Abstract
Intestinal-type gastric carcinoma exhibits a multistep carcinogenic sequence from adenoma to carcinoma with a gradual increase in genomic alterations. But the roles of microRNAs (miRNA) in this multistage cascade are not fully explored. To identify differentially expressed miRNA (DEM) during early gastric carcinogenesis, we performed miRNA microarray profiling with 24 gastric cancers and precursor lesions (7 early gastric cancer [EGC], 3 adenomas with high-grade dysplasia, 4 adenomas with low-grade dysplasia, and 10 adjacent normal tissues). Alterations in the expression of 132 miRNA were detected; these were categorized into three groups based on their expression patterns. Of these, 42 miRNAs were aberrantly expressed in EGC. Five miRNA (miR-26a, miR-375, miR-574-3p, miR-145, and miR-15b) showed decreased expression since adenoma. Expression of two miRNA, miR-200C and miR-29a, was down-regulated in EGCs compared to normal mucosa or adenomas. Six miRNA (miR-601, miR-107, miR-18a, miR-370, miR-300, and miR-96) showed increased expression in gastric cancer compared to normal or adenoma samples. Five representative miRNAs were further validated with RT-qPCR in independent 77 samples. Taken together, these results suggest that the dysregulated miRNA show alterations at the early stages of gastric tumorigenesis and may be used as a candidate biomarker.
Introduction
A subset of gastric cancer (intestinal-type gastric cancer by Lauren’s histologic classification) exhibits a gradual development through a multistep carcinogenic sequence from non-neoplastic atrophic and metaplastic gastric mucosa to adenoma with low-grade dysplasia (LGD), followed by adenoma with high-grade dysplasia (HGD) and eventually develops into invasive carcinoma1,2. A recent report by Min et al. showed a gradual increase in genomic alterations, including somatic nucleotide variation, gene fusion, and copy number variation, from LGD to carcinoma3.
Aside from the accumulation of genetic alterations, epigenetic changes such as DNA methylation and aberrant gene expression by non-coding RNAs are other key players involved in carcinogenesis4. MicroRNAs (miRNAs) are abundant non-coding RNA molecules of 18–25 nucleotides that inhibit translation or promote degradation of messenger RNAs (mRNAs) with complementary sequences. miRNAs are estimated to regulate the expression of 30–60% of human genes and are known to modulate cell development, differentiation, proliferation, and apoptosis. Hence, alterations in their expression are associated with human diseases such as cancer5,6. Depending on its mRNA target, the miRNA may function as a tumor suppressor or promoter of tumorigenesis7.
In colorectal and esophageal adenocarcinomas, miRNA profiles of cancer and their precursor lesions such as Barrett’s esophagus or colorectal adenoma are well described8–15. These profiles have the potential to be used as diagnostic biomarkers for early cancer detection and therapeutic targets for cancer prevention. Only a single study has reported the gradual increase in miR-106a, a member of miR-17 family, during the multistep gastric carcinogenesis using real-time quantitative polymerase chain reaction (RT-qPCR)16. However, there are no reports on miRNA profiles in gastric precursor lesions or during multistep carcinogenesis.
To identify miRNA expression signatures through a multistep carcinogenic sequence, we performed NanoString miRNA expression assays in normal gastric mucosa, LGDs, HGDs, and intestinal-type early gastric cancers (EGCs) and subsequently validated five miRNAs using independent sample sets with RT-qPCR. In addition, we used RNA sequencing data from our previous study to investigate the correlation between the expression of miRNAs and their target mRNAs.
Results
Altered miRNA expression in normal gastric mucosa, adenoma, and EGC
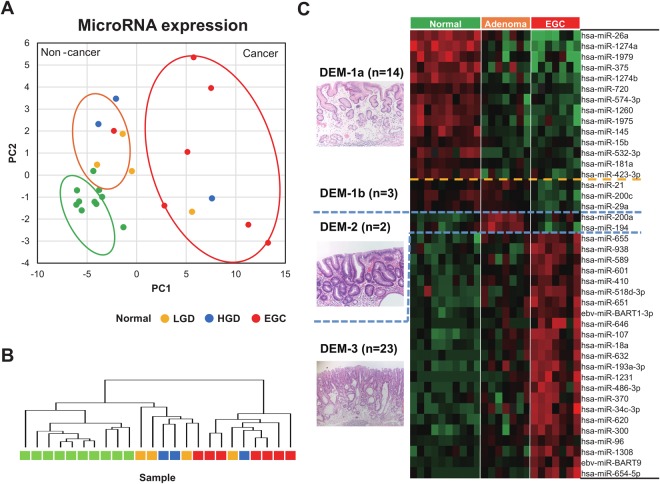
We performed miRNA expression arrays by using NanoString to identify DEM patterns in normal gastric mucosa, adenoma with LGD and HGD, and EGC (Table 1). We identified 132 DEMs among three groups (p-value < 0.01, average expression >5, and fold change > = 2.5 or < = 0.4). DEMs were categorized into three groups based on the changing patterns of miRNA expression. The DEM-1 group exhibited a decrease in the miRNA expression from adenoma lesion (DEM-1a, n = 16) or EGC (DEM-1b, n = 7) compared to normal gastric mucosa. DEM-2 group comprised cases with highest miRNA expression levels in adenomas (n = 3). DEM-3 group showed an upregulated miRNA expression in EGC as compared with normal mucosa or adenomas (n = 98) (Supplementary Table 1).
Table 1.
Demographics of the discovery cohorts.
| Pathology | Sample No. | Gender | Age | Size (cm) | Invasion Depth | Lymphatic invasion | H. pylori infection | EBV infection |
|---|---|---|---|---|---|---|---|---|
| EGC | CST04 | M | 75 | 1.2 | LP | No | Yes | No |
| EGC | CST95 | M | 63 | 1.8 | LP | No | Yes | No |
| EGC | CST22 | F | 69 | 4.2 | LP | No | Yes | No |
| EGC | CST36 | M | 75 | 2.2 | MM | No | Yes | No |
| EGC | CST58 | F | 65 | 3.0 | MM | No | Yes | No |
| EGC | CST78 | M | 66 | 3.2 | MM | No | Yes | No |
| EGC | CST29 | M | 72 | 4.5 | MM | No | Yes | No |
| HGD | HST92 | F | 74 | 0.4 | Yes | No | ||
| HGD | HST87 | M | 64 | 1.4 | Yes | No | ||
| HGD | HST85 | M | 63 | 2.3 | No | No | ||
| LGD | LST09 | F | 63 | 1.1 | Yes | No | ||
| LGD | LST34 | F | 75 | 2.1 | Yes | No | ||
| LGD | LST20 | M | 74 | 2.6 | Yes | No | ||
| LGD | LST72 | M | 64 | 3.0 | Yes | No |
Principal component analysis (PCA) showed that the cancer and non-cancer samples were separated along the PCA1 axis, whereas the normal gastric mucosa and adenoma samples were differentially located along the PC2 axis (Fig. 1A). Unsupervised PCA results showed that normal and adenoma samples showed similar expression patterns, but EGC samples tended to cluster to a separate group (Fig. 1B).
Figure 1.
The expression profiles of miRNA for LGD, HGD, and EGC. (A) Principal component analysis of the whole set of miRNAs. (B) Results of un-supervised clustering analysis. (C) Differentially expressed miRNAs (DEM) among three groups. DEM-1 with highest expression levels in normal control mucosa, DEM-2 with highest miRNA expression levels in adenoma, and DEM-3 with upregulated miRNA in EGC with their representative histopathologic findings.
We found that 42 miRNAs were aberrantly expressed (false discovery rate [FDR] <0.01) in early gastric tumorigenesis (Fig. 1C). Among the 42 DEMs, we selected 13 miRNAs that showed patterns identical or similar to those reported in previous miRNA studies (Table 2). In comparison to normal gastric mucosa samples, adenoma samples showed a decrease in the expression of five miRNAs (miR-26a, miR-375, miR-574-3p, miR-145, and miR-15b), which are known to be down-regulated in gastric cancer17–21. The expression of two miRNAs (miR-200C and miR-29a), which are known to be down-regulated in gastric cancer22,23, decreased in EGCs as compared with normal mucosa or adenomas. In addition, there was an increase in the expression of six miRNAs (miR-601, miR-107, miR-18a, miR-370, miR-300, and miR-96), which are known to be upregulated in gastric cancer24–35, in EGCs as compared with normal or adenoma samples (Fig. 1C and Table 2).
Table 2.
Differentially expressed miRNAs showing identical patterns as in the previous miRNA studies.
| DEM group | miRNA | Fold change adenoma/normal | Fold change egc/normal | Fold change egc/adenoma | FDR | Up- or down- regulation | Reference |
|---|---|---|---|---|---|---|---|
| DEM-1a | hsa-miR-26a | 0.33 | 0.07 | 0.22 | 5.25E-05 | Down | 17 |
| hsa-miR-375 | 0.45 | 0.15 | 0.33 | 9.73E-03 | Down | 18, 46– 48 | |
| hsa-miR-574-3p | 0.32 | 0.26 | 0.80 | 2.58E-04 | Down | 19 | |
| hsa-miR-145 | 0.24 | 0.20 | 0.85 | 1.98E-03 | Down | 20 | |
| hsa-miR-15b | 0.48 | 0.47 | 0.98 | 1.98E-03 | Down | 21 | |
| DEM-1b | hsa-miR-200c | 0.73 | 0.26 | 0.36 | 4.43E-03 | Down | 22 |
| hsa-miR-29a | 0.91 | 0.39 | 0.43 | 4.60E-03 | Down | 23 | |
| DEM-3 | hsa-miR-601 | 2.53 | 4.69 | 1.85 | 1.98E-03 | Up | 24 |
| hsa-miR-107 | 2.98 | 6.31 | 2.12 | 5.14E-03 | Up | 25– 28, 41 | |
| hsa-miR-18a | 2.03 | 3.46 | 1.70 | 5.89E-03 | Up | 29– 31 | |
| hsa-miR-370 | 2.02 | 5.65 | 2.80 | 4.38E-03 | Up | 32, 33 | |
| hsa-miR-300 | 2.13 | 4.58 | 2.15 | 1.98E-03 | Up | 34 | |
| hsa-miR-96 | 1.92 | 2.62 | 1.36 | 3.23E-03 | Up | 35 |
Additionally, we validated two novel miRNAs which have been established as tumor suppressor or suppressor of epithelial-to-mesenchymal transition (miR-655) in hepatocellular36 and esophageal squamous cell carcinoma37,38, and reported as oncogenic (miR-938) miRNA in colorectal cancers39, but their roles have not been studied in gastric cancer. Unexpectedly, we found that expressions of miR-655 decreased from normal to adenoma and carcinoma, suggesting tumor suppressive role in gastric cancer. Meanwhile, miR-938 was up-regulated in EGCs compared to normal gastric mucosa and adenomas, suggesting oncogenic function in gastric cancer (Supplementary Fig. 1).
Validation of results and correlation between miRNA and target genes
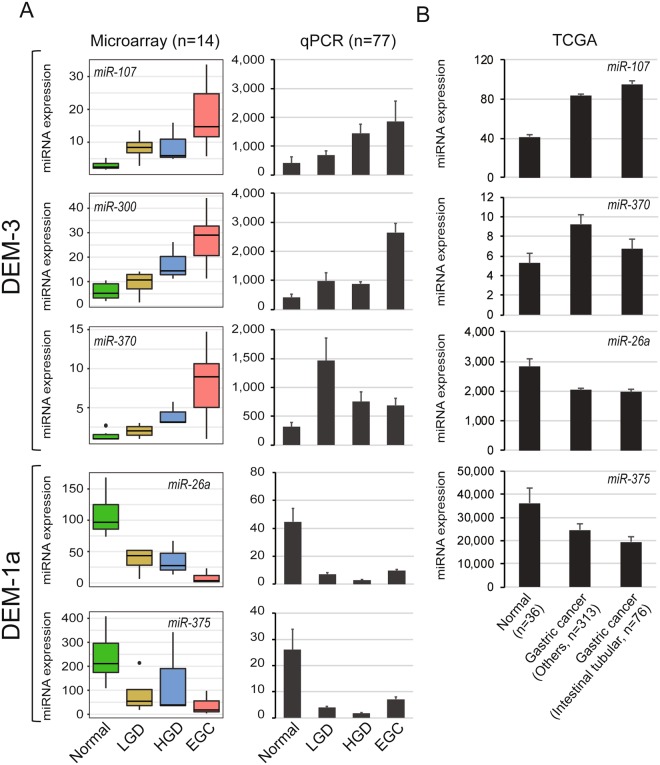
For further experimental validation, we measured the expression of five miRNAs (miR-107, miR-300, and miR-370 from DEM-3; miR-26a and miR-375 from DEM-1a) in 77 independent samples using RT-qPCR, owing to the obvious difference (fold change >4) in their expression between normal and EGC group in the miRNA microarray analysis. We observed a progressive increase in the expression of miR-107 and miR-300 from normal to adenoma and EGC in microarray analysis; these results were subsequently validated in an independent sample set with RT-qPCR. In comparison to the normal gastric mucosa, adenomas and EGCs showed a decrease in the expression of miR-26a and miR-375 in both microarray and qPCR assays (Fig. 2A). However, we failed to confirm the gradual increase in the expression of miR-370 in the validation set. From TCGA datasets, we found that 4 miRNAs (miR-107, miR-370, miR-26a and miR-375) were dysregulated in gastric cancer compared to normal gastric mucosa and also showed differential gene expression levels between tubular adenocarcinoma (well- and moderately differentiated, intestinal-type by Lauren) group (n = 76) and other types of gastric cancer group (n = 313) (Fig. 2B).
Figure 2.
Stepwise changes in the expression of miRNAs during early gastric carcinogenesis. (A) The expression levels of five miRNAs were measured using miRNA array and real-time RT-PCR. (B) The expression patterns of four miRNAs were also identified in TCGA dataset.
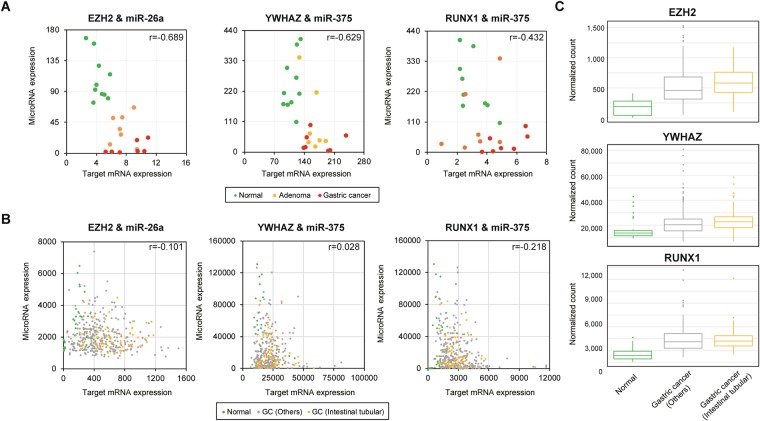
To determine the impact of these miRNAs on target genes, we calculated the correlation coefficient between the expression of miRNAs and their target genes using our previous RNA sequencing data. A negative correlation was observed between miR-26a and EZH2 (r = −0.689), miR-375 and YWHAZ (r = −0.629), and miR-375 and RUNX1 (r = −0.432) (Fig. 3A). We also found weak negative correlation between miR-26a and EZH2 (r = −0.101), miR-375 and RUNX1 (r = −0.218) from TCGA dataset (Fig. 3B). Three target genes (EZH2, YWHAZ and RUNX1) were upregulated in gastric cancer compared to normal gastric mucosa. Moreover, their normalized mRNA levels also showed slight difference between well- and moderately differentiated tubular, intestinal-type gastric cancer group compared to other types of cancers (Fig. 3C). We also performed correlation analyses with miR107, miR300, and miR370 with their target genes and only miR375 and miR26a showed high correlations with their target genes (Supplementary Fig. 2).
Figure 3.
Spearman’s correlation between miRNA and well-known target genes in samples from normal, LGD, HGD, and EGC. (A) Negative correlation between miR-26a and EZH2 (***P = 2.87E-4, r = -0.689). Negative correlation between miR-375 and YWHAZ (*P = 1.28E−3, r = −629) and RUNX1 (*P = 3.61E−2, r = −0.432) in microarray data. (B) Negative correlation between miR-26a and EZH2 (*P = 2.18E-2, r = -0.101). Negative correlation between miR-375 and YWHAZ (P = 0.288, r = 0.028) and RUNX1 (***P = 5.00E-6, r = -0.218) observed in TCGA data. (C) Expression of miRNA and their target gene mRNA expression patterns in TCGA dataset.
Discussion
The pathogenesis of gastric cancer involves multistep genetic and epigenetic alterations, which predispose cells to neoplastic transformation1–3. Given the importance of miRNAs in the regulation of cell growth and viability, miRNA dysregulation is believed to be closely correlated with the development and progression of gastric cancer4. In the stomach, previous research on miRNA dysregulation was focused on gastric cancer itself6,7,18,20,22,23,26,27,29,33,40 and only one study has been conducted to explore the role of miRNA during the histologic progression from gastric adenoma to carcinoma without non-tumorous controls, warranting more research16. To our best knowledge, this is the first study to evaluate and compare the expression profile of miRNAs in non-tumor tissue, LGD, HGD, and EGC, using a high-throughput screening array. The aim of our study was to screen alterations in miRNA expressions during the stepwise gastric carcinogenesis and we demonstrated the role of miRNAs during the stepwise gastric carcinogenesis and confirmed their expressions with RT-qPCR.
Although previous reports have revealed several molecular alterations in gastric cancer such as DNA mutations, copy number variations, mRNA expression changes, and miRNA alterations, most of these studies were conducted by comparing gastric cancer tissues or cell lines with normal gastric samples, without adenoma samples. Zhu et al.16 investigated the miR-106a expression in gastric dysplasia and gastric samples using in situ hybridization. The frequency and extent of miR-106a expression was found to show a gradual increase along the histologic progression from mild, moderate, and severe dysplasia to EGC. However, they failed to evaluate the expression of other miRNAs. In the present study, we identified alterations in miRNA expressions using normal, LGD, HGD, and EGC samples. Overall, the expression of miRNAs showed distinct patterns as compared with the expression pattern of mRNAs. In comparison to the EGC samples, samples from the non-cancerous group (normal and adenoma samples), showed similar miRNA expression pattern. Within the non-cancerous group, the normal and adenoma samples were separated based on their expression patterns.
The expression of miR-107 has been reported to increase in the serum and tissues of gastric cancer patients and gastric cancer cell lines. miR-107 acts as an oncogene and regulates gastric cancer development and progression by targeting NF1, DICER1, FOXO1, and CDK825,27,28,41. We found a gradual increase in the expression of miR-107 with the histologic progression from LGD to EGC. Microarray and RT-qPCR results revealed that the expression of miR-107 was significantly upregulated in HGD and EGC as compared with the normal mucosa (**p = 0.006 and *p = 0.031, respectively).
Overexpression of miR-300 promotes cell cycle progression, cell proliferation, and invasion in several cancers, including gastric cancer34, liver cancer40, and osteosarcoma42. miR-300 displays the potential to be used in the treatment, diagnosis, and prognosis of gastric cancer, as its expression decreased in gastric cancer following chemotherapy or exposure to radiation43.
In gastric cancer, miR-370 showed altered expression and acted as either an oncogene or a tumor suppressor. Previous studies have shown that the up-regulation of miR-370 resulted in the progression of gastric carcinoma via suppression of transforming growth factor beta receptor II (TGFβRII) or FOXO132,33. Although we observed a relative increase in the expression of miR-370 in LGD, it showed a weak correlation with the expression of its target gene, FOXO3.
The expression of miR-26a is frequently aberrant in many tumors such as gastric cancer, bladder tumor, breast cancer, oral squamous cell carcinoma, and Burkitt lymphoma44. miR-26a is known to be significantly down-regulated in gastric cancer and suppresses tumor growth and metastasis by targeting FGF9 gene17. Furthermore, miR-26a improves the sensitivity of gastric cancer cells to cisplatin-based chemotherapies by targeting NRAS and E2F245. We found a decrease in the expression of miR-26a from LGD and a strong negative correlation between the expression of miR-26a and its known target EZH2 (r = −0.689).
Previous studies have demonstrated that miR-375 inhibits cell proliferation of gastric cancer cells by repressing JAK2, ERBB2, and YWHAZ18,46–48. We found a significant decrease in miR-375 expression in LGD, HGD, and EGC, as evident from the microarray and RT-qPCR results. Moreover, miR-375 expression was inversely correlated (r = −0.629) with the expression of YWHAZ (14-3-3ζ), a member of the 14-3-3 family of proteins, which has been implicated in the initiation and progression of cancers and is a potential biomarker for gastric cancer.
Additionally, we validated two novel miRNAs which have been known as tumor suppressive (miR-655) and as oncogenic (miR-938) miRNA in gastrointestinal cancers other than gastric37–39,49. Although they did not show any stepwise elevation during multistep carcinogenesis, we could prove that they worked as tumor suppressive and oncogenic in gastric cancers. These results also prove that those novel miRNAs are important in gastric cancers, but do not work in multistep carcinogenesis, so they were not detected in our DEM miRNA groups.
Our study has several limitations. First, the sample size in miRNA array experiments is quite small (n = 24). To overcome this limitation, we replicated our microarray results in 77 FFPE samples and TCGA datasets. Secondly, the effects of altered miRNA on their target mRNA were not investigated. It is widely accepted that miRNAs have multiple -sometimes hundreds- of targets and the main approach to explore connections between miRNAs and their targets has been focused on the most significant target for each miRNA50. In the present study, we selected target genes that have been known to interact with miRNA in the previous studies by experimental investigation to confirm the relationship between miRNA and target mRNA. Further in vitro experiments using cell lines and larger scale studies are recommended to confirm our results and to analyze the potential effects of those deregulated miRNA.
In conclusion, we found that the upregulated (miR-107 and miR-300) or down-regulated (miR-26a and miR-375) miRNAs show alteration at early stages of gastric tumorigenesis and may be used as candidate biomarkers. Further investigation is needed to elucidate the exact role of these miRNAs in early gastric carcinogenesis.
Materials and Methods
Ethics statement
Fresh tumor and non-tumor samples were obtained by forceps biopsy at Samsung Comprehensive Cancer Center. Informed consent was obtained from all individuals who participated in this study. The study protocol was approved by the institutional review board of Samsung Medical Center (IRB 2010-09-020-008) and all experiments were performed in accordance with the approved guidelines and regulations.
Samples
Fresh tumor samples from 14 patients were obtained during endoscopy by forceps biopsy and were used as a test set (seven well to moderately differentiated intestinal-type EGCs, three HGDs, and four LGDs) (Table 1). No patient had prior chemo or radiation therapy. All lesions were completely removed further with an endoscopic submucosal dissection technique after tissue acquisition with forceps biopsy, and the resected specimens were reviewed by two pathologists. The pathologic diagnosis of histological grade (LGD, HGD, or EGC) was made based on the review of both forceps biopsy and endoscopic submucosal dissection specimens to ensure histological homogeneity as previously described3. Tumors mixed with components of different histological grades were excluded from the study. Non-tumor gastric samples more than 2 cm apart from the tumor were used as a reference. All non-tumor samples showed intestinal metaplasia. All fresh tissue samples were snap frozen in liquid nitrogen immediately after collection and stored at −80 °C until use. Helicobacter pylori infection status was determined using both urea breath test and histology, while Epstein-Barr virus status was determined by the Epstein-Barr encoding region in situ hybridization.
In addition, a total of 77 formalin-fixed paraffin-embedded (FFPE) samples were collected and used as a validation set (21 well/moderately differentiated intestinal-type EGCs, 22 HGDs, 24 LGDs, and 10 non-tumor tissues). Informed consent was obtained from all individuals who participated in the study, and the study protocol was approved by the institutional review board.
miRNA microarray by NanoString
For the nCounter analysis, 10 consecutive tissue sections (4-μm thick) from archival FFPE tissues of precursor lesions (LGD and HGD) and carcinoma were used. Total miRNA was isolated using the Qiagen miRNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total miRNA samples were analyzed using the nCounter Human miRNA Expression Assay kit (NanoString, Seattle, WA) according to manufacturer’s instructions. Briefly, 100 ng RNA was incubated in the presence of miRNA-specific capture and reporter probes and non-hybridized probes were removed, followed by immobilization of the purified hybridized complexes. The abundance of specific target molecules was subsequently quantified on the nCounter Digital Analyzer by counting the individual fluorescent barcodes and assessing the target molecules, as previously described51.
Differentially expressed miRNA analysis and correlation with mRNA
To identify the differentially expressed miRNAs (DEMs) among LGDs, HGDs, and EGCs, we calculated analysis of variance (ANOVA) p-value, average of miRNA expression within the same group, and fold change by comparing expression levels among the three groups. DEMs were defined by a p-value < 0.01, average expression >5 in at least one group, and |log2(fold change)| >1. The adjusted p-value was calculated using Benjamini and Hochberg algorithm in R. We next performed a k-means clustering to categorize DEMs into groups using expression patterns. To quantify the mRNA expression level, we counted the number of aligned fragments for each gene using HTSeq-0.6.1 with parameters (−s no, −r pos, −f bam, −m intersection-non-empty, and −t exon) according to the Ensembl transcript annotation (GRCh37 version) and calculated the fragments per kilobase per million mapped read (FPKM) values of each gene. Correlation between miRNA and mRNA expression was calculated by Spearman’s correlation using R. miRNA and mRNA expression data from The Cancer Genome Atlas (TCGA) datasets52 were also used to validate the patterns of DEMs and correlation between expression of miRNA and target mRNA expression.
Real-time quantitative PCR analysis with FFPE samples
Ten serial paraffin cuts obtained in an Eppendorf tube were deparaffinized in xylene. Total RNA was isolated using RNeasy Micro Kit (Qiagen, Germany) according to the manufacturer’s instructions. RNA concentrations were measured using NanoDrop (Thermo Scientific, USA). Total RNA from each sample was reverse transcribed with the TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher, USA). Reverse transcription was performed with the following thermal cycling parameters: 30 minutes at 16 °C, 30 minutes at 42 °C, and 5 minutes at 85 °C (Bio-Rad).
The miRNA expression was determined with TaqMan MicroRNA primer/probe sets. All qPCR reactions were performed with 7900 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Gene expressions for hsa-miR-375 (Assay ID, 000564), hsa-miR-370 (Assay ID, 002275), hsa-miR-26a (Assay ID, 000405), hsa-miR-300 (Assay ID, 241035), hsa-miR-1260 (Assay ID, 002896), and hsa-miR-107 (Assay ID, 000443), hsa-miR-655 (Assay ID, 001612), and hsa-miR-938 (Assay ID, 002181) were quantified by TaqMan microRNA Assays (Applied Biosystems) according to manufacturers’ protocol and normalized by U6 snRNA (Assay ID 001973). PCR amplification of target genes and quantification of PCR products were performed by ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems). Differences in the expression were determined by the relative quantification method; the Ct values of the test genes were normalized to the Ct values of the endogenous control U6 snRNA. The fold change was calculated using the equation 2−ΔΔCt.
Electronic supplementary material
Acknowledgements
This work was supported by the Samsung Biomedical Research Institute (grant # SBRI C-B1-110 to B.H.M.), the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (grant # NRF-2017R1D1A1B03032449 to K.M.K.), the NRF grant funded by the Korean government (MSIP; grant # 2012R1A5A2A44671346 to J.-I.K.), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare (grant # HI13C2148 to J.-I.K.).
Author Contributions
J.H., B.H.M. and J.J. designed, planned, and performed the experiments, analyzed the results, and wrote the manuscript. S.Y.K., H.B. and S.S.J. analyzed the results and performed the experiments. J.I.K. and K.M.K. conceptualized and supervised the project, analyzed the results, and wrote the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinha Hwang, Byung-Hoon Min and Jiryeon Jang contributed equally.
Contributor Information
Jong-Il Kim, Email: jongil@snu.ac.kr.
Kyoung-Mee Kim, Email: kkmkys@skku.edu.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32782-8.
References
- 1.Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut. 1999;45:784–90. doi: 10.1136/gut.45.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rugge M, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–6. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min BH, et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. J Pathol. 2016;240:304–314. doi: 10.1002/path.4777. [DOI] [PubMed] [Google Scholar]
- 4.Ishimoto T, et al. Current perspectives toward the identification of key players in gastric cancer microRNA dysregulation. Int J Cancer. 2016;138:1337–49. doi: 10.1002/ijc.29627. [DOI] [PubMed] [Google Scholar]
- 5.Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143:35–47 e2. doi: 10.1053/j.gastro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2013;10:109–18. doi: 10.1038/nrgastro.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WK, et al. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29:5761–71. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 8.Gattolliat CH, et al. MicroRNA and targeted mRNA expression profiling analysis in human colorectal adenomas and adenocarcinomas. Eur J Cancer. 2015;51:409–20. doi: 10.1016/j.ejca.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Li L, et al. Sequential expression of miR-182 and miR-503 cooperatively targets FBXW7, contributing to the malignant transformation of colon adenoma to adenocarcinoma. J Pathol. 2014;234:488–501. doi: 10.1002/path.4407. [DOI] [PubMed] [Google Scholar]
- 10.Nagy ZB, et al. Colorectal adenoma and carcinoma specific miRNA profiles in biopsy and their expression in plasma specimens. Clin Epigenetics. 2017;9:22. doi: 10.1186/s13148-016-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaby O, et al. Dynamic changes in microRNA expression profiles reflect progression of Barrett’s esophagus to esophageal adenocarcinoma. Carcinogenesis. 2015;36:521–7. doi: 10.1093/carcin/bgv023. [DOI] [PubMed] [Google Scholar]
- 12.Slattery ML, et al. MicroRNA profiles in colorectal carcinomas, adenomas and normal colonic mucosa: variations in miRNA expression and disease progression. Carcinogenesis. 2016;37:245–61. doi: 10.1093/carcin/bgv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streppel MM, et al. MicroRNA 223 is upregulated in the multistep progression of Barrett’s esophagus and modulates sensitivity to chemotherapy by targeting PARP1. Clin Cancer Res. 2013;19:4067–78. doi: 10.1158/1078-0432.CCR-13-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, et al. MicroRNA expression signatures during malignant progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Prev Res (Phila) 2013;6:196–205. doi: 10.1158/1940-6207.CAPR-12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, et al. MicroRNA expression signatures in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:5744–52. doi: 10.1158/1078-0432.CCR-09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu M, Zhang N, He S. Similarly up-regulated microRNA-106a in matched formalin-fixed paraffin-embedded and fresh frozen samples and the dynamic changes during gastric carcinogenesis and development. Pathol Res Pract. 2014;210:909–15. doi: 10.1016/j.prp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Deng M, et al. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One. 2013;8:e72662. doi: 10.1371/journal.pone.0072662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, et al. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–93. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 19.Su Y, et al. Aberrant expression of microRNAs in gastric cancer and biological significance of miR-574-3p. Int Immunopharmacol. 2012;13:468–75. doi: 10.1016/j.intimp.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JJ, et al. Reverse Correlation between MicroRNA-145 and FSCN1 Affecting Gastric Cancer Migration and Invasion. PLoS One. 2015;10:e0126890. doi: 10.1371/journal.pone.0126890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia L, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 22.Tang H, et al. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res. 2013;19:5602–12. doi: 10.1158/1078-0432.CCR-13-1326. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, et al. miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-A. BMB Reports. 2014;47:39–44. doi: 10.5483/BMBRep.2014.47.1.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–70. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, et al. miR-107 regulates tumor progression by targeting NF1 in gastric cancer. Sci Rep. 2016;6:36531. doi: 10.1038/srep36531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayremlou N, Mozdarani H, Mowla SJ, Delavari A. Increased levels of serum and tissue miR-107 in human gastric cancer: Correlation with tumor hypoxia. Cancer Biomark. 2015;15:851–60. doi: 10.3233/CBM-150529. [DOI] [PubMed] [Google Scholar]
- 27.Song YQ, et al. MicroRNA-107 promotes proliferation of gastric cancer cells by targeting cyclin dependent kinase 8. Diagn Pathol. 2014;9:164. doi: 10.1186/s13000-014-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, et al. MicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancer. J Cell Mol Med. 2011;15:1887–95. doi: 10.1111/j.1582-4934.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YJ, et al. MicroRNA-18a modulates P53 expression by targeting IRF2 in gastric cancer patients. J Gastroenterol Hepatol. 2016;31:155–63. doi: 10.1111/jgh.13041. [DOI] [PubMed] [Google Scholar]
- 30.Tsujiura M, et al. Circulating miR-18a in plasma contributes to cancer detection and monitoring in patients with gastric cancer. Gastric Cancer. 2014;18:271–279. doi: 10.1007/s10120-014-0363-1. [DOI] [PubMed] [Google Scholar]
- 31.Wu W, et al. MicroRNA-18a modulates STAT3 activity through negative regulation of PIAS3 during gastric adenocarcinogenesis. Br J Cancer. 2013;108:653–61. doi: 10.1038/bjc.2012.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo SS, et al. Overexpression of miR-370 and downregulation of its novel target TGFbeta-RII contribute to the progression of gastric carcinoma. Oncogene. 2012;31:226–37. doi: 10.1038/onc.2011.226. [DOI] [PubMed] [Google Scholar]
- 33.Fan C, et al. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521–6. doi: 10.1016/j.biopha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Shen Z, et al. The up-regulation of miR-300 in gastric cancer and its effects on cells malignancy. Int J Clin Exp Med. 2015;8:6773–83. [PMC free article] [PubMed] [Google Scholar]
- 35.Tang X, et al. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988–98. doi: 10.1093/nar/gkt1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, et al. MicroRNA-655-3p functions as a tumor suppressor by regulating ADAM10 and beta-catenin pathway in Hepatocellular Carcinoma. J Exp Clin Cancer Res. 2016;35:89. doi: 10.1186/s13046-016-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harazono Y, et al. miR-655 Is an EMT-suppressive microRNA targeting ZEB1 and TGFBR2. PLoS One. 2013;8:e62757. doi: 10.1371/journal.pone.0062757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Mir-655 up-regulation suppresses cell invasion by targeting pituitary tumor-transforming gene-1 in esophageal squamous cell carcinoma. J Transl Med. 2013;11:301. doi: 10.1186/1479-5876-11-301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Li CF, Li YC, Jin JP, Yan ZK, Li DD. miR-938 promotes colorectal cancer cell proliferation via targeting tumor suppressor PHLPP2. Eur J Pharmacol. 2017;807:168–173. doi: 10.1016/j.ejphar.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Luo H, Du J, Liu Y. MicroRNA-300 plays as oncogene by promoting proliferation and reducing apoptosis of liver cancer cells by targeting MDC1. International Journal Of Clinical And Experimental Pathology. 2016;9:1231–1239. [Google Scholar]
- 41.Li F, et al. Upregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014;588:538–44. doi: 10.1016/j.febslet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Xue Z, et al. Up-Regulation of MiR-300 Promotes Proliferation and Invasion of Osteosarcoma by Targeting BRD7. PLoS One. 2015;10:e0127682. doi: 10.1371/journal.pone.0127682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.He J, et al. Modulation of microRNAs by ionizing radiation in human gastric cancer. Oncol Rep. 2014;32:787–93. doi: 10.3892/or.2014.3246. [DOI] [PubMed] [Google Scholar]
- 44.Gao J, Liu QG. The role of miR-26 in tumors and normal tissues (Review) Oncol Lett. 2011;2:1019–1023. doi: 10.3892/ol.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen L, Cheng F, Zhou Y, Yin C. MiR-26a enhances the sensitivity of gastric cancer cells to cisplatin by targeting NRAS and E2F2. Saudi J Gastroenterol. 2015;21:313–9. doi: 10.4103/1319-3767.166206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen ZY, Zhang ZZ, Liu H, Zhao EH, Cao H. miR-375 inhibits the proliferation of gastric cancer cells by repressing ERBB2 expression. Exp Ther Med. 2014;7:1757–1761. doi: 10.3892/etm.2014.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, et al. Snail-regulated MiR-375 inhibits migration and invasion of gastric cancer cells by targeting JAK2. PLoS One. 2014;9:e99516. doi: 10.1371/journal.pone.0099516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura Y, et al. Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer. 2013;108:1324–31. doi: 10.1038/bjc.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv ZD, et al. miR-655 suppresses epithelial-to-mesenchymal transition by targeting Prrx1 in triple-negative breast cancer. J Cell Mol Med. 2016;20:864–73. doi: 10.1111/jcmm.12770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Peter ME. Targeting of mRNAs by multiple miRNAs: the next step. Oncogene. 2010;29:2161–4. doi: 10.1038/onc.2010.59. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, et al. High-throughput sequencing and copy number variation detection using formalin fixed embedded tissue in metastatic gastric cancer. PLoS One. 2014;9:e111693. doi: 10.1371/journal.pone.0111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cancer Genome Atlas Research, N Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.