Abstract
Purpose
Both laboratory markers and radiographic findings in the setting of spinal infection can be nonspecific in determining the presence or absence of active infection, and can lag behind both clinical symptoms and antibiotic response. Magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI) has been shown to be helpful in evaluating brain abscesses but has not been commonly used in evaluating spinal infections. We aimed to correlate findings on DWI of the spine to results of microbiological sampling in patients with suspected spinal infection.
Methods
Patients who underwent MRI with DWI for suspicion of spine infection and microbiological sampling from 2002 to 2010 were identified and reviewed retrospectively in this IRB approved study. In addition to DWI, scans included sagittal and axial T1, fast-spin echo (FSE) T2, and post-gadolinium T1 with fat saturation. Regions of interest were drawn on apparent diffusion coefficient (ADC) maps in the area of suspected infection, and ADC values were correlated with microbiological sampling.
Results
Of 38 patients with suspected spine infection, 29 (76%) had positive microbiological sampling, and 9 (24%) had negative results. The median ADC value was 740 × 10−6 mm2/sec for patients with positive microbiological sampling and 1980 × 10−6 mm2/sec for patients with negative microbiological sampling (p < 0.001). Using an ADC value of 1250 × 10−6 mm2/sec or less as the cut-off value for a positive result for spine infection, sensitivity was 66%, specificity was 88%, positive predictive value was 95%, negative predictive value was 41% and accuracy was 70%.
Conclusion
In patients with suspected spine infection, ADC values on DWI are significantly reduced in those patients with positive microbiological sampling compared to patients with negative microbiological sampling. DWI of the spine correlates well with the presence or absence of spinal infection and may complement conventional MRI.
Keywords: Spinal infection, diffusion weighted imaging, MRI, radiology-pathology correlation
INTRODUCTION
Spine infections often present a diagnostic challenge due to non-specific symptoms such as fever, malaise, and back or neck pain[1]. MRI is the most sensitive imaging tool to detect early infection. However, MR findings of discitis/osteomyelitis often lag behind the clinical symptoms and also lag behind in the healing phase with persistent abnormalities[2, 3]. Findings on MRI suggestive of infection can be non-specific, especially in the post-operative spine where abscesses, hematomas, and seromas can have a similar appearance[4–6]. DWI has been useful in the diagnosis of cerebral infections where abscesses have been shown to have low ADC values[7–11], but is currently not commonly clinically used for the evaluation of spinal infections.
Previous studies have investigated the role of DWI in differentiating infectious vertebral body changes from malignant or degenerative changes[12–14], and one case report demonstrated a spinal abscess with qualitatively reduced diffusion[15]. Additionally, DWI has been used to guide patient management in the setting of spinal infection, such as in site selection for biopsy or decision-making of surgical drainage[16]. However, to our knowledge, no study has quantitatively validated the utility of DWI as a diagnostic test for spinal infections outside of the vertebral body, and no standards have been developed for ADC values representative of spinal infection. In this study, we correlated findings on DWI of the spine to results of microbiological or pathological sampling in patients with suspected spinal infection. The goals of this study were to assess the sensitivity and specificity of DWI in diagnosing spinal infections, and to identify an ADC threshold value that makes the diagnosis of infection more likely. We hypothesize that DWI in this context will complement conventional MRI in challenging cases to help identify or exclude active infection.
METHODS
Patients
In this cross-sectional study, the electronic medical records were retrospectively searched to identify all patients who underwent spine MRI with DWI for clinical suspicion of spine infection and had concurrent microbiological and/or pathological sampling from 2002 – 2010. Both inpatients and outpatients were included. Exclusion criteria included the presence of metallic implants that rendered DWI un-interpretable due to artifact or recent postoperative state (6 weeks or less) where the presence of blood products would potentially confound DWI. All patients clinically suspected of having spine infection who had both MRI with DWI and biopsy results were otherwise included in this study. Patient medical history including prior spinal surgery, pertinent concurrent laboratory tests (white blood cell count (WBC), elevated sedimentation rate (ESR), C-reactive protein (CRP)), and concurrent antibiotic therapy was also obtained from the medical records. The study was designed and carried out in accordance with the Standards for Reporting Diagnostic Accuracy Studies (STARD 2015[17]), and with approval of the institutional review board.
Imaging & Analysis
All MR imaging was performed at UCSF on 1.5 tesla MR units (Signa, GE Medical Systems, Milwaukee, WI, USA; Achieva, Philips, Amsterdam, Netherlands) and consisted of sagittal and axial T1 (TR 500–600 ms, TE 8–16 ms), sagittal and axial FSE T2 (TR 3000–4000 ms, TE 102–108 ms), and axial and sagittal post-gadolinium T1 (gadopentate dimeglumine (Magnevist, Schering) 0.1 mmol/L per kilogram of body weight) with fat saturation. Sagittal and/or axial DWI was acquired in three directions (single shot echo planar: TR 2 x pulse-pulse interval; TE 15 ms; FOV 22 cm; 256×144; 5.0-mm slice thickness with 0.5 mm gap; b-values 0 and 400 sec/mm2). Two board-certified neuroradiologists with combined 15 years experience in spine and diffusion imaging blinded to the clinical data retrospectively evaluated the conventional MR and diffusion-weighted images, in consensus. Regions of interest (ROI)[18] were drawn on the apparent diffusion coefficient (ADC) maps within areas of abnormal T2 signal and/or enhancement suspicious for infection and analyzed for mean ADC values using software available on the scanner. Spine infection can involve various structures including vertebral marrow, intervertebral discs, joints, epidural compartment, and paraspinal soft tissues. One or more of these spaces were involved in the patients included in this study. If the conventional MRI findings spanned multiple compartments of the spine, the area with the lowest ADC value was chosen for analysis, and an ROI was placed within the center of the abnormality. The ROI size depended on the size of the area involved, however the mean ROI size was 7 mm2 and ranged up to 13 mm2.
Biopsy & Specimen Analysis
Infection was defined by the presence of a pathogenic organism in microbiological and/or pathological samples, which were obtained at UCSF by using standard CT-guided techniques targeting the area of MR imaging abnormality as previously described[19], and targeted the area included in the ROI when possible. Samples were routinely processed for microbiological culture and/or pathology by the UCSF Departments of Pathology and Laboratory Medicine as clinically indicated.
Statistical Analysis
Using the results of microbiological and/or pathological testing as the clinical reference standard, a Receiver Operating Characteristic (ROC) curve was generated to evaluate the ability of DWI to diagnose spinal infection. From this curve, several empiric ADC values were chosen as cutoff values for a positive test. These empirically chosen values were based on prior studies evaluating the ADC values of brain abscess, which found that brain abscesses had mean ADC values of 1000 × 10−6 mm2/sec or less[7, 9–11]. Using these ADC values, the specificity, sensitivity, positive and negative predictive values, and diagnostic accuracy of DWI in diagnosing spinal infection were determined. Additionally, in those patients where pertinent corresponding laboratory values were available, the specificity, sensitivity, positive and negative predictive values, and diagnostic accuracy of abnormal laboratory values in diagnosing spinal infection were determined.
To assess whether ADC values in patients with microbiological and/or pathological sampling positive for infection were significantly different from patients with sampling negative for the presence of infection, the Mann-Whitney U test was used. A Chi-squared test was used to evaluate whether the presence or absence of concurrent antibiotic therapy correlated to the presence or absence of active infection on microbiological and/or pathological sampling. All statistical analysis was performed using GraphPad Prism (version 7.0 for Mac; GraphPad Software) (available at: http://www.graphpad.com). Two-sided p values of <0.01 were considered statistically significant.
RESULTS
Thirty-eight patients with clinically suspected spinal infection underwent MRI with DWI and microbiological sampling and/or pathological sampling between April 2002 and December 2010 (Table 1). All 38 patients had findings on standard MRI concerning for infection (areas of T1 hypointense and T2 hyperintense signal abnormality and/or enhancement in disc, vertebrae, and/or paraspinal soft tissue), and were read as suspicious for infection in the final MRI report.
Table 1.
Patient Characteristics
| Characteristic | All patients (n = 38) | |
|---|---|---|
| Gender | females | 15 (39.5%) |
| males | 23 (60.5%) | |
| Age (y) | median | 58.5 |
| range | 27–87 | |
| Antibiotics* | yes | 27 (71%) |
| no | 11 (29%) | |
| Abnormal labs** | yes | 22 (59%) |
| no | 15 (41%) | |
| Prior spine surgery | yes | 12 (32%) |
| no | 26 (68%) | |
| Immunocompromised | organ transplant | 13 (34%) |
| chronic steroids | 5 (13%) | |
| diabetes | 5 (13%) | |
| neutropenia | 1 (3%) | |
| Additional risk factors | IV drug abuse | 1 (3%) |
| malignancy | 6 (16%) | |
| renal failure | 3 (8%) | |
| Lyme disease | 1 (3%) |
At time of MRI
Elevated ESR, CRP and/or WBC
Six patients had findings concerning for osteomyelitis-discitis, 6 patients had epidural fluid collections, 2 patients had sacroiliac joint involvement, and 9 patients had paraspinal fluid collections. Additionally, 15 patients had involvement of multiple spinal compartments, which was most commonly osteomyelitis-discitis plus involvement of the epidural space and/or the paraspinal soft tissues.
Twenty-nine patients (76%) had positive results on microbiological sampling, and 9 patients (24%) had negative results. Among the 29 patients with positive microbiological results, 27 (93%) had pyogenic infections (17 Staphylococcus aureus, 4 Streptococcus spp., 1 Staphylococcus epidermidis, 1 Escherichia coli, 1 Enterococcus, 1 Pseudomonas, 1 gram-positive rod, 1 gram-positive cocci), 1 (4%) had infection with Mycobacterium tuberculosis and 1 (4%) had infection with Mucor. In the patients from whom Staphylococcus epidermidis, gram-positive cocci, and gram-positive rods were cultured, pathologic evaluation yielded osteomyelitis in each case.
Of the 9 patients with negative microbiological results, 5 (56%) were on antibiotics at the time of MRI and tissue sampling, and 4 were treated in full despite negative cultures. Eight of these 9 patients had concurrent pathology performed in addition to microbiology, and none revealed pathologic evidence of infection in addition to negative microbiological results. None of the 9 patients experienced worsening of clinical symptoms or radiographic findings.
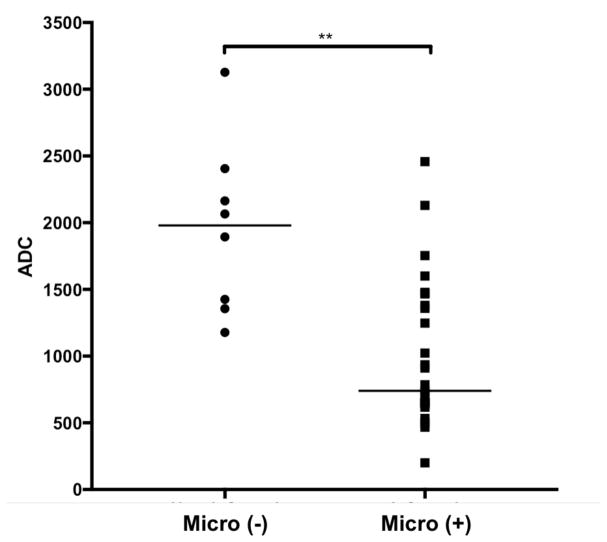
Figures 1 and 2 show examples of DWI in patients with positive and negative microbiological sampling, respectively. The median ADC value was 740 × 10−6 mm2/sec for patients with positive microbiological sampling and 1980 × 10−6 mm2/sec for patients with negative microbiological sampling; the distributions in the two groups differed with high statistical significance (Mann–Whitney U = 32, n1 = 29, n2 = 9, two-tailed p < 0.001). Figure 3 shows the distribution of ADC values in both patient groups. In the 29 patients with positive microbiological sampling, the locations of imaging abnormalities can be broken down as follows: 13 (45%) patients had transcompartmental abnormalities involving the vertebral body and intervertebral disc as well as the epidural and/or paraspinal soft tissues, 6 (21%) patients had infections confined to the vertebral body and intervertebral disc, 6 (21%) patients had predominant involvement of the paraspinal soft tissues, and 4 (14%) had predominant involvement of the epidural space.
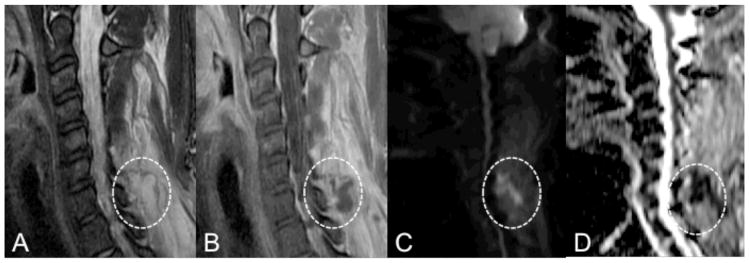
Figure 1.
MRI with DWI of the cervical spine in a 30-year-old female with a history of IV drug use who presented with neck pain and fevers. Sagittal T2 and post-gadolinium T1-weighted MR images (A, B) demonstrate diffuse inflammatory changes of the posterior paraspinal muscles with a focal rim enhancing fluid collection (circles) at the level of C7. DWI (C) demonstrates high intensity within the collection (circle) with corresponding low ADC (D, circle) concerning for abscess. The ADC value within the fluid collection measures 800 × 10−6 mm2/sec. Pathological examination of the fluid collection was consistent with abscess, and microbiology yielded Staphylococcus aureus.
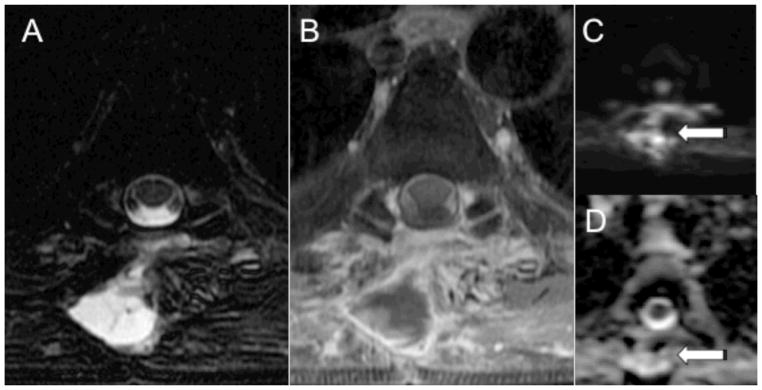
Figure 2.
MRI with DWI of the thoracic spine in a 65-year-old male with history of liver and renal transplant who presented with back pain after removal of thoracic masses. Axial T2 and post-gadolinium T1-weighted MR images (A, B) demonstrate a rim enhancing fluid collection in the paraspinal tissues at T5. DWI (C) demonstrates high intensity within the paraspinal collection (arrow) with corresponding high ADC (D, arrow). The ADC value measures 3268 × 10−6 mm2/sec. Microbiology of the fluid collection was negative.
Figure 3.
Distribution of ADC values in patients with positive microbiological sampling (Micro +) and in patients with negative microbiological sampling (Micro -) (two-tailed p < 0.001).
Using an ADC value of 1250 × 10−6 mm2/sec or less as the cut-off value for a positive result for spine infection, sensitivity was 66%, specificity was 88%, positive predictive value was 95%, negative predictive value was 41% and accuracy was 70%. Table 2 shows the specificity, sensitivity, accuracy, and positive and negative predictive values of DWI in diagnosing spinal infection using different empirically chosen ADC values as cutoff values for a positive test result. The area under the ROC curve was 0.862. In comparison, the calculated sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy for having at least one abnormal laboratory value suggestive of infection (elevated WBC, elevated ESR, elevated CRP) in our cohort of patients were 62%, 50%, 82%, 27% and 59%, respectively. There was no correlation between the detection of infection on pathology and/or microbiology and the presence or absence of concurrent antibiotic therapy at the time of sampling (Chi squared test = 1.377, p = 0.24061).
Table 2.
Performance of DWI for diagnosing spinal infection using different maximum ADC (mm2/sec) values as cutoffs for a positive test.
| ADC | Sensitivity | Specificity | Accuracy | PPV* | NPV** |
|---|---|---|---|---|---|
| 1000 | 62% | 100% | 70% | 100% | 42% |
| 1250 | 66% | 88% | 70% | 95% | 41% |
| 1600 | 86% | 63% | 81% | 89% | 56% |
| 2130 | 93% | 38% | 81% | 84% | 60% |
| 2460 | 97% | 13% | 78% | 80% | 50% |
PPV: positive predictive value
NPV: negative predictive value
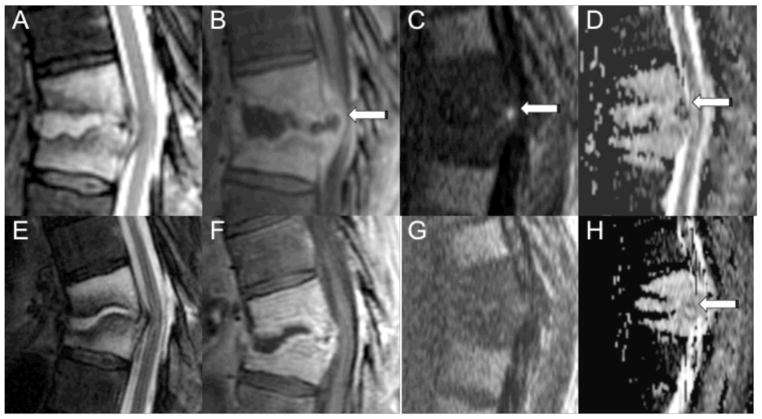
Two of the patients with positive microbiological sampling underwent follow-up spine MRI with DWI after antibiotic treatment. Both patients had epidural abscesses that initially were reduced in diffusion (ADC values of 200 × 10−6 mm2/sec and 468 × 10−6 mm2/sec). Follow-up DWI after antibiotic treatment demonstrated normalization of diffusion findings (ADC values of 1556 × 10−6 mm2/sec after 3 weeks of therapy and 4021 × 10−6 mm2/sec after 6 weeks of therapy, respectively). Figure 4 demonstrates the change in DWI in one of these patients. Three patients were unable to receive gadolinium-based contrast due to renal insufficiency. Figure 5 demonstrates an example from one of these patients, where an epidural fluid collection with corresponding low ADC values yielded Staphylococcus Aureus on percutaneous sampling.
Figure 4.
MRI with DWI of the thoracic spine in a 64-year-old male with a history of thoracic radiation for esophageal cancer who presented with back pain before (A–D) and after four weeks of antibiotic therapy (E–H). Sagittal MR images demonstrate abnormal T2 hyperintensity (A, E) and enhancement (B, F) involving the T9 and T10 vertebral bodies with a rim enhancing fluid collection involving the T9-T10 disc extending into the epidural space (arrow). Before antibiotic therapy, DWI (C) shows focal hyperintensity within the ventral epidural space with associated low ADC (D) of 200 × 10−6 mm2/sec. Microbiology of the epidural fluid collection yielded Viridans group streptococci. DWI (G) after four weeks of antibiotic therapy shows resolution of the focal hyperintensity, and the ADC (H) is now relatively elevated (1556 × 10−6 mm2/sec).
Figure 5.
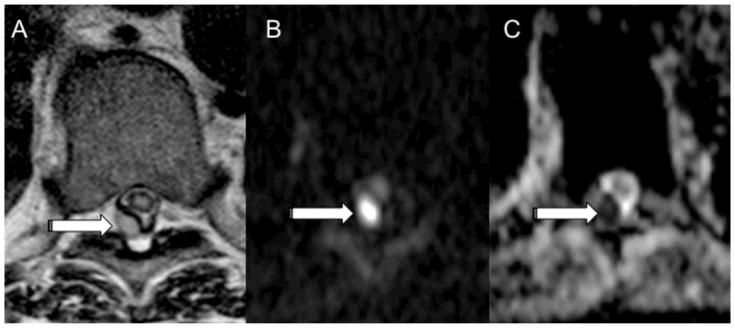
MRI with DWI of the thoracic spine in a 57-year-old male with a history of renal failure who presented with back pain and fevers. Axial MR images demonstrate a T2 hyperintense (A) posterior epidural fluid collection at T8-T9 (arrow). The patient was unable to receive gadolinium due to renal failure. DWI (B) demonstrates focal high intensity in the posterior epidural space at T8-T9 (arrow) with corresponding low ADC (C, arrow) concerning for an epidural abscess. The ADC value in the area of epidural reduced diffusion measured 648 × 10−6 mm2/sec. Microbiology of the epidural fluid collection yielded Staph aureus.
DISCUSSION
MRI is the preferred diagnostic modality for detection of spinal infections. However, MRI findings in pyogenic and atypical infections may be relatively non-specific, especially in the post-operative spine, and can lag behind the clinical symptoms during the initial phase and lag behind during the healing phase[20, 21]. Ill-defined T1 hypointense and T2 hyperintense signal within the vertebral marrow and intervertebral disc space are compatible with inflammatory exudates or edema along with loss of end-plate definition. Loss of disc space height is a characteristic but late finding. Post-contrast sequences show enhancement of the disc and adjacent end-plates. A conclusive diagnosis often requires invasive biopsy and culture[4–6]. Definitive diagnosis is imperative for optimal clinical management, to avoid subjecting patients to unnecessary long-term antibiotics and to avoid contributing to bacterial antibiotic resistance.
DWI has been useful in distinguishing brain abscesses from necrotic neoplasms[8, 7, 10, 9], but has not been commonly used in the evaluation of spinal infection due to previous technical limitations of DWI of the spine related to the small size of the spine and involuntary motion from CSF pulsations that require much more optimization of these DWI pulse sequences when compared to DWI of the brain[22]. DWI is based on the random movement of water molecules within tissues. The calculated ADC relates the average displacement of a molecule over an area to the observation time, with higher values indicating more mobile water. In abscesses, the high cellularity and viscosity of pus cause impeded water mobility and thus low ADC values and reduced diffusion[23]. With an average acquisition time of 3 to 4 minutes, the addition of a DWI sequence will not significantly lengthen a standard spine MR protocol.
Our study correlated the findings on DWI of the spine with the results of microbiological sampling in patients with suspected spinal infection and found a statistically significant difference in ADC values between patients with positive and negative sampling results, with median ADC values lower in the patients with positive sampling across a variety of different pathogenic bacterial and fungal organisms. Prior studies evaluating the ADC values of brain abscesses found that brain abscesses had mean ADC values less than 1000 × 10−6 mm2/sec[10, 11, 7, 9]. When applied to this study, an ADC value was highly specific for spine infection, however the sensitivity was only 62%. While we do not propose a strict cut off value for which infection can be entirely ruled in or excluded based on our data, an ADC value of less than 1250 yielded a positive predictive value of 95% in our cohort with additional parameters that are more reliable than laboratory values alone.
Previous studies have investigated the role of DWI in differentiating infectious vertebral body bone marrow changes from malignant or degenerative changes[12, 14, 13]. For example, Balliu et al evaluated DWI in 16 patients with osteoporotic compression fracture, 15 patients with neoplastic endplate bone marrow infiltration, and 14 patients with infectious endplate bone marrow infiltration and found high mean ADC values for the acute osteoporotic fractures, and lower mean ADC values for the infectious lesions, but no statistically significant difference in mean ADC values between malignant and infectious lesions[13]. These studies did not evaluate spinal infection outside of the bone marrow.
Eastwood et al described a case of spinal epidural abscess with qualitatively reduced diffusion[15], and in a review on DWI of the spine, Thurnher and Bammer described an unpublished series of patients with spinal infections in whom all epidural abscesses also demonstrated qualitatively reduced diffusion with corresponding low ADC values[22]. Although these reports support the use of DWI in evaluating spinal infection, particularly epidural abscess, they have not quantitatively validated the utility of DWI as a diagnostic test for spinal infections, and no standards have been developed for ADC values representative of spinal infection. Our sample size of 38 patients, while limited, is clinically relevant as the largest available study to date evaluating DWI data with pathologic sampling as a gold standard in patients with suspected spinal infection. Our study confirmed the utility of DWI in helping to diagnose spinal infection, and suggests that an ADC value of less than 1250 × 10−6 mm2/sec may be concerning for spine infection. In cases where gadolinium-based contrast cannot be administered due to underlying renal insufficiency, diffusion weighted images may be especially helpful in discriminating between infected and non-infected spinal fluid collections.
In addition, in the two patients who underwent follow-up DWI after long-term antibiotic therapy, we noted improvement and near normalization of DWI changes and ADC values on the follow-up DWI despite persistent changes on the conventional MRI sequences. This may be of greatest clinical utility – the ability to confirm adequacy of medical treatment in the presence of ongoing imaging abnormalities. Additional prospective studies with DWI before, during, and after antibiotic treatment are warranted to further evaluate the role of DWI in following response to antibiotic treatment in spine infections.
Limitations of this study include small sample size, a heterogeneous patient cohort including a variety of spinal infections, and a skewed population with high positive predictive values. Due to the retrospective nature of the study, the MR imaging protocols were not standardized. In addition, many of the patients were already on antibiotics at the time of MRI and DWI, which may affect the sensitivity of DWI to infection as the timeline and correlation of ADC values and antibiotic therapy is not established. However, statistical analysis of our population demonstrated that, in spite of antibiotic use, there was no correlation between the detection of infection on pathology and/or microbiology and the presence or absence of concurrent antibiotic therapy at the time of sampling. The use of microbiological sampling as the clinical reference standard also has limitations as sampling errors and indolent infections may yield negative cultures. However, no other reference standard for spinal infection currently exists.
CONCLUSION
Spine infection is difficult to diagnose. MR findings often lag behind the clinical symptoms and lag behind during the healing phase resulting in uncertainty in initiating treatment as well as knowing when treatment has been adequate and can therefore be stopped. DWI has been useful in the evaluation of cerebral infections, but is not commonly used in diagnosing spine infection. The current study, which is the largest available to the best of the authors’ knowledge, has quantitatively validated the utility of DWI as a diagnostic test for spinal infections both inside and outside of the vertebral body. Additionally, this study has suggested standards for ADC values representative of spinal infection, which have not been previously documented in the literature. This study found that in patients with suspected spine infection, ADC values on DWI are significantly lower in patients with positive microbiological sampling than in patients with negative microbiological sampling. An ADC value of less than 1250 × 10−6 mm2/sec may be concerning for spine infection. ADC values can normalize during effective treatment of spine infection. DWI of the spine correlates well with the presence or absence of spinal infection by microbiological sampling and may also be a useful marker for response to antibiotic therapy, complementing conventional MRI when the clinical picture is equivocal.
Footnotes
DISCLOSURES
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Schofferman L, Schofferman J, Zucherman J, Gunthorpe H, Hsu K, Picetti G, et al. Occult infections causing persistent low-back pain. Spine (Phila Pa 1976) 1989;14(4):417–9. doi: 10.1097/00007632-198904000-00014. [DOI] [PubMed] [Google Scholar]
- 2.DeSanto J, Ross JS. Spine infection/inflammation. Radiologic clinics of North America. 2011;49(1):105–27. doi: 10.1016/j.rcl.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 3.James SL, Davies AM. Imaging of infectious spinal disorders in children and adults. European journal of radiology. 2006;58(1):27–40. doi: 10.1016/j.ejrad.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Hong SH, Choi JY, Lee JW, Kim NR, Choi JA, Kang HS. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009;29(2):599–612. doi: 10.1148/rg.292085137. 29/2/599 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Tins BJ, Cassar-Pullicino VN. MR imaging of spinal infection. Semin Musculoskelet Radiol. 2004;8(3):215–29. doi: 10.1055/s-2004-835362. [DOI] [PubMed] [Google Scholar]
- 6.Gillams AR, Chaddha B, Carter AP. MR appearances of the temporal evolution and resolution of infectious spondylitis. AJR Am J Roentgenol. 1996;166(4):903–7. doi: 10.2214/ajr.166.4.8610571. [DOI] [PubMed] [Google Scholar]
- 7.Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, et al. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14(9):1113–6. doi: 10.1016/s0730-725x(96)00237-8. S0730-725X(96)00237-8 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Chang KH, Song IC, Kim HD, Seong SO, Kim YH, et al. Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol. 1998;171(6):1487–90. doi: 10.2214/ajr.171.6.9843275. [DOI] [PubMed] [Google Scholar]
- 9.Han KT, Choi DS, Ryoo JW, Cho JM, Jeon KN, Bae KS, et al. Diffusion-weighted MR imaging of pyogenic intraventricular empyema. Neuroradiology. 2007;49(10):813–8. doi: 10.1007/s00234-007-0264-7. [DOI] [PubMed] [Google Scholar]
- 10.Chang SC, Lai PH, Chen WL, Weng HH, Ho JT, Wang JS, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging. 2002;26(4):227–36. doi: 10.1016/s0899-7071(02)00436-9. S0899707102004369 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Lai PH, Ho JT, Chen WL, Hsu SS, Wang JS, Pan HB, et al. Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol. 2002;23(8):1369–77. [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi Y, Ohtori S, Yamashita M, Yamauchi K, Suzuki M, Orita S, et al. Diffusion magnetic resonance imaging to differentiate degenerative from infectious endplate abnormalities in the lumbar spine. Spine (Phila Pa 1976) 2011;36(3):E198–202. doi: 10.1097/BRS.0b013e3181d5ff05. [DOI] [PubMed] [Google Scholar]
- 13.Balliu E, Vilanova JC, Pelaez I, Puig J, Remollo S, Barcelo C, et al. Diagnostic value of apparent diffusion coefficients to differentiate benign from malignant vertebral bone marrow lesions. European journal of radiology. 2009;69(3):560–6. doi: 10.1016/j.ejrad.2007.11.037. S0720-048X(07)00596-7 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Pui MH, Mitha A, Rae WI, Corr P. Diffusion-weighted magnetic resonance imaging of spinal infection and malignancy. J Neuroimaging. 2005;15(2):164–70. doi: 10.1177/1051228404274306. 15/2/164 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Eastwood JD, Vollmer RT, Provenzale JM. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol. 2002;23(3):496–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Moritani T, Kim J, Capizzano AA, Kirby P, Kademian J, Sato Y. Pyogenic and non-pyogenic spinal infections: emphasis on diffusion-weighted imaging for the detection of abscesses and pus collections. The British journal of radiology. 2014;87(1041):20140011. doi: 10.1259/bjr.20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology. 2015;277(3):826–32. doi: 10.1148/radiol.2015151516. [DOI] [PubMed] [Google Scholar]
- 18.Tolvanen T, Yli-Kerttula T, Ujula T, Autio A, Lehikoinen P, Minn H, et al. Biodistribution and radiation dosimetry of [(11)C]choline: a comparison between rat and human data. European journal of nuclear medicine and molecular imaging. 2010;37(5):874–83. doi: 10.1007/s00259-009-1346-z. [DOI] [PubMed] [Google Scholar]
- 19.Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218(1):211–4. doi: 10.1148/radiology.218.1.r01ja06211. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;43(2):172–9. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski TJ, Layton KF, Berbari EF, Steckelberg JM, Huddleston PM, Wald JT, et al. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. AJNR Am J Neuroradiol. 2007;28(4):693–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Semin Roentgenol. 2006;41(4):294–311. doi: 10.1053/j.ro.2006.07.003. S0037-198X(06)00048-4 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008;29(4):632–41. doi: 10.3174/ajnr.A1051. ajnr.A1051 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]