Abstract
Objective
Obesity and obesity-related metabolic disorders are major health problems worldwide. The most effective obesity intervention is bariatric surgery. This study tested the hypothesis that bariatric surgery alters phospholipid metabolism in the gastrointestinal tract to favor a metabolically healthy gut microbiota profile and therapeutic intervention of phospholipid metabolism in the gastrointestinal may have similar metabolic benefits.
Methods
The first study compared plasma levels of the bioactive lipid metabolites lysophospholipid and trimethylamine N-oxide (TMAO) as well as gut microbiota profile in high fat/carbohydrate (HFHC) diet-fed C57BL/6 mice with or without vertical sleeve gastrectomy (VSG) and in Pla2g1b−/− mice with group 1B phospholipase A2 gene inactivation. The second study examined the effectiveness of the non-absorbable secretory phospholipase A2 inhibitor methyl indoxam to reverse hyperglycemia and hyperlipidemia in HFHC diet-fed C57BL/6 mice after diabetes onset.
Results
Both bariatric surgery and PLA2G1B inactivation were shown to reduce lysophospholipid content in the gastrointestinal tract, resulting in resistance to HFHC diet-induced alterations of the gut microbiota, reduction of the cardiovascular risk factors hyperlipidemia and TMAO, decreased adiposity, and prevention of HFHC diet-induced diabetes. Importantly, treatment of wild type mice with methyl indoxam after HFHC diet-induced onset of hyperlipidemia and hyperglycemia effectively restored normal plasma lipid and glucose levels and replicated the metabolic benefits of VSG surgery with diabetes remission and TMAO reduction.
Conclusion
These results provided pre-clinical evidence that PLA2G1B inhibition in the digestive tract may be a viable alternative option to bariatric surgery for obesity and obesity-related cardiometabolic disorder intervention.
Keywords: Phospholipase A2, Gut microbiota, Cardiometabolic disease, Bariatric surgery, Lysophospholipid
Abbreviations: HFHC, High fat high carbohydrate; LPC, Lysophosphatidylcholine; LPCAT3, Lysophosphatidylcholine acyltransferase-3; PLA2G1B, Group 1B phospholipase A2; TMA, Trimethylamine; TMAO, Trimethylamine N-oxide; TMSP, 3-Trimethylsilyl 2,2,3,3-d4 propionate; VSG, Vertical sleeve gastrectomy
Highlights
-
•
Bariatric surgery reduces bioactive lipid metabolites lysophospholipids and TMAO.
-
•
Group 1b Phospholipase A2 inactivation mimics benefits of bariatric surgery.
-
•
Group 1b phospholipase A2 inhibition induces diabetes remission.
1. Introduction
The increasing prevalence of obesity and its associated cardiometabolic diseases has limited successful treatment options, thereby demanding the discovery of novel intervention strategies to combat these debilitating disorders and reduce the burden of health cost. The current strategy for obesity management includes lifestyle modifications, pharmacologic therapy, and bariatric surgery [1]. Lifestyle modification is usually the first line of intervention but is generally ineffective due to high relapse rates. Pharmacotherapy for obesity has been disappointing so far, and some of the obesity drugs have been associated with serious side effects [2]. The most effective obesity intervention currently is bariatric surgery [2]. Because of potential risks associated with surgical procedures, the development of novel therapeutics that can mimic the metabolic benefits of bariatric surgery is highly desirable [3], [4]. A critical barrier to achieving this goal is the insufficient knowledge of the mechanisms underlying the metabolic benefits of bariatric surgery.
The most commonly practiced bariatric procedures are the Roux-en Y gastric bypass and vertical sleeve gastrectomy (VSG). The metabolic benefits of both procedures have been attributed to a combination of nutrient transport bypassing the proximal intestine with rapid delivery to the distal intestine, and altering enterohepatic circulation to increase circulating bile acids that improve insulin sensitivity [5], [6], [7], [8], [9]. Another mechanism underlying the metabolic benefits of bariatric surgery is changes in the gut microbiota, which can alter energy yield from food and affect several nutrient metabolism pathways in the distal small intestine and colon [10]. The relationship between bariatric surgery and gut microbiota changes leads to the hypothesis that manipulating the gut microbiota may be a viable strategy to mimic the metabolic benefits of bariatric surgery [11], [12].
Current approaches aimed at gut microbiota manipulation include dietary modification, prebiotic/probiotic/symbiotic supplementation, and fecal microbiota transplant [12]. Unfortunately, dietary modification has high relapse rate while prebiotic and probiotic supplementation may cause unexpected weight gain [13]. Although fecal microbiota transplant shows promising results, the gut microbiota can adapt to environment dictated by the diet that is characteristic of metabolic dysfunction phenotype within hours and days after high fat feeding [14], [15], [16]. Thus, repeated fecal microbiota transplant is necessary to achieve sustained metabolic benefits, which adds to social and ethical concerns that limit its long term use for chronic metabolic disease management [17]. Accordingly, the development of pharmacotherapy that can mimic the effect of bariatric surgery on gut microbiota is critical for effective management of the global public health crisis associated with obesity and obesity-related metabolic diseases [18].
A recent survey of metabolomic and lipidomic changes associated with bariatric surgery in humans revealed that successful diabetes remission is associated with reduced sphingomyelin and lysophospholipid levels [19]. In view of previous studies showing that reducing intestinal phosphatidylcholine hydrolysis to lysophosphatidylcholine (LPC) via group 1B phospholipase A2 (PLA2G1B) inhibition suppresses the onset of diet-induced obesity and hyperglycemia in mice [20], [21], the goals of this study is to determine whether PLA2G1B inactivation also alters gut microbiota, and more importantly whether PLA2G1B inhibitors may be a viable therapeutic option instead of bariatric surgery for diabetes intervention to reverse hyperglycemia, hyperinsulinemia, and hyperlipidemia after their onset.
2. Methods
2.1. Animals
Wild type C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME) were used for all bariatric surgery experiments. For experiments with Pla2g1b−/− mice, the Pla2g1b−/− mice were backcrossed with C57BL/6J mice originally obtained from Jackson Laboratories for >10 generations to generate Pla2g1b−/− mice in C57BL/6J background [22]. Breeding colonies of Pla2g1b+/+ and Pla2g1b−/− mice were maintained in our institutional animal facility and littermates were used for all experiments. All animals were maintained in a temperature- and humidity-controlled room with a 12-hr light/dark cycle. The animals were maintained on rodent chow (LM485; Harlan-Teklad, Madison, WI) with free access to water. Male mice at the age of 12–14 weeks were used for all experiments except for the cohousing studies when 2 female mice placed in the same cage were studied due to aggressive nature of male mice. The animals were fed either the rodent chow or a high fat-high carbohydrate (HFHC) diet (D12331; Research Diets, New Brunswick, NJ). In selected experiments, the mice were administered drinking water containing the antibiotics vancomycin (0.5 g/L) and bacitracin (1 g/L) during the 8-week HFHC dietary feeding period as described [23]. All experimental animal protocols were approved by the institutional animal care and use committee at the University of Cincinnati in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
2.2. PLA2G1B inhibition studies
The pan-secretory phospholipase A2 inhibitor methyl indoxam, which is cell impermeable and cannot be absorbed through the intestinal mucosa [21], was used to test the effect of PLA2G1B inhibition on metabolic diseases. Methyl indoxam was synthesized by Intogen Biosciences (Telangana, India) according to the procedure described [24]. After gas chromatography confirmation of >95% purity and in vitro inhibition of PLA2G1B activity, the methyl indoxam was added to the HFHC diet at a concentration to yield a dosage of 90 mg kg−1. Body weights were recorded at the beginning as well as throughout the experimental feeding period. Body fat mass was measured in conscious mice using proton magnetic resonance spectroscopy (EchoMRI-100; Echo Medical Systems).
2.3. Vertical sleeve gastrectomy surgery
VSG surgery was performed in C57BL/6J mice after feeding the HFHC diet to induce obesity and glucose intolerance as described [25]. Briefly, the mice were anesthetized with isofluorane inhalation. The lateral 80% of the stomach was excised leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly. A sham procedure without stomach excision was performed similarly with manual pressure along a vertical line between esophageal sphincter and the pylorus. The mice were fed Osmolite One Cal liquid diet one day before surgery for acclimation and then three days after the operation prior to re-introduction of the HFHC diet. Food consumption was assessed daily for 4 consecutive days 3 weeks after the recovery period and was repeated again 3 weeks later as described [26]. Intestinal expression of lysophosphatidylcholine acyltransferase-3 (LPCAT3) was assessed by isolating total RNA from the ileum for RT-PCR quantification of its mRNA. The primers used for amplification of LPCAT3 mRNA are: 5′-CCAGGGAAGATGCCAAACAG-3′ and 5′-GTGTAGCCCACCAGGTAGACAAG-3’. Cyclophilin mRNA amplification with primers: 5′-TCATGTGCCAGGGTGGTGAC-3′ and 5′-AACTTCAGTCTTGGCAGTGC-3′ was used as the normalization control.
2.4. Postprandial glucose tolerance test
Mice were fasted overnight and then fed 4 ml-kg−1 body weight of a bolus HFHC meal containing 2.6 mM egg phosphatidylcholine, 13.33 mM triolein, and 2.6 mM cholesterol in a saline solution containing 50% w/v glucose. Blood was obtained before and at different times after administration of the test meal. Blood glucose levels were measured with an Accu-Chek glucometer (Roche Applied Science, Indianapolis, IN).
2.5. Plasma chemistry
Animals were fasted overnight prior to blood draw for plasma chemistry measurements. Plasma cholesterol and triglyceride levels were measured using the Infinity Triglyceride and Cholesterol Assay Kits from Thermo Fisher Scientific (Middletown, NJ). Glucose levels were measured with an Accu-Chek glucometer, and insulin levels were determined by the UltraSensitive Rat Insulin ELISA kit (Crystal Chem, Chicago, IL). Homeostatic model assessment of insulin resistance was calculated from fasting glucose and insulin levels using the formula [glucose (mg/dL) x insulin (ng/ml)]/22.5.
2.6. Lysophosphatidylcholine assays
Plasma LPC concentrations were determined by enzymatic assay as described [22]. To measure LPC content in the digestive tract, the entire small intestine was collected and flushed with phosphate-buffered saline and then flash frozen at −80 °C. The intestine was thawed in buffer containing 50 mM Tris-Cl, pH 7.4, 150 mM NaCl, and 10 mM EDTA. Protein concentration was determined by the Lowry method. Samples with similar protein levels were extracted three times with water saturated n-butanol. The butanol fractions were dried under N2 stream and then rehydrated in LC-MS grade methanol containing 1 mM ammonium formate and 0.2% formic acid in autosampler vials. The concentration of LPC with unsaturated fatty acyl (14:0, 16:0, 18:0, 20:0, 22:0, 24:0, and 26:0), monounsaturated fatty acyl (16:1, 18:1, 20:1, 22:1, and 24:1), and polyunsaturated fatty acyl (18:2, 18:3, 18:4, 20:2, 20:3, 20:4, 20:5, 22:2, 22:3,22:4, 22:5, and 22:6) moieties in each sample was quantified by LC-MS at the Wayne State University Lipidomics Core.
2.7. Plasma TMAO quantification
All the plasma test samples were thawed on ice on the day of data collection. Once thawed, samples were filtered at 12,000 × g for 60 min at 4 °C using pre-washed 3-kDa spin filters (NANOSEP 3K, Pall Life Sciences). The NMR buffer containing 100 mM phosphate buffer in D2O, pH 7.3, and 1.0 mM TMSP (3-Trimethylsilyl 2,2,3,3-d4 propionate) was added to plasma filtrate up to 600 μL. The final TMSP concentration in each sample was determined based on the ratio of plasma filtrate and NMR buffer. The experiments were conducted using 550 μL samples in 103.5 mm × 5 mm NMR tubes (Bruker). One-dimensional 1H NMR spectra were acquired using noesypr1d (or noesygppr1d) pulse sequence on a Bruker Avance II 600 MHz spectrometer for each sample. For a representative sample, two dimensional data, 1H-1H total correlation spectroscopy (TOCSY) and 1H-13C heteronuclear single quantum coherence (HSQC) were collected for metabolites assignment conformation. All the data were processed using Topspin 3.5 software (Bruker Analytik, Rheinstetten, Germany). The chemical shifts for Trimethylamine (TMA) and TMAO were assigned on the 2.89 ppm (s) and 3.28 ppm (s) based on 1D 1H, 2D TOCSY and HSQC NMR experiments with reference spectra found in databases, Human Metabolome Database (HMDB) [27], and Chenomx® NMR Suite profiling software (Chenomx Inc. version 8.1). The concentrations of the metabolites were calculated using Chenomx software based on the internal standard, TMSP.
2.8. Gut microbiota analysis
Genomic DNA was extracted from whole cecum of fasted mice using the Powersoil DNeasy Kit from Mo Bio Laboratories (Carlsbad, CA). The samples were amplified using the 16S rRNA gene V4 variable region PCR primers set 341/785 using the HotStar Taq Plus Master Mix Kit (Qiagen) under the following conditions: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s, and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. After amplification, PCR products were checked in 2% agarose gel to determine the success of amplification and the relative intensity of bands. Multiple samples were pooled together (e.g., 100 samples) in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads, and purified PCR product was used to prepare Illumina DNA library. Sequencing was performed at MR DNA (Shallowater, TX, USA) on a MiSeq following the manufacturer's guidelines. Sequence data were processed using MR DNA analysis pipeline (MR DNA, Shallowater, TX, USA). In summary, sequences were joined, depleted of barcodes then sequences <150 bp removed, sequences with ambiguous base calls removed. Sequences were de-noised, OTUs generated, and chimeras removed. Operational taxonomic units (OTUs) were defined by clustering at 3% divergence (97% similarity). Final OTUs were taxonomically classified using BLASTn against a curated database derived from RDPII and NCBI.
2.9. Statistics
All results are expressed as means ± standard error with sample size of N ≥ 6 in each group. Sample size for each experiment was estimated based on previous studies documenting that 6 mice in each group is sufficient to yield significant differences in body weight, adiposity, and plasma lipid, glucose, insulin, and LPC levels [22]. Mice were not randomized prior to assigning to experimental groups. No samples, mice, or data points were excluded from the reported analyses. Metabolic and physiological parameters were not performed in a blinded manner, but intestinal LPC and gut microbiota levels were analyzed by core facility personnel in a blinded manner. Statistical analysis was performed using SigmaPlot version 13.0 software (SysStat Software, San Jose, CA). Normality was examined using the Shipiro-Wilk test. Data with equal variance based on Brown-Forsythe analysis were evaluated by Student's t test for studies comparing 2 groups or by one way ANOVA for comparison of multiple groups. Data with unequal variance were evaluated using the Mann–Whitney test. Differences at P < 0.05 were considered statistically significant.
3. Results
3.1. Bariatric surgery alters gut microbiota and reduces plasma lysophospholipid and TMAO levels
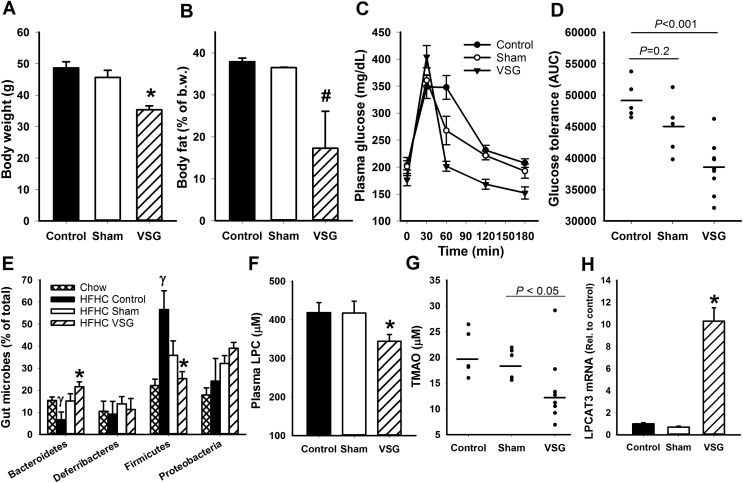
Vertical sleeve gastrectomy (VSG) surgery was performed in C57BL/6J mice obtained from Jackson Laboratories after feeding them a high fat-high carbohydrate (HFHC) diet for two weeks. Metabolic parameters were monitored for eight additional weeks after VSG surgery when the animals were maintained on the same HFHC diet. As expected, VSG surgery reduced body weight and body fat mass (Figure 1A,B) as well as improved glucose tolerance in response to a lipid-sucrose rich mixed meal (Figure 1C,D). Similar to results reported by others [25], food intake after recovery from surgery was comparable between sham- and VSG-operated animals (2.49 ± 0.13 vs 2.47 ± 0.07 gm/day). Feeding of the HFHC diet induced changes in gut microbiota with reduced percentage of Bacteriodetes and increased percentage of Firmicutes, but these changes were overcame by VSG surgery and led to a favorable gut microbiota profile similar to that observed in chow-fed mice (Figure 1E). Additionally, we found that VSG surgery also lowered plasma levels of the cardiometabolic risk factor LPC in HFHC diet-fed mice (Figure 1F). The lower levels of Firmicutes, a major gut bacteria that utilizes the LPC metabolic product choline to generate another cardiometabolic risk factor TMAO [28], [29], [30], resulted in reduced levels of TMAO in HFHC diet-fed mice after VSG surgery (Figure 1G).
Figure 1.
Relationship between VSG surgery improvement of adiposity and glucose tolerance with changes in plasma LPC and TMAO levels and gut microbiota in C57BL/6 mice. (A,B) Body weight and adiposity, measured as % body fat, in HFHC-fed mice without surgery (N = 6) or with sham (N = 6) or VSG procedures (N = 8). (C) Blood glucose levels in response to a bolus lipid-glucose meal in HFHC-fed mice without surgery (N = 6) or with sham (N = 6) or VSG procedures (N = 8). (D) Area under the curve analysis of the glucose tolerance test data reported in C. (E) Distribution of major bacterial phyla in the cecum of chow- or HFHC-fed mice without surgery (N = 6) or with sham (N = 6) or VSG procedures (N = 7). (F) Plasma LPC levels in HFHC-fed mice without surgery (N = 6) or with sham (N = 6) or VSG procedures (N = 8). (G) Plasma TMAO levels in control (N = 5), sham- (N = 5) and VSG-operated (N = 8) mice that were fed the HFHC diet for 8 weeks. (H) Expression levels of LPCAT3 in ileal intestine of control (N = 3), sham- (N = 3), and VSG-operated (N = 5) mice. Data are presented as mean ± s.e.m. *P < 0.01 and #P < 0.05 indicate differences from control group and the sham-operated group by one way ANOVA (A,B,C,E,G, H) or Mann–Whitney rank sum test (D and F). γ indicates differences from chow-fed mice at P < 0.05.
While the VSG-induced TMAO reduction reflects alteration in the gut microbiota, the mechanism underlying the reduction of LPC levels after VSG surgery is unknown. It is unlikely that VSG surgery interferes with LPC generation from phospholipid digestion because this operation does not affect dietary fat uptake and triglyceride accumulation in enterocytes [31], a process that requires lipid digestion in the intestinal lumen [32], [33]. Therefore, we examined if VSG surgery alters LPC usage in the intestine and found that VSG surgery resulted in ∼10-fold activation of LPCAT3, an enzyme that is responsible for acylation of LPC to phosphatidylcholine (Figure 1H).
3.2. PLA2G1B inactivation alters gut microbiota and lowers lysophospholipid and TMAO levels
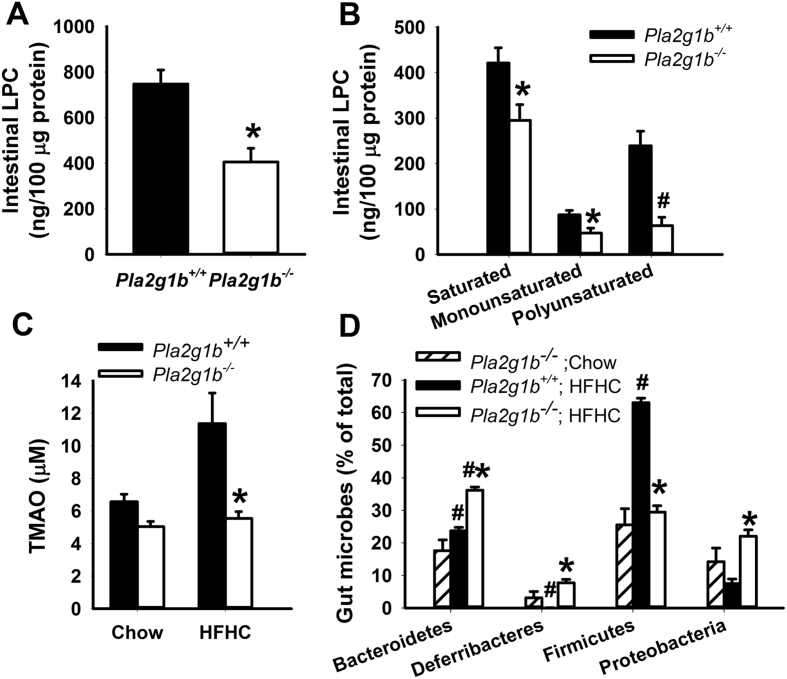
The relationship between intestinal phospholipid metabolism and gut microbiota was explored by comparing gut phospholipid metabolites and microbiota composition in the cecum of chow- and HFHC diet-fed Pla2g1b−/− mice, an animal model with genetic inactivation of group 1B phospholipase A2, the main enzyme responsible for phospholipid conversion to lysophospholipid in the gastrointestinal tract [34]. Results showed significant reduction of LPC levels in the intestine of Pla2g1b−/− mice (Figure 2A). Analysis of fatty acyl composition of the LPC revealed that 3 classes of LPC, including those with saturated, monounsaturated, and polyunsaturated fatty acyl groups [35], [36] were all reduced in the absence of PLA2G1B (Figure 2B). The reduction of phospholipase A2 digested products in the gastrointestinal tract also decreased plasma TMAO levels in HFHC-fed Pla2g1b−/− mice to levels observed in chow-fed mice (Figure 2C). Furthermore, Bacteriodetes levels were higher in HFHC-fed Pla2g1b−/− mice compared to Pla2g1b+/+ mice. Importantly, PLA2G1B inactivation in Pla2g1b−/− mice also resulted in resistance to HFHC-induced elevation of Firmicutes levels (Figure 2D), thereby resulting in a metabolically favorable gut microbiota that mimics those observed in HFHC-fed mice after VSG surgery. These results indicate that gut microbiota composition is influenced by intestinal phospholipid metabolism and the metabolites generated from PLA2G1B-mediated phospholipid digestion are responsible for the elevated Firmicutes levels that have been linked to diabetes and cardiovascular disease risk.
Figure 2.
Influence of PLA2G1B gene inactivation on intestinal LPC and plasma TMAO levels and gut microbiota in C57BL/6J mice. (A) Intestinal LPC levels in HFHC-fed Pla2g1b+/+ and Pla2g1b−/− mice (N = 6 in each group). (B) Levels of saturated, monounsaturated, and polyunsaturated LPC in intestine of Pla2g1b+/+ (filled bars) and Pla2g1b−/− (open bars) mice. (C) Plasma TMAO levels in chow-fed Pla2g1b+/+ mice (N = 6), and Pla2g1b−/− mice (N = 5), HFHC-fed Pla2g1b+/+ (N = 10), and HFHC-fed Pla2g1b−/− (N = 5) mice. Data from Pla2g1b+/+ mice are shown in filled bars and Pla2g1b−/− mice are shown in open bars. (D) Distribution of major bacterial phyla in the cecum of chow-fed Pla2g1b−/− mice (striped bars, N = 3) and HFHC-fed Pla2g1b+/+ (filled bars, N = 6) and HFHC-fed Pla2g1b−/− (open bars, N = 6) mice. Data are presented as mean ± s.e.m. *P < 0.01 by t test compared to wild type mice on same diet; #P < 0.001 compared to mice with same genotype on chow diet.
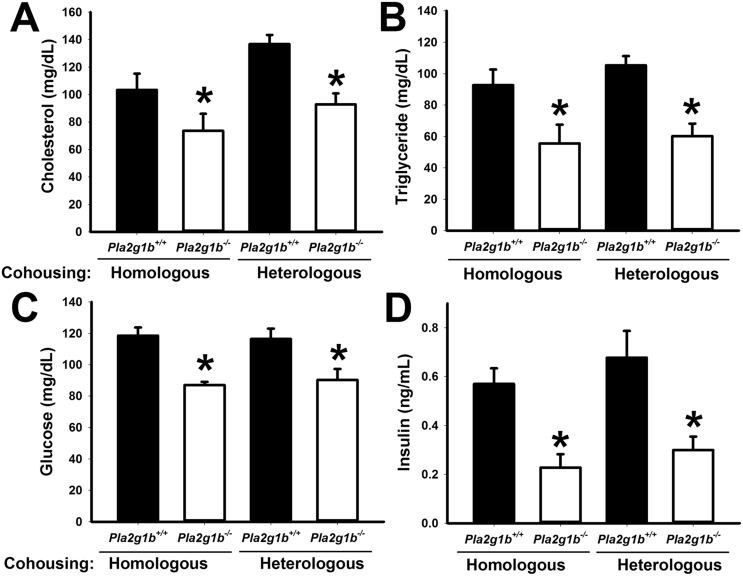
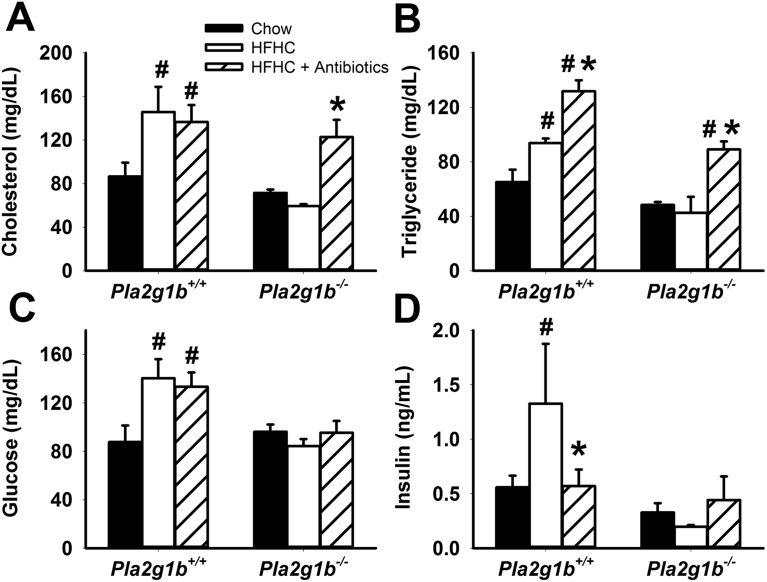
The hypothesis that gut microbiota composition is influenced by metabolites generated from PLA2G1B was substantiated by additional experiments comparing the metabolic phenotype of Pla2g1b+/+ and Pla2g1b−/− mice when the animals were cohoused together in the same cage where coprophagous behavior is expected to result in similar microbiota transplant. Results showed that the metabolic phenotype of elevated plasma cholesterol, triglyceride, glucose, and insulin levels persisted in HFHC diet-fed Pla2g1b+/+ mice regardless of whether they were cohoused with Pla2g1b+/+ or Pla2g1b−/− mice (Figure 3A–D). These results are consistent with prior observations that the gut microbiota adapt rapidly to the microenvironment dictated by nutrients derived from the diet and the host genotype [15], [37]. Importantly, the results also showed that metabolic benefits of PLA2G1B inactivation persisted and Pla2g1b−/− mice remained protected against HFHC diet-induced hyperlipidemia, hyperglycemia, and hyperinsulinemia regardless of the genotype of their cohousing partner (Figure 3A–D). Thus, these data provided additional support for the hypothesis that LPC derived from PLA2G1B digestion of phospholipids in the intestinal lumen is an important determinant of the gut microbiota composition and metabolic health. Additional studies showed that whereas plasma cholesterol and triglyceride levels in Pla2g1b−/− mice were higher with antibiotics treatment with levels comparable to those observed in Pla2g1b+/+ mice (Figure 4A,B), fasting plasma glucose and insulin levels remained lower in Pla2g1b−/− mice and comparable to those observed in chow-fed mice after antibiotic treatment (Figure 4C,D). Taken together, these results indicated that PLA2G1B-derived LPC may have direct influence in regulation of plasma glucose and insulin levels independent of gut microbiota but the protective effects of PLA2G1B inactivation against diet-induced hyperlipidemia are due to the effects on gut microbiota.
Figure 3.
Influence of cohousing on metabolic phenotype of Pla2g1b+/+and Pla2g1b−/−mice. Two female Pla2g1b+/+ and Pla2g1b−/− mice of the same (homologous; N = 4 per group) or different (heterologous; N = 6 per group) genotype were housed in the same cage and fed the HFHC diet for 8 weeks. Plasma cholesterol (A), triglyceride (B), glucose (C), and insulin (D) levels were measured after a 6 h fasting period. Data are presented as mean ± s.e.m. *P < 0.05 by t test compared to Pla2g1b+/+ mice.
Figure 4.
Influence of antibiotics treatment on metabolic phenotype of Pla2g1b+/+and Pla2g1b−/−mice. Pla2g1b+/+ and Pla2g1b−/− mice were fed a normal chow diet (solid bars, N = 4), or a HFHC diet without antibiotics (open bars, N = 4) or with vancomycin and bacitracin (hatched bars, N = 6) for 8 week. Plasma cholesterol (A), triglyceride (B), glucose (C), and insulin (D) levels were measured after a 6 h fasting period. Data are presented as mean ± s.e.m. *P < 0.05 by t test compared to HFHC diet-fed mice of the same genotype without antibiotics treatment; #P < 0.05 compared to chow-fed mice of the same genotype.
3.3. Phospholipase A2 inhibition reverses hyperlipidemia, hyperglycemia, and hyperinsulinemia in diet-induced diabetic mice
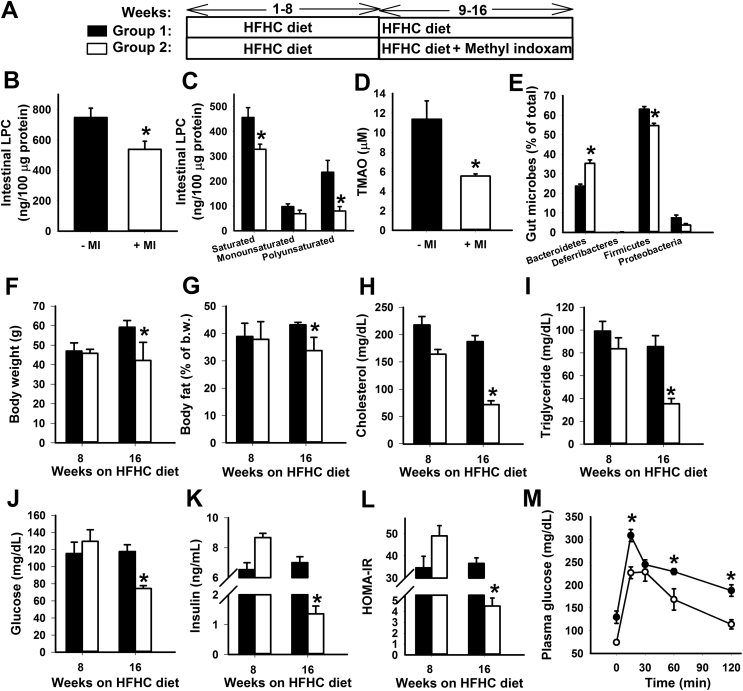
The resistance of Pla2g1b−/− mice to HFHC diet-induced changes in gut microbiota profile mimics the effect of bariatric surgery, thereby suggesting that PLA2G1B inhibition may be an intervention strategy for obesity and diabetes treatment after their onset. To test this hypothesis, wild type C57BL/6J mice were placed on HFHC diet for 8 weeks until obesity, hyperlipidemia, and hyperglycemia were observed. The pan-secretory phospholipase A2 inhibitor methyl indoxam was added to the HFHC diet and fed to half the animals while the other half of the mice continued to be fed the HFHC diet but without methyl indoxam for an additional 8 weeks (Figure 5A). Methyl indoxam is not cell permeable and is not absorbed by the intestinal mucosa, hence can be used to assess its therapeutic potential via PLA2G1B inhibition in the intestinal lumen. The inclusion of methyl indoxam in the diet did not influence food intake [21] but abrogated the HFHC-induced increase of intestinal LPC and plasma TMAO levels, resulting in levels similar to those observed in chow-fed animals (Figure 5B,C,D). Analysis of gut microbiota composition revealed that methyl indoxam treatment significantly reduced HFHC diet-induced increase in Firmicutes levels, albeit to less extent as that observed with VSG surgery. However, methyl indoxam invoked higher levels of Bacteriodetes compared to VSG treatment and chow-fed animals (Figure 5E). As a consequence, methyl indoxam treatment also led to a metabolic favorable Bacteroidetes/Firmicutes ratio similar to that observed with VSG surgery. The reduction of intestinal LPC along with the improvement of Bacteroidetes/Firmicutes profile as a consequence of methyl indoxam treatment also prevented the additional body weight gain and adiposity with the HFHC diet (Figure 5F,G). Importantly, methyl indoxam therapy after onset of metabolic diseases normalized plasma cholesterol and triglyceride levels (Figure 5H,I) to levels observed in chow-fed mice (Figure 4A,B) as well as eradicated hyperglycemia, hyperinsulinemia, insulin resistance, and glucose intolerance induced by the HFHC diet (Figure 5J–M).
Figure 5.
Diabetes and hyperlipidemia remission in HFHC-fed mice with methyl indoxam therapy. (A) Schematics showing the timeline for HFHC feeding and initiation of methyl indoxam therapy in C57BL/6J wild type mice. (B,C) Total LPC levels and levels of LPC with saturated, monounsaturated, and polyunsaturated fatty acyl moieties in HFHC fed wild type mice with or without methyl indoxam therapy beginning at week 8. (D) Plasma TMAO levels in HFHC fed wild type mice with or without methyl indoxam therapy beginning at week 8. (E) Distribution of major bacterial phyla in the cecum of HFHC-fed wild type with or without methyl indoxam therapy beginning at week 8. (F) Body weight, (G) adiposity, (H) plasma cholesterol, (I) plasma triglyceride, (J) fasting plasma glucose, and (K) fasting plasma insulin levels in group 1 mice with HFHC feeding for 16 weeks and in group 2 mice with HFHC feeding for 16 weeks with methyl indoxam therapy beginning at week 8. (L) Homeostatic assessment of insulin resistance index calculated from data shown in panels I and J. (M) Plasma glucose levels in response to a bolus lipid-glucose meal in HFHC-fed C57BL/6J without (filled symbols) or with (open symbols) methyl indoxam therapy beginning at week 8. All data are presented as mean ± s.e.m. from N = 6 in each group. *P < 0.01 by t test compared to mice without methyl indoxam therapy.
4. Discussion
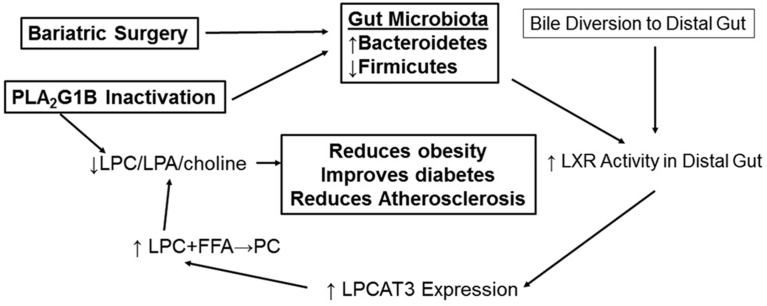
The effectiveness of bariatric surgery for metabolic disease intervention has prompted an extensive search of a pharmacological approach that can mimic its metabolic benefits without the risk associated with surgical procedures. A major impediment of these efforts is the incomplete information regarding the mechanism(s) underlying the bariatric surgery benefits. One hypothesis is that bariatric surgery-induced gut microbiota changes may be responsible for its metabolic benefits. Another possibility is that bariatric surgery alters circulating bile acid composition to improve insulin sensitivity. These two possibilities are not mutually exclusive since bile acid composition can be modified by bacteria in the gut [38]. In fact, diverting bile flow from the gallbladder directly to the ileum has been shown to confer comparable metabolic benefits as bariatric surgery in mice [9]. The mechanism underlying the metabolic improvement of bile diversion has been attributed to increased levels of the FXR antagonist tauro-β-muricholic acid [9]. The metabolic benefits of intestinal FXR inhibition have also been noted previously [39]. The inhibition of FXR activity is expected to reduce the levels of the LXRα inhibitor small heterodimer partner SHP-1 thereby activating LXR signaling in the intestine [40]. In view of the known metabolic benefits associated with intestinal-specific LXR activation [40], the metabolic benefits of VSG surgery, gut microbiota modification, and bile diversion may at least in part be due to elevated expression the LXR responsive LPCAT3 that mediates LPC conversion to phosphatidylcholine (Figure 6).
Figure 6.
Schematic diagram depicting a unifying model for the metabolic benefits of bariatric surgery, PLA2G1B inactivation, and bile diversion. This diagram depicts the impact of bariatric surgery and bile diversion in promoting LPCAT3 activity to reduce levels of LPC and its metabolites lysophosphatidic acid (LPA) and choline whereas PLA2G1B inactivation reduces these metabolites by reducing their production, both of which are expected to reduce obesity, improve diabetes, and reduce atherosclerosis.
Another approach to lower LPC levels in the gastrointestinal tract is through inhibition of its generation by phospholipase A2 inhibition [22], [41]. We have reported previously that PLA2G1B inactivation suppresses the onset of HFHC diet-induced obesity and hyperglycemia [20]. The reduction of diet-induced body weight gain in Pla2g1b−/− mice was attributed to reduction of lysophospholipids to alleviate their inhibition of hepatic fatty acid β-oxidation postprandially [41]. The current study extended on these earlier observations and showed that PLA2G1B inhibition not only suppressed diet-induced onset of hyperglycemia and hyperlipidemia, but importantly also reversed and normalized plasma glucose and lipid levels after diabetes onset in HFHC diet-fed mice. Interestingly, PLA2G1B inhibition did not reverse adiposity but prevented additional increase in body fat mass. The ability of methyl indoxam to limit additional body weight gain is likely due to reducing postprandial lysophospholipid content thereby limiting additional excessive nutrients transported to adipose tissues for storage, via mechanism similar to its prevention of obesity onset [41]. Importantly, despite the lack of obesity improvement, PLA2G1B inhibition effectively normalized plasma lipid and glucose levels in this diet-induced diabetic mouse model. Thus, the metabolic benefits of PLA2G1B inhibition are consistent with the benefits of bariatric surgery, which is known to improve diabetes independently of fat mass loss. One mechanism by which PLA2G1B inhibition improves metabolic phenotype may be due to its modulation of gut microbiota similar to that observed with bariatric surgery or through bile diversion (Figure 6).
In addition to diabetes remission, the current study showed that both bariatric surgery and PLA2G1B inhibition reduced plasma levels of TMAO, a major risk factor of atherosclerosis and cardiovascular disease [28]. These results are consistent with previous reports that metabolites of dietary phosphatidylcholine (and likely biliary phosphatidylcholine) are major sources of TMA and its oxidative product TMAO [28], [42]. Interestingly, while the phosphatidylcholine metabolites together with gut microbes are necessary for TMA production [28] and TMA-producing bacteria also influences choline availability for TMA production [43], our data showed that PLA2G1B inactivation also alters gut microbiota profile suggesting that metabolites from phospholipid hydrolysis also influences gut microbiota composition. Since VSG surgery also reduced PLA2G1B metabolites, the alteration of gut microbiota with bariatric surgery may at least in part be mediated via altered phospholipid metabolism in the digestive tract. A schematic diagram depicting the inter-relationship between PLA2G1B inactivation, gut microbiota, bile diversion, and LPC generation in improvement of diabetes is shown in Figure 6.
5. Conclusion
In summary, while genetic and pharmacological inactivation of PLA2G1B have been shown previously to prevent dietary onset of obesity and diabetes [20], [21], this study found that therapeutic PLA2G1B intervention has similar metabolic benefits as bariatric surgery with normalization of plasma lipid, glucose, insulin, and TMAO levels after HFHC-induced onset of these metabolic disorders. Thus, this pre-clinical study highlighted the potential of PLA2G1B inhibitor as an alternative option to bariatric surgery for management of obesity and obesity-related diabetes and cardiovascular disease.
Author contributions
J.G.C. carried out the physiological studies including animal diet studies and phenotyping experiments and was responsible for collecting all the data. E.K performed the VSG surgery and participated in analysis of VSG effects. N.H. and D.G.K. participated in the gut microbiota studies with sample collection, verification, and analysis of the microbiota data. M.W. and L.R-R. were responsible for the TMAO analysis. D.Y.H. designed and conceptualized the experimental approaches, analyzed the data, and wrote the paper.
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) grant RO1 DK112657. The authors acknowledge the NMR-Based Metabolomics Core at Cincinnati Children's Hospital Medical Center for TMAO analyses. Intestinal LPC quantification was performed by the Lipidomics Core Facility at Wayne State University which was supported by the National Center for Research Resources, NIH grant S10RR027926.
Conflict of interest
None.
References
- 1.American Diabetes Association Obesity management for the treatment of type 2 diabetes. Diabetes Care. 2016;39(Suppl. 1):S47–S51. doi: 10.2337/dc16-S009. [DOI] [PubMed] [Google Scholar]
- 2.Polonsky K.S., Klein S. Gastric banding to treat obesity: band-aid or breakthrough. Nature Clinical Practice Endocrinology & Metabolism. 2008;4:421. doi: 10.1038/ncpendmet0889. [DOI] [PubMed] [Google Scholar]
- 3.Miras A.D., Le Roux C.W. Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery. International Journal of Obesity. 2014;38:325–333. doi: 10.1038/ijo.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miras A.D., Le Roux C.W. Metabolic surgery in a pill. Cell Metabolism. 2017;25:985–987. doi: 10.1016/j.cmet.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Miras A.D., Le Roux C.W. Mechanisms underlying weight loss after bariatric surgery. Nature Reviews Gastroenterology & Hepatology. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad N.N., Pfalzer A., Kaplan L.M. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. International Journal of Obesity. 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patti M.E., Houten S.M., Bianco A.C., Bernier R., Larsen P.R., Holst J.J. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli R., Kirby M., Setchell K.D., Jha P., Klustaitis K., Woollett L.A. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn C.R., Albaugh V.L., Cai S., Cheung-Flynn J., Williams P.E., Brucker R.M. Bile acid diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nature Communications. 2015;6:7715. doi: 10.1038/ncomms8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 11.Wu G.D. The gut microbiome, its metabolome, and their relationship to health and disease. Nestle Nutritional Institution Workshop Series. 2016;84:103–110. doi: 10.1159/000436993. [DOI] [PubMed] [Google Scholar]
- 12.Kootte R.S., Vrieze A., Holleman F., Dallinga-Thie G.M., Zoetendal E.G., de Vos W.M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obesity and Metabolism. 2012;14:112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 13.Chouraqui J.P., Grathwohl D., Labaune J.M., Hascoet J.M., de Montgolfier I., Leclaire M. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. American Journal of Clinical Nutrition. 2008;87:1365–1373. doi: 10.1093/ajcn/87.5.1365. [DOI] [PubMed] [Google Scholar]
- 14.Turnbaugh P.J., Backhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host & Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmody R.N., Gerber G.K., Luevano J.M., Jr, Gatti D.M., Somes L., Svenson K.L. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host & Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science Translational Medicine. 2009;1 doi: 10.1126/scitranslmed.3000322. 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daloiso V., Minacori R., Refolo P., Sacchini D., Craxi L., Spagnolo A.G. Ethical aspects of fecal microbiota transplantation. European Review for Medical and Pharmacological Sciences. 2015;19:3173–3180. [PubMed] [Google Scholar]
- 18.Kakkar A.K., Dahiya N. Drug treatment of obesity: current status and future prospects. European Journal of Internal Medicine. 2015;26:89–94. doi: 10.1016/j.ejim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Arora T., Velagapudi V.R., Pournaras D.J., Welbourn R., Le Roux C.W., Oresic M. Roux-en-Y gastric bypass surgery induces early plasma metabolomic and lipidomic alterations in humans associated with diabetes remission. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huggins K.W., Boileau A.C., Hui D.Y. Protection against diet-induced obesity and obesity-related insulin resistance in Group 1B PLA2-deficient mice. American Journal of Physiology. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hui D.Y., Cope M.J., Labonte E.D., Chang H.-T., Shao J., Goka E. The Phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. British Journal of Pharmacology. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labonté E.D., Kirby R.J., Schildmeyer N.M., Cannon A.M., Huggins K.W., Hui D.Y. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–941. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang I., Park Y.J., Kim Y.-R., Kim Y.N., Ka S., Lee H.Y. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagen-like peptide 1 in diet-induced obesity. The FASEB Journal. 2015;29:2397–2411. doi: 10.1096/fj.14-265983. [DOI] [PubMed] [Google Scholar]
- 24.Singer A.G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. Journal of Biological Chemistry. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 25.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilham D., Labonté E.D., Rojas J.C., Jandacek R.J., Howles P.N., Hui D.Y. Carboxyl ester lipase deficiency exacerbates dietary lipid absorption abnormalities and resistance to diet-induced obesity in pancreatic triglyceride lipase knockout mice. Journal of Biological Chemistry. 2007;282:24642–24649. doi: 10.1074/jbc.M702530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wishart D.S., Tzur D., Knox C., Eisner R., Guo A.C., Young N. HMDB: the human metabolome database. Nucleic Acids Research. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., DuGar B. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs B., Muller K., Paasch U., Schiller J. Lysophospholipids: potential markers of diseases and infertility. Mini Reviews in Medicinal Chemistry. 2012;12:74–86. doi: 10.2174/138955712798868931. [DOI] [PubMed] [Google Scholar]
- 30.Bennett B.J., De Aguiar Vallim T.Q., Wang Z., Shih D.M., Meng Y., Gregory J.C. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabolism. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefater M.A., Sandoval D.A., Chambers A.P., Wilson-Perez H.E., Hofmann S.M., Jandacek R.J. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:939–949. doi: 10.1053/j.gastro.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay K., Starr J.R., Lawn R.M., Ellsworth J.L. Phosphatidylcholine hydrolysis is required for pancreatic cholesterol esterase- and phospholipase A2-facilitated cholesterol uptake into intestinal Caco-2 cells. Journal of Biological Chemistry. 1997;272:13380–13389. doi: 10.1074/jbc.272.20.13380. [DOI] [PubMed] [Google Scholar]
- 33.Richmond B.L., Boileau A.C., Zheng S., Huggins K.W., Granholm N.A., Tso P. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2 deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- 34.Hui D.Y. Intestinal phospholipid and lysophospholipid metabolism in cardiometabolic disease. Current Opinion in Lipidology. 2016;27:507–512. doi: 10.1097/MOL.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navab M., Chattopadhyay A., Hough G., Meriwether D., Fogelman S.I., Wagner A.C. Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. The Journal of Lipid Research. 2015;56:871–887. doi: 10.1194/jlr.M056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navab M., Hough G., Buga G.M., Su F., Wagner A.C., Meriwether D. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. The Journal of Lipid Research. 2013;54:3403–3418. doi: 10.1194/jlr.M042051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ussar S., Griffin N.W., Bezy O., Fujisaka S., Vienberg S., Softic S. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metabolism. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin G., Kolida S., Marchesi J.R., Want E., Sidaway J.E., Swann J.R. In vitro modeling of bile acid processing by the human fecal microbiota. Frontiers in Microbiology. 2018;9:1153. doi: 10.3389/fmicb.2018.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang C., Xie C., Li J., Krausz K.W., Shi J., Brocker C.N. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nature Communications. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nature Reviews Drug Discovery. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 41.Labonté E.D., Pfluger P.T., Cash J.G., Kuhel D.G., Roja J.C., Magness D.P. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. The FASEB Journal. 2010;24:2516–2524. doi: 10.1096/fj.09-144436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang W.H.W., Wang Z., Shrestha K., Borowski A.G., Wu Y., Troughton R.W. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic ststolic heart failure. Journal of Cardiac Failure. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romano K.A., Vivas E.I., Amador-Noguez D., Rey F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6 doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]