Abstract
Purpose
Follistatin-like protein 1 (FSTL1) is a secreted glycoprotein that has been shown to play a role in various types of cancer. However, the clinical significance and function of FSTL1 in breast cancer have not been reported. We investigated the role of FSTL1 in breast cancer in this study.
Methods
Enzyme-linked immunosorbent assays, western blot analysis, and reverse transcription polymerase chain reaction were used to monitor the expression of FSTL1 in breast cancer tissue and in serum samples from breast cancer patients. We employed a 4T1 breast cancer model and Fstl1+/− mice for in vivo studies. Hematoxylin and eosin staining, western blot analysis, and RNA sequencing were used to analyze the effect of FSTL1 on primary tumor growth and lung metastasis.
Results
We demonstrated that the expression of FSTL1 is reduced in both the breast cancer tissue and the serum of breast cancer patients. We showed that reduced levels of FSTL1 in serum correlate with elevated expression of Ki-67 and epidermal growth factor receptor (EGFR) in cancer tissues. Moreover, lowered expression of FSTL1 was associated with decreased survival in breast cancer patients. Experiments on the Fstl1+/− mouse model established that FSTL1 deficiency had no effect on primary tumor growth, but increased the lung metastases of breast cancer cells, resulting in reduced survival of tumor-bearing mice. RNA sequencing found significantly reduced expression of Egln3 and increased expression of EGFR in Fstl1+/− mice. Thus, our results suggest that FSTL1 may affect the expression of EGFR through Egln3, inhibiting the proliferation of breast cancer cells at lung metastatic sites.
Conclusion
In conclusion, we suggest a suppressor role of FSTL1 in breast cancer lung metastasis. Furthermore, FSTL1 may represent a potential prognostic biomarker and a candidate therapeutic target in breast cancer patients.
Keywords: Breast neoplasms, Follistatin-related proteins, Neoplasm metastasis, Tumor suppressor genes
INTRODUCTION
Breast cancer is one of the most frequent malignant diseases among women. Its incidence has been steadily increasing in China [1]. Triple-negative breast cancer (TNBC) accounts for only 10% to 20% patients [2]. However, due to its aggressive behavior and resistance to therapy, the survival rate of TNBC patients, for whom there is currently no effective treatment, is less than 30%. Finding a targeted therapy for these patients is therefore urgently needed. Epidermal growth factor receptor (EGFR), a receptor tyrosine kinase of the ErbB receptor family, is abnormally activated in many epithelial tumors, including TNBC. Cancer cells become addicted to EGFR signaling, providing a strong rationale for the development of EGFR-targeting drugs. Studies have shown that 70% to 80% of TNBC patients are positive for EGFR, which has been used as an indicator for adverse TNBC prognosis [3]. Notably, the proliferation of TNBC cells has been found to be inhibited by anti-EGFR antibodies [4]. Thus, EGFR and its downstream signaling molecules can be used as targets for the treatment of TNBC [4].
Follistatin-like protein 1 (FSTL1, also known as TSC-36, or FRP) is a secreted glycoprotein [5]. It was initially cloned from the mouse osteoblastic cell line MC3T3-E1 and has been shown to be upregulated by transforming growth factor β1 (TGF-β1) [5]. FSTL1 belongs to the matricellular group of proteins but does not form part of the extracellular matrix structure. Recent reports indicate that FSTL1 is involved in embryonic development [6], rheumatoid arthritis [7], and cardiovascular disease [8]. FSTL1 has also been shown to regulate lung development and distal lung epithelial differentiation by antagonizing bone morphogenetic protein 4 signaling [6].
FSTL1 also plays an important role in epithelial cancers. However, its function in tumorigenesis is controversial. Overexpression of FSTL1 is associated with poor prognosis in glioblastoma patients [9] and promotes the growth and progression of castration-resistant prostate cancer [10]. A recent study found that FSTL1 also functions as an oncogene in esophageal squamous cell carcinoma development and metastasis [11]. In contrast, FSTL1 is downregulated in nasopharyngeal carcinoma [12], lung cancer [13], and endometrial and ovarian cancers [14]. FSTL1 has been shown to possess antiangiogenic activity in pancreatic cancer [15] and predicts a poor prognosis in metastatic clear-cell renal cell carcinoma [16]. In ovarian cancer and endometrial cancer, FSTL1 promotes apoptosis and inhibits cell proliferation and migration [14]. Thus, these studies demonstrate the existence of organ-specific roles for FSTL1 in cancer. However, the clinical significance and functional role of FSTL1 in breast cancer remain largely unknown.
Egnl3 encodes prolyl hydroxylase domain protein 3 (PHD3) and has been identified as a key regulator of hypoxia-inducible factors. A tumor-suppressive effect of Egln3 has been reported in several types of cancer [17]. Recent studies demonstrated that PHD3 regulates EGFR signaling by promoting EGFR endocytosis, inhibiting EGFR signaling and cell proliferation [18,19]. However, the relationship of Egln3 and FSTL1 has not been reported.
This is the first study to show that the expression of FSTL1 is downregulated in breast cancer, and that this downregulation is associated with reduced survival of breast cancer patients. The goal of this study was to establish the role of FSTL1 in breast cancer growth and lung metastasis. We demonstrated that FSTL1 deficiency has no effect on primary tumor growth but promotes lung metastases. We found that FSTL1 in the lung microenvironment regulates Egln3 expression which, in turn, has been shown to inhibit EGFR signaling and tumor growth. To our knowledge, this is the first study to analyze the role of FSTL1 in breast cancer metastasis in vivo.
METHODS
Patients and samples
Blood samples were collected from breast cancer patients who underwent surgery at the Cancer Hospital of Huanxing Chaoyang District Beijing. Normal sera were obtained from healthy individuals. A total of 94 serum samples from breast cancer patients and 21 samples from age-matched healthy women were used in this study. The clinical parameters of patients, including Ki-67, EGFR, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and p53, were measured. Our research investigated the characteristics of the patients and controls according to the Beijing Administration Office. This study was approved by the Cancer Hospital of Huanxing Chaoyang District Beijing (IRB number: 2017-01). Informed consent was obtained, in writing, from all patients.
Measurement of serum FSTL1 levels by enzyme-linked immunosorbent assay
Blood samples were collected at the first admission, before patients received any therapy. Blood was collected by venipuncture and left untouched for 1 hour to allow coagulation. Two hours later, the tubes were centrifuged at 4,000×g for 10 minutes. The plasma supernatant was aliquoted into 2 mL tubes and stored at −20℃ until analysis. Serum FSTL1 concentrations were determined using enzyme-linked immunosorbent assay (ELISA) kits (Human FSTL1 ELISA Kit; Boster, Wuhan, China) as instructed by the manufacturer.
Cell culture
The 4T1 cell line was a kind gift from the Ning's Lab, College of Life Sciences, Nankai University (Tianjin, China). Cells were grown in RPMI-1640 (Thermo Fisher Scientific, Waltham, USA) containing 10% fetal bovine serum (complete medium) and 1% streptomycin/penicillin at 37℃ and 5% CO2.
Animal models
Fstl1flox/+ mice of a mixed background (129Sv/C57BL6) were purchased from Model Animal Research Center of Nanjing University. To meet the requirements for breast cancer study, we have backcrossed Fstl1flox/+ mice to a Balb/c genetic background for more than 10 generations. All animal experiments were performed in accordance with the Administration Regulations on Laboratory Animals of Beijing Municipality. Normal female Balb/c mice (6–8 weeks) were purchased from Vital River Laboratory Animal Technology Company of Beijing in China. All animals were allowed free access to food and water and were maintained under pathogen-free conditions.
Breast tumors and metastases were generated in Fstl1+/− mice and their wide type (WT) littermates by injection of 106 4T1 cells into a mammary fat pad. Body weight was monitored for 4 weeks. Tumor volumes were measured with a caliper once per week. The mice were euthanized at day 7, 14, 21, or 28 to determine the weight and size of the primary tumors. Thirty-one days after injection of the tumor cell line the mice were euthanized, and their lungs were collected. Metastases were visible to the naked eye as nodules. Lung tissues were sectioned, and the number of metastases was determined.
Mouse genotyping was performed using genomic DNA isolated from mouse tails. The primer set of FSTL1 we used was: forward, 5′-TCCCACCTTCGCCTCTAACT-3′; reverse, 5′-GAACTCTGCGGCTGCTCTG-3′. Agarose gel electrophoresis showed fragments of either 560 bp or 350 bp, which were produced from the WT or the null allele, respectively.
Western blot analysis
The lung and breast tissues were lysed in RIPA buffer (Applygen, Beijing, China) containing a phosphatase inhibitor. Human FSTL1 antibody (AF1694; R&D Systems, Minneapolis, USA), Mouse FSTL1 antibody (AF1738; R&D Systems), and antibodies recognizing the following proteins were used: Phospho-p44/42 mitogen-activated protein kinase (MAPK) (#4370; Cell Signaling Technology, Boston, USA), β-actin (#4970; Cell Signaling Technology). Western blot analyses were performed following standard protocols as described previously [20].
Quantitative real-time reverse transcription polymerase chain reaction and transcriptome analysis
Total cellular RNA was extracted from breast cancer tissue using Trizol reagent (Thermo Fisher Scientific). Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis was performed as previously described [20]. The primer sets we used were as follows: FSTL1, forward, 5′-GAGCAATGCAAACCTCACAAG-3′; reverse, 5′-CAGTGTCCATCGTAATCAACCTG-3′; β-actin, forward, 5′-CATGTACGTTGCTATCCAGGC-3′; reverse, 5′-CTCCTTAATGTCACGCACGAT-3′; Egln3, forward, 5′-AGGCAATGGTGGCTTGCTATC-3′; reverse, 5′-GCGTCCCAATTCTTATTCAGGT-3′.
For transcriptome analysis of lung metastases, we utilized lung tissue from WT and Fstl1+/− mice. Sequencing and bioinformatic analysis was conducted by the Beijing Genomics Institute (Shenzhen, China). Shortly, purified poly-A-containing messenger RNA molecules and the cleaved RNA fragments were copied into first strand complementary DNA (cDNA) using reverse transcriptase and random primers. This was followed by second strand cDNA synthesis using DNA polymerase I and RNase H. These cDNA fragments were appended with a single ‘A’ base, which allowed ligation to an adapter. The products were then purified and enriched with PCR amplification. We then quantified the PCR yield by Qubit and pooled the samples to make a single strand DNA circle (ssDNA circle), which gave the final library. DNA nanoballs (DNBs) were generated from the circular ssDNA by means of rolling circle replication, to increase the fluorescent signals during the sequencing process. The DNBs were loaded into patterned nanoarrays and pair-end reads of 100 bp were generated on a BGISEQ-500 (The Beijing Genomics Institute, Shenzhen, China) platform for the subsequent data analysis study. During this step, the BGISEQ-500 platform combines the DNA nanoball-based nanoarrays and stepwise sequencing using the Combinational Probe-Anchor Synthesis Sequencing Method.
Hematoxylin and eosin staining and immunohistochemistry
Human breast cancer samples were obtained from the Cancer Hospital of Huanxing Chaoyang District Beijing and were fixed in 4% paraformaldehyde (PFA) for 24 to 48 hours. Mouse metastatic lung tissue was dissected and fixed in PFA for 48 hours, and then stored in 70% alcohol. All samples were embedded in paraffin, and 5 µm sections were obtained using a Paraffin slicer (RM2235; Leica, Heidelberg, Germany). For hematoxylin and eosin (H&E) staining, lung tissue was stained with H&E. For immunohistochemistry, sections were deparaffinized and rehydrated, then incubated with primary antibodies (FSTL1, AF1694 and AF1738; R&D Systems) at 4℃ overnight, followed by a horseradish peroxidase secondary antibody (donkey anti-goat IgG H&L, ab97110; Abcam, Cambridge, UK) at 37℃ for 1 hour. Samples were developed using a diaminobenzidine solution (ZsBio, Beijing, China) for 2 minutes at room temperature. Sections were then dehydrated and affixed to coverslips.
Statistical analysis
Statistical analysis was performed using Prism 5 (GraphPad Software, La Jolla, USA). All results were reported as mean±standard error of the mean and were considered statistically significant at p<0.05.
RESULTS
Clinical characteristics of breast cancer patients
In this study, 94 breast cancer patients were included. The clinicopathological characteristics of the patients along with their treatments are shown in Table 1. We first measured the serum levels of FSTL1 by ELISA and analyzed possible correlation of the levels of FSTL1 with clinical parameters (Table 2). We found expression of FSTL1 to be significantly decreased in patients with Ki-67≤30 compared to patients with Ki-67>30 (p=0.039). In addition, EGFR-positive patients had lower levels of FSLT1 than EGFR-negative patients (p=0.004). In contrast, we found no correlation between FSTL1 and ER (p=0.388), PR (p=0.482), HER2 (p=0.300), p53 (p=0.300), grades 1–2 tumors (p=0.091) or T status (p=0.790). In conclusion, the correlation of FSTL1 with Ki-67 and EGFR suggest that FSTL1 may represent a potential biomarker in breast cancer patients.
Table 1. Patient demographic and clinical characteristics (n=94).
| Variable | No. (%) |
|---|---|
| Grade | |
| 1 | 5 (5) |
| 2 | 55 (59) |
| 3 | 13 (14) |
| Unknown | 21 (22) |
| Ki-67 (%) | |
| ≤ 14 | 21 (22) |
| > 14, ≤ 30 | 32 (34) |
| > 30 | 27 (29) |
| Unknown | 14 (15) |
| ER | |
| Negative | 20 (21) |
| Positive | 63 (67) |
| Unknown | 11 (12) |
| PR | |
| Negative | 19 (20) |
| Positive | 64 (68) |
| Unknown | 11 (12) |
| HER2 | |
| Negative | 31 (33) |
| Positive | 26 (28) |
| Unknown | 37 (39) |
| Subtype | |
| Luminal | 65 (69) |
| Triple-negative | 6 (6) |
| HER2-enriched | 9 (10) |
| Unknown | 14 (15) |
| N stage | |
| 0 | 31 (33) |
| ≥1 | 33 (35) |
| Unknown | 30 (32) |
| EGFR | |
| Negative | 38 (40) |
| Positive | 26 (28) |
| Unknown | 30 (32) |
| p53 | |
| Negative | 19 (20) |
| Positive | 12 (13) |
| Unknown | 63 (67) |
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; EGFR=epidermal growth factor receptor.
Table 2. Comparisons of serum marker levels according to various clinical/laboratory parameters.
| Variable | FSTL1 (pg/mL) Mean±SE | p-value |
|---|---|---|
| Grade | 0.091 | |
| I+II (good+medium) | 4,513 ± 273.8 | |
| III (poor) | 3,675 ± 433.0 | |
| Ki-67 | 0.039 | |
| ≤ 30 | 4,550 ± 242.1 | |
| > 30 | 3,607 ± 415.3 | |
| ER | 0.388 | |
| Negative | 3,920 ± 536.0 | |
| Positive | 4,362 ± 231.8 | |
| PR | 0.482 | |
| Negative | 3,973 ± 547.5 | |
| Positive | 4,340 ± 232.2 | |
| HER2 | 0.300 | |
| Negative | 4,507 ± 475.3 | |
| Positive | 3,753 ± 489.6 | |
| Classification | 0.767 | |
| Luminal | 4,226 ± 226.6 | |
| Others | 4,057 ± 665.3 | |
| Subtype I | 0.263 | |
| Triple-positive | 3,324 ± 958.6 | |
| Others | 4,265 ± 225.4 | |
| Subtype II | 0.573 | |
| HER2-enriched | 4,546 ± 913.0 | |
| Others | 4,150 ± 222.3 | |
| N status | 0.790 | |
| Negative | 4,242 ± 360.4 | |
| Positive | 4,370 ± 316.0 | |
| EGFR | 0.004 | |
| Negative | 4,772 ± 300.5 | |
| Positive | 3,412 ± 330.2 | |
| p53 | 0.300 | |
| Negative | 4,507 ± 475.3 | |
| Positive | 3,753 ± 489.6 |
FSTL1=follistatin-like protein 1; SE=standard error; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; EGFR=epidermal growth factor receptor.
Decreased levels of FSTL1 in breast cancer tissue and serum of breast cancer patients
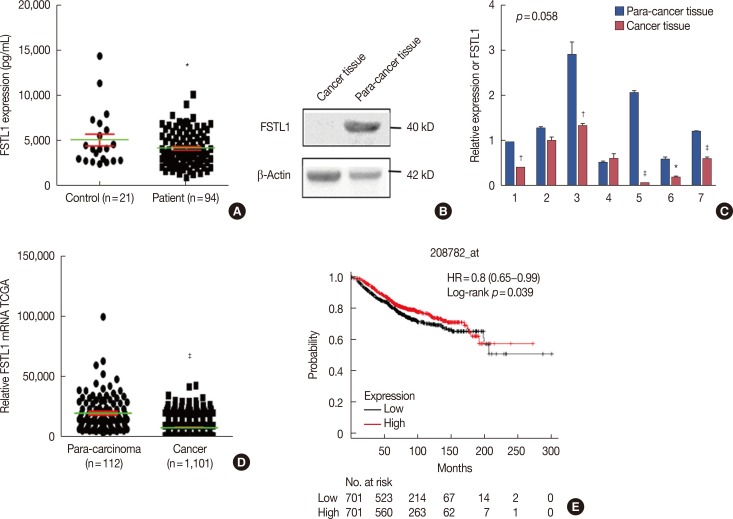
As determined by ELISA, the levels of FSTL1 were 4,221±1,920 pg/mL (mean±standard deviation) in breast cancer patients and 5,119±3,112 pg/mL (mean±standard deviation) in tumor-free control individuals (Figure 1A). This represents a statistically significant decrease in serum levels of FSTL1 in breast cancer patients compared to healthy controls (p=0.045). Consistent with this finding, lowered expression of FSTL1 was also identified in 11 breast cancer tissues, compared to para-cancer tissue, by western blot analysis or RT-PCR (Figure 1B and 1C). To confirm our observations, we analyzed the transcriptional levels of FSTL1 in 1,101 breast cancer tumor tissues and 112 para-carcinoma tissues of breast cancer patients from the Cancer Genome Atlas (TCGA). This analysis confirmed that the levels of FSTL1 were reduced in tumor tissue (FSTL1=7,823) compared to para-carcinoma tissue (FSTL1=19,660) (p<0.001) (Figure 1D). Finally, to increase the significance of our results, we investigated correlations between patient FSTL1 levels and clinical breast cancer behavior. Kaplan-Meier survival analysis of data from a public database (TCGA 2017) showed that reduced expression of FSTL1 was associated with a worse overall survival of breast cancer patients (p=0.039) (Figure 1E). Together, our results revealed that the levels of FSTL1 were reduced both in cancer tissue and in the serum of breast cancer patients, and that a lowered level of FSTL1 expression correlates with reduced survival in breast cancer patients.
Figure 1. The expression of follistatin-like protein 1 (FSTL1) in breast cancer patients. (A) Serum levels of FSTL1 were determined by enzyme-linked immunosorbent assay in breast cancer patients (n=94) and healthy controls (n=21). (B) Western blot analysis of FSTL1 expression in cancer tissue and marched para-carcinoma tissue (n=4). (C) Real-time reverse transcription polymerase chain reaction analysis of eight paired breast carcinoma samples for the expression of FSTL1. (D) Relative expression of FSTL1 messenger RNA in cancer tissue (n=1,101) and marched para-carcinoma tissue (n=112) in breast cancer patients from the Cancer Genome Atlas (TCGA) cohort. (E) Kaplan-Meier survival analysis of public database (TCGA 2017) showed low expression of FSTL1 was associated with a worse overall survival of breast cancer patients. Each bar represents mean±SD for triplicate experiments.
HR=hazard ratio. *p<0.05; †p<0.01; ‡p<0.001, unpaired t-test.
FSTL1 heterozygosity does not impact the growth of primary 4T1 tumors
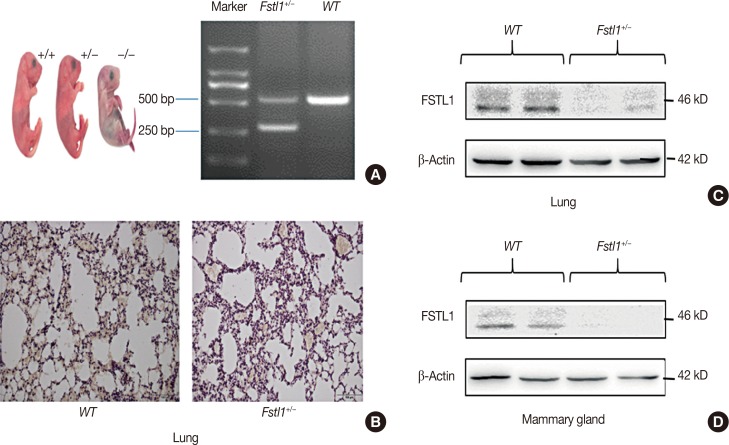
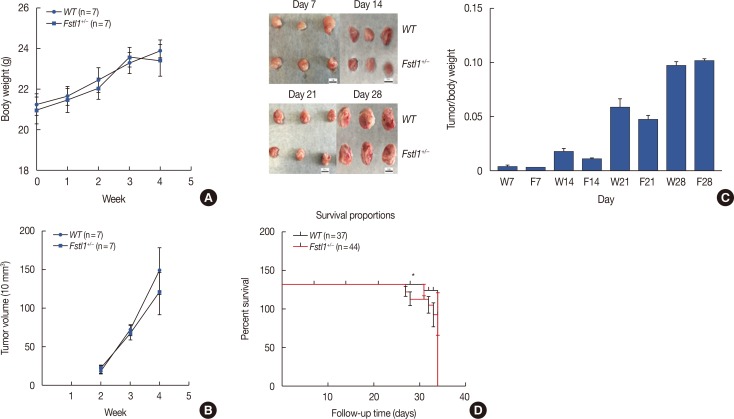
To further study the biological function of FSTL1 in breast cancer, we used Fstl1+/− mice in our experiments, as Fstl1+/− mice died shortly after birth. Genotyping was applied to determine the subtypes of mice (Figure 2A). Immunohistochemistry and western blotting confirmed the decrease in FSTL1 protein levels in lung tissue (Figure 2B and 2C) and in mammary tissue (Figure 2D). To investigate the role of FSTL1 in breast cancer progression in vivo, we injected 4T1 cancer cells into the mammary fat pad of WT and Fstl1+/− mice. Tumor volume and body weight were measured once per week (Figure 3A and 3B). We demonstrated that the growth of primary tumors, and the ratio of tumor volume to weight, was similar in WT and Fstl1+/− mice (Figure 3C). However, when we compared the survival of 4T1 tumor-bearing WT and Fstl1+/− mice, we found that Fstl1+/− mice showed reduced survival (p<0.05) (Figure 3D). These findings established that, while FSTL1 deficiency does not impact the growth of primary tumors, it does result in the reduced survival of 4T1 tumor-bearing animals.
Figure 2. Generation of Fstl1+/− mice. (A) Genotyping of mice. (B) Histological analysis of follistatin-like protein 1 (FSTL1) in lung tissue of wild type and Fstl1+/− mice (n=3) by immunohistochemical (IHC) staining (IHC for FSTL1, ×400). (C) Western blot analysis of FSTL1 in lung tissue of WT and Fstl1+/− mice. (D) Western blot analysis of FSTL1 in mammary tissue of WT and Fstl1+/− mice.
bp =base pair.
Figure 3. Growth of primary 4T1 tumors in wild type (WT) and Fstl1+/−mice. (A) Body weight was monitored in WT and Fstl1+/−mice for 4 weeks after the injection of 4T1 cancer cells (n=7). (B) Tumor volume was measured for 3 weeks (n=7). (C) The dissected tumors at day 7, 14, 21, and 28 (n=3). Tumors were dissected, and their weights and tumor volumes were measured. (D) Survival analysis of WT (n=37) and Fstl1+/−mice (n=44). Each bar represents the mean±SD for triplicate experiments.
W=WT; Fstl1+/−. *p<0.05, unpaired t-test.
Fstl1+/− mice display increased lung metastases in a 4T1 tumor model
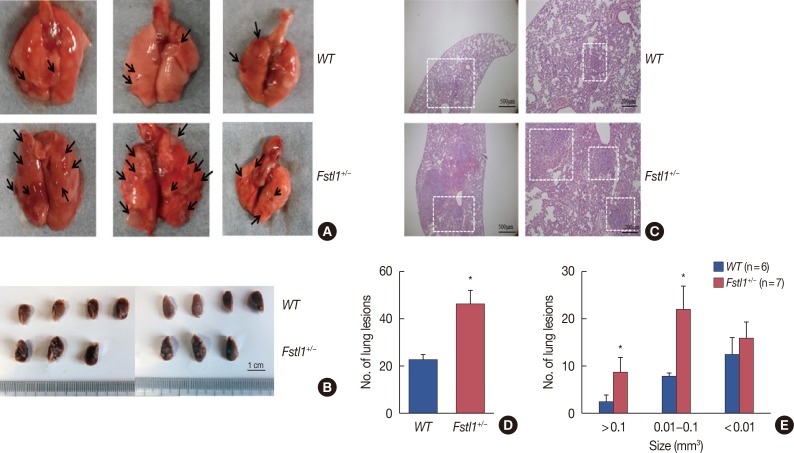
To establish whether FSTL1 deficiency affects the metastatic spread of 4T1 cells, we dissected lung tissue from tumor-bearing mice 31 days after the injection of 4T1 cells. We found a significant increase in the number of lung metastatic lesions in Fstl1+/− mice (Figure 4A and 4B). H&E staining confirmed increased metastases in Fstl1+/− mice (Figure 4C). Metastatic lesions were counted in the lungs of WT (23.330±2.404) and Fstl1+/− mice (47.003±2.400) per whole lung (p=0.010) (Figure 4D). The size of metastatic nodules was monitored, and the number of macro (>0.1 mm3) and intermediate-sized (0.01–0.1 mm3) lung metastases in Fstl1+/− mice was significantly increased. However, the number of micro (<0.01 mm3) lung metastases was not increased in Fstl1+/− mice (Figure 4E). In summary, we demonstrated that 4T1 tumor-bearing Fstl1+/− mice had an increased number and size of metastatic foci in lung tissues.
Figure 4. Increased lung metastasis in Fstl1+/−mice. (A) Thirty-one days after injection of 4T1 cancer cells, the mice were euthanized and analyzed for lung metastasis. The arrows were pointed to the lung metastasis in fresh lung tissue. (B) Thirty-one days after injection of 4T1 cancer cells, the mice were euthanized and analyzed for lung metastasis of formalin fixation. (C) Representative H&E stained images of the lung. (D) Number of lung metastatic foci were assessed in wild type (WT) (n=6) and Fstl1+/−mice (n=7). (E) Number and size of lung metastatic foci were assessed in WT (n=6) and Fstl1+/−mice (n=7). Each bar represents mean±SD for triplicate experiments.
*p<0.05, unpaired t-test.
FSTL1 regulates lung metastatic tumor growth by inhibiting EGFR signaling
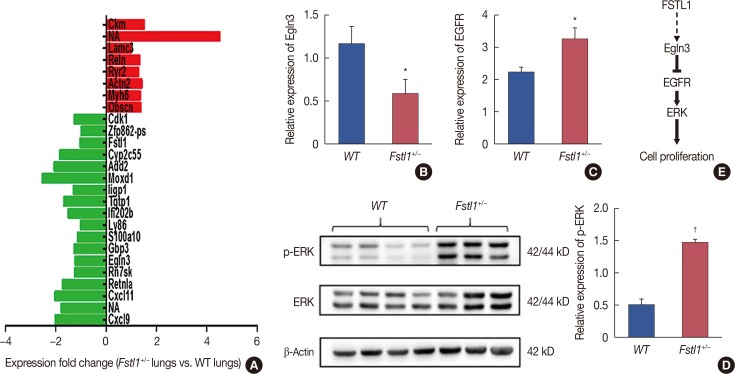
To further explore the role of FSTL1 in the metastatic spread of breast cancer cells, we profiled metastatic lung tissue from WT and Fstl1+/− mice by sequencing and bioinformatic analysis (Beijing Genomics Institute, Shenzhen, China). We found several genes to be differentially-expressed in the two groups (Figure 5A), with eight genes being upregulated, and 18 genes downregulated, in FSTL1-deficient mice. One of the downregulated genes, Egln3, is regulated by hypoxia and plays an important role in tumor progression [17,21,22]. Recent studies also demonstrated that Egln3 regulates EGFR to restrain tumor growth. We confirmed by RT-PCR that Egln3 is downregulated (Figure 5B) and that EGFR was increased in Fstl1+/− mice (Figure 5C). Consistent with this, the phosphorylation of MAPK was increased in Fstl1+/− mice (Figure 5D). Thus, we hypothesize that FSTL1 deficiency results in the downregulation of Egln3 coupled with the activation of EGFR signaling and increased cancer cell proliferation (Figure 5E).
Figure 5. The epidermal growth factor receptor (EGFR) signaling in lung metastatic tumor tissue of Fstl1+/−mice. (A) Differential gene expression in lung metastatic tumor tissue of wild type (WT) (n=3) and Fstl1+/−mice (n=3). (B) Relative expression of Egln3 was decreased in lung metastatic tumor tissue of Fstl1+/−mice by real-time reverse transcription polymerase chain reaction (n=7). (C) Relative expression of EGFR was increased in lung metastatic tumor tissue of Fstl1+/−mice by transcription analysis (n=7). (D) Western blot analysis of phosphorylation of extracellular signal-regulated kinase (p-ERK) expression in lung metastatic tumor tissue of WT and Fstl1+/−mice. (E) Hypothetical model of follistatin-like protein 1 (FSTL1)-regulated cancer cell proliferation. Each bar represents the mean±SD for triplicate experiments.
*p<0.05; †p<0.001, unpaired t-test.
DISCUSSION
The role of FSTL1 in cancer is controversial, and it has been reported to have both oncogenic and tumor-suppressor activity in various cancer types [9,14,23]. However, the role of FSTL1 in breast cancer remains unknown.
This study demonstrated that the expression of FSTL1 is reduced both in breast cancer tissue and in the serum of breast cancer patients and showed that patients with low levels of FSTL1 expression have significantly reduced overall survival. To the best of our knowledge, this is the first study to measure FSTL1 in human serum and to show that FSTL1 plays a role in breast cancer progression. Long-term studies are required to confirm whether FSTL1 serum levels can serve as a prognostic/predictive marker in breast cancer patients.
The mechanism underlying FSTL1 downregulation in breast cancer is currently unknown. Lau et al. [11] recently found that amplification of the FSTL1 gene on chromosome 3q correlates with an increased expression of FSTL1 protein. The expression of FSTL1 has been shown to be upregulated by TGF-β [5]. Additional regulatory mechanisms leading to FSTL1 inactivation include miR-27a [24] and histone deacetylase 5 [25].
In this study, we investigated the correlation between serum concentrations of FSTL1, Ki-67 and EGFR. Ki-67 is a biomarker of cancer cell proliferation, indicates poor prognosis, and has been shown to have an adverse effect on overall survival and disease-free survival. Similarly, overexpression of EGFR has been reported to lead to tumor progression and is associated with tumor size and poor clinical outcomes [26].
FSTL1 inhibits cell proliferation through smad3 in MBA-MD-231 cells, as observed previously [27]. To explore the function of FSTL1 in vivo, we employed FSTL1 deficient mice. In this study, we demonstrated that FSTL1 deficiency reduces the survival of 4T1 tumor-bearing mice (Figure 3). However, we have shown that FSTL1 deficiency has no effect on the growth of primary tumors, suggesting that the inhibitory effects of FSTL1 on cell proliferation is cancer-cell-type specific, reflecting differences between in vitro and in vivo studies. We assumed that WT and Fstl1+/− mice would have similar lengths of latency after the injection of sufficient (106), but not limited number (102–105) of 4T1 cells into the mammary fat pad [28]. Consistently, in this study we found that FSTL1 deficiency does not alter the growth of primary 4T1 tumors.
Studies have shown that metastasis is the leading cause of breast cancer-related death. In our study, FSTL1 deficiency significantly increased the formation of lung metastases. Both the number and the size of metastatic lung lesions were increased in Fstl1+/− mice, suggesting that FSTL1 regulates both metastatic seeding and the proliferation of cancer cells after seeding. This indicates that FSTL1 deficiency provides a suitable microenvironment for lung metastasis. Accordingly, FSTL1 has been shown to inhibit invasion and metastasis [13], and to block cell proliferation and induce apoptosis [12]. In addition, FSTL1 may regulate immune responses [29], contributing to rapid colonization of tumor cells that metastasize to lung tissue.
Consistent with our findings, previous reports have indicated that FSTL1 inhibits invasion and metastasis in lung cancer [13], ovarian, and endometrial carcinoma [14], as well as in nasopharyngeal carcinoma [12]. In addition, FSTL1 has been shown to suppress cell proliferation and induce apoptosis in nasopharyngeal carcinoma. In contrast, FSTL1 has been shown to function as a pro-metastatic gene in both prostate cancer [30] and esophageal squamous cell carcinoma [11]. Little is known concerning the mechanism by which FSTL1 inhibits cell proliferation. We found that the expression of Egln3 is downregulated in metastatic lung tissue of Fstl1+/− mice. Egln3 regulates EGFR internalization and signaling and thus sustains the growth of tumors [18]. We demonstrated that phosphorylation of extracellular signal-regulated kinase is increased in Fstl1+/− lungs, suggesting that blocking FSTL1 downregulates Egln3 and promotes proliferation through EGFR/MAPK signaling. However, the mechanism by which FSTL1 regulates Egln3 expression remains to be determined.
In conclusion, we demonstrated that FSTL1 is downregulated in breast cancer tissue and in serum, and that it may therefore serve as a useful biomarker for breast cancer. In vivo studies suggest that FSTL1 deficiency promotes breast cancer metastasis to the lung. Thus, FSTL1 may be an important prognostic and predictive biomarker in breast cancer.
ACKNOWLEDGMENTS
We thank Dr. Wen Ning (College of Life Sciences, Nankai University) for the 4T1 cell line.
Footnotes
The present study was supported by National Natural Science Foundation of China (grant no. 81602532); Beijing Natural Science Foundation (grant no. 7164238, 7152016); Beijing Municipal Commission of Education (grant no. KM201410025002); Beijing Municipal Organization Department Talents Project (grant no. 2015000020124G113); Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (grant no. IDHT20170516).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, et al. A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364. doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peddi PF, Ellis MJ, Ma C. Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer. 2012;2012:217185. doi: 10.1155/2012/217185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, et al. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Wang Y, Xu N, Wei Q, Wu M, Li X, et al. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R17. doi: 10.1186/ar3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, et al. Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res. 2008;14:2978–2987. doi: 10.1158/1078-0432.CCR-07-4821. [DOI] [PubMed] [Google Scholar]
- 10.Su S, Parris AB, Grossman G, Mohler JL, Wang Z, Wilson EM. Up-regulation of follistatin-like 1 by the androgen receptor and melanoma antigen-a11 in prostate cancer. Prostate. 2017;77:505–516. doi: 10.1002/pros.23288. [DOI] [PubMed] [Google Scholar]
- 11.Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, et al. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res. 2017;77:5886–5899. doi: 10.1158/0008-5472.CAN-17-1411. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Xiao X, Huang T, Du C, Wang S, Mo Y, et al. Epigenetic inactivation of follistatin-like 1 mediates tumor immune evasion in nasopharyngeal carcinoma. Oncotarget. 2016;7:16433–16444. doi: 10.18632/oncotarget.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Han HB, Zhang ZQ. Suppression of lung cancer cell invasion and metastasis by connexin43 involves the secretion of follistatin-like 1 mediated via histone acetylation. Int J Biochem Cell Biol. 2011;43:1459–1468. doi: 10.1016/j.biocel.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson G, Hager JH, Volik S, Hariono S, Wernick M, Moore D, et al. Genome scanning with array CGH delineates regional alterations in mouse islet carcinomas. Nat Genet. 2001;29:459–464. doi: 10.1038/ng771. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Han X, Yu Y, Ding Y, Ni C, Liu W, et al. A genetic polymorphism affects the risk and prognosis of renal cell carcinoma: association with follistatin-like protein 1 expression. Sci Rep. 2016;6:26689. doi: 10.1038/srep26689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatzimichael E, Dasoula A, Shah R, Syed N, Papoudou-Bai A, Coley HM, et al. The prolyl-hydroxylase EGLN3 and not EGLN1 is inactivated by methylation in plasma cell neoplasia. Eur J Haematol. 2010;84:47–51. doi: 10.1111/j.1600-0609.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 18.Garvalov BK, Foss F, Henze AT, Bethani I, Gräf-Höchst S, Singh D, et al. PHD3 regulates EGFR internalization and signalling in tumours. Nat Commun. 2014;5:5577. doi: 10.1038/ncomms6577. [DOI] [PubMed] [Google Scholar]
- 19.Henze AT, Garvalov BK, Seidel S, Cuesta AM, Ritter M, Filatova A, et al. Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat Commun. 2014;5:5582. doi: 10.1038/ncomms6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng X, Zheng R, Zhang Y, Qiao M, Liu L, Jing P, et al. An activated sympathetic nervous system affects white adipocyte differentiation and lipolysis in a rat model of Parkinson's disease. J Neurosci Res. 2015;93:350–360. doi: 10.1002/jnr.23488. [DOI] [PubMed] [Google Scholar]
- 21.Su Y, Loos M, Giese N, Hines OJ, Diebold I, Görlach A, et al. PHD3 regulates differentiation, tumour growth and angiogenesis in pancreatic cancer. Br J Cancer. 2010;103:1571–1579. doi: 10.1038/sj.bjc.6605936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology. 2010;138:606–615. doi: 10.1053/j.gastro.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Bae K, Park KE, Han J, Kim J, Kim K, Yoon KA. Mitotic cell death caused by follistatin-like 1 inhibition is associated with up-regulated Bim by inactivated Erk1/2 in human lung cancer cells. Oncotarget. 2016;7:18076–18084. doi: 10.18632/oncotarget.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi DL, Shi GR, Xie J, Du XZ, Yang H. MicroRNA-27a inhibits cell migration and invasion of fibroblast-like synoviocytes by targeting follistatin-like protein 1 in rheumatoid arthritis. Mol Cells. 2016;39:611–618. doi: 10.14348/molcells.2016.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsou PS, Wren JD, Amin MA, Schiopu E, Fox DA, Khanna D, et al. Histone deacetylase 5 is overexpressed in scleroderma endothelial cells and impairs angiogenesis via repression of proangiogenic factors. Arthritis Rheumatol. 2016;68:2975–2985. doi: 10.1002/art.39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 27.An J, Wang L, Zhao Y, Hao Q, Zhang Y, Zhang J, et al. Effects of FSTL1 on cell proliferation in breast cancer cell line MDA MB 231 and its brain metastatic variant MDA MB 231 BR. Oncol Rep. 2017;38:3001–3010. doi: 10.3892/or.2017.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 2016;166:47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2014;103:111–120. doi: 10.1093/cvr/cvu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25(1A):183–191. [PubMed] [Google Scholar]