levels and substrate selection (RER) towards fat oxidation on day 1 compared to day 2 and 5. We speculate that this increase in circulating levels of FA in fasting recovery from glycogen-depleting exercise (day 1) exerts a dampening effect on whole body insulin sensitivity. From a physiological point of view, dampening the sensitivity in non-exercised muscle while maintaining the sensitivity near normal or above in the exercised muscles would help sparring glucose for storage and indeed help reload the glycogen storage in the prior exercised muscle. As our study design does not allow for a final conclusion on this matter (due to the lack of randomization of experimental days/diet), further studies are warranted to study the isolated effect of prolonged exercise on whole body insulin sensitivity.
The observation of sustained AMPK α1 activity and phosphorylation of the fat oxidation promoting enzyme ACC and phosphorylation of the inhibitory site 1 and 2 on PDH in the present study provide a cellular mechanism in skeletal muscle that directs intracellular glucose towards glycogen synthesis in a situation in which increased glucose oxidation would be expected. This molecular mechanism could explain previous observations of substantially increased fat oxidation in the post exercise period, an effect that can last for several hours or even days after cessation of exercise [52], [53], [54], [55], [56]. Notably, elevated fat oxidation in exercise recovery is even seen when dietary intake of carbohydrates is increased [52], [55], [56], a situation in which high carbohydrate oxidation otherwise would be expected.
Previous studies have reported that AMPK is involved in regulation of muscle glycogen content without providing underlying mechanisms. For example, chronic activation of AMPK by missense mutation of AMPK γ3 or γ1 leads to excessive accumulation of muscle glycogen [21], [22], [23], [24], [25]. In line, deletion of either AMPK γ3 or functional AMPK α2 is associated with reduced glycogen storage following exercise [21], [57], [58]. The essential role of AMPK in glycogen supercompensation seen in the present study adds significantly to this view, because the AMPK ablation was induced acutely allowing the resulting phenotype to be under minimal if any influence by secondary cellular compensation (e.g. mitochondrial dysfunction as seen in all other of the above mentioned AMPK deficient models). In the transgenic mouse model used in the current study, both catalytic subunits were deleted acutely in skeletal muscle. Accordingly, this does not allow for investigating the relative contribution of AMPK α1 and α2 in the regulation of glycogen supercompensation. Genetic deletion of both isoforms was chosen as previous mouse models with genetic deletion of one of the isoforms have resulted in a compensatory increase abundance of the remaining isoform [59], complicating the overall physiological interpretation to the role of AMPK.
5. Conclusion
To conclude, a single bout of exercise induces temporal cellular alterations potentially offsetting the otherwise tight feedback inhibition of glycogen synthesis and glucose uptake by glycogen, ultimately allowing muscle glycogen to become supercompensated in the post exercise period.
The potential translational value of these mechanistic insights should be appreciated considering the outmost importance of pre-competition muscle glycogen content for endurance exercise performance [60] – driving athletes to seek optimal glycogen supercompensation regimes in preparation for competition. Furthermore, impaired insulin-stimulated glycogen synthesis is a hallmark in muscle insulin resistance as observed in various diseased conditions like type 2 diabetes [61], and the present findings may indeed be applicable in therapeutic strategies.
Author contributions
J.R.H and J.F.P.W. conceived and designed the experiments and drafted the manuscript. J.R.H., L.B., M.F.R., J.F., and J.B.B performed experiments in mice and analyzed samples. K.S., B.V. and M.F. provided genetic mouse models. J.R.H, M.B.H., E.A.R., B.K., and J.F.P.W carried out the human glycogen supercompensation study. J.R.H., M.B.H., J.B.B., N.J.F., J.F.H., B.L.P., and D.E.J. analyzed samples from this study. All authors interpreted the results, read and edited the manuscript and approved the final version of the manuscript. J.F.P.W is the guarantor of this work and takes responsibility for the integrity of the data.
Grants
This study was supported by grants from the Danish Council for Independent Research Medical Sciences (FSS 6110-00498B to JFPW), The Novo Nordisk Foundation (NNF 16OC0023046, NNF 170OC00272224 to JFPW, NNF -12009-2015 to JFPW), The Lundbeck Foundation (R221-2016-0027/ R180-2014-3887 to JFPW), Ministry of Culture Denmark (FPK 2016-0027 to JFPW), The research program “Physical activity and nutrition for improvement of health” funded by the University of Copenhagen Excellence Program for Interdisciplinary Research (to BK, JFPW and EAR). Prof. Jørgen Jensen was supported by a visiting professorship provided by the Danish Diabetes Academy supported by the Novo Nordisk Foundation. This work was supported by an NHMRC Project grant (APP1122376). B.L.P. and D.E.J. are funded by NHMRC fellowships. The contents of the published material are solely the responsibility of the individual authors and do not reflect the view of any of the funding bodies.
Acknowledgement
We appreciate the great assistance from Betina Bolmgren and Irene Bech Nielsen, Section of Molecular Physiology, Department of Nutrition, Exercise and Sports, University of Copenhagen. The authors acknowledge prof. D. G. Hardie (Divison of Cell Signalling & Immunology, College of Life Sciences, University of Dundee Scotland, UK), prof. O. Pedersen (Steno Hospital, Gentofte, DK), prof. C. MacKintosh (University of Dundee, Scotland, UK) and Dr. L. J. Goodyear (Joslin Diabetes Center, Boston, MA, USA) for providing antibodies. Founder HAS-Cre+/- mice were kindly provided by prof. Karyn Esser (University of Kentucky, Lexington, Kentucky, USA) [62]. The authors would like to acknowledge the inspiration and the competent insights given by the late Prof. Bengt Saltin at the initiation of this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.07.001.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Table S1: Proteomic analyses of targeted proteins involved in glucose uptake, glycolysis, TCA Cycle, fatty acid β-oxidation, PDH and oxidative phosphorylation. Data are presented as log2 ratio for exercise leg relative to resting leg (Ex/Re) (n = 5). The number observations for up- and down-regulation of proteins are indicated by arrows.
Supplemental I: Supplemental Experimental Procedures. Supplemental I provides a detailed description of experimental procedures and analyses applied in the human one-legged glycogen supercompensation study and animal experiments.
Supplemental III: Full List of Human Muscle MS repository. Quantification of peptides is given as log2 ratio for exercise leg relative to resting leg (Ex/Re) (n = 5). Supplemental II provides a full list of proteins detected and is presented as log2 ratio for the exercised leg relative to the rested control leg.
Figs1.
Supplemental II – Supplemental Figures containing GS protein expression, GS maximal activity, PDH-Eα phosphorylation (site 1 and 2) and expression and representative western blots. Blood samples were obtained before the clamp (−30 min and 0 min) and every 20 min during the clamp and analyzed for plasma FFA concentration (Fig. S1). Substrate selection was evaluated at a whole body level as RER based on indirect calorimetry (Fig. S2). Muscle biopsies were obtained before (basal state) and under insulin-stimulated conditions (following 120 min insulin infusion and investigated for protein expression of glycogen synthase (GS) (Fig. S3) as well as enzyme activity of GS under saturating and hence maximal conditions (in the presence of 8 mM G6P) (Fig. S4), PDH-Eα site 1 and 2 phosphorylation (Figs. S5 and S6) and PDH-Eα protein expression (Fig. S7). Muscle samples collected during the human glycogen supercompensation regime were investigated for phosphorylation and expression of key metabolic proteins by use of immunoblotting with site-specific antibodies (n = 8–9) and representative blots are given in Fig. S8. Protein expression of mitochondrial proteins in quadriceps muscle from WT and inducible AMPK α double KO mice (imdKO) was investigated by immunoblotting of muscle lysates (n = 6–8) and a representative western blot is provided in Fig. S9. ∗p ≤ 0.5 and ∗∗∗p ≤ 0.001 for effect of insulin infusion/time. †p ≤ 0.005 and †††p ≤ 0.001 for effect of leg. ¤¤p ≤ 0.01 for significant different from day 1. Main effect is given by horizontal line. Coomassie staining was used to verify even protein transfer.
References
- 1.Roach P.J., Depaoli-roach A.A., Hurley T.D., Tagliabracci V.S. Glycogen and its metabolism: some new developments and old themes. Biochemical Journal. 2012;441(3):763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo R.A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 3.Danforth W.H. Glycogen synthase activity in skeletal muscle – Interconversion of two forms and control of glycogen synthesis. Journal of Biological Chemistry. 1965;240(2):588–593. [PubMed] [Google Scholar]
- 4.Bergström J., Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210(5033):309–310. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- 5.Leloir L.F., Olavarria J.M., Goldemberg S.H., Carminatti H. Biosynthesis of glycogen from uridine diphosphate glucose. Archives of Biochemistry and Biophysics. 1959;81(2):508–520. doi: 10.1016/0003-9861(59)90232-2. [DOI] [PubMed] [Google Scholar]
- 6.Friedman D.L., Larner J. Studies on UDPG-alpha-glucan transglucosylase. III. Interconversion of two forms of muscle UDPG-alpha-glucan transglucosylase by a phosphorylation- dephosphorylation reaction sequence. Biochemistry. 1963;2:669–675. doi: 10.1021/bi00904a009. [DOI] [PubMed] [Google Scholar]
- 7.Jensen J., Rustad P.I., Kolnes A.J., Lai Y.-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Frontiers in Physiology. 2011;2(December):112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prats C., Helge J.W., Nordby P., Qvortrup K., Ploug T., Dela F. Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. Journal of Biological Chemistry. 2009;284(23):15692–15700. doi: 10.1074/jbc.M900845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen A.J.T., Hingst J.R., Friedrichsen M., Kristensen J.M., Højlund K., Wojtaszewski J.F.P. Dysregulation of muscle glycogen synthase in recovery from exercise in type 2 diabetes. Diabetologia. 2015;58(7):1569–1578. doi: 10.1007/s00125-015-3582-z. [DOI] [PubMed] [Google Scholar]
- 10.Roach P.J., Takeda Y., Larner J. Rabbit skeletal muscle glycogen synthase. I. Relationship between phosphorylation state and kinetic properties. Journal of Biological Chemistry. 1976;251(April 10):1913–1919. [PubMed] [Google Scholar]
- 11.Lai Y.-C., Stuenaes J.T., Kuo C.-H., Jensen J. Glycogen content and contraction regulate glycogen synthase phosphorylation and affinity for UDP-glucose in rat skeletal muscles. American Journal of Physiology – Endocrinology and Metabolism. 2007;293:E1622–E1629. doi: 10.1152/ajpendo.00113.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hardie D.G. AMP-activated protein kinase as a drug target. Annual Review of Pharmacology and Toxicology. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 13.McBride A., Ghilagaber S., Nikolaev A., Hardie D.G. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metabolism. 2009;9(1):23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polekhina G., Gupta A., Michell B.J., Van Denderen B., Murthy S., Feil S.C. AMPK β subunit targets metabolic stress sensing to glycogen. Current Biology. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 15.Oligschlaeger Y., Miglianico M., Chanda D., Scholz R., Thali R.F., Tuerk R. The recruitment of AMP-activated protein kinase to glycogen is regulated by autophosphorylation. Journal of Biological Chemistry. 2015;290(18):11715–11728. doi: 10.1074/jbc.M114.633271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojtaszewski J.F.P., MacDonald C., Nielsen J.N., Hellsten Y., Hardie D.G., Kemp B.E. Regulation of 5’AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. American Journal of Physiology – Endocrinology and Metabolism. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- 17.Wojtaszewski J.F.P., Jørgensen S.B., Hellsten Y., Grahame Hardie D., Richter E.A. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- 18.Holmes B.F., Kurth-Kraczek E.J., Winder W.W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. Journal of Applied Physiology. 1999;87(5):1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 19.Cokorinos E.C., Delmore J., Reyes A.R., Albuquerque B., Kjøbsted R., Jørgensen N.O. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metabolism. 2017;25(5):1147–1159.e10. doi: 10.1016/j.cmet.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Myers R.W., Guan H.P., Ehrhart J., Petrov A., Prahalada S., Tozzo E. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science. 2017;357(6350):507–511. doi: 10.1126/science.aah5582. [DOI] [PubMed] [Google Scholar]
- 21.Barnes B.R., Marklund S., Steiler T.L., Walter M., Hjälm G., Amarger V. The 5’-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. Journal of Biological Chemistry. 2004;279(37):38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- 22.Barré L., Richardson C., Hirshman M.F., Brozinick J., Fiering S., Kemp B.E. Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. American Journal of Physiology – Endocrinology and Metabolism. 2007;292(3):E802–E811. doi: 10.1152/ajpendo.00369.2006. [DOI] [PubMed] [Google Scholar]
- 23.Costford S.R., Kavaslar N., Ahituv N., Chaudhry S.N., Schackwitz W.S., Dent R. Gain-of-Function R225W mutation in human AMPKγ3 causing increased glycogen and decreased triglyceride in skeletal muscle. PLoS One. 2007;2(9):e903. doi: 10.1371/journal.pone.0000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milan D., Jeon J.T., Looft C., Amarger V., Robic A., Thelander M. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science (New York, N.Y.) 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 25.Schönke M., Myers M.G., Zierath J.R., Björnholm M. Skeletal muscle AMP-activated protein kinase γ1 H151R overexpression enhances whole body energy homeostasis and insulin sensitivity. American Journal of Physiology – Endocrinology and Metabolism. 2015;309(7):E679–E690. doi: 10.1152/ajpendo.00195.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birk J.B., Wojtaszewski J.F.P. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. The Journal of Physiology. 2006;577(Pt 3):1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii N., Hayashi T., Hirshman M.F., Smith J.T., Habinowski S.A., Kaijser L. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochemical and Biophysical Research Communications. 2000;273(3):1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 28.Wojtaszewski J.F.P., Nielsen P., Hansen B.F., Richter E.A., Kiens B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. The Journal of Physiology. 2000;528(1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z.P., McConell G.K., Michell B.J., Snow R.J., Canny B.J., Kemp B.E. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. American Journal of Physiology – Endocrinology and Metabolism. 2000;279(5):E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 30.Kjøbsted R., Pedersen A.J.T., Hingst J.R., Sabaratnam R., Birk J.B., Kristensen J.M. Intact regulation of the AMPK signaling network in response to exercise and insulin in skeletal muscle of male patients with type 2 diabetes - Illumination of AMPK activation in recovery from exercise. Diabetes. 2016;65(5):1219–1230. doi: 10.2337/db15-1034. [DOI] [PubMed] [Google Scholar]
- 31.Kjøbsted R., Munk-Hansen N., Birk J.B., Foretz M., Viollet B., Björnholm M. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes. 2016 doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 32.Kjøbsted R., Treebak J.T., Fentz J., Lantier L., Viollet B., Birk J.B. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes. 2015;64(6):2042–2055. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 33.Wojtaszewski J.F.P., Hansen B.F., Kiens B., Richter E.A. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46(11):1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman N.J., Parker B.L., Chaudhuri R., Fisher-Wellman K.H., Kleinert M., Humphrey S.J. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metabolism. 2015;22(5):922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry O.H., Passonneau J.V. Academic Press, Inc; London: 1972. A flexible system of enzymatic analysis. [Google Scholar]
- 36.Bouskila M., Hunter R.W., Ibrahim A.F.M., Delattre L., Peggie M., van Diepen J.A. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metabolism. 2010;12(5):456–466. doi: 10.1016/j.cmet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Derave W., Hansen B.F., Lund S., Kristiansen S., Richter E.A. Muscle glycogen content affects insulin-stimulated glucose transport and protein kinase B activity. American Journal of Physiology – Endocrinology and Metabolism. 2000;279(5):E947–E955. doi: 10.1152/ajpendo.2000.279.5.E947. [DOI] [PubMed] [Google Scholar]
- 38.Jensen J., Aslesen R., Ivy J.L., Brørs O. Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle. American Journal of Physiology. 1997;272(4 Pt 1):E649–E655. doi: 10.1152/ajpendo.1997.272.4.E649. [DOI] [PubMed] [Google Scholar]
- 39.Wojtaszewski J.F.P., Hansen B.F., Gade J., Kiens B., Markuns J.F., Goodyear L.J. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 40.Thong F.S.L., Derave W., Kiens B., Graham T.E., Ursø B., Wojtaszewski J.F.P. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51(3):583–590. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- 41.Frøsig C., Sajan M.P., Maarbjerg S.J., Brandt N., Roepstorff C., Wojtaszewski J.F.P. Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle. Journal of Physiology. 2007;3:1289–1301. doi: 10.1113/jphysiol.2007.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher J.S., Gao J., Han D.-H., Holloszy J.O., Nolte L.A. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. American Journal of Physiology – Endocrinology and Metabolism. 2002;282(1):E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill H.M., Lally J.S., Galic S., Thomas M., Azizi P.D., Fullerton M.D. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57(8):1693–1702. doi: 10.1007/s00125-014-3273-1. [DOI] [PubMed] [Google Scholar]
- 44.Fentz J., Kjøbsted R., Birk J.B., Jordy A.B., Jeppesen J., Thorsen K. AMPKα is critical for enhancing skeletal muscle fatty acid utilization during in vivo exercise in mice. The FASEB Journal. 2015;29(5):1725–1738. doi: 10.1096/fj.14-266650. [DOI] [PubMed] [Google Scholar]
- 45.O'Neill H.M., Maarbjerg S.J., Crane J.D., Jeppesen J., Jørgensen S.B., Schertzer J.D. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proceedings of the National Academy of Sciences of the USA. 2011;108(38):16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen J., Jebens E., Brennesvik E.O., Ruzzin J., Soos M., Engebretsen E.M.L. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. American Journal of Physiology – Endocrinology and Metabolism. 2006;290(1):E154–E162. doi: 10.1152/ajpendo.00330.2005. [DOI] [PubMed] [Google Scholar]
- 47.Ivy J.L., Katz A.L., Cutler C.L., Sherman W.M., Coyle E.F. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. Journal of Applied Physiology (Bethesda, Md.: 1985) 1988;64:1480–1485. doi: 10.1152/jappl.1988.64.4.1480. [DOI] [PubMed] [Google Scholar]
- 48.Kuo C.H., Hunt D.G., Ding Z., Ivy J.L. Effect of carbohydrate supplementation on postexercise GLUT-4 protein expression in skeletal muscle. Journal of Applied Physiology. 1999;87(6):2290–2295. doi: 10.1152/jappl.1999.87.6.2290. [DOI] [PubMed] [Google Scholar]
- 49.Ren J.M., Semenkovich C.F., Gulve E.A., Gao J., Holloszy J.O. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. Journal of Biological Chemistry. 1994;269(20):14396–14401. [PubMed] [Google Scholar]
- 50.Kraniou G.N., Cameron-smith D., Hargreaves M., Giorgos N. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. Journal of Applied Physiology. 2006;101:934–937. doi: 10.1152/japplphysiol.01489.2005. [DOI] [PubMed] [Google Scholar]
- 51.Hansen B.F., Asp S., Kiens B., Richter E.A. Glycogen concentration in human skeletal muscle: effect of prolonged insulin and glucose infusion. Scandinavian Journal of Medicine and Science in Sports. 1999;9(4):209–213. doi: 10.1111/j.1600-0838.1999.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 52.Bielinski R., Schutz Y., Jequier E. Energy metabolism during the postexercise recovery in man. American Journal of Clinical Nutrition. 1985;42(1):69–82. doi: 10.1093/ajcn/42.1.69. [DOI] [PubMed] [Google Scholar]
- 53.Maehlum S., Grandmontagne M., Newsholme E.A., Sejersted O.M. Magnitude and duration of excess postexercise oxygen consumption in healthy young subjects. Metabolism. 1986;35(5):425–429. doi: 10.1016/0026-0495(86)90132-0. [DOI] [PubMed] [Google Scholar]
- 54.Wolfe R.R., Klein S., Carraro F., Weber J.M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. American Journal of Physiology. 1990;258(2 Pt 1):E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 55.Kiens B., Richter E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. American Journal of Physiology. 1998;275(2 Pt 1):E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 56.Kimber N.E., Heigenhauser G.J.F., Spriet L.L., Dyck D.J. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. The Journal of Physiology. 2003;548(Pt 3):919–927. doi: 10.1113/jphysiol.2002.031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mu J., Barton E.R., Birnbaum M.J. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on “lazy mice”. Biochemical Society Transactions. 2003;31(Pt 1):236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- 58.Fritzen A.M., Lundsgaard A.-M., Jeppesen J., Christiansen M.L.B., Biensø R., Dyck J.R.B. 5′-AMP activated protein kinase α 2 controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase 4. The Journal of Physiology. 2015;593(21):4765–4780. doi: 10.1113/JP270821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jørgensen S.B., Viollet B., Andreelli F., Frøsig C., Birk J.B., Schjerling P. Knockout of the α2 but not α1, 5′-AMP-activated protein kinase isoform abolishes 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside- but not contraction-induced glucose uptake in skeletal muscle. Journal of Biological Chemistry. 2004;279(2):1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 60.Ahlborg B., Bergström J., Ekelund L.-G., Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiologica Scandinavica. 1967;70(2):129–142. [Google Scholar]
- 61.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2) doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy J.J., Srikuea R., Kirby T.J., Peterson C.A., Esser K.A. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skeletal Muscle. 2012;2(1):8. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Proteomic analyses of targeted proteins involved in glucose uptake, glycolysis, TCA Cycle, fatty acid β-oxidation, PDH and oxidative phosphorylation. Data are presented as log2 ratio for exercise leg relative to resting leg (Ex/Re) (n = 5). The number observations for up- and down-regulation of proteins are indicated by arrows.
Supplemental I: Supplemental Experimental Procedures. Supplemental I provides a detailed description of experimental procedures and analyses applied in the human one-legged glycogen supercompensation study and animal experiments.
Supplemental III: Full List of Human Muscle MS repository. Quantification of peptides is given as log2 ratio for exercise leg relative to resting leg (Ex/Re) (n = 5). Supplemental II provides a full list of proteins detected and is presented as log2 ratio for the exercised leg relative to the rested control leg.