Abstract
Paeonia suffruticosa ‘Shima Nishiki’ is a very precious double-color cultivar because of its distinctive and colorful flowers. However, our understanding of the underlying mechanisms of its double-color formation is limited. The present study investigated the soluble sugar content, cell sap pH value and anatomical structure, anthocyanin composition and content and expression patterns of genes related to anthocyanin biosynthesis in the red and pink petals of the ‘Shima Nishiki’ cultivar. Here, we found that soluble sugar content, cell sap pH and the shape of outer epidermal cells were not the key factors that determine double-color formation. Five different anthocyanins were detected in both the red and pink petals, and the pelargonidin-3,5-di-O-glucoside (Pg3G5G) and pelargonidin-3-O-glucoside (Pg3G) contents in the red petals were significantly higher than those in the pink petals at every developmental stage. In addition, these gene expression patterns suggested that the significant differential expression of the dihydroflavonol 4-reductase gene (PsDFR) gene might play a key role in double-color formation. These results will provide a valuable resource for further studies unraveling the underlying genetic mechanisms of double-color formation in P. suffruticosa ‘Shima Nishiki’.
Keywords: P. suffruticosa ‘Shima Nishiki’, Double color, Anthocyanins, Gene expression
Introduction
Tree peony (Paeonia suffruticosa Andr.) is a highly popular ornamental plant that is valued for its large and attractive flowers of various colors (Ji et al. 2012; Zhang et al. 2015b). Among the many flower colors of tree peony, double color is extremely rare. P. suffruticosa ‘Shima Nishiki’ is a well-known double-color cultivar that has red and pink petals on the same flower. Importantly, the double color of this cultivar is stably inherited through ramets or grafting. There is no doubt that the showy color phenotype of this flower has great ornamental and commercial value (Zhao and Tao 2015). Therefore, the cultivar that has this trait is considered to be a valuable material for flower color breeding (Lim et al. 2011; Noman et al. 2017).
Several studies on flower color in tree peony have been performed, many of which have focused on color indices and qualitative and quantitative analyses of anthocyanins in monochromatic flowers, such as red, pink, white, yellow and purple flowers. Among these studies, Wang et al. (2001) conducted a comparative study on Zhongyuan (130, Chinese) and Daikon Island (37, Japanese) tree peony cultivars to analyze anthocyanin compositions in the petals in relation to flower colors. Furthermore, Zhang et al. (2007) investigated the nonblotched and blotched parts of 35 cultivars of Xibei tree peony to explore the pigment compositions of these cultivars by high-performance liquid chromatography (HPLC) analysis. Other investigations have involved anatomical observations (Yang et al. 2015), assessment of soluble sugar content (Zhang et al. 2015a) and expression analysis of genes associated with the anthocyanin biosynthetic pathway. Among these investigations, in P. suffruticosa ‘Luoyang Hong’, Zhang et al. (2014) performed temporal expression analysis of anthocyanin biosynthetic genes in on-plant and in-vase developing flowers at different developmental stages. In P. delavayi, Shi et al. (2015) performed quantitative real-time-polymerase chain reaction (qRT-PCR) analysis of the candidate differentially expressed genes (DEGs) likely involved in flavonoid biosynthesis in purple-red and yellow petals. However, studies on flower color in double-color tree peony cultivars are limited. Furthermore, some studies on flower color in other ornamental plants, such as P. lactiflora Pall., Rosa hybrida Hort., Anagallis monelli L. and Rhododendron simsii Planch., have also been performed focusing on the determination of cell sap pH (Quintana et al. 2007; Schmitzer et al. 2010; Nakatsuka et al. 2015; Zhao et al. 2016; Kim et al. 2018). All these factors may influence flower color in P. suffruticosa ‘Shima Nishiki’; hence, a thorough investigation of these factors may be helpful in understanding the mechanisms underlying double-color petal coloration.
Among these factors, various studies have confirmed that flavonoids, particularly anthocyanins, play a critical role in the determination of flower color in various ornamental plants (Weiss 2000; Nishihara and Nakatsuka 2011; Davies et al. 2012). In P. suffruticosa, the anthocyanin compositions of differently colored petals have been extensively studied (Hosoki et al. 1991). The differences in petal color can mainly be attributed to the content of six anthocyanins: cyanidin-3-O-glucoside (Cy3G), cyanidin-3,5-di-O-glucoside (Cy3G5G), peonidin-3-O-glucoside (Pn3G), peonidin-3,5-di-O-glucoside (Pn3G5G), pelargonidin-3-O-glucoside (Pg3G) and pelargonidin-3,5-di-O-glucoside (Pg3G5G). Several studies on flower color in P. suffruticosa ‘Shima Nishiki’ have been performed (Sakata et al. 1995; Fan et al. 2009); however, only a rough determination of the anthocyanin content of the red petals has been carried out. Furthermore, the underlying mechanisms of double-color formation in this particular cultivar have not yet been fully elucidated.
To more thoroughly explore the determinants of flower color variation between red and pink petals in P. suffruticosa ‘Shima Nishiki’, we observed the shape of the outer epidermal cells at the full bloom stage. Furthermore, we performed a series of measurements of color indices, soluble sugar content, cell sap pH value and anthocyanin composition and content at four flowering periods in this cultivar. Subsequent expression analysis of the anthocyanin biosynthetic genes and correlation analysis of several main factors that typically influence flower color were also performed. Our primary aim, based on these findings, was to more precisely determine the mechanisms underlying double-color formation in P. suffruticosa ‘Shima Nishiki’.
Materials and methods
Plant materials
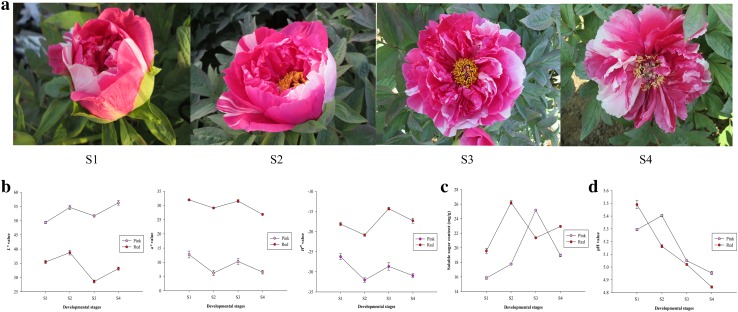
Tree peony (P. suffruticosa ‘Shima Nishiki’) was grown in the experimental nursery of Forestry College, Shandong Agricultural University, Tai’an City (36°18′N, 117°13′E), Shandong Province, China. The ‘Shima Nishiki’ cultivar with double-color flowers (red and pink petals) was selected as the experimental material. Red- and pink-petal samples were collected from the double-color petals of various plants at the end of April and could be divided into four developmental stages (Guo et al. 2004), namely, the soft bud stage (S1), initial bloom stage (S2), full bloom stage (S3) and senescent stage (S4) (Fig. 1a). Some petal samples were immediately used for anatomical observations, and the others were immediately frozen in liquid nitrogen and then stored at − 80 °C for further analysis after the measurement of color indices.
Fig. 1.
Flowers of P. suffruticosa ‘Shima Nishiki’ (a) and color indices (b), soluble sugar content (c) and cell sap pH value (d) of the red and pink petals. S1: soft bud stage; S2: initial bloom stage; S3: full bloom stage; S4: senescent stage
Color indices measurement
The color indices in the middle of the upper epidermis of the red and pink petals were measured using a CM-5 spectrophotometer (Konica Minolta Holdings, Inc., Japan). The petal colors were expressed as three main color parameters [lightness (L*), the ratio of red/magenta and green (a*) and hue angle (H0)]. Three points on each petal were measured. At every developmental stage, the flower color of red/pink-petal samples was determined for five replicates, and each replicate was collected from the double-color petals of at least three plants.
Soluble sugar content measurement
Soluble sugar content was measured using the method of Zhang et al. (2015a). A total of 0.1 g of petals of each sample was placed into a test tube and extracted with 5 mL of distilled water in a water bath at 100 °C for 30 min. After filtering, the supernatant was collected. This step was repeated twice, and distilled water was then added to a final volume of 10 mL. Subsequently, the soluble sugar content of each sample was measured using the sulfuric acid anthrone method at a wavelength of 630 nm. The measurements were made on five replicates.
Cell sap pH determination
A total of 7 g of petals of each sample was ground with quartz and centrifuged at 13,000 rpm for 20 min. This centrifugation step was repeated twice, and the supernatant was immediately transferred to a new, 10 mL centrifuge tube. The pH of the solution was measured using a PHS-3C pH meter (Shanghai INESA Scientific Instrument Co. Ltd., China). The determinations were made in five replicates.
Anatomical observations
Following the method of Stabentheiner et al. (2010) with some modifications, 0.25 cm2 (0.5 cm × 0.5 cm) of tissues of the red and pink petals was first fixed with 3.5% glutaraldehyde solution for 24 h at 4 °C and then postfixed with 1% osmic acid solution for 4 h. Next, the fixed tissues were dehydrated by using sequential ethanol concentrations (30, 50, 70, 80, 90 and 100%) with 20 min of exposure per concentration, and the ethanol was then replaced by isoamyl acetate. After dehydration, the samples were dried with liquid carbon dioxide by a critical-point drying method. Subsequently, the dry samples were mounted with tape to a specimen stage and sputter coated with gold. Finally, the shape of the outer epidermal cells of the samples was observed by a JSM-6610LV scanning electron microscope (JEOL Ltd., Japan) at magnifications of 200×, 1000× and 5000×.
Qualitative and quantitative analyses of anthocyanins
Anthocyanin extraction was performed according to Zhao et al. (2014). In total, 0.3 g of petals of each sample was extracted with 5 mL of acidic methanol solution (CH3OH:HCl:H2O = 70:0.1:29.9, v/v/v) at 4 °C in the dark for 24 h. Qualitative and quantitative analyses of anthocyanins were performed using HPLC, and the specific method of determination was modified from that of Yang et al. (2015). Cy3G5G, Pg3G5G, Pn3G5G, Cy3G, Pg3G and Pn3G were used as standards for accurate quantitative analysis of anthocyanins. By comparing the retention time and peak area with those of the six anthocyanin standards at a wavelength of 520 nm, the anthocyanin component and accumulation of each sample could be precisely determined. Anthocyanin content was determined in five replicates.
Gene expression analysis
Based on previous studies related to the flower color of tree peony (Zhang et al. 2014, 2015c), seven structural genes in the anthocyanin biosynthetic pathway, namely, chalcone synthase (PsCHS), chalcone isomerase (PsCHI), flavanone 3-hydroxylase (PsF3H), flavonoid 3′-hydroxylase (PsF3′H), anthocyanin O-methyltransferase (PsAOMT), dihydroflavonol 4-reductase (PsDFR) and anthocyanidin synthase (PsANS), were selected as candidate genes in this study. The expression levels of the seven genes were analyzed using qRT-PCR with a Bio-Rad CFX96™ real-time system (Bio-Rad, USA). The cDNA was synthesized with 1 µg of total RNA using 5 × All-In-One RT MasterMix (with an AccuRT Genomic DNA Removal Kit) (ABM, Canada). The qRT-PCR experiments were performed using SYBR® Premix Ex Taq™ (Tli RNaseH Plus) (TaKaRa, Japan) with three replicates. The Psubiquitin gene was used as an internal control to normalize the expression data in this study. All gene-specific primers used in this study are shown in Table 1. The relative expression levels of genes were calculated using the 2−ΔΔCt method (Schmittgen and Livak 2008), and the expression level of each gene in the pink petals at S1 was used as the control (Zhao et al. 2015).
Table 1.
Primer sequences of the genes used for qRT-PCR
| Genes’ name | Forward primer (5ʹ–3ʹ) | Reverse primer (5ʹ–3ʹ) |
|---|---|---|
| PsCHS | AGCAGAGAACAACAAAGGGTCACG | TCAGCACCGACAATAACCGCAG |
| PsCHI | TCCCACCTGGTTCTTCTA | AACTCTGCTTTGCTTCCG |
| PsF3H | CCCAAGGTAGCCTACAACCAA | GAAAATCCCCCAGTCTTCACA |
| PsF3′H | AACTTGTTCACGGCAGGGACT | GGCTTGGGCTAGGATTTTAGG |
| PsDFR | TTCATCGGTTCATGGCTTGTC | AATGGGTATCCGCTTTTGGC |
| PsANS | GCCCTCACTTTCATCCTCCACAAC | AAAACTGCCCACGAAATCCTTACCT |
| PsAOMT | CGAGAGTGCAACTGAACCAA | GAGCCATTGATGAAGCTGGT |
| Psubiquitin | GACCTATACCAAGCCGAAG | CGTTCCAGCACCACAATC |
Statistical analysis
All data are presented as the mean ± standard error of at least three biological replicates. Variance and correlation analyses were performed using SPSS software ver. 17.0 (SPSS Inc., USA). P values of < 0.05 were considered to be statistically significant.
Results
Color indices
The color indices were measured and expressed as L*, a* and H0 values (Fig. 1b). At the four developmental stages (S1, S2, S3 and S4), the L* values of the pink/red-petal samples increased from S1 to S2 and from S3 to S4, respectively. However, they both decreased from S2 to S3. Moreover, the L* values of the pink-petal samples were consistently higher than those of the red-petal samples at every developmental stage. Compared with the L* values, the a*/H0 values of these two samples showed the opposite trend.
Soluble sugar content
The soluble sugar content of the red and pink petals at different developmental stages was measured (Fig. 1c). At S3, the soluble sugar content of the pink-petal samples was higher than that of the red-petal samples, with a highest value of 25.16 mg/g. However, at S1, S2 and S4, the opposite pattern was observed in these two samples, with the red-petal samples showing the highest value (26.21 mg/g). Moreover, the correlation coefficient between soluble sugar content and a* was only 0.403, and the P value was 0.323 (Table 2). These results indicate that no significant correlation occurs between soluble sugar content and flower color.
Table 2.
Correlation analysis of a* and several main factors influencing flower color (cell sap pH, soluble sugar and four main anthocyanins) in P. suffruticosa ‘Shima Nishiki’
| Cell sap pH | Soluble sugar | Pg3G5G | Pg3G | Pn3G5G | Pn3G | |
|---|---|---|---|---|---|---|
| a* | ||||||
| Correlation coefficient | − 0.017 | 0.403 | 0.852** | 0.849** | 0.67 | 0.621 |
| P valuea | 0.968 | 0.323 | 0.007 b | 0.008 b | 0.069 | 0.100 |
aThe significance (*P < 0.05, **P < 0.01) was calculated by two-tailed test
bThe bold data indicate the statistical significance of relationships
Cell sap pH
The cell sap pH values of the red and pink petals at different developmental stages ranged between 4.84 and 5.49 (Fig. 1d). The pH values of the red-petal samples all exhibited a downward trend from S1 to S4. However, the pink-petal samples had the highest pH value at S2, and the pH values of the pink-petal samples also exhibited a decreasing trend during the other three developmental stages. Moreover, the pH values of the pink-petal samples were consistently higher than those of the red-petal samples during the other three developmental stages, except for S1. The correlation coefficient between cell sap pH value and a* was only 0.017, and the P value was 0.968 (Table 2). These results indicate that cell sap pH and flower color are not significantly correlated.
Anatomical structures
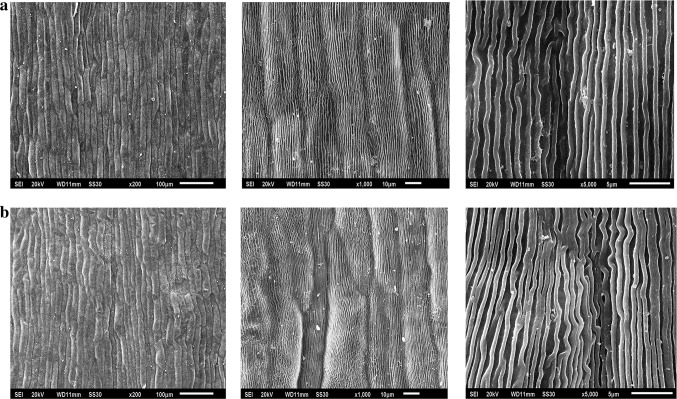
The outer epidermal cell shape of the red and pink petals at the full bloom stage was observed. The shapes of the pink-petal samples (Fig. 2a) were highly similar to those of the red-petal samples (Fig. 2b), and both types of petal samples were densely and unevenly papillated at a magnification of 200×, indicating that their light absorption characteristics are highly similar.
Fig. 2.
The shape of outer epidermal cells of the pink petals (a, from left to right) and the red petals (b, from left to right) of P. suffruticosa ‘Shima Nishiki’ at magnifications of ×200, ×1000 and ×5000
Qualitative and quantitative analyses of anthocyanins
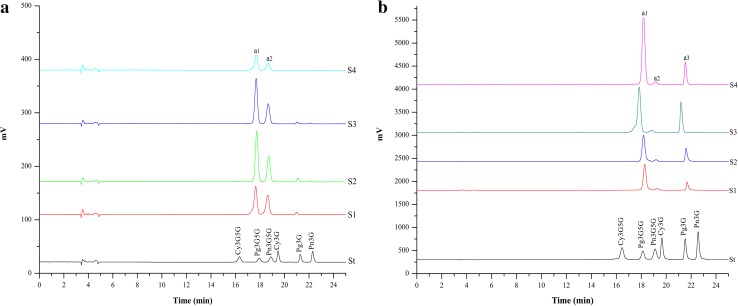
Based on the retention times and peak areas of these six anthocyanin standards at 520 nm, the anthocyanin composition and content of the red and pink petals were measured at different developmental stages using HPLC, respectively. Figure 3a, b shows that the chromatographic peaks of the pink- and red-petal samples from S1 to S4 were essentially similar, but differed in terms of peak area. The anthocyanin composition and content results are shown in Table 3. The compositions of the red- and pink-petal samples at different developmental stages were identical, and five anthocyanins (Cy3G5G, Pg3G5G, Pn3G5G, Pg3G and Pn3G) were detected in both types of petal samples. However, some differences in pigment compositions were noted between the two petal colors. Pg3G5G, Pn3G5G and Pg3G were the major anthocyanins in the red-petal samples, whereas Pg3G5G and Pn3G5G were dominant in the pink-petal samples. With regard to anthocyanin content, the total anthocyanins (TA) content in the red-petal samples was always significantly higher than that in the pink-petal samples at every developmental stage. Furthermore, the average Pg3G5G and Pg3G contents in the red-petal samples were 14.79- and 75.23-fold higher than those in the pink-petal samples, respectively.
Fig. 3.
HPLC chromatograms of the anthocyanins of the pink petals (a) and red petals (b) of P. suffruticosa ‘Shima Nishiki’ (detected at 520 nm) from S1 to S4. St indicates the HPLC chromatograms for a combination of the six anthocyanin standards. a1, a2 and a3 indicate Pg3G5G, Pn3G5G and Pg3G, respectively
Table 3.
Anthocyanin composition and content of red and pink petals of P. suffruticosa ‘Shima Nishiki’ during flower development
| Sample | Anthocyanin content (µg/g) | ||||||
|---|---|---|---|---|---|---|---|
| Cy3G5G | Pg3G5G | Pn3G5G | Cy3G | Pg3G | Pn3G | TA | |
| Reda | |||||||
| S1 | 1.75 ± 0.01 | 1748.42 ± 16.00 | 107.12 ± 2.08 | – | 275.43 ± 3.00 | 3.30 ± 0.19 | 2136.02 ± 12.62 |
| S2 | 2.86 ± 0.05 | 2189.49 ± 32.06 | 128.78 ± 1.81 | – | 514.67 ± 30.13 | 6.78 ± 0.41 | 2842.58 ± 53.33 |
| S3 | 3.34 ± 0.13 | 2854.14 ± 13.86 | 115.61 ± 6.69 | – | 855.59 ± 6.09 | 8.95 ± 0.22 | 3837.64 ± 24.15 |
| S4 | 1.15 ± 0.10 | 3761.17 ± 67.70 | 104.25 ± 1.86 | – | 647.28 ± 21.85 | 18.06 ± 1.41 | 4531.91 ± 82.12 |
| Pinkb | |||||||
| S1 | 0.63 ± 0.01 | 137.44 ± 2.56 | 87.69 ± 1.05 | – | 5.37 ± 0.24 | 1.22 ± 0.05 | 232.35 ± 3.42 |
| S2 | 0.97 ± 0.07 | 259.53 ± 2.91 | 107.34 ± 9.52 | – | 16.20 ± 0.69 | 1.63 ± 0.08 | 385.68 ± 10.71 |
| S3 | 0.73 ± 0.06 | 227.16 ± 3.72 | 94.51 ± 1.46 | – | 7.15 ± 0.18 | 1.90 ± 0.03 | 331.45 ± 3.21 |
| S4 | 0.45 ± 0.06 | 89.31 ± 1.22 | 49.58 ± 1.71 | – | 1.76 ± 0.22 | 0.57 ± 0.04 | 141.66 ± 2.38 |
aRed: red petals of Paeonia suffruticosa ‘Shima Nishiki’
bPink: pink petals of Paeonia suffruticosa ‘Shima Nishiki’
Correlation analysis between a* and anthocyanin content showed that the correlation coefficients between a* and Pg3G5G, Pg3G, Pn3G5G and Pn3G were 0.852, 0.849, 0.670 and 0.621, and the P values were 0.007, 0.008, 0.069 and 0.100, respectively (Table 2). These results indicate that a significant correlation exists between flower color and two of the anthocyanins (Pg3G5G and Pg3G).
Expression analysis of anthocyanin biosynthetic genes
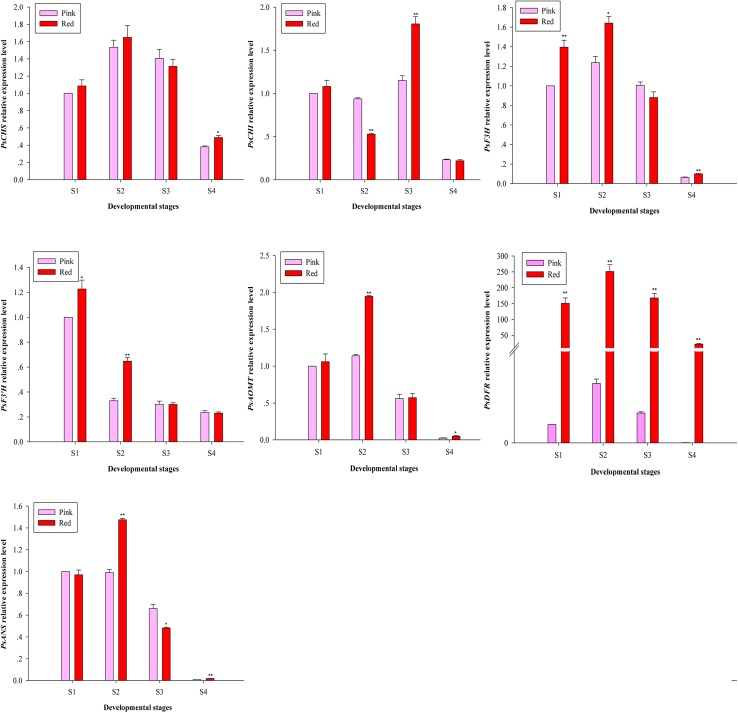
To further explore the molecular mechanisms of double-color formation in P. suffruticosa ‘Shima Nishiki’, the expression patterns of seven structural genes in the anthocyanin biosynthetic pathway were analyzed using qRT-PCR (Fig. 4).
Fig. 4.
Expression analysis of anthocyanin biosynthetic structural genes in P. suffruticosa ‘Shima Nishiki’ at the four developmental stages. Asterisks indicate significant differences (*P < 0.05, **P < 0.01)
Among the seven genes, the expression levels of PsCHS, PsF3H, PsAOMT, PsDFR and PsANS in the red- and pink-petal samples displayed similar trends with flower development, peaking at S2 and then decreasing from S2 to S4. Slight differences in the expression level of PsCHI were detected in comparison to the five genes mentioned above, and both types of petal samples reached the maximum levels at S3. Moreover, the expression level of PsF3′H in the red- and pink-petal samples exhibited a general downward trend from S1 to S4.
In terms of petal color, PsCHS, PsCHI, PsF3H, PsF3′H PsAOMT,and PsANS generally showed higher expression levels in the red-petal samples than in the pink-petal samples at three developmental stages. Moreover, only PsDFR showed significantly higher expression levels in the red-petal samples at every developmental stage. The expression level of PsDFR in the red-petal samples was 197.73-, 72.52-, 101.20- and 1276.12-fold higher than that in the pink-petal samples from S1 to S4, respectively.
Discussion
To explore the causes of double-color formation in P. suffruticosa ‘Shima Nishiki’, a quantitative evaluation of flower color was performed as the first step in this study. Many studies on flower color have been performed using chroma meter due to its relative accuracy and scientificity. In P. suffruticosa, Yang et al. (2015) found that the L* values of ‘Xiaguang’ gradually increased, whereas the corresponding a* and H0 values decreased with flower development. Moreover, in Rhododendron mucronulatum, petal color indices (L*, a* and H0 values) fluctuated during flower development (Li et al. 2008). In our study, L*, a* and H0 values were also used to assess flower color changes, and the results were basically consistent with the above-mentioned results. In terms of the differences between the two petal colors, our results showed that the red-petal samples all had significantly higher a*/H0 values and lower L* values than the pink-petal samples from S1 to S4.
We then further explored and analyzed the causes of color variations in double-color flowers based on four main aspects that influence flower color. In terms of the shape of the outer epidermal cells, it is generally believed that a conical shape increases the proportion of incident light that enters the epidermal cells, enhancing the intensity of their color (Noda et al. 1994). In our study, there were no detectable differences in shape between the red- and pink-petal samples. The results showed that the shape of the outer epidermal cells does not play a key role in double-color formation. In terms of vacuolar pH, Stewart et al. (1975) found that the pH value of the petals of most plants ranges from 2.5 to 7.5. In the present study, the cell sap pH value of the pink- and red-petal samples ranged from 4.95 to 5.40 and 4.84–5.49, respectively, which corroborates the findings of Stewart et al. (1975). Additionally, correlation analysis between cell sap pH and flower color indicated that cell sap pH is not a key factor determining the double-color phenotype. Soluble sugar can be used to produce anthocyanins (Zheng et al. 2009; Zhang et al. 2015a); thus, its content may influence petal color. However, correlation analysis between soluble sugar content and flower color showed that soluble sugar content also is not a key factor influencing the double-color phenotype.
In many ornamental plants, the composition or content differences in anthocyanins are factors dictating flower color variation. In Lycoris longituba, He et al. (2011) found that four anthocyanins (Cy3So, Cy3XyGlc, Cy3Sa and Pg3XyGlc) occurred at various amounts in the petals of 44 cultivars with different petal colors. Moreover, Shi et al. (2014) found that cyanidin-3-O-glucoside is the main anthocyanin in Magnolia sprengeri, with a 26-fold higher content in the red petals than in the white petals. Furthermore, it was found that Pn3G5G and Cy3G5G were the main anthocyanins influencing flower color in P. ostii with white, light-pink and deep-pink flowers (Gao et al. 2016). In the present study, the red- and pink-petal samples generally had the same anthocyanin composition, whereas significant differences in anthocyanin content (especially in Pg3G5G and Pg3G) were observed. Moreover, correlation analysis of anthocyanin content and flower color indicated that Pg3G and Pg3G5G, which had the highest correlation coefficients and the lowest P values, should strongly influence double-color formation.
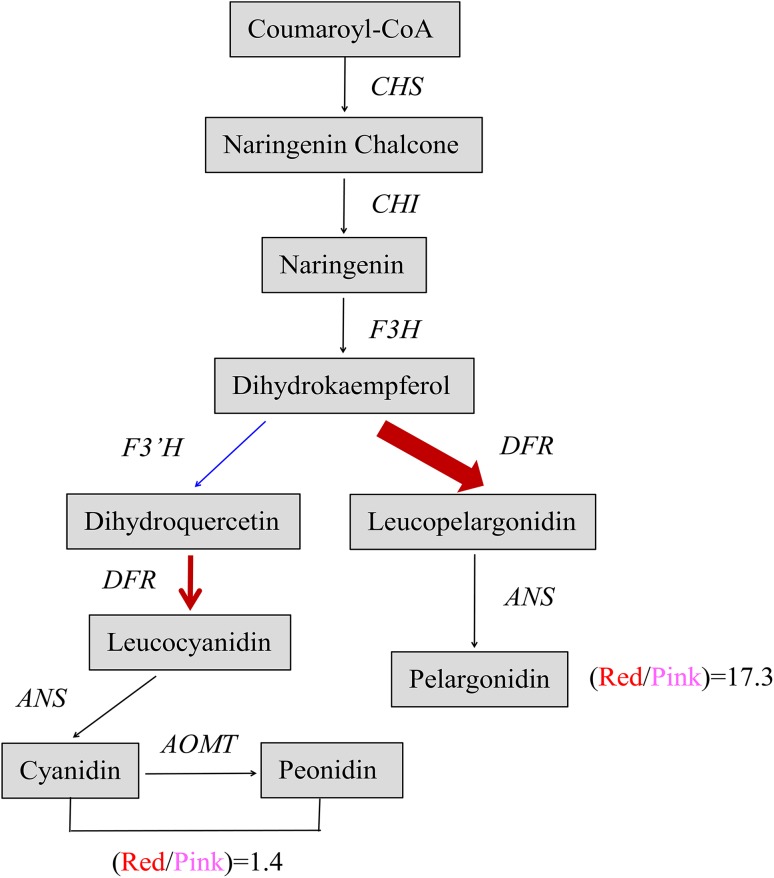
Anthocyanin biosynthesis is regulated by a series of structural genes and regulatory genes, among which the significant differential expression of specific structural genes is directly associated with flower color variations (Nakatsuka et al. 2005; Grotewold 2006). For example, in Prunus persica f. versicolor, CHS and F3H exhibited significantly higher expression levels in the red petals than in the white petals and were confirmed to play key roles in the flower color variations of variegated flowers (Chen et al. 2014). In P. suffruticosa ‘Jinrong’, PsCHS, PsF3H, PsDFR, and PsANS were expressed at a significantly higher level in the purple-spotted area than in the white nonspotted area (Zhang et al. 2015c). In our study, only PsDFR showed significantly higher expression levels in the red-petal samples than in the pink-petal samples at the four developmental stages. However, this pattern of differential expression was not observed for the eight other genes. In the red- and pink-petal samples, the expression level of PsF3′H was very low, whereas that of PsDFR was high, causing a large portion of the dihydrokaempferol (DHK) to move downstream toward the Pg branch and a smaller portion to move toward the Cy and Pn branch. Furthermore, the higher expression of PsDFR in the red-petal samples than in the pink-petal samples would catalyze a much larger portion of the DHK to move toward the Pg branch in the former than in the latter. These findings indicate that PsDFR plays an extremely critical role in mediating double-color formation (Fig. 5).
Fig. 5.
The biochemical link between the activity of PsDFR and anthocyanin content in P. suffruticosa ‘Shima Nishiki’. Red lines indicate the expression level of PsDFR in the red or white petals, and the blue line indicates that of PsF3′H. “Red/Pink = 1.4” indicates the average content ratio of three glycosides of cyanidin and peonidin in the red- and pink-petal samples at the four development stages, and “Red/Pink = 17.3” indicates that of two glycosides of pelargonidin
In conclusion, from a physiological perspective, the markedly higher accumulation of Pg3G5G and Pg3G in the red petals is responsible for the flower color differences in P. suffruticosa ‘Shima Nishiki’. Moreover, at the molecular level, the significant differential expression of PsDFR directly determines the differences in anthocyanin content between the red and pink petals, thereby mediating double-color formation. The results of this study provide valuable resources for further studies aimed at unraveling the underlying genetic mechanisms of double-color formation in P. suffruticosa ‘Shima Nishiki’.
Acknowledgements
This project was funded by the National Science Foundation of China (NSFC) (31700622).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Lanyong Zhao, Email: zlynd369@163.com.
Zongda Xu, Email: xuzoda@163.com.
References
- Chen YN, Mao Y, Liu HL, Yu FX, Li SX, Yin TM. Transcriptome analysis of differentially expressed genes relevant to variegation in peach flowers. PLoS One. 2014;9:e90842. doi: 10.1371/journal.pone.0090842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Albert NW, Schwinn KE. From landing lights to mimicry: the molecular regulation of flower coloration and mechanisms for pigmentation patterning. Funct Plant Biol. 2012;39:619–638. doi: 10.1071/FP12195. [DOI] [PubMed] [Google Scholar]
- Fan JL, Zhu WX, Shen JW, Ma HL. Determination of the contents of anthocyanins and flavonols in petals of red flowered cultivars of Zhongyuan tree peony. North Hortic. 2009;10:191–194. [Google Scholar]
- Gao LX, Yang HX, Liu HF, Yang J, Hu YH. Transcriptome changes underlying the flower color intensity variation in Paeonia ostii. Front Plant Sci. 2016;6:1205. doi: 10.3389/fpls.2015.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- Guo WW, Dong L, Wang LY, Chen RX, Liu AQ. The postharvest characteristics and water balance of some cultivars of tree-peony cut flowers. Sci Silvae Sin. 2004;40:89–93. [Google Scholar]
- He QL, Shen Y, Wang MX, Huang MR, Yang RZ, Zhu SJ, Wang LS, Xu YJ, Wu RL. Natural variation in petal color in Lycoris longituba revealed by anthocyanin components. PLoS One. 2011;6:e22098. doi: 10.1371/journal.pone.0022098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki T, Hamada M, Kando T, Moriwaki R, Inaba K. Comparative study of anthocyanins in tree peony flowers. J Jpn Soc Hortic Sci. 1991;60:395–403. doi: 10.2503/jjshs.60.395. [DOI] [Google Scholar]
- Ji LJ, Wang Q, da Silva JAT, Yu XN. The genetic diversity of Paeonia L. Sci Hortic. 2012;143:62–74. doi: 10.1016/j.scienta.2012.06.011. [DOI] [Google Scholar]
- Kim DH, Park S, Lee JY, Ha SH, Lim SH. Enhancing flower color through simultaneous expression of the B-peru and mPAP1 transcription factors under control of a flower-specific promoter. Int J Mol Sci. 2018;19:309. doi: 10.3390/ijms19010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Wang LS, Shu YQ, Xu YJ, Zhang J. Pigments composition of petals and floral color change during the blooming period in Rhododendron mucronulatum. Acta Hortic Sin. 2008;35:1023–1030. [Google Scholar]
- Lim SH, Kim JK, Kim DH, Sohn SH, Lee JY, Kim YM, Ha SH. Flower color modification by manipulating flavonoid biosynthetic pathway. Kor J Hort Sci Technol. 2011;29:511–522. [Google Scholar]
- Nakatsuka T, Nishihara M, Mishiba K, Yamamura S. Temporal expression of flavonoid biosynthesis—related genes regulates flower pigmentation in gentian plants. Plant Sci. 2005;168:1309–1318. doi: 10.1016/j.plantsci.2005.01.009. [DOI] [Google Scholar]
- Nakatsuka A, Hitomi M, Tsuma M, Ito A, Mizuta D, Kobayashi N. Effect of anthocyanin profile and petal pH on flower coloration in evergreen azalea. Acta Hortic. 2015;48:357–362. doi: 10.17660/ActaHortic.2015.1104.53. [DOI] [Google Scholar]
- Nishihara M, Nakatsuka T. Genetic engineering of flavonoid pigments to modify flower color in floricultural plants. Biotechnol Lett. 2011;33:433–441. doi: 10.1007/s10529-010-0461-z. [DOI] [PubMed] [Google Scholar]
- Noda K, Glover BJ, Linstead P, Martin C. Flower color intensity depends on specialized cell shape controlled by a Myb–related transcription factor. Nature. 1994;369:661–664. doi: 10.1038/369661a0. [DOI] [PubMed] [Google Scholar]
- Noman A, Aqeel M, Deng JM, Khalid N, Sanaullah T, Shuilin H. Biotechnological advancements for improving floral attributes in ornamental plants. Front Plant Sci. 2017;8:530. doi: 10.3389/fpls.2017.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Albrechtová J, Griesbach RJ, Freyre R. Anatomical and biochemical studies of anthocyanidins in flowers of Anagallis monelli L. (Primulaceae) hybrids. Sci Hortic. 2007;112:413–421. doi: 10.1016/j.scienta.2007.01.024. [DOI] [Google Scholar]
- Sakata Y, Aoki N, Tsunematsu S, Nishikouri H, Johjima T. Petal coloration and pigmentation of tree peony bred and selected in Daikon Island (Shimane Prefecture) J Jpn Soc Hortic Sci. 1995;64:351–357. doi: 10.2503/jjshs.64.351. [DOI] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schmitzer V, Veberic R, Osterc G, Stampar F. Color and phenolic content changes during flower development in groundcover Rose. J Am Soc Hortic Sci. 2010;135:195–202. [Google Scholar]
- Shi SG, Yang M, Zhang M, Wang P, Kang YX, Liu JJ. Genome-wide transcriptome analysis of genes involved in flavonoid biosynthesis between red and white strains of Magnolia sprengeri pamp. BMC Genom. 2014;15:706. doi: 10.1186/1471-2164-15-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi QQ, Zhou L, Wang Y, Li K, Zheng BQ, Miao K. Transcriptomic analysis of Paeonia delavayi wild population flowers to identify differentially expressed genes involved in purple-red and yellow petal pigmentation. PLoS One. 2015;10:e0135038. doi: 10.1371/journal.pone.0135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabentheiner E, Zankel A, Pölt P. Environmental scanning electron microscopy(ESEM)—a versatile tool in studying plants. Protoplasma. 2010;246:89–99. doi: 10.1007/s00709-010-0155-3. [DOI] [PubMed] [Google Scholar]
- Stewart RN, Norris KH, Asen S. Microspectrophotometric measurement of pH and pH effect on color of petal epidermal cells. Phytochemistry. 1975;14:937–942. doi: 10.1016/0031-9422(75)85162-4. [DOI] [Google Scholar]
- Wang LS, Shiraishi A, Hashimoto F, Aoki N, Shimizu K, Sakata Y. Analysis of petal anthocyanins to investigate flower coloration of Zhongyuan (Chinese) and Daikon Island (Japanese) tree peony cultivars. J Plant Res. 2001;114:33–43. doi: 10.1007/PL00013966. [DOI] [Google Scholar]
- Weiss D. Regulation of flower pigmentation and growth: multiple signaling pathways control anthocyanin synthesis in expanding petals. Physiol Plant. 2000;110:152–157. doi: 10.1034/j.1399-3054.2000.110202.x. [DOI] [Google Scholar]
- Yang Q, Yuan T, Sun XB. Preliminary studies on the changes of flower color during the flowering period in two tree peony cultivars. Acta Hortic Sin. 2015;42:930–938. [Google Scholar]
- Zhang JJ, Wang LS, Shu QY, Liu ZA, Li CH, Zhang J, Wei XL, Tian DK. Comparison of anthocyanins in non–blotches and blotches of the petals of Xibei tree peony. Sci Hortic. 2007;114:104–111. doi: 10.1016/j.scienta.2007.05.009. [DOI] [Google Scholar]
- Zhang C, Wang WN, Wang YJ, Gao SL, Du DN, Fu JX, Dong L. Anthocyanin biosynthesis and accumulation in developing flowers of tree peony (Paeonia suffruticosa) ‘Luoyang Hong’. Postharvest Biol Technol. 2014;97:11–22. doi: 10.1016/j.postharvbio.2014.05.019. [DOI] [Google Scholar]
- Zhang C, Fu JX, Wang YJ, Gao SL, Du DN, Wu F, Guo J, Dong L. Glucose supply improves petal coloration and anthocyanin biosynthesis in Paeonia suffruticosa ‘Luoyang Hong’ cut flowers. Postharvest Biol Technol. 2015;101:73–81. doi: 10.1016/j.postharvbio.2014.11.009. [DOI] [Google Scholar]
- Zhang YX, Zhan L, Gai SP, Liu CY, Lu S. Cloning and expression analysis of the R2R3- PsMYB1 gene associated with bud dormancy during chilling treatment in the tree peony (Paeonia suffruticosa) Plant Growth Regul. 2015;75:667–676. doi: 10.1007/s10725-014-9968-y. [DOI] [Google Scholar]
- Zhang YZ, Cheng YW, Ya HY, Xu SZ, Han JM. Transcriptome sequencing of purple petal spot region in tree peony reveals differentially expressed anthocyanin structural genes. Front Plant Sci. 2015;6:964. doi: 10.3389/fpls.2015.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DQ, Tao J. Recent advances on the development and regulation of flower color in ornamental plants. Front Plant Sci. 2015;6:261. doi: 10.3389/fpls.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DQ, Jiang Y, Ning CL, Meng JS, Lin SS, Ding W, Tao J. Transcriptome sequencing of a chimaera reveals coordinated expression of anthocyanin biosynthetic genes mediating yellow formation in herbaceous peony (Paeonia lactiflora Pall.) BMC Genom. 2014;15:689. doi: 10.1186/1471-2164-15-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DQ, Tang WH, Hao ZJ, Tao J. Identification of flavonoids and expression of flavonoid biosynthetic genes in two coloured tree peony flowers. Biochem Biophys Res Commun. 2015;459:450–456. doi: 10.1016/j.bbrc.2015.02.126. [DOI] [PubMed] [Google Scholar]
- Zhao DQ, Wei MR, Liu D, Tao J. Anatomical and biochemical analysis reveal the role of anthocyanins in flower coloration of herbaceous peony. Plant Physiol Biochem. 2016;102:97–106. doi: 10.1016/j.plaphy.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Zheng YJ, Li T, Liu HT, Pan QH, Zhan JC, Huang WD. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009;58:251–260. doi: 10.1007/s10725-009-9373-0. [DOI] [Google Scholar]