Abstract
Three-dimensional (3D) cultures use the property of some cells to self-organize in matrices and generate structures that can be programmed to represent an organ or a pathology. Organoid cultures are the 3D cultivation of source tissue (ranging from cells to tissue fragments) in a support matrix and specialized media that nearly resembles the physiological environment. Depending on the source tissue, growth factors, and inhibitors provided, organoids can be programmed to recapitulate the biology of a system and progression of pathology. Organoids are genetically stable, and genetically amenable, making them very suitable tools to study tissue homeostasis and cancer. In this review, we focus on providing recent technical advances from published literature to efficiently use organoids as a tool for disease modeling and therapeutics. Also, we discuss stem cell biology principles utilized to generate multiple organoids and their characteristics, with a brief description of methodology. A major theme of this review is to expand organoid applications to the study disease progression and drug response in different cancers. We also discuss shortcomings, limitations, and advantages of developed 3D cultures, with the rationale behind the methodology.
Keywords: Organoids, 3D-Culture, Tumoroid, Experimental Models, Stem Cell Culture, Differentiation, Adult Stem Cells, Induces Pluripotent Stem Cells
Graphical abstract
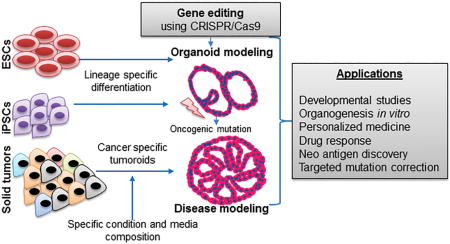
INTRODUCTION
The past decade has seen tremendous development in disease modeling and generating accurate experimental models that mimic biological processes, from co-culture techniques to 3D printed scaffolds and organoid culture [1–5]. Generating precise experimental models is essential for understanding basic biology, disease development, and therapy responses, but generating the complex biological environment of an organ to study development, or disease progression and therapy, is nevertheless a challenging task.
Over time, various tools have been employed to generate experimental models that can recapitulate human biology (or at least some of its properties). Conventional cultures include growing transformed cells derived from biological tissues in monolayer cultures. These are easy to culture and amenable to experimental modifications. Though these transformed cell lines allow study of human cancer cells, because they have spent years in vitro since establishment, they tend to acquire an undefined and complex mutational background [6]. Monolayer cultures are also two-dimensional (2D) and do not represent the tissue architecture and cellular heterogeneity found in tissues or tumors of their origin [6, 7].
Animal models make for some of the drawbacks of 2D cultures since they include stroma, vasculature, and immune components. They can be engineered to generate transgenic disease models to recapitulate pathogenesis using molecular biology tools and breeding strategies. Another experimental use of these animals is generating patient-derived xenografts or tumor xenografts. These models are compelling but are also resource intensive and time-consuming to develop. Moreover, the genomic profile of animal models does not precisely match with human profiles [8].
The first reports that described 3D culture systems discussed models that allow for long-term expansion of single mouse adult intestine [9], stomach [1], liver [10], and pancreas [10]. The first breakthrough experiments in the field of 3D culture were performed using Lgr5+ stem cells (SCs) or intestinal crypt cells in 2009 [9]; this study demonstrated that SCs could be used to generate stable, near-physiological epithelia when supplied with growth factors and proteins close to endogenous stem cell niche components [9].
The idea of a system that recapitulates a holistic microenvironment of normal biology ex vivo while proving experimental ease and feasibility of cell lines lead to the development of 3D culture methods. Cellular behaviors in vivo depend on environmental signals and contacts with neighboring cells and the extracellular matrix. 3D cultures allow for these signals to some extent and hence serve as an experimental system closer to normal biology.
Organoid 3D-cultures can be formed using a variety of source materials, from spheroids derived from SCs/progenitor cells/iPSCs (induced pluripotent stem cells) to tissue segments to whole organ explants [4, 5, 9, 11]. Due to the differences in source material used and the 3D environment and scaffold provided, there are differences in the types of cultures generated, and it is this diversity that poses a challenge to define these organoids. Although the definition of organoids is still evolving, for this review, we use the term to address cultures that recapitulate in vivo architecture, maintain SCs or progenitor pool, and exhibit multi-lineage heterogeneity.
Several approaches used to study a broad range of developmental and cellular processes have been comprehensively covered in reviews elsewhere [11, 12]. Our focus herein is on the most recent developments in organoid culture for major organs and cancers, including representation of their microenvironments and SCs (niches) on an appropriate scaffold. We also present our views on their implications for the development and testing of therapeutics.
PREREQUISITES TO ORGANOID GENERATION: WHERE DO WE START?
Organoids have become a powerful tool for research and are becoming familiar to everyday lab practice, but there are certain key pieces of information necessary to consider before organoid development. Paramount amongst these are, the selection of appropriate sources of organ or cells (iPSCs, Adult SCs, Organ chunks and embryonic SCs), and appropriate protocols that employ necessary growth factors and morphogens. The correct matrices essential for multiple stages of organoid development must also be determined. Then, once organoids develop, media composition must be determined to maintain structure and retain the functional characteristics of the organ of intent. In the following sections, we discuss such considerations and how they affect the generation of organoids.
Source tissue: Beginning decides the end
Organoids can be cultured from embryonic stem cells (ESCs), adult stem cells (AdSCs), iPSCs, and tissue fragments. Development of hPSCs and human ESC culture techniques in parallel to 3D culture systems has helped researchers recapitulate the successful differentiation and development of endodermal (lung, stomach, liver, small intestine) and ectodermal (brain and retina) tissues in vitro, and has opened up new avenues for further research (Fig. 1) [1, 11, 13–15].
Fig. 1. Representation of the organoids generated, and the media compositions required.
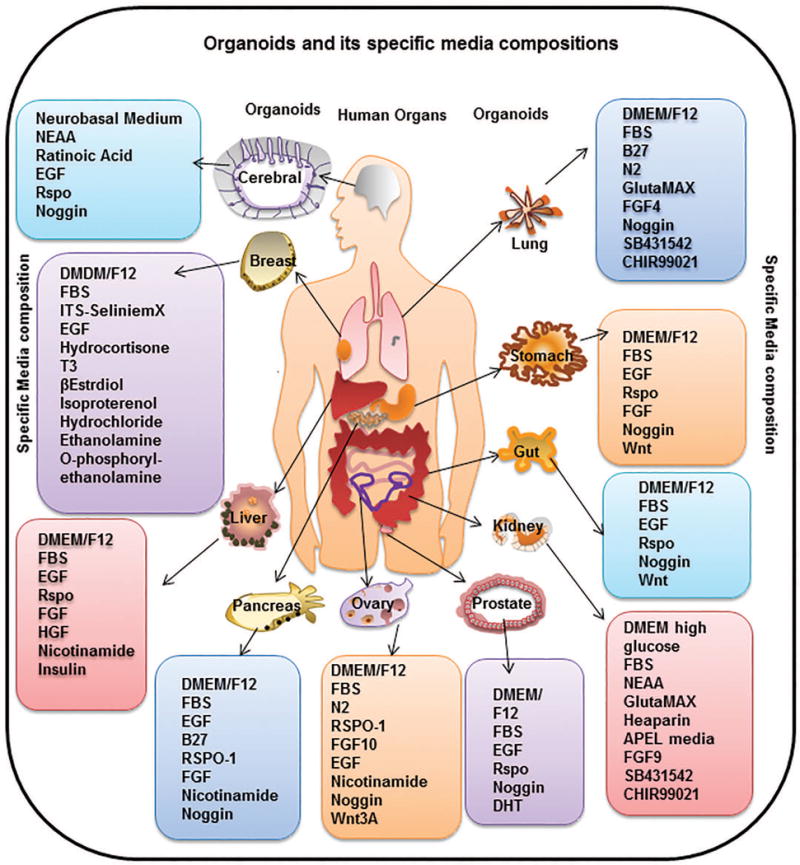
These hosts of organoids have been generated from different source materials, including iPSCs, AdSC, embryonic tissues or cells and adult tissue explants. Different media compositions are required for each type of source material used and the type of differentiation to be achieved (organ specific), which is elaborated in detail in the text. Specifically, cerebral organoids need a stepwise incubation of PSC in neural induction media (DMEM-F12, N2 supplement, GlutaMAX supplement, MEM-NEAA, heparin) followed by cerebral induction media (DMEM-F12, Neurobasal medium, N2 supplement, insulin, GlutaMAX supplement, MEM-NEAA, penicillin-streptomycin, 2-mercaptoethanol, B27 supplement). Mammary organoids can be developed from tissue fragments using media composed of DMEM/F12, FBS, ITS Selenite media supplement, FGF2, FGF10 for mouse or EpiCult B medium supplemented with hydrocortisone, insulin, FGF10, HGF for humans. Liver organoids can be generated by mixing tissue fragments in DMEM/F12 media supplemented with FBS, EGF, RSPO1, FGF, HGF, Nicotinamide, and insulin. Pancreatic organoids need a media comprising of DMEM/F12, B27 supplement, Nicotinamide, Noggin, EGF, FGF and RSPO1. Ovarian organoids are generated by seeding fallopian epithelial cells in matrigel with media comprising AdDMEM/F12, Wnt3A, RSPO1, HEPES, GlutaMAX, B27, N2 Supplement, EGF, noggin, FGF10, Nicotinamide, Y-27632, and SB431542. Prostate organoids need a media containing DMEM/F12, B27 Supplement, N-acetylcysteine, EGF, Noggin, RSPO1, A83-01, and DHT. Kidney organoids need a media containing DMEM high glucose, FBS, NEAA, GlutaMAX, Heaparin, APEL media, FGF9, SB431542 and CHIR99021. Gut or intestinal organoids need a media composition of DMEM/F12, FBS, B27, EGF, RSPO1, Noggin and Wnt. Specific cultivation of stomach organoids need media composition same as intestinal organoids with addition of FGF. Lung organoids can be generated and grown in media containing DMEM/F12, FBS, B27, N2 Supplement, GlutaMAX, FGF4, Noggin, SB431542 and CHIR99021.
Abbreviation used are: Y-27632:ROCK inhibitor, SB431542:TGF-β R Kinase Inhibitor IV, ITS: Insulin Transferrin-Sodium, NEAA: Non Essential Amino Acid Culture Supplement, EGF: Epidermal Growth Factor, RSPO1: R-spondin-1, Wnt3A: Wingless-Type MMTV Integration Site Family Member 3A, T3: Triiodothyronine, FBS: Fetal Bovine Serum, FGF: Fibroblast Growth Factor, HGF: Hepatocyte Growth factor, DMEM/F12: Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12, DHT: Dihydrotestosterone, CHIR99021: glycogen synthase kinase 3 inhibitor.
Recent attention has focused on using hPSCs or patient tissue samples via the process of reprogramming adult somatic cells into iPSCs by ectopic expression of pluripotency transcription factors [3]. These cells are then transformed into organoids by using the signaling pathways involved in modeling germ layer formation and induction of organ primordia, Wnt, EGF, Retinoid, and TGFβ/BMP [3]. iPSC-derived organoids have been generated from brain [16], lung [3], intestine [17], stomach [18], eyes [19], and kidney [20]. Organoids have also been generated using AdSCs or adult primary tissue and then expanded long-term in vitro. These studies also build upon the available knowledge of stem cell niche requirements and generated their media with a base composition of Wnt, RSPONDIN, EGF, and Noggin, and include mouse and human pancreas, liver, intestine, stomach, prostate, fallopian tube, and salivary gland organoids [1, 3, 13, 14, 21–26] [27]. Such availability of different sources determines the necessary media components.
Matrix selection: The Bed Where They Lay
The primary purpose of matrix is to provide structural support similar to extracellular matrix (ECM) that is capable of instigating necessary instructive signaling. ECM components like laminin, fibronectin, and collagen engage the integrin receptors of cells and tissues that they support to maintain cell identity and functions [28]. ECM is also a vital component of stem cell niche and provides instructive signaling for cell polarization, retention, and mobilization [29]. Nanofibrous structures that provide such cues and can be modified to allow simulation of various cell behaviors appear to be ideal materials for the organoid matrix.
Matrigel, although undefined, is the most commonly used matrix. Matrigel contains gelatinous mixtures of extracellular matrix components, including laminin, collagen type IV, entactin, and heparan sulfate proteoglycans, as well as some growth factors, such as TGF-β and FGF [30, 31]. For instance, a primary culture of pancreatic ductal cells has been made possible by seeding SCs or tissue fragments in Matrigel as submerged cultures that support the growth of epithelial cell cultures [1], whereas long-term organoid cultures that include both epithelial and mesenchymal components have been successfully performed using an air-liquid interface method [1, 8]. Since matrigel has a heterogeneous composition, it does not allow easy manipulation of the matrix to facilitate various morphogenetic processes.
Alternatively, 3D scaffolds can be designed synthetically to incorporate specific spatiotemporal cues to produce designer ECMs. For example, RGD (Arg-Gly-Asp) peptide which is known to bind integrins (e.g., β1 and β3) and enhance cell-matrix interactions and focal adhesion, enhances metabolic activity in cells [32]. However, such scaffolds are synthetically designed and hence lack the critical dynamic properties that are present in the cell driven modeling of matrigel. Another option to recapitulate ECM is to engineer bio-inert matrices by chemically/enzymatically crosslinking signaling proteins at adhesive or proteolytically cleavable sites [25].
A recent study reported that matrix stiffness has an impact on organoid growth and stem cell signaling in intestinal organoids. The authors reported employing a synthetic scaffold design, using a polyethylene glycol backbone with more consistent and chemically defined synthetic hydrogel that allowed stem cell expansion and organoid formation [33]. Similarly, artificial scaffolds can be created by attaching microenvironment signals such as ECM components and cell-to-cell interaction proteins to an artificial scaffold [33] and various techniques may be tried as described below.
Designing techniques
Microcontact printing
direct depositing of ECM, cells or proteins on partially polymerized hydrogel substrate, often by a poly (dimethylsiloxane) (PDMS) stamp using soft lithography techniques [34].The stamp is made by Nanolithography strategies like electrospinning, nano-imprint lithography, and selective etching to deposit nanofibrous structures like nanopits, nanopillars, or nanochannels on different matrix substrates [34].
Amongst these, electron-beam lithography is used to produce patterns at nanoscale resolutions. Electrospinning produces ultra-fine fibers that form randomly oriented fibrous meshes appropriate for tissue engineering applications [34]. To generate natural shape and dimensions similar to natural basement membrane fiber and pore sizes, nano-imprint lithography and selective etching are used where rigid molds or chemical etchants are used to modify matrix polymer [25, 34]. Such matrices have been shown to allow Human ESC self-renewal and mesenchymal stem cell differentiation [35].
Bio-printing
A biomaterial with living cells is precisely positioned in an additive layer-by-layer approach to generate a 3D biological structure that mimics the structural and functional properties of native tissues and organs [36]. A recent study reported generation of a beating heart organoid that could respond to electrical and chemical cues by altering its beating patterns. Human liver, muscle, and blood micro vessel organoids have also been created using bio-printing [34]. Despite all this progress, we still have a very limited ability to print in high resolution and maintain long-term cell functionality of the bio links. It is also a challenge to achieve relevant and controllable cell densities.
Researchers have also devised a bottom-up approach to achieve a microscale spatial control of cell-cell interaction. Microscale cell-laden constructs are individually assembled, and then controlled multi-construct organization is induced to assemble spatially controlled cell aggregates [37]. In addition to bio-printing, bottom-up approaches present an exciting possibility to design controlled stem cell niches or the construction of multi-tissue organoid systems.
Media Requirements and Properties of Generated Organoids
Developmental biology studies have long revealed that morphogen gradient decides cell fate during embryo development. 3D organoids have been used to intelligently apply that knowledge when deciding on which media components will be necessary for specific organoid generation. We will further describe media requirements and the rationale behind several organoids that have been developed and used for modeling disease. Certain media components are common and vital for maintaining several organoids. Most organoid media concoctions have a basic composition of AdMEM, HEPES, Nacetyl-l-cysteine, and EGF. Wnt activation and FGF promote lineage specific growth. Nacetyl-l-cysteine is a substrate in the synthesis of glutathione [38], and glutathione concentrations provide a rich reductive capacity for the cells in organoids. HEPES provides a buffered pH environment, and EGF facilitates growth and maintenance of SCs [1, 8, 11, 39, 40]. N2 and B27 are defined mixes of growth factors that replace or complement FBS in cultures and facilitate growth of neuronal and other cell lineages respectively [1, 11, 16, 39, 41, 42].
Brain Organoids
3D brain tissues or mini brains can now be generated using pluripotent stem cells (PSC). These tissues, also called cerebral organoids, are created by first driving PSC to a neural progenitor lineage and then providing a supportive 3-dimensional microenvironment for them, where they can self-organize spontaneously into the early embryonic brain [42]. A recent study reported the successful formation of brain organoids from human PSC, comprising the timely amalgamation of several previously published methodologies to accomplish successful differentiation of PSC into neural progenitors and progress further down the neural lineage [16, 43].
Eiraku et al. reported in 2008 a unique 3D culture condition that showed differentiation of ESC into embryoid bodies and then into self-organized apicobasal polarized cortical tissue [44]. He used FGF, Wnt, and BMP factors to model his 3D aggregate cultures. Since then, multiple reports have built upon this methodology [5, 16, 41–43, 45, 46]. More recent study reported development of heterogeneous brain organoids, naming them “cerebral organoids”. They used a similar approach and differentiated embryonic cells to neuroectoderm and then incubated them in differentiation media in Matrigel, finally allowing them to grow and form cerebral organoids in differentiation media supplemented with retinoic acid as Matrigel droplets in a spinning bioreactor [42].
A recent report described a novel developmental model of 3D brain-like tissue by applying an interdisciplinary approach, involving seeding cells within a biomaterial scaffold to assemble microstructural features representative of native tissue [43]. This technique sought to recapitulate the structural features formed during development of the forebrain cerebral cortex, including gray matter (containing cell bodies) and white matter (containing neuronal axons). The study used silk protein to design scaffold, which provided spatial separation of cell bodies and neural processes. This lead to the development of a suitable matrix for growth of 3D compartmentalized neuronal networks that could recapitulate the properties of the native cortex and to establish the suitable conditions for neural growth [16, 46].
Lung Organoids
During embryonic development, the endoderm produces a primitive gut tube along which the lung, thyroid, and organs lining the gastrointestinal (GI) tract emerge. The lung arises from cells expressing the transcription factor NKX2.1 (TTF-1; Thyroid Transcription Factor 1) in the ventral wall of the anterior foregut endoderm [47]. Hence, currently available protocols include discrete steps to differentiate human PSC through initial definitive endoderm (DE) specification, then the anterior foregut endoderm (AFE), and finally into ventral anterior foregut endoderm (VAFE) and NKX2.1 expressing lung progenitors [47, 48]. Each step uses stage-specific growth factors to recapitulate the signaling pathways involved in lung development. Of importance, since organoids develop from tissue-specific SCs or progenitors, hPSC differentiation into these cell types has been employed as a strategy to develop organoids [47, 49] .
Lung organoids are mostly derived from primary respiratory cells and cell lines [47, 49]. A recent study demonstrated that primary basal cells harvested from mouse and human lungs could self-organize into organoids, called tracheospheres or bronchospheres, when cultured in a 3D ALI [49]. These organoids are derived from basal cells expressing p63 and NGFR, which proliferate to establish a layer of basal cells in a spherical organization, and that are covered on the luminal side by a second layer of a differentiated goblet and ciliated cells[49].
Thus far, two studies have reported the generation of lung organoids in vitro from hPSCs. The first showed that purified Carboxypeptidase M (CPM) expressing cells in 3D conditions, supplemented with alveolar-related growth factors and human lung fibroblasts, produced alveolar epithelial spheroids [28, 49]. These spheroids contained cells expressing NKX2.1 and CPM, as well as differentiated cells that stained positive for AQP5 (Aquaporin 5) and SFPTC (Surfactant Protein C), markers of type 1 and 2 alveolar epithelial cells (AEC1s and AEC2s), respectively [50, 51].
The second study performed a step-by-step differentiation of hPSCs and reported multi-lineage organoids with epithelial and mesenchymal components. By stimulating the Hedgehog (HH) signaling pathway during spheroid generation, the authors could enhance NKX2.1 expression and expand spheroids in media containing FGF10 [47, 50]. This allowed VAFE spheroids to grow into more complex structures that the authors called human lung organoids (HLOs). HLOs persisted in culture for over 100 days and developed organized proximal airway-like epithelial tubules containing numerous cell types found in the native airway epithelium, including basal, ciliated, and club cells and that were surrounded by smooth muscle actin (SMA)- expressing mesenchymal tissue maintaining early bi-potent alveolar progenitor cells [47, 50, 51].
Mammary Organoids
Mammary acinus contains extensive stromal and extracellular matrix compartments, the composition of which changes depending on signals such as growth factors and hormonal changes. Even monolayer cultures of mammary epithelial cells can form functional tubular structures when provided with the required environmental cues; for example, upon transplantation into the gland-free fat pads of mice [52]. It would be logical to infer that either the systemic factors or cellular microenvironment that surrounds the mammary epithelial cells confer the cues that drive functional differentiation of mammary epithelial tissue, suggesting the importance of providing a matrix to the cells in culture that resembles their biological ECM. Michalopoulos and Pitot (1976) were the first to use floating collagen gels to provide an extracellular environment to hepatocytes that could maintain their functional and morphological identities in culture for a short period [53]. Emerman and Pitelka further adapted this idea in the 1970s, and in 2013, Mroue and Bissell cultured mouse primary mammary epithelial cells that retained functional differentiation using both floating collagen-I (Col-I) gels and laminin-rich ECM gels (lrECM) [23, 52]. Mammary epithelial cells grown on floating collagen gels were found to reorganize and form secretory structures that express milk proteins de novo.
Another observation made by Mroue et al. highlighted the significance of the composition of ECM provided in deciding the fate of cells in culture [23]. They reported that mammary organoids, when cultured on floating collagen gels, contained mammary epithelial cell (MECs) clusters, which exhibit basoapical polarity and cellular junctions, and expressed the milk protein β-casein. These lacked expression of whey acidic protein (WAP) and did not form luminal alveolar structures, both essential features of the mammary gland [23].
In a recent report, Linnemann et al. published alternative 3D culture conditions for the expansion of TDLU-like structures (Terminal Ductal lobular Units) from primary human cells [54]. The advantage of this method is that it includes conditions that support the growth of single cells at high efficiency. Its drawbacks are that it incorporates chemical agents (Rho-associated protein kinase inhibitor, forskolin) and serum that perturb intracellular signaling in nonphysiological ways [23]. The relative merits and failings must be carefully considered before deciding the appropriate model system to use in future study.
Liver Organoids
Two epithelial cell types, hepatocytes and ductal cells, chiefly compose liver [55]. Hepatocyte-like cells have been generated by differentiating human embryonic stem (hES) cells and human induced pluripotent stem (hiPS) cells. However, because of the genetic and epigenetic aberrations that occur during the reprogramming process [56, 57], the use of these models for translational research and regenerative medicine [58] remains limited. Generation of liver organoids overcomes these limitations; the organoids are generated by using the Wnt, BMP, RA, HGF, and FGF signaling pathways that regulate the embryonic development of liver [59].
Liver organoids were generated by mixing SCs and tissue fragments with Matrigel and providing growth factors like EGF, HGF, FGF, and RSPO1. Such conditions allowed liver cells to self-organize into organoids resembling embryonic liver buds [60]. These organoids were Keratin positive and expressed progenitor cell markers. The authors also reported that generation of human liver organoids requires inhibition of TGFβ signaling. Replacement of Notch and RSPO by dexamethasone and BMP also allowed differentiation of these organoids into hepatocytes [60].
Pancreatic Organoids
Two functionally distinct compartments make up the pancreas: ductal and acinar cells consist of the exocrine compartment, and the Islets of Langerhans make up the endocrine compartment. The genes and molecular pathways regulating the embryonic development of the two compartments are evolutionarily conserved and include FGF, HGF, Wnt, BMP, RA, and TGFβ. These pathways promote survival, proliferation, and migration of the progenitor pools that generate these two distinct compartments [1, 9, 10, 14, 61]. Developmental studies and knowledge acquired from 3D cultures of the stomach and intestine allowed researchers to culture, expand, and differentiate mouse and human primary pancreatic tissue [9–11]. Providing an ECM and a microenvironment that includes growth factors essential during development of pancreas (Noggin, EGF, FGF and R-spondin-1(Rspo1)) is necessary for the long-term expansion of the adult pancreatic tissue in these 3D culture systems [10]. Using similar approach, we developed murine pancreatic organoids from wild-type C57BL/6 murine pancreas which show histology similar to pancreatic ducts (Fig. 2.) [62]. A similar approach was also used to generate models to study pancreatic tumors and for which tumor organoids were generated from KC (Kras; PdxCre) and KPC (Kras;p53; PdxCre) autochthonous animal models for PDAC and were shown to represent PDAC progression histologically [62].
Fig. 2. General scheme of generating organoids and representative figures of organoids generated by our laboratory.
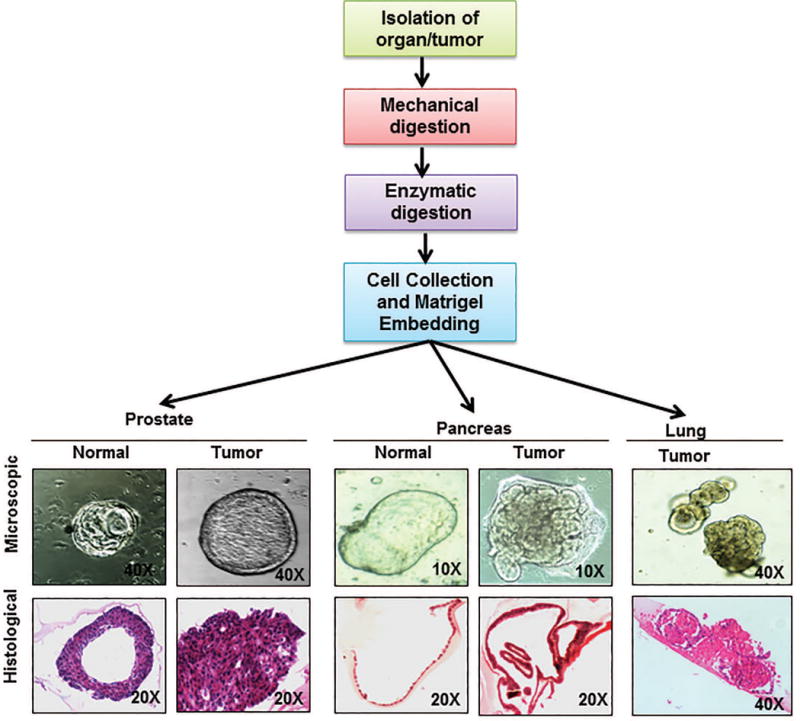
The flowchart represents the scheme of organoid isolation which is modified for each organoid according to the organ or tissue architecture to generate submerged organoids. Briefly, desired source tissue (progenitor cells or tissue fragments) is isolated from host by mincing the organ and then subjecting it to enzymatic digestion. The digestion media composition and the digestion protocol are decided depending on the host tissue. The digestion media usually contains a mixture or Dispase and Collagenase or Collagenase alone and can take from 30 mins to 4-6 hours. Following digestion, the cells are mixed in the matrix (like matrigel or collagen) suitable for the desired organoids. A suitable media is overlaid once the matrix solidifies. Once generated, organoids grow in ductal like morphologies like their human counter parts. Picture panels depict the microscopic pictures of organoids generated in our lab from normal and cancerous prostate and pancreas as well as lung cancer organoids (upper panel) along with hematoxylin and eosin stained sections of the same (lower panel) depicting the difference in organization of cells in each of these organoids. Figure magnifications are mentioned on each of the figures.
Another study used neonatal wild-type C57BL/6 mice pancreatic tissue to generate organoids by the air-liquid interface culture method. These grew progressively for more than 30 days as cystic structures carrying an epithelial layer and surrounded by fibroblasts [63]. α-smooth muscle actin–positive (SMA+) stromal cells were observed in association with these cystic epithelial organoids, which could be readily infected with adenovirus and were predominantly comprised of E-cadherin–positive (E-cad+) and Pdx1+ ductal epithelium with PCNA+ proliferating cells. [1, 59]. Somatostatin and insulin were also found to be expressed in rare, islet-like regions not always associated with ductal structures; sporadic immunoreactivity was also reported for glucagon and amylase occasionally [59].
Intestinal organoids
First intestinal crypt cultures were generated in 1992 by Evans et al. which could last one to two weeks on collagen1 coated plates [64]. Later in 2009 air-liquid interface with collagen support matrix was used to generate intestinal crypt cultures which could last long, but both these culture conditions required mesenchymal fibroblast feeder layer to allow growth [65]. In 2009 Sato et al. published a submerged Matrigel model allowing Lgr5+ cells or crypt fractions to be cultured into exclusively epithelial organotypic intestinal structures, also referred to as enteroids [66]. Growth factors that simulate the paracrine signaling environment surrounding SCs were supplemented in a media concoction for these enteroids [66]. Since Wnt signaling is essential for crypt proliferation and EGF and noggin are vital for intestinal stem cell growth, RSpondin-1 (Wnt agonist), EGF and recombinant Noggin were included in the media [9]. Laminin rich Matrigel was used as the choice of matrix considering basement of intestinal crypt is enriched in laminin (α1 and α2). Isolation of these enteroids follows a general scheme of mincing tissue or intestine and subjecting it to enzymatic digestion. The digested fraction is centrifuged, and intestinal crypts are isolated from it [39, 66]. These crypts are then embedded in Matrigel for submerged cultures or seeded in ALI cultures [39, 65, 66].
Enteroids typically constitute cystic epithelium with an inward-facing apical side and a progenitor pool containing crypt-like invagination [40]. These organoids once established resulted in structures containing Lgr5+ intestinal SCs and other differentiated cells localized corresponding to the in vivo organization with SCs and Paneth cells at the bottom of budding structures, and mature enterocytes to the central cyst structure [14, 39, 65, 66].
Extrinsic factors provided in media serve as deciding factors for stem cell self-renewal in mini-gut organoids. Above mentioned minimally essential niche factors are enough for mouse small intestinal organoids, whereas additional niche factors are required for other organoids. Like, Wnt-3A is necessary for mouse colon organoids, and addition of p38 inhibitor and TGF-β inhibitor is required for human intestinal and colonic organoids [67]. Human colonic organoid cultures can also be generated with Wnt-3A, prostaglandin E2, and nicotinamide additions in the minimally essential niche factors containing medium [68]. To enhance efficiency and include multiple biological parameters, several refinements have been made to this system, including Wnt3A withdrawal used to facilitate differentiation; the use of the Rho kinase inhibitor Y-27632 to avoid anoikis during tissue preparation, passaging and revival; using GSK3β kinase inhibitors and valproic acid to promote a stem-like state; and myofibroblast feeder layers to replace Wnt and R-spondin from medium [69].
These organoid cultures for gut have immense potential as preclinical models and have been utilized to study various gut disorders like microvillous atrophy and multiple intestinal attricia and common diseases like inflammatory bowel disease (IBD) [70–72]. These studies have provided a better understanding of the diseases and revealed novel therapeutic targets. Transplantation studies with in vitro–expanded organoids derived from a single Lgr5+ intestinal SC onto damaged mucosa have shown recovery from IBD in mouse models, and hence transplantation studies with organoids are also a promising avenue [26]. Normal human intestinal organoids can be transformed to tumor organoids by CRISPR-Cas9 genome editing and introducing five colorectal cancer (CRC) mutations (in APC, KRAS, SMAD4, TP53, and PIK3CA) [73]. Organoids hence provide a promising model for CRC studies.
Ovarian and Fallopian Tube Organoids
Fallopian tube model systems have traditionally facilitated ovarian development and ovarian cancer studies, and a similar trend was followed in 3D culture systems. Recent studies have demonstrated the existence of AdSC in the human fallopian tube epithelium that give rise to differentiated epithelial cells in complex 3D organoids in vitro [74].
Fallopian tube organoids are derived from mucosal cells isolated from fallopian tube epithelia. These cells are isolated and seeded in monolayer culture, progenitor cells amongst them are favored in this culture while being supplemented with the minimal organoid media discussed before which additions of, Wnt3A, human RSPO1 medium, HEPES, GlutaMAX, B27, N2, human EGF (all from Invitrogen), noggin, FGF10 (nicotinamide, ROCK inhibitor (Y-27632) and TGF-β R Kinase Inhibitor IV (SB431542) [74]. Upon reaching 70 % confluency these cells could be embedded in matrigel for 3 D organoid culture in minimal organoid media supplemented with HEPES, GlutaMAX, B27, N2, nicotinamide and TGF-β R Kinase Inhibitor IV [74]. These organoids were reported to recapitulate the in vivo tissue architecture; they could depict growth and be maintained in culture long term. The culture protocol and growth conditions are similar on a gross scale to the intestinal tract, skin, and liver [1, 14, 15, 21, 75]. Active Wnt signaling works towards maintaining the stem cell population in these organoids, achieved by activating Wnt target genes Lgr4, 5, and 6, all of which are a subfamily of leucine-rich, repeat-containing G protein-coupled receptors that can strongly amplify Wnt signals [74]. The R-spondin family of proteins also acts as an Lgr receptor agonist. Similarly, the growth capacity of organoids is modulated by Wnt3A and R-spondin-1 (RSPO1) [74]. This organoid model faithfully mimics the normal physiology and anatomy of the human fallopian tube and provides a platform for future investigations into the regulatory mechanisms involved in its cellular renewal and pathology. Overall growth rates for these organoids during long-term culture remained constant, with passaging every 2–3 weeks [74]. This method yielded expandable, stable organoid cultures in all healthy tissue samples, with minimal variations in sphere formation potential and growth rate between donors or between distal and proximal tubal regions.[74]
Prostate Organoids
The prostate gland produces seminal fluid in the male reproductive system and is composed of a pseudostratified epithelium of luminal, basal and rare neuroendocrine cells. Additionally, prostate development and homeostasis, as well as prostate cancer initiation and progression, requires androgen receptor (AR) signaling [76]. Most in vitro studies are performed using cell lines, and most of these do not have an intact AR signaling pathway, making them weak models to represent healthy prostate and cancer tissue [76, 77]. Lack of suitable in vitro model systems is an obstacle for prostate cancer research. Although robust in vivo models are available, these are often expensive, time-consuming, and technically daunting.
Another study by Chua et al. recently demonstrated the development of organoids from sorted luminal cells, but these 3D cultures with a limited growth potential [75]. Although these were AR-responsive, the medium used in this study included undefined components like fetal calf serum and had a plating efficiency of 0.2% to 0.3% [75, 77].
Drost et al. developed a testosterone-responsive prostate organoid culture system by adapting and optimizing the culture conditions previously employed to establish mouse and human colon and intestinal organoid cultures. Different compounds and growth factors, including epidermal growth factor (EGF), Noggin, and R-spondin 1, were added to the generic organoid medium to allow for the establishment of long-term mouse and human prostate organoid cultures. These submerged prostate organoid culture systems contain multipotent progenitor cells in both the luminal and basal lineages that can be propagated long term. AR signaling is indispensable for organoids to functionally recapitulate prostate and was maintained in these organoids. This study concluded that organoids derived from human or mouse prostate cancer recapitulate genetically and phenotypically the tumor from which they were derived [77].
This method was further adapted by our lab to generate prostate and prostate cancer organoids from mouse tissues (Fig. 2). The main procedure involves dissection and digestion of tissue, followed by subsequent embedding, plating, and organoid passaging. We plated organoids are plated in Matrigel and cultured them in a defined prostate culture medium, after which they were sub-cultured and frozen. These organoids can be cryopreserved once established and are genetically and phenotypically stable. Prostate tumor organoid media includes DMEM/F12 supplemented with B27, N-acetylcysteine, EGF, Noggin, R-spondin 1, A83-01, and DHT [22, 77].
LIMITATIONS: NEED FOR INTERVENTION
The potentials organoids hold for future study and use are innumerable, but they present some limitations, as does any technology. The organoids established so far need to be characterized and studied to the extent that exact recapitulation of in vivo development is possible. Tissue maturation is one of the limitations associated with retinal and cerebral organoids, where early events display intact organization, but the organized tissue fails to develop into a functionally mature organ. However, intestinal organoids produce Lgr5+ SCs, implying movement towards a mature intestine [9]. One solution to the maturation problem may be by growing a mature organ followed by transplantation. Another limitation of organoids is non-vascularization due to limitations in nutrient supply, which may be solved by spinning bioreactors that provide better nutrient exchange. Vascularization may also be achieved by co-culturing with endothelial cells that can generate vascular-like networks. Some researchers have generated hybrid cultures with organoids to incorporate different cell types to generate more insightful models.[78]
Another limitation to organoid cultures is the limited presence of stromal components, including immune components, and this hinders organoid use in modeling inflammation and drug penetration studies [15]. Organoid cultures are also heterogeneous with no reliable means of synchronizing size shape and viability. This, unfortunately, leads to complications in data analysis and study design. Although all these limitations stand in the way of organoid applications, they can be overcome by better understanding of ECM components and live cell imaging techniques that facilitate the analysis of the co-cultures or hybrid cultures of these organoids.
PROSPECTIVE APPLICATIONS: MAKING THE MOST OF A TREMENDOUS TOOL
Normal organoids derived from SCs and specific organs can be used for their molecular impact on organogenesis study. Here we discuss different areas of biological science research that organoids have applications in. (Fig. 3).
Fig. 3. Potential applications of organoids generated.
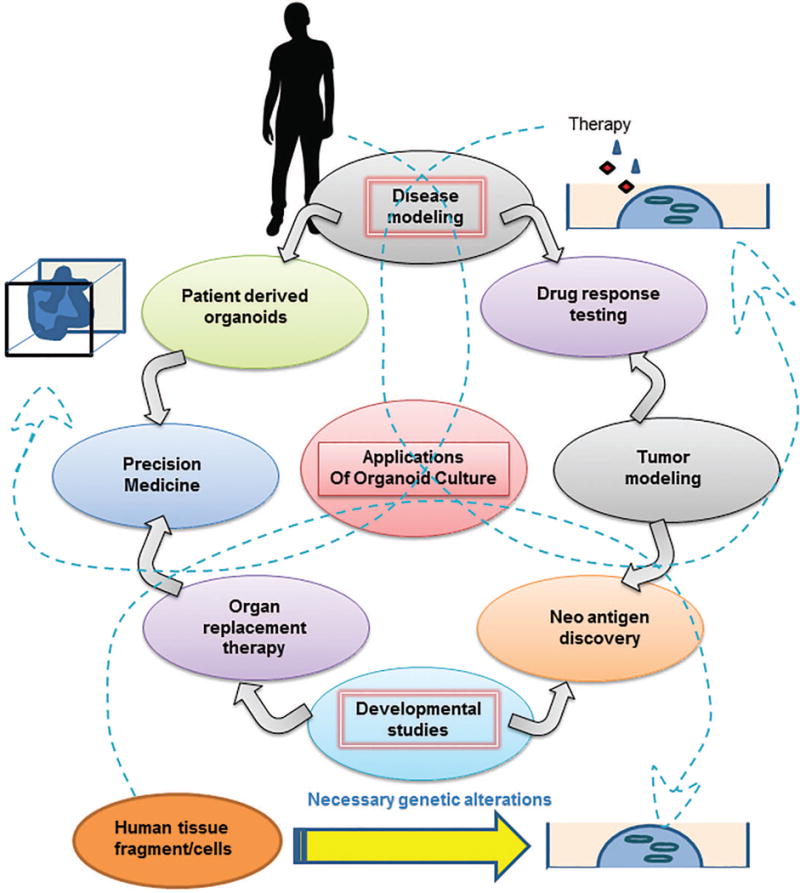
The figure presents prospective applications of organoid culture tool for advancement of biological research. The arrows in the figure represent the flow of information from tumor modeling, disease modeling and developmental biology studies towards therapeutic interventions. To expand since organoids, represent tissue homeostasis in vitro, they can be used to model pathologies by inducing desired mutations or exposing them to necessary stimulus or pathogens. Following which the pathogenesis and disease development can be studied. Such studies facilitate further research to study drug response or generate organoids directly from patients to device personalized therapeutic strategy as represented by the arrows emerging from disease modeling bubble. Similarly, modeling cancer initiation and progression in organoids can facilitate therapeutic response studies and discovery of new oncogenic proteins or antigens that can be targeted illustrated by arrows emerging from Tumor modeling bubble. Additionally, lineage tracing studies or organ development studies using organoids have immense potential for the field of organ replacement therapy and can help neo-antigen discovery for cancer research (arrows emerging from developmental biology studies bubble). The dashed arrows represent the overlapping domains amongst these applications as explained above.
Organoid developed for tissue modeling
Organoids hold an advantage over traditional techniques to solve unanswered questions in developmental biology because of the accessibility of model systems, especially for human models. For example, the unique division mode of neural SCs has been studied using human brain organoids [15, 16, 42]. Similarly, the differences in tissue morphogenesis and timing between humans and rodents have been studied using retinal organoids. Furthermore, organoids may be used to study processes that differ in model organisms and humans, such as GI tract development, and to model adult homeostasis. Specifically, the role played by the crypt niche in self-renewal and differentiation of SCs has been studied using intestinal organoids. Regenerative events in adult organs, such as liver and stomach, have also been closely recapitulated by organoids derived from adult progenitors.
Organoids developed for regenerative medicine and transplantation studies
Organoids also provide an alternative for cell and whole organ transplantation by providing autologous tissue. Organ transplant procedures with high demands and low success rates, such as renal transplants, may be improved using corresponding organoids. Successful transplantation of kidney organoids in adult mice has been already achieved by Taguchi et al., paving the way for a promising future for organoid transplantation [20, 79]. Stem cell therapies are being aided by retinal organoids in clinical trials to replace degenerating cell types. On similar lines, intestinal organoids are also under investigation to treat damaged and diseased colon.
Organoids used for therapy response in pathologies
The failure rate of traditional models of therapy testing translating to patient treatment regimens has launched a hunt for more reliable and physiological models, such as patient-derived organoids for drug screenings and drug discovery studies. Yin et al. recently reported use of primary intestinal organoids for modeling retroviral infection and antiviral therapy in an attempt to address the lack of potent antiviral therapies [80]. In another study, Ogawa et al. used patient-derived cholangiocyte organoids to correct the misfolded CFTR protein (cystic fibrosis transmembrane conductance regulator) [81].Using a similar strategy, Schwank et al. used CRISPER/Cas9 to repair the function of CFTR in intestinal stem cell organoids. Such studies highlight the utility of organoids in disease modeling and therapeutic testing [70]. All these studies suggest that 3D organoids models are still developing but have immense potential for modeling various diseases and use in therapy design.
Organoids as a tool to study cancer
Organoid studies have emerged as valuable tool for cancer research. Modeling cancer environment in vitro is an arduous task, and conventional cultures fail to do so. Patient organoids recapitulate gene expression profiles and histological features of tumor of origin and hence are attractive tools for developing precise treatment strategies for patients, enhancing of precision medicine front [8]. These tumor organoids can be genetically characterized and modified to allow multiple drug screening and treatment strategies to be tested and hence consequently preventing toxicity. These miniature tools can help in deciding the most efficient and effective treatment strategy for the patient to improve overall survival. Broutier et al. [21] in a recent study used organoids derived from patient biopsies to generate three types of liver cancer organoids: hepatocellular carcinoma (HCC), cholangiocarcinoma (CC) and combined HCC/CC cancer (CHC). They use these organoids to study the diseases and test therapeutic response.
Additionally, Huang et al., in a recent study generated pancreatic ductal organoids and induced mutations in them to successfully model pancreatic ductal adenocarcinoma. They then used these organoids to test therapeutic efficacy of a histone methyl transferase inhibitor, working along the idea of disease modeling and therapy [82]. Skardal et al. established 3D liver organoids to model colorectal cancer metastasis and were able to test therapies on metastatic disease [78]. Van de Wetering et al. established tumor organoid cultures from 20 colorectal carcinoma patients representing most genetic subtypes of colorectal cancer generating a living biobank [83]. In these and other examples, we can see that organoid technology provides an opportunity to bridge the gap between patient-derived cell lines and xenograft mouse models, generating a link between cancer, genetic, and patient trials to make possible better and personalized therapy designs.
CONCLUSIONS AND PERSPECTIVES: TOWARDS MODELING ACCURATE HOMEOSTASIS AND DISEASE
Organoid cultures are accessible and physiologically relevant models to study biology. They can be derived from multiple sources, and they maintain stem cell or progenitor population. [1, 11, 40, 59, 84]. These models are robust in recapitulating in vivo tissue biology and have shown to be reliable in testing therapeutic response. They have the capacity to serve as a platform for translational research and high throughput preclinical screenings. Organoid technology has worked successfully with current research methodologies and found its niche.
More development will be seen in 3D organoid systems that will compensate for the limitations the technology still suffers, however. Despite this, we hope to see the extensive use of organoids in many more avenues. Patient-derived organoids provide an opportunity to develop personalized treatment regimens for patients since biopsies can be an excellent source of disease site tissues and normal tissues for deep sequencing. This would, in turn, reveal causal mutations and phenotypic profile to generate therapeutic approaches tailored to each patient. Once organoids from patients are generated, they can be used to test efficacy or resistance to proposed regimens. Additionally, organoids generated from healthy tissues can be used to weed out toxicity and other undesired effects of the proposed therapy.
Organoid studies are being used for disease modeling for developmental disorders, cancer, degeneration, and other infectious diseases [39, 40, 70, 78, 80, 85–88]. This may also be achieved by introducing patient mutations in human PSCs, using genome-editing techniques, by generating organoids or inserting mutations directly into organoids. Organoids are also being employed for screening drugs, testing for efficacy and toxicity by modeling different degenerative conditions such as liver fibrosis or cystic kidney diseases where effective treatment regimens are required. If successful, this approach could lead to reduction of the use of animal testing, which would be reserved only for studies requiring whole-organism readouts.
Organoids have found their place in everyday research and to date have significantly supplemented our knowledge and ability to model diseases. We have seen an exponential increase in their usage and application since first introduction. Their reliability, robustness, and amenability for research have yielded enormous downstream applications, highlighting their role in recapitulating homeostasis and diseases. These characteristics make organoids extremely exciting and promising technology that hold a promising future for therapeutics.
SIGNIFICANCE STATEMENT.
3-D organoid culture is a coming of age technology to recapitulate normal biology and pathology in vitro. Use of 3-D organoids has become a common practice in modern-day research and rightfully so, owing to the robust physiological relevance provided by this tool. Hence, it becomes imperative to critically analyze the development protocol and the biology behind the generation of these organoids to make sure their utilization is justified for any study. This review focuses on elaborating the biology behind the generation of organoids from specific vital organs along with a brief description of methodology, limitations, and applications of organoid culture.
Acknowledgments
We would like to thank Dr. Adrian E. Koesters, Research Editor at UNMC, for her editorial contribution to this manuscript. We also greatly appreciate kind technical help of Drs. Rachagani, Seshacharyulu, Lakshmanan and Ms. Kavita Mallya. The authors on this manuscript are, in parts, supported by the grants from the National Institutes of Health (RO1 CA210637, RO1CA206444, RO1 CA183459, RO1 GM113166 and UO1 CA185148) and Garima Kaushik is supported by University of Nebraska Medical Center Graduate Studies Fellowship.
Footnotes
AUTHOR CONTRIBUTIONS
G.K., M.P.P., and S.K.B.: wrote the manuscript, summarized the contents, and organized the graphical overview, final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST
The authors indicated no potential conflict of interest.
References
- 1.Baker LA, Tiriac H, Clevers H, et al. Modeling pancreatic cancer with organoids. Trends Cancer. 2016;2:176–190. doi: 10.1016/j.trecan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell JJ, Davidenko N, Caffarel MM, et al. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One. 2011;6:e25661. doi: 10.1371/journal.pone.0025661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dye BR, Hill DR, Ferguson MA, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4 doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huch M. Building stomach in a dish. Nat Cell Biol. 2015;17:966–967. doi: 10.1038/ncb3211. [DOI] [PubMed] [Google Scholar]
- 5.Lindborg BA, Brekke JH, Vegoe AL, et al. Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl Med. 2016;5:970–979. doi: 10.5966/sctm.2015-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Stein WD, Litman T, Fojo T, et al. A Serial Analysis of Gene Expression (SAGE) database analysis of chemosensitivity: comparing solid tumors with cell lines and comparing solid tumors from different tissue origins. Cancer Res. 2004;64:2805–2816. doi: 10.1158/0008-5472.can-03-3383. [DOI] [PubMed] [Google Scholar]
- 8.Neal JT, Kuo CJ. Organoids as Models for Neoplastic Transformation. Annu Rev Pathol. 2016;11:199–220. doi: 10.1146/annurev-pathol-012615-044249. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.Huch M, Bonfanti P, Boj SF, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 12.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 13.Bals R, Beisswenger C, Blouquit S, et al. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J Cyst Fibros. 2004;3(Suppl 2):49–51. doi: 10.1016/j.jcf.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Fatehullah A, Tan SH, Barker N, et al. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster MA, Knoblich JA, Lancaster MA, et al. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 17.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCracken KW, Cata EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Takasato M, Er PX, Becroft M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 21.Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calderon-Gierszal EL, Prins GS. Directed Differentiation of Human Embryonic Stem Cells into Prostate Organoids In Vitro and its Perturbation by Low-Dose Bisphenol A Exposure. PLoS One. 2015;10:e0133238. doi: 10.1371/journal.pone.0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mroue R, Bissell MJ. Three-dimensional cultures of mouse mammary epithelial cells. Methods Mol Biol. 2013;945:221–250. doi: 10.1007/978-1-62703-125-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 25.Yin X, Mead Benjamin E, Safaee H, et al. Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 27.Drost J, Clevers H. Translational applications of adult stem cell-derived organoids. Development. 2017;144:968–975. doi: 10.1242/dev.140566. [DOI] [PubMed] [Google Scholar]
- 28.Vazin T, Schaffer DV. Engineering strategies to emulate the stem cell niche. Trends Biotechnol. 2010;28:117–124. doi: 10.1016/j.tibtech.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell-niche engineering. J Clin Invest. 2010;120:60–70. doi: 10.1172/JCI41158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes CS, Postovit LM, Lajoie GA, et al. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 31.Sokol ES, Miller DH, Breggia A, et al. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18:19. doi: 10.1186/s13058-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raza A, Ki CS, Lin CC. The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D. Biomaterials. 2013;34:5117–5127. doi: 10.1016/j.biomaterials.2013.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 34.Perl A, Reinhoudt DN, Huskens J. Microcontact Printing: Limitations and Achievements. Advanced Materials. 2009;21:2257–2268. [Google Scholar]
- 35.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 37.Du Y, Lo E, Ali S, et al. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinander R, Anderson C, Morgenstern R. Identification of N-acetylcysteine as a new substrate for rat liver microsomal glutathione transferase. A study of thiol ligands. J Biol Chem. 1994;269:71–76. [PubMed] [Google Scholar]
- 39.Cao L, Kuratnik A, Xu W, et al. Development of intestinal organoids as tissue surrogates: cell composition and the epigenetic control of differentiation. Mol Carcinog. 2015;54:189–202. doi: 10.1002/mc.22089. [DOI] [PubMed] [Google Scholar]
- 40.Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Hubert CG, Rivera M, Spangler LC, et al. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016;76:2465–2477. doi: 10.1158/0008-5472.CAN-15-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelava I, Lancaster MA, Lancaster MA, et al. Stem Cell Models of Human Brain Development. Cell Stem Cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Ihrie RA, Shah JK, Harwell CC, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Van Haute L, De Block G, Liebaers I, et al. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guseh JS, Bores SA, Stanger BZ, et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaughan MB, Ramirez RD, Wright WE, et al. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima M, Kawanami O, Jin E, et al. Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int. 1998;48:944–953. doi: 10.1111/j.1440-1827.1998.tb03865.x. [DOI] [PubMed] [Google Scholar]
- 51.Green MD, Chen A, Nostro MC, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emerman JT, Pitelka DR, Mroue R, et al. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 53.Michalopoulos G, Sattler G, Sattler C, et al. Interaction of chemical carcinogens and drug-metabolizing enzymes in primary cultures of hepatic cells from the rat. Am J Pathol. 1976;85:755–772. [PMC free article] [PubMed] [Google Scholar]
- 54.Linnemann JR, Miura H, Meixner LK, et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development. 2015;142:3239–3251. doi: 10.1242/dev.123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duncan AW, Hickey RD, Paulk NK, et al. Ploidy reductions in murine fusion-derived hepatocytes. PLoS Genet. 2009;5:e1000385. doi: 10.1371/journal.pgen.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, Liu J, Liu Y, et al. Generation, characterization and potential therapeutic applications of mature and functional hepatocytes from stem cells. J Cell Physiol. 2013;228:298–305. doi: 10.1002/jcp.24150. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z, Sun M, Yuan Q, et al. Generation of functional hepatocytes from human spermatogonial stem cells. Oncotarget. 2016;7:8879–8895. doi: 10.18632/oncotarget.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayart E, Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther. 2013;13:73–92. doi: 10.2174/1566523211313020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hindley CJ, Cordero-Espinoza L, Huch M. Organoids from adult liver and pancreas: Stem cell biology and biomedical utility. Dev Biol. 2016;420(2):251–261. doi: 10.1016/j.ydbio.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 60.Broutier L, Andersson-Rolf A, Hindley CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11:1724–1743. doi: 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- 61.Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanteti R, Mirzapoiazova T, Riehm JJ, et al. Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma. Cancer Biol Ther. 2018:1–12. doi: 10.1080/15384047.2017.1416937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 64.Evans GS, Flint N, Somers AS, et al. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 65.Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 67.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 68.Jung P, Sato T, Merlos-Suarez A, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 69.Neal JT, Kuo CJ, Zhang HC, et al. Organoids as Models for Neoplastic Transformation. Annu Rev Pathol. 2016;11:199–220. doi: 10.1146/annurev-pathol-012615-044249. [DOI] [PubMed] [Google Scholar]
- 70.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Wiegerinck JI, Yntema C, Brouwer HJ, et al. Incidence of calcaneal apophysitis in the general population. Eur J Pediatr. 2014;173:677–679. doi: 10.1007/s00431-013-2219-9. [DOI] [PubMed] [Google Scholar]
- 72.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 73.Matano M, Date S, Shimokawa M, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 74.Kessler M, Hoffmann K, Brinkmann V, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989. doi: 10.1038/ncomms9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chua CW, Shibata M, Lei M, et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol. 2014;16:951–961. 951–954. doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pienta KJ, Abate-Shen C, Agus DB, et al. The current state of preclinical prostate cancer animal models. Prostate. 2008;68:629–639. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drost J, Karthaus WR, Gao D, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11:347–358. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skardal A, Devarasetty M, Rodman C, et al. Liver-Tumor Hybrid Organoids for Modeling Tumor Growth and Drug Response In Vitro. Annals of biomedical engineering. 2015;43:2361–2373. doi: 10.1007/s10439-015-1298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gouon-Evans V. The race for regeneration: Pluripotent-stem-cell-derived 3D kidney structures. Cell Stem Cell. 2014;14:5–6. doi: 10.1016/j.stem.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin Y, Bijvelds M, Dang W, et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral research. 2015;123:120–131. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Ogawa M, Ogawa S, Bear CE, et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:853–861. doi: 10.1038/nbt.3294. [DOI] [PubMed] [Google Scholar]
- 82.Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weeber F, van de Wetering M, Hoogstraat M, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A. 2015;112:13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh AJ, Cook RS, Sanders ME, et al. Drug response in organoids generated from frozen primary tumor tissues. Scientific reports. 2016;6:18889. doi: 10.1038/srep18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nantasanti S, de BA, Rothuizen J, et al. Concise Review: Organoids Are a Powerful Tool for the Study of Liver Disease and Personalized Treatment Design in Humans and Animals. Stem Cells Transl Med. 2016;5:325–330. doi: 10.5966/sctm.2015-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuratnik A, Giardina C. Intestinal organoids as tissue surrogates for toxicological and pharmacological studies. Biochemical pharmacology. 2013;85:1721–1726. doi: 10.1016/j.bcp.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 88.Fan Y, Tajima A, Goh SK, et al. Bioengineering Thymus Organoids to Restore Thymic Function and Induce Donor-Specific Immune Tolerance to Allografts. Molecular therapy: the journal of the American Society of Gene Therapy. 2015;23:1262–1277. doi: 10.1038/mt.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


