Abstract
OBJECTIVE:
The objective of this study was to characterize morbidity, acuity, and maternal risks associated with preeclampsia across hospitals with varying obstetric volumes.
METHODS:
This retrospective cohort analysis used a large administrative data source, the Perspective database, to characterize the risk for preeclampsia from 2006 to 2015. Hospitals were classified as having either low (≤1,000), moderate (1,001–2,000) or high (≥2,000) delivery volume. The primary outcomes included preeclampsia, antihypertensive administration, comorbidity, and related severe maternal morbidity. Severe maternal morbidity was estimated using criteria from the Centers for Disease Control and Prevention. Comorbidity was estimated using an obstetric comorbidity index. Univariable comparisons were made with chi-squared test. Adjusted log linear regression models were fit to assess factors associated with severe morbidity with risk ratios with 95% confidence intervals as the measures of effect. Population weights were applied to create national estimates.
RESULTS:
Of 36,985,729 deliveries included, 1,414,484 (3.8%) had a diagnosis of preeclampsia. Of these, 779,511 (2.1%) had mild, 171,109 (0.5%) superimposed, and 463,864 (1.3%) severe preeclampsia. The prevalence of mild, superimposed and severe preeclampsia each increased over the study period with severe and superimposed preeclampsia as opposed to mild preeclampsia increasing the most proportionately (53.2% and 102.5% versus 10.8%, respectively). Use of anti-hypertensives used to treat severe range hypertension increased with use of intravenous labetalol increasing 31.5%, 43.2% and 36.1% at low, medium, and high volume hospitals. Comorbid risk also increased across hospital volume settings as did risk for severe maternal morbidity.
CONCLUSIONS:
Preeclampsia is increasing across obstetric care settings with preeclamptic patients demonstrating increasing comorbid risk, increased risk for severe morbidity, and more frequent need for treatment of acute hypertension.
Keywords: preeclampsia management, obstetric volume, maternal morbidity
INTRODUCTION
Preeclampsia is a leading cause of severe maternal morbidity and mortality in the United States,1,2 and the incidence of preeclampsia appears to be rising.3 Maternal mortality reviews have demonstrated that many maternal deaths attributable to preeclampsia are preventable.4,5 Major safety and quality improvement initiatives, such as the Severe Hypertension Bundle from the National Partnership for Maternal Safety, have been developed to reduce maternal risk by improving preparedness for, recognition of, and responses to severe hypertension.6
Understanding how hospital delivery volume relates to preeclampsia and related risk for severe morbidity may be important in improving patient care and reducing risk. If preeclampsia-related morbidity were found to be increasing across hospital volume settings, this would support the need for quality improvement initiatives focused on this issue at all hospitals. Efforts to improve safety culture in diagnosing and managing hypertension could lead to meaningfully improved outcomes. Specifically, knowing how risk and outcomes have changed differentially based on hospital volume would add to knowledge on obstetric safety and support uptake of recommended interventions.
Given that (i) preeclampsia risk may vary based on hospital volume, and (ii) it is unclear to what degree outcomes vary across hospitals with different delivery volumes, the purpose of this study was to characterize preeclampsia severity and outcomes across obstetric volume settings in the US.
METHODS
The Perspective database was used for this analysis. Perspective is maintained by Premier Incorporated (Charlotte, NC) and includes patient demographics, hospital characteristics, and discharge diagnosis codes, as well as medications and devices administered during acute care hospitalizations for both teaching and non-teaching hospitals. Within Perspective, 100% of hospitalizations for individual hospitals are reported. Ninety five quality assurance and validation checks are performed on data each year prior to being released.7 Perspective is routinely used for research on trends on medications and device use during delivery hospitalizations. This database was created for national quality and utilization benchmarking and includes approximately 15% of discharges from non-federal institutions in the US; data is contributed voluntarily with the number of hospitals included varying temporally.8–14 The Columbia University Institutional Review Board deemed the study exempt given that all data are deidentified.
Women 15 to 54 years of age were included in this analysis if they were admitted for a delivery hospitalization with an associated preeclampsia diagnosis from January 2006 through March 2015. Patients with preeclampsia were identified based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and subcategorized as mild, severe, and superimposed (ICD-9-CM codes 642.4x, 642.5x, and 642.7x, respectively). Prior analyses have shown ICD-9-CM codes for preeclampsia to have moderate sensitivity.15–17 Delivery hospitalizations were identified based on billing codes (ICD-9-CM 650 and V27.x) that ascertain more than 95% of deliveries.18
For each hospital, we calculated the number of delivery hospitalizations and divided this by years in which a hospital had at least one delivery. Hospitals were categorized as low (≤1000 deliveries per year), medium (1001 to 2000 deliveries per year), or high volume (>2000 deliveries per year). Prior analyses have used varying obstetric volume cutoffs;5,19,20 the volume categories chosen for use in this analysis represent easily interpretable and clinically meaningful distinctions in obstetric volume.
We compared demographic and hospital characteristics for women delivery at low, medium and high volume centers. Hospital characteristics included location (urban versus rural), teaching status (teaching versus nonteaching), geographic region (Midwest, Northeast, South, West), and hospital size based on the number of beds (fewer than 400, 400 to 600, or greater than 600 beds). Demographic characteristics included maternal age at delivery (<20, 20–24, 25–29, 30–34, or ≥35 years), maternal race (white, black, Hispanic, other), marital status (married, single, unknown), year of delivery (2006 to 2015), and insurance status (commercial, Medicare, Medicaid, uninsured, and unknown).
Among women diagnosed with preeclampsia, we analyzed measures of acuity, risk, and adverse outcomes. First, we evaluated temporal trends by hospital volume for mild, severe, and superimposed preeclampsia. Second, as a measure of acuity of care, we evaluated temporal trends in the administration of antihypertensive medications to preeclamptic women by hospital volume. We analyzed the use of (i) any the three following first-line agents used to treat severe range hypertension:6,21 intravenous labetalol, intravenous hydralazine, oral nifedipine, and (ii) intravenous labetalol alone. Third, we evaluated temporal trends in risk for severe morbidity for women diagnosed with preeclampsia by hospital volume. Severe morbidity was measured based on criteria from the Centers for Disease Control and Prevention (CDC). The CDC definition of severe maternal morbidity includes 21 diagnoses including shock, stroke, heart failure, transfusion, and other conditions all identified using ICD codes.22 Additionally, because the most common diagnosis in the severe morbidity composite is transfusion (ICD-9-CM 99.0x) a sensitivity analysis was performed excluding transfusion and restricted to the remaining 20 conditions representative of non-transfusion severe morbidity.
Fourth, we evaluated temporal trends in comorbid risk by hospital volume categories for women with preeclampsia as measured by an obstetric comorbidity index.23 This comorbidity index provides weighted scores for comorbidity for individual patients based on the presence of specific diagnosis codes and demographic factors present in administrative data. Higher scores are associated with increased risk for severe morbidity. In the initial study validating the comorbidity index, patients with the lowest score of 0 had a 0.68% risk of severe morbidity whereas a score of >10 was associated with a risk of severe morbidity of 10.9%.23 This comorbidity index was subsequently validated in an external population.24 Because the comorbidity index includes preeclampsia, we modified this scoring system excluding these preeclampsiadiagnoses for the present analysis.
Demographic comparisons and temporal trends were evaluated using the chi-square test. Adjusted risk ratios (RR) for severe morbidity with 95% confidence interval (CI) as the measure of effect accounting for demographic, hospital, and preeclampsia diagnosis as well as obstetric volume were derived from fitting marginal log-linear models based on the method of generalized estimating equations that accounts for the effect of patients clustered within hospitals. We hypothesized that all of the major demographic and hospital factors were of potential significance and included them in the multivariable model. Specific preeclampsia diagnosis (mild, severe, or superimposed) was included in the model. Sampling weights, provided in the Perspective data, were applied to the 5,683,559 deliveries in Perspective based on the study inclusion criteria in this analysis for all outcomes to create national estimates. Weights in Perspective were derived from the 1998 American Hospital Association Annual Survey and validated by the 1998 National Hospital Discharge Survey and have been used in outcomes analyses across a number of medical and surgical specialties.25–28 All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 36,985,729 weighted delivery hospitalizations were included in the analysis. Of these, 1,414,484 (3.8%) had an associated diagnosis of preeclampsia including 779,511 (2.1%) hospitalizations complicated by mild preeclampsia, 463,864 (1.3%) by severe preeclampsia, and 171,109 (0.5%) by superimposed preeclampsia. Demographics and hospital characteristics were compared for patients at low, medium and high volume centers (Table 1). Severe preeclampsia was more common at high and medium volume hospitals than low volume hospitals (1.4%, 1.2%, versus 0.8%, respectively, P<0.01). A higher proportion of deliveries were to white women at low volume hospitals compared to moderate and high-volume hospitals (63.1% versus 53.8% versus 50.7%, P<0.01). Low-volume hospitals accounted for a larger proportion of deliveries in the Midwest than the Northeast, South and West (34.5%, versus 13.1%, 32.9%, and 19.6%, respectively, p<0.01).
Table 1.
Patient demographics
| Hospital volume | Low | Medium | High volume | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| All patients | 6,060,219 | 9,848,592 | 21,076,918 | |||
| Preeclampsia | ||||||
| Mild | 124,240 | 2.1% | 217,170 | 2.2% | 438,101 | 2.1% |
| Superimposed | 18,480 | 0.3% | 43,530 | 0.4% | 109,099 | 0.5% |
| Severe | 50,550 | 0.8% | 118,010 | 1.2% | 295,304 | 1.4% |
| None | 5,866,949 | 96.8% | 9,469,882 | 96.2% | 20,234,414 | 96.0% |
| Year | ||||||
| 2006 | 719,378 | 11.9% | 1,061,284 | 10.8% | 2,450,516 | 11.6% |
| 2007 | 703,628 | 11.6% | 1,059,683 | 10.8% | 2,576,821 | 12.2% |
| 2008 | 651,816 | 10.8% | 1,081,4561, | 11.0% | 2,494,216 | 11.8% |
| 2009 | 621,680 | 10.3% | 1,101,984 | 11.2% | 2,326,592 | 11.0% |
| 2010 | 617,865 | 10.2% | 1,074,237 | 10.9% | 2,214,579 | 10.5% |
| 2011 | 623,445 | 10.3% | 1,105,997 | 11.2% | 2,169,245 | 10.3% |
| 2012 | 667,687 | 11.0% | 1,074,698 | 10.9% | 2,161,441 | 10.3% |
| 2013 | 651,643 | 10.8% | 1,084,139 | 11.0% | 2,157,556 | 10.2% |
| 2014 | 647,377 | 10.7% | 989,393 | 10.0% | 2,079,409 | 9.9% |
| 2015 (1Q) | 155,700 | 2.6% | 215,721 | 2.2% | 447,543 | 2.1% |
| Hospital bed size | ||||||
| <400 | 5,644,095 | 93.1% | 7,322,780 | 74.4% | 10,535,882 | 50.0% |
| 400–600 | 416,002 | 6.9% | 2,268,087 | 23.0% | 5,927,645 | 28.1% |
| >600 | 121 | 0.0% | 257,724 | 2.6% | 4,613391 | 21.9% |
| Age, years | ||||||
| 15–17 | 183,901 | 3.0% | 269,097 | 2.7% | 509,939 | 2.4% |
| 18–24 | 2,065,989 | 34.1% | 2,988,940 | 30.3% | 5,677,785 | 26.9% |
| 25–34 | 3,092,836 | 51.0% | 5,182,145 | 52.6% | 11,502,097 | 54.6% |
| ≥35 | 717,492 | 11.8% | 1,408,410 | 14.3% | 3,387,097 | 16.1% |
| Insurance status | ||||||
| Medicare | 59,929 | 1.0% | 56,924 | 0.6% | 151,609 | 0.7% |
| Medicaid | 2,933,981 | 48.4% | 5,067,371 | 51.5% | 8,302,961 | 39.4% |
| Private | 2,758,532 | 45.5% | 4,118,637 | 41.8% | 11,502,097 | 54.6% |
| Uninsured | 134,710 | 2.2% | 273,587 | 2.8% | 483,347 | 2.3% |
| Other | 173,068 | 2.9% | 332,073 | 3.4% | 692,990 | 3.3% |
| Race | ||||||
| White | 3,824,774 | 63.1% | 5,296,931 | 53.8% | 10,691,383 | 50.7% |
| Black | 498,111 | 8.2% | 1,550,501 | 15.7% | 2,589,118 | 12.3% |
| Other | 1,731,701 | 28.6% | 2,994,685 | 30.4% | 7,788,574 | 37.0% |
| Unknown | 5,632 | 0.1% | 6,475 | 0.1% | 7,843 | 0.0% |
| Hospital Location | ||||||
| Rural | 804,267 | 13.3% | 394,254 | 4.0% | 173,352 | 0.8% |
| Urban | 5,255,951 | 86.7% | 9,454,339 | 96.0% | 20,903,566 | 99.2% |
| Marital Status | ||||||
| Married | 2,907,368 | 48.0% | 4,530,468 | 46.0% | 13,802,083 | 65.5% |
| Unmarried | 2,455,919 | 40.5% | 3,795,766 | 38.5% | 7,274,835 | 34.5% |
| Unknown | 696,932 | 11.5% | 1,522,358 | 15.5% | 2,585,985 | 12.3% |
| Hospital Region | ||||||
| Northeast | 795,333 | 13.1% | 1,850,894 | 18.8% | 3,167,228 | 15.0% |
| Midwest | 2,087,936 | 34.5% | 2,687,089 | 27.3% | 3,206,611 | 15.2% |
| South | 1,991,239 | 32.9% | 3,932,455 | 39.9% | 8,485,621 | 40.3% |
| West | 1,185,710 | 19.6% | 1,378,154 | 14.0% | 6,218,457 | 29.5% |
| Teaching | ||||||
| Non-teaching | 5,675,807 | 93.7% | 8,160,304 | 82.9% | 15,823,813 | 75.1% |
| Teaching | 384,411 | 6.3% | 1,688,288 | 17.1% | 5,253,105 | 24.9% |
Hospitals were defined as low volume (<1000 deliveries per year), medium volume (1000 to 2000 deliveries per year), and high volume (>2000 deliveries per year). All comparisons by demographic variable by volume category were statistically significant (p<0.01). All data presented are weighted estimates.
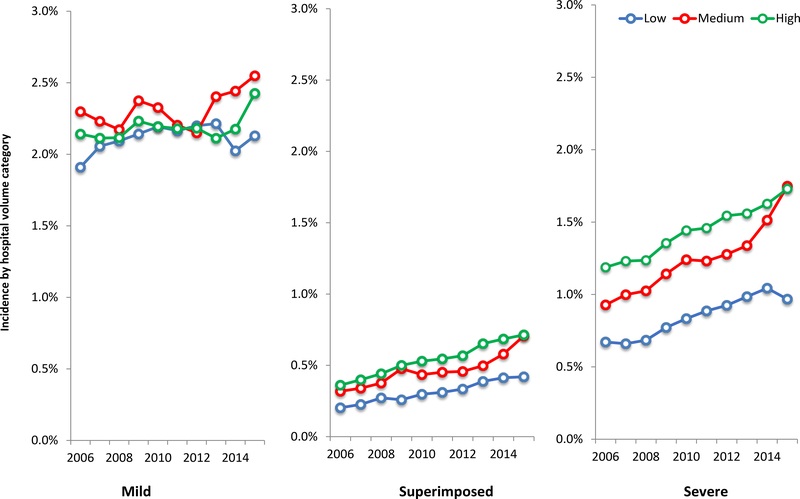
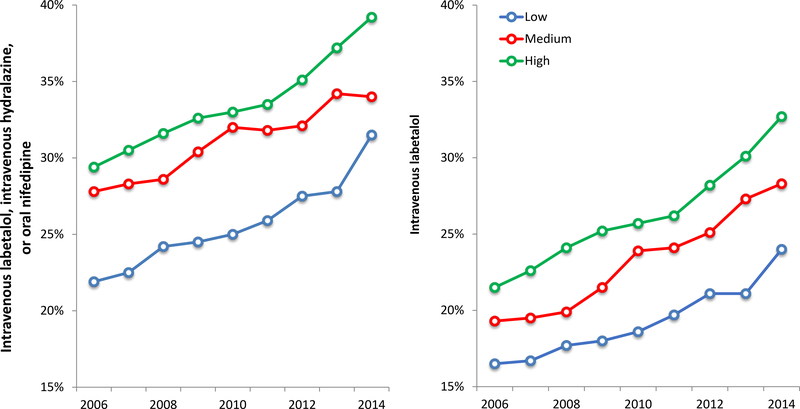
Over the course of the study period, the risk for mild, severe, and superimposed preeclampsia all increased across low, medium, and high-volume hospitals (Figure 1). From 2006 to 2015, mild preeclampsia increased by 11.5%, 10.9% and 13.2%, severe preeclampsia increased by 44.0%, 88.4%, and 45.7%, and superimposed preeclampsia increased by 107.1%, 122.2%, and 97.8%, at low, medium, and high-volume hospitals, respectively. Over this study period, the proportion of preeclamptic patients receiving antihypertensive medications also increased. Receipt of intravenous labetalol increased from 16.5% to 23.4% of deliveries at low volume hospitals, 19.3% to 32.1% at medium volume hospitals, and 21.5% to 33.2% at high volume hospitals (p<0.01 for all). Rates of use of any first-line medication to treat severe hypertension (intravenous labetalol, intravenous hydralazine, or oral nifedipine) similarly increased (Figure 2).
Figure 1. Mild, superimposed and severe preeclampsia incidence by hospital volume.
Legend: The figure demonstrates incidence of mild, superimposed and severe preeclampsia for delivery hospitalizations by year at low (≤1000 deliveries per year), medium (1001 to 2000 deliveries per year), and high volume (>2000 deliveries per year) hospitals.
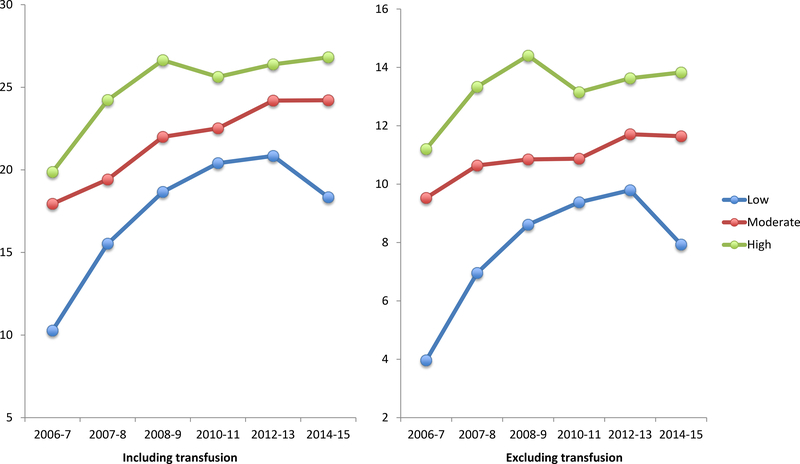
Figure 2. Severe morbidity associated with preeclampsia per 10,000 deliveries at low, medium, and high volume hospitals.
Legend: The figure demonstrates severe morbidity risk based on CDC criteria associated with preeclampsia per 10,000 delivery hospitalizations by year at low (≤1000 deliveries per year), medium (1001 to 2000 deliveries per year), and high volume (>2000 deliveries per year) hospitals. Transfusion is included as a measure of severe morbidity in the left figure and excluded in the right figure.
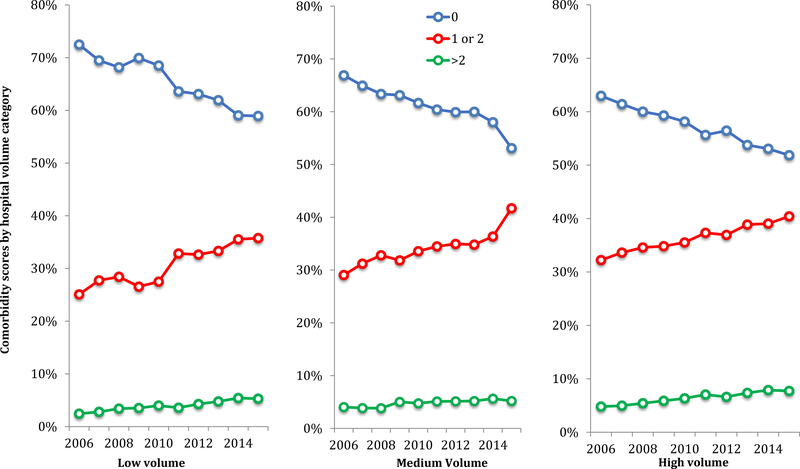
The proportion of women with preeclampsia with the lowest comorbid risk (a comorbidity score of 0) decreased over the study period from 72.5% to 58.9% at low volume hospitals, 66.9% to 53.1% at medium volume hospitals, and 63.0% to 51.9% at high volume hospitals (Figure 3). The proportion of women with a comorbidity score of 1 or 2 increased from 25.1% to 35.8% at low volume hospitals, from 29.1% to 41.7% at medium volume hospitals, and from 32.2% to 40.4% at high volume hospitals (p<0.01 for all). Finally, the proportion of women with a score >2 increased from 2.4% to 5.3% at low volume hospitals, 4.0% to 5.2% at medium volume hospitals, and 4.8% to 7.7% at high volume hospitals (p<0.01 for all).
Figure 3. Proportion of preeclamptic patients receiving antihypertensives based on hospital volume.
Legend: The figure demonstrates the proportion of patients with a preeclampsia diagnosis receiving antihypertensives by hospital volume category: low (≤1000 deliveries per year), medium (1001 to 2000 deliveries per year), and high volume (>2000 deliveries per year).
Over the course of the study period, severe morbidity associated with preeclampsia became more common on a population basis (Figure 4). Per 10,000 deliveries, severe morbidity associated with preeclampsia increased over the study period from 10.3 to 18.3 at low volume hospitals, 17.9 to 24.2 for medium volume hospitals, and 19.9 to 26.9 for high volume hospitals (p<0.01 for all). For morbidity excluding transfusion, morbidity increased over the study period increased from 4.0 to 7.9 at low volume hospitals, 9.5 to 13.8 at medium volume hospitals, and 11.2 to 13.8 for high volume hospitals (p<0.01 for all). Increased population based risk for severe morbidity was due to both increased prevalence of preeclampsia diagnoses and higher rates of severe morbidity when a preeclampsia diagnosis was present (Supplementary Figure 1).
Figure 4. Comorbidity index scores by year for patients with preeclampsia delivering at low, medium and high volume hospitals.
Legend: The figure demonstrates the proportion of patients with preeclampsia delivering at low volume hospitals with obstetric comorbidity index scores of 0, 1–2, or >2. The obstetric comorbidity index is a measure, based on medical, obstetric, and demographic factors, of risk for severe morbidity. In calculating comorbidity scores preeclampsia diagnoses were excluded.
The adjusted multivariable analysis is demonstrated in Table 2 and includes demographics, patient factors, and hospital factors. In the adjusted multivariable analysis, factors associated with increased risk for severe morbidity among women with preeclampsia included severe and superimposed preeclampsia compared to mild preeclampsia (risk ratio (RR) 2.57, 95% confidence interval (CI) 2.44–2.71, RR 1.45 95% CI 1.36–1.57, respectively), the years 2009, 2010, 2011, 2012, 2013, and 2014 with 2006 as a referent, and both teenage and advanced maternal age compared to maternal age 25 to 34 years (RR 1.20 95% CI 1.08–1.33, RR 1.27 95% CI 1.19–1.35). Obstetric delivery volume was not significantly associated with severe morbidity risk when high volume and medium volume hospitals were compared to low volume hospitals (RR 0.97 95% CI 0.86–1.09, RR 1.00 0.86–1.09, respectively).
Table 2.
Adjusted model for risk for severe morbidity among preeclamptic patients
| Adjusted risk ratio | 95% confidence interval | |
|---|---|---|
| Year | ||
| 2006 | 1.00 | Reference |
| 2007 | 1.06 | 0.96–1.16 |
| 2008 | 1.07 | 0.96–1.18 |
| 2009 | 1.23 | 1.10–1.36 |
| 2010 | 1.18 | 1.07–1.31 |
| 2011 | 1.18 | 1.07–1.31 |
| 2012 | 1.16 | 1.06–1.28 |
| 2013 | 1.22 | 1.10–1.35 |
| 2014 | 1.12 | 1.00–1.26 |
| 2015 (1Q) | 1.06 | 0.89–1.25 |
| Obstetric delivery volume | ||
| <1000 | 1.00 | Reference |
| 1000-<2000 | 1.00 | 0.86–1.09 |
| ≥2000 | 0.97 | 0.86–1.09 |
| Age | ||
| 15–17 | 1.20 | 1.08–1.33 |
| 18–24 | 1.00 | Reference |
| 25–34 | 1.03 | 0.98–1.09 |
| ≥35 | 1.27 | 1.19–1.35 |
| Insurance status | ||
| Medicare | 1.57 | 1.35–1.84 |
| Medicaid | 1.07 | 1.02–1.12 |
| Private | 1.00 | Reference |
| Uninsured | 1.12 | 0.98–1.29 |
| Other | 1.02 | 0.93–1.13 |
| Race | ||
| White | 1.00 | Reference |
| Black | 1.36 | 1.28–1.44 |
| Other | 1.14 | 1.09–1.20 |
| Unknown | 0.88 | 0.30–2.57 |
| Hospital Location | ||
| Rural | 1.01 | 0.86–1.19 |
| Urban | 1.00 | Reference |
| Marital Status | ||
| Married | 1.00 | Reference |
| Single | 1.03 | 0.98–1.08 |
| Unknown | 1.05 | 0.94–1.16 |
| Hospital Region | ||
| Northeast | 1.00 | Reference |
| Midwest | 0.83 | 0.71–0.97 |
| South | 0.88 | 0.77–1.0 |
| West | 0.91 | 0.78–1.05 |
| Preeclampsia diagnosis | ||
| Mild preeclampsia | 1.00 | Reference |
| Severe preeclampsia | 2.57 | 2.44–2.71 |
| Superimposed preeclampsia | 1.46 | 1.36–1.57 |
| Teaching | ||
| Non-teaching | 1.00 | Reference |
| Teaching | 0.98 | 0.89–1.08 |
All factors in this table are included in the adjusted analysis. Estimates are based on weighted data.
DISCUSSION
This analysis demonstrated that incidence of preeclampsia continues to rise across a range of obstetric care settings. The proportionately largest increases in diagnoses were of superimposed and severe preeclampsia. Hypertensive disorders are among the most common medical problem encountered in pregnancy29 with a wide range of both pregnancy-specific characteristics and maternal pre-existing features contributing to increasing incidence. Factors associated with increased preeclampsia may include more common pre-existing hypertension, diabetes, obesity, delay in childbearing, and the use of artificial reproductive technologies and the associated increase in multi-fetal gestation.30–33 While prenatal administration of aspirin may reduce preeclampsia risk,34 it is unclear to what degree this intervention will offset larger trends in increased population-based risk.
Along with increased risk for preeclampsia, our analysis demonstrated increased use of first line anti-hypertensive agents used to treat severe hypertension during delivery hospitalizations. These data support rising acuity involved in the care of preeclamptic women either because of increased surveillance and treatment of severe range blood pressure, increased risk for severe range blood pressure, or both. Protocols directed at prompt recognition and treatment of hypertensive crisis have demonstrated maternal benefit35 and the ACOG Task Force on Hypertension and the National Partnership for maternal safety have provided recommendations regarding antihypertensive management.6,21 Our data support that administration of first-line antihypertensive medications to treat preeclampsia is becoming a routine part of inpatient obstetrical care.
On a population basis, severe morbidity associated with preeclampsia increased over the study period across hospital volume setting secondary to both increased incidence of preeclampsia and increased risk associated with the diagnosis. This study found that after excluding diagnoses of preeclampsia from the obstetric comorbidity index, women with preeclampsia had higher comorbidity scores over the study period. The degree to which increasing maternal morbidity from preeclampsia is associated with other comorbid risk factors is an important avenue of future research. Given that more than half of all hospitals providing obstetric care deliver <1000 deliveries per year22 and that our study found increasing incidence, risk, and acuity for preeclampsia across obstetric volume settings, targeted maternal safety protocols and quality improvement initiatives across all hospitals will be required to reduce maternal risk. These findings demonstrate that low volume centers are not necessarily low risk. Outcomes at low-volume hospitals may be critically important given that as of 2008, 58% of hospitals providing obstetrical care performed less than 1000 deliveries a year, and an additional 21% performed 1000 to 2000 deliveries a year. 22 While centers that perform <500 deliveries per year are responsible for delivering less than 10% of the obstetric population, they may have the least administrative resources dedicated to maternal safety.36
These findings build on prior analyses of preeclampsia performed with the Perspective data by our group. Ananth et al. found that from 2006–2012 condition-specific risk for stroke, pulmonary edema, sepsis, renal and heart failure among other conditions were all more common in the setting of severe preeclampsia. Additionally, morbidity associated with severe preeclampsia varied significantly within hospital volume categories and risk for severe morbidity was decreased for hospitals with higher compared to lower rates of severe preeclampsia.20 Additionally, we have evaluated rates of antihypertensive use for delivery hospitalizations for patients with severe, mild, and superimposed preeclampsia diagnoses (under review, Obstetrics and Gynecology). The current analysis adds to these findings by evaluating comorbidity, use of antihypertensives, temporal trends in incidence, severe morbidity across all preeclampsia diagnoses, and overall population based risk stratified by obstetric volume. For clinicians, practical implications of these findings include that no matter which setting one practices, preeclampsia is increasing and patients with preeclampsia are at higher a priori risk for adverse maternal outcomes based on comorbidity. Why women receiving Medicare insurance were at increased risk for preeclampsia is unclear and may be due to unmeasured confounding.
Several limitations are important to consider when interpreting our data. The data analyzed was collected from an administrative database. Information on individual hospitals protocols and guidelines for acute hypertensive management was unavailable. While there is no data we are evaluating temporal trends in sensitivity of ICD-9-CM codes, given the implementation of hypertensive bundles and the greater awareness of maternal risk, the temporal changes seen across our study period could be in part due to improved reporting or ICD-9-CM capturing of both risk factors and outcomes. A second limitation of this analysis is that this administrative dataset does not provide information on hospital resources, infrastructure and staffing, all of which contribute to maternal outcomes and risk. While we are able to include general hospital characteristics such as location, region, and teaching status, more granular data is not available. Third, although our analysis demonstrated increased risk for severe morbidity with and without transfusion, direct causality from preeclampsia could not be demonstrated as individual chart review was not performed. Fourth, the population weights in this analysis are applied based on hospital characteristics and are not specifically designed for an obstetric population. Strengths of this study include the relatively long study period as well as the ability to include a geographically diverse set of hospitals and patients.
In summary, the findings from this analysis demonstrate increased risk of preeclampsia across hospital care settings, along with increased acuity, comorbid risk, and risk for severe morbidity. That these trends have occurred across varied obstetric settings supports the need for universal adoption of interventions to reduce maternal risk from preeclampsia.
Supplementary Material
Acknowledgments
Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
REFERENCES
- 1.Callaghan WM. Overview of maternal mortality in the United States. Seminars in perinatology 2012;36:2–6. [DOI] [PubMed] [Google Scholar]
- 2.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstetrics and gynecology 2017;130:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstetrics and gynecology 2005;106:1228–34. [DOI] [PubMed] [Google Scholar]
- 5.Snowden JM, Cheng YW, Emeis CL, Caughey AB. The impact of hospital obstetric volume on maternal outcomes in term, non-low-birthweight pregnancies. American journal of obstetrics and gynecology 2015;212:380 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus Bundle on Severe Hypertension During Pregnancy and the Postpartum Period. Obstetrics and gynecology 2017;130:347–57. [DOI] [PubMed] [Google Scholar]
- 7.Stulberg J, Delaney C, Neuhauser D, Aron D, Fu P, Koroukian S. Adherence to surgical care improvement project measures and the asssociation with postoperative infections. JAMA 2010;303:2479–85. [DOI] [PubMed] [Google Scholar]
- 8.Fang M, Maselli J, Lurie J, Lindenauer P, Auerbach A. Use and outcomes of venous thromboembolism prophyalxis after spinal fusion surgery. J Thromb Haemost 2011;9:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritch J, Kim J, Lewin S, et al. Venous thromboembolism and use of prophylaxis among women undergoing laparoscopic hysterectomy. Obstetrics and gynecology 2011;117:1367–74. [DOI] [PubMed] [Google Scholar]
- 10.Wright J, Lewin S, Shah M, et al. Quality of venous thromboembolism prophylaxis in patients undergoing oncologic surgery. Ann Surg 2011;253:1140–6. [DOI] [PubMed] [Google Scholar]
- 11.Zacharia BE, Youngerman BE, Bruce SS, et al. Quality of Postoperative Venous Thromboembolism Prophylaxis in Neuro-oncologic Surgery. Neurosurgery 2016. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran S, Herbers P, Khoury J, et al. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage? Stroke 2015;46:369–75. [DOI] [PubMed] [Google Scholar]
- 13.Kulik A, Rassen JA, Myers J, et al. Comparative effectiveness of preventative therapy for venous thromboembolism after coronary artery bypass graft surgery. Circ Cardiovasc Interv 2012;5:590–6. [DOI] [PubMed] [Google Scholar]
- 14.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 2012;255:821–9. [DOI] [PubMed] [Google Scholar]
- 15.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. American journal of epidemiology 2007;166:117–24. [DOI] [PubMed] [Google Scholar]
- 16.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. American journal of obstetrics and gynecology 2006;194:992–1001. [DOI] [PubMed] [Google Scholar]
- 17.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatric and perinatal epidemiology 2005;19:460–71. [DOI] [PubMed] [Google Scholar]
- 18.Kuklina E, Whiteman M, Hillis S, Jameieson D, Meikle S, Posner S. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J 2008;12:469–77. [DOI] [PubMed] [Google Scholar]
- 19.Janakiraman V, Lazar J, Joynt KE, Jha AK. Hospital volume, provider volume, and complications after childbirth in U.S. hospitals. Obstetrics and gynecology 2011;118:521–7. [DOI] [PubMed] [Google Scholar]
- 20.Ananth CV, Lavery JA, Friedman AM, Wapner RJ, Wright JD. Serious maternal complications in relation to severe pre-eclampsia: a retrospective cohort study of the impact of hospital volume. BJOG 2017;124:1246–53. [DOI] [PubMed] [Google Scholar]
- 21.Sadler LC, Austin DM, Masson VL, et al. Review of contributory factors in maternity admissions to intensive care at a New Zealand tertiary hospital. American journal of obstetrics and gynecology 2013;209:549 e1–7. [DOI] [PubMed] [Google Scholar]
- 22.Simpson KR. An overview of distribution of births in United States hospitals in 2008 with implications for small volume perinatal units in rural hospitals. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN / NAACOG 2011;40:432–9. [DOI] [PubMed] [Google Scholar]
- 23.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstetrics and gynecology 2013;122:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe A, Lix LM, Johnson JA, et al. Validation of an obstetric comorbidity index in an external population. BJOG 2015;122:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varda BK, Johnson EK, Clark C, Chung BI, Nelson CP, Chang SL. National trends of perioperative outcomes and costs for open, laparoscopic and robotic pediatric pyeloplasty. J Urol 2014;191:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dagenais J, Leow JJ, Haider AH, et al. Contemporary Trends in the Management of Renal Trauma in the United States: A National Community Hospital Population-based Analysis. Urology 2016;97:98–104. [DOI] [PubMed] [Google Scholar]
- 27.Brown K, Lundborg P, Levinson J, Yang H. Incidence of peptic ulcer bleeding in the US pediatric population. J Pediatr Gastroenterol Nutr 2012;54:733–6. [DOI] [PubMed] [Google Scholar]
- 28.Allard CB, Gelpi-Hammerschmidt F, Harshman LC, et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol Oncol 2015;33:496 e11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. American journal of hypertension 2008;21:521–6. [DOI] [PubMed] [Google Scholar]
- 30.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstetrics and gynecology 2009;113:1075–81. [DOI] [PubMed] [Google Scholar]
- 31.Wang YA, Chughtai AA, Farquhar CM, Pollock W, Lui K, Sullivan EA. Increased incidence of gestational hypertension and preeclampsia after assisted reproductive technology treatment. Fertility and sterility 2016;105:920–6.e2. [DOI] [PubMed] [Google Scholar]
- 32.Jeyabalan A Epidemiology of preeclampsia: impact of obesity. Nutrition reviews 2013;71 Suppl 1: S18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianco A, Stone J, Lynch L, Lapinski R, Berkowitz G, Berkowitz RL. Pregnancy outcome at age 40 and older. Obstetrics and gynecology 1996;87:917–22. [DOI] [PubMed] [Google Scholar]
- 34.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017;377:613–22. [DOI] [PubMed] [Google Scholar]
- 35.Clark SL, Christmas JT, Frye DR, Meyers JA, Perlin JB. Maternal mortality in the United States: predictability and the impact of protocols on fatal postcesarean pulmonary embolism and hypertension-related intracranial hemorrhage. American journal of obstetrics and gynecology 2014;211:32.e1–9. [DOI] [PubMed] [Google Scholar]
- 36.Friedman AM, Ananth CV, Huang Y, D’Alton ME, Wright JD. Hospital delivery volume, severe obstetrical morbidity, and failure to rescue. American journal of obstetrics and gynecology 2016;215:795 e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.