Abstract
To investigate the prognostic value of heart-type fatty acid binding protein (H-FABP) in patients with stable coronary heart disease (SCHD). A total of 1,071 patients with SCHD were prospectively enrolled in this Taiwan multicenter registry study, followed for 24 months. The cut-off value of H-FABP, 4.143 ng/mL, was determined using receiver operating characteristic curves. The primary cardiovascular (CV) outcome was composite CV events, defined as cardiovascular or cerebrovascular death, myocardial infarction (MI), stroke, angina related-hospitalization, PAOD-related hospitalization and heart failure. Secondary outcomes included CV or cerebrovascular death, nonfatal MI, nonfatal stroke, and acute heart failure-related hospitalization. We found that the high H-FABP group had more than a two-fold higher rate of primary CV outcomes than the low H-FABP group (32.36% vs. 15.78%, p < 0.001). Eleven patients (4.82%) of the high H-FABP group died during the 24 months of follow-up, compared to only one patient (0.12%) in the low H-FABP group. The acute heart failure-related hospitalization rate was also significantly higher in the high H-FABP group (3.5% vs. 0.95%, p < 0.005). The results remained significant after adjusting for baseline covariates. In conclusion, H-FABP was an independent predictor for CV outcomes in the patients with SCHD, mainly in CV death and acute heart failure-related hospitalization.
Introduction
Ischemic heart disease and stroke have been the leading causes of death globally in the past decades, and the mortality rate from these diseases is gradually increasing. In addition to traditional cardiovascular (CV) risk factors such as smoking, type 2 diabetes mellitus (T2DM), hypertension (HTN) and dyslipidemia, researchers have investigated potential novel biomarkers, for instance, copeptin1, pentraxin-32 and heart-type fatty acid binding protein (H-FABP) to predict the clinical course and CV outcomes. In particular, H-FABP has been widely studied in patients with acute coronary syndrome (ACS), and it has been suggested to increase diagnostic sensitivity and possibly predict long-term survival3.
H-FABP is a human protein that is encoded by the fatty acid binding protein 3 (FABP3) gene and is located on chromosome 1p32-p35. It is a cytoplasmic protein which was first isolated from ischemic rat hearts in 1988, and was identified as being released from injured myocardium4,5. Associations between H-FABP and ACS6–8, acute kidney injury9, post-cardiac surgery10, acute pulmonary embolism11, acute ischemic stroke12, severe sepsis13, acute heart failure14, hypothyroidism15 and hyperthyroidism16 have been reported over the past decades. On the other hand, H-FABP has also been used to assess perioperative cardiac risk17,18. However, the prognostic implication of H-FABP in patients with stable coronary heart disease (SCHD) is unknown. The aim of this study was to investigate the prognostic value of H-FABP in CV outcomes in patients with SCHD.
Results
Patients
A total of 1,072 SCHD patients from the National Taiwan Biosignature Research (NTBR) cohort study were enrolled and followed for 24 months or until a CV event. At 24 months, 207 cardiovascular events had occurred, including 12 CV deaths, 24 nonfatal myocardial infarction (MI), 6 nonfatal strokes and 16 acute heart failure-related hospitalizations (Table 1).
Table 1.
Cardiovascular Events in 24 months.
| Events | Number of case (%) |
|---|---|
| Total CV events | 207 |
| CV or cerebrovascular death | 12 (5.8%) |
| Nonfatal myocardial infarction | 24 (12%) |
| Nonfatal stroke | 6 (3%) |
| Angina-related hospitalization | 132 (64%) |
| PAOD-related hospitalization | 14 (7%) |
| Heart failure Acute heart failure-related hospitalization Aortic dissection Brady- or tachyarrhythmia |
19 (9.2%) 16 (7.7%) 1 (0.5%) 2 (1%) |
CV = cardiovascular, MI = myocardial infarction, PAOD = peripheral arterial occlusive disease.
The cut-off value of H-FABP (4.143 ng/mL) was determined by receiver operating characteristic curves (ROC) curve analysis (Fig. 1) between the patients with and without CV events from the blood sample obtained at enrollment. The baseline characteristics revealed that the patients with a high level of H-FABP had higher rates of HTN, but lower rate of family history of premature coronary artery disease (CAD). Except for a lower level of serum high-density lipoprotein cholesterol (HDL-C), patients with a high level of H-FABP had significantly higher blood glucose, systolic blood pressure (SBP), serum creatinine, high sensitivity C-reactive protein (hs-CRP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) than those with a low level of H-FABP (Table 2).
Figure 1.
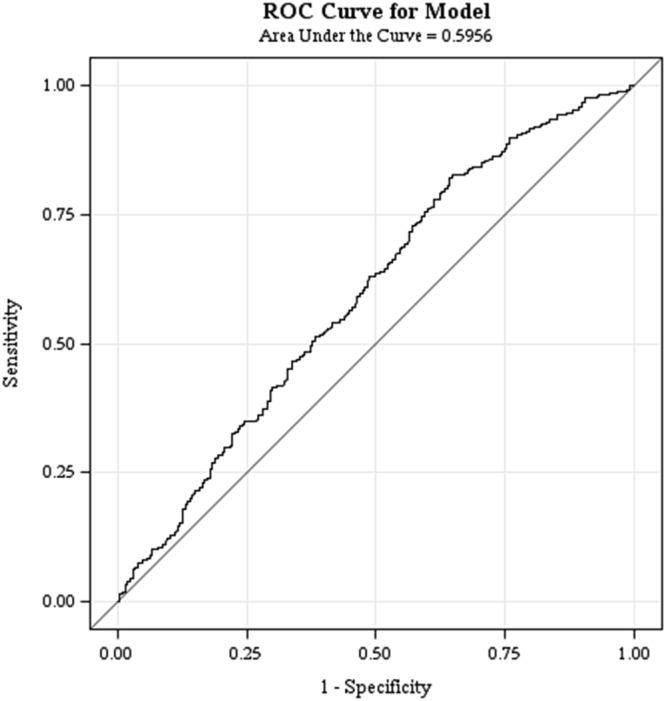
Receiver operating characteristic curve (ROC) analysis plot with area under the curve, sensitivity and specificity of H-FABP in prediction of total cardiovascular events.
Table 2.
Baseline characteristics of patients with stable coronary heart disease.
| Total | (%) | H-FABP < 4.143 ng/mL | H-FABP ≧ 4.143 ng/mL | p | |||
|---|---|---|---|---|---|---|---|
| n | n | (%) | n | (%) | |||
| Male gender | 1071 | 913 (85.25%) | 843 | 718 (85.17%) | 228 | 195 (85.53%) | 0.894 |
| Hypertension | 1071 | 698 (65.17%) | 843 | 531 (62.99%) | 228 | 167 (73.25%) | 0.004 |
| Diabetes | 1071 | 408 (38.1%) | 843 | 283 (33.57%) | 228 | 125 (54.82%) | <0.001 |
| Smoking | 1071 | 603 (56.3%) | 843 | 482 (57.18%) | 228 | 121 (53.07%) | 0.267 |
| Family history of premature CAD | 1071 | 246 (22.97%) | 843 | 213 (25.27%) | 228 | 33 (14.47%) | 0.001 |
| Previous stroke | 1071 | 28 (2.61%) | 843 | 22 (2.61%) | 228 | 6 (2.63%) | 0.985 |
| 1-vessel disease | 1071 | 596 (55.65%) | 843 | 482 (57.18%) | 228 | 114 (50%) | 0.138 |
| 2-vessel disease | 1071 | 165 (15.41%) | 843 | 124 (14.71%) | 228 | 41 (17.98%) | 0.118 |
| 3-vessel disease | 1071 | 21 (1.96%) | 843 | 16 (1.9%) | 228 | 5 (2.19%) | 0.699 |
| Median (IQRs) | Median (IQRs) | Median (IQRs) | |||||
| Age, year | 1071 | 64.9 (57.2–74.3) | 843 | 63.3 (56.5–71.6) | 228 | 72.5 (62.2-81.0) | <0.001 |
| BMI (kg/m2) | 1070 | 25.9 (23.7-28.3) | 842 | 26.0 (23.7–28.4) | 228 | 25.6 (23.4–28.4) | 0.555 |
| Systolic BP, mmHg | 1071 | 130 (119–114) | 843 | 130 (119–140) | 228 | 131 (120–147) | 0.016 |
| Diastolic BP, mmHg | 1071 | 74 (66–83) | 843 | 75 (67–83) | 228 | 73 (65–83) | 0.112 |
| Glucose, mg/dL | 1065 | 106 (95–131) | 839 | 105 (94–126) | 226 | 114 (97–143) | 0.002 |
| Hemoglobin, g/dL | 1019 | 13.6 (12.4–14.9) | 796 | 14.0 (12.8–15.1) | 223 | 12.4 (11.0–13.7) | <0.001 |
| LDL-C, mg/dL | 1066 | 90 (73–111) | 841 | 90 (74–112) | 225 | 91 (72–108) | 0.322 |
| HDL-C, mg/dL | 1065 | 40 (35–48) | 840 | 41 (35–48) | 225 | 38 (33–45) | 0.007 |
| Serum creatinine, mg/dL | 1066 | 1.03 (0.87–1.28) | 839 | 0.98 (0.83–1.14) | 227 | 1.50 (1.19–2.36) | <0.001 |
| eGFR, mL/min/1.73 m2 | 1066 | 74 (59–90) | 839 | 79 (66–95) | 227 | 68 (28-63) | <0.001 |
| hs-CRP, mg/dL | 779 | 0.14 (0.07–0.31) | 611 | 0.13 (0.07–0.27) | 168 | 0.22 (0.10–0.58) | 0.004 |
| NT-pro BNP, pg/mL | 1071 | 171 (66–460) | 843 | 141 (58–367) | 228 | 334 (109–880) | 0.001 |
Results are expressed as percentage or medians (IQRs).
BMI = body mass index; BP = blood pressure; CAD = coronary artery disease; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein -cholesterol; LDL-C = low-density lipoprotein-cholesterol; hs-CRP = high sensitivity C-reactive protein; NT-pro BNP = N-terminal pro-brain natriuretic peptide.
Primary outcomes
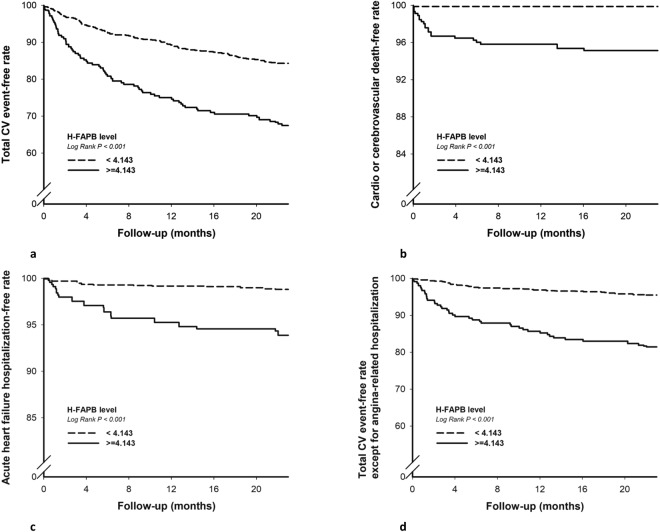
After 24 months of follow up, the high H-FABP group had more than a two-fold higher rate of primary CV events than the low H-FABP group (32.36% vs. 15.78%, p < 0.001) (Table 3). The Kaplan-Meier curves of the two groups were significantly separated from the beginning of the study to 24 months (Fig. 2a).
Table 3.
Clinical outcomes in 24 months.
| All (n = 1,071) | H-FABP < 4.143 ng/mL, (n = 843) | H-FABP ≧ 4.143 ng/mL, (n = 228) | p | |
|---|---|---|---|---|
| Primary outcome | ||||
| Total CV events, n (%) | 207 (19.33%) | 133 (15.78%) | 74 (32.46%) | <0.001 |
| Secondary outcome | ||||
| CV or cerebrovascular death, n (%) | 12 (1.12%) | 1 (0.12%) | 11 (4.82%) | <0.001 |
| Nonfatal myocardial infarction, n (%) | 24 (2.24%) | 16 (1.9%) | 8 (3.51%) | 0.145 |
| Nonfatal stroke, n (%) | 6 (0.56%) | 3 (0.36%) | 3 (1.32%) | 0.085 |
| Acute heart failure-related hospitalization, n (%) | 16 (1.49%) | 8 (0.95%) | 8 (3.51%) | 0.005 |
| Total CV events except for angina-related hospitalization, n (%) | 80 (7.47%) | 38 (4.51%) | 42(18.00%) | <0.001 |
CV = cardiovascular.
Figure 2.
Kaplan–Meier survival curves analysis showing total cardiovascular (CV) event-free rate (a), CV or cerebrovascular death-free rate (b), acute heart failure hospitalization-free rate (c), and total CV event-free rate except for angina-related hospitalization (d) in patients with serum H-FABP ≧ 4.143 ng/mL and H-FABP < 4.143 ng/mL (all p < 0.001).
Secondary outcomes
A total of 11 deaths (4.82%) occurred in the high H-FABP group, compared with only one (0.12%) in the low H-FABP group (Table 3 and Fig. 2b). In addition, the high H-FABP group had a significantly higher rate of acute heart failure-related hospitalizations (3.5%) compared to the low H-FABP group (0.95%) (Table 3 and Fig. 2c). Although statistically non-significant, there was also a trend of higher rate of nonfatal MI and nonfatal stroke in the high H-FABP group (Table 3). There were 80 patients with total CV events except for “angina-related hospitalization”, 38 patients in H-FABP group, 42 patients in H-FABP group (4.51% vs 32.46%, p < 0.001). The difference between these two groups remained significant (Fig. 2d).
In multivariate Cox proportional hazards analysis adjusted for age, sex, body mass index (BMI), serum creatinine, estimated glomerular filtration rate (eGFR), HDL-C, hemoglobin (Hb), blood glucose, hs-CRP, NT-proBNP, SBP, smoking, family history of premature CAD, history of hypertension and diabetes mellitus, high H-FABP level was still an independent prognostic risk factor for CV events (HR 2.93, 95% CI 1.95–4.394, p < 0.001). In addition, a high level of H-FABP also predicted CV death (HR 22.89, 95% CI 2.16–242.55, p = 0.009) and acute heart failure-related hospitalizations (HR 5.16, 95% CI 1.096–24.324, p = 0.038) in the 24-month follow-up period, even after adjusted for other covariates (Table 4).
Table 4.
Univariate and multivariable logistic Cox-proportional regression analysis models for clinical outcomes.
| Univariate* | Multivariate** | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Primary outcome | ||||
| Total CV events | 2.35 (1.77–3.13) | <0.001 | 2.93 (1.95–4.39) | <0.001 |
| Secondary outcome | ||||
| CV or cerebrovascular death | 41.75 (5.39–323386) | 0.004 | 22.89 (2.16–242.55) | 0.009 |
| Nonfatal myocardial infarction | 1.96 (0.84–4.58) | 0.120 | 2.62 (0.80–8.59) | 0.112 |
| Nonfatal stroke | 3.91 (0.79–19.35) | 0.085 | 1.56 (0.06–38.62) | 0.786 |
| Acute heart failure related hospitalization | 3.90 (1.46–10.39) | 0.007 | 5.16 (1.10–24.32) | 0.038 |
CV = cardiovascular.
*Non-adjusting.
**Adjusting for significant variables in univariate analysis, which including age, gender, body mass index, serum creatinine, estimated glomerular filtration rate, high-density lipoprotein, low-density lipoprotein, hemoglobin, fasting glucose, high sensitivity C-reactive protein, N-terminal pro-brain natriuretic peptide, systolic blood pressure, history of hypertension, smoking and diabetes, family history of premature CAD.
Discussion
This study is the first prospective cohort study to demonstrate that a higher serum H-FABP level (≧4.143 ng/mL) is an independent predictor for CV events, particularly for cardio- and cerebrovascular death and acute heart failure-related hospitalizations in patients with SCHD. Our result was concordant with the Takahata study19, which also found that H-FABP level was increased in association with greater numbers of cardiovascular risk factors. In addition, Takahata study noted higher H-FABP level was an independent risk factor for all-cause and cardiovascular deaths in 3,503 subjects who participated in a community-based health checkup in a 7-year follow-up.
The early diagnosis of acute MI is still challenging for emergency physicians despite the wide application of myoglobin and high-sensitivity cardiac troponin (cTn) in emergency rooms, because the elevation of most myocardial injury serum markers are delayed by at least 2–4 hours after an ischemic insult. In 2000, an experimental study of ligation of the left main coronary artery in mice demonstrated that the concentration of H-FABP at 4 hours could be used to stratify MI compared to cTn at 48 hours20. In addition, Okamoto et al. reported that H-FABP is more sensitive than myoglobin and creatinine kinase isoenzyme MB for the diagnosis of acute MI in the early phase21. In 2006, O’Donoghue et al. reported an association between an elevated level of H-FABP and increased risks of death and major cardiac events in patients with ACS22. Collinson et al.23 compared the diagnostic performances of cTn-I, H-FABP and copeptin in low-risk patients presenting with chest pain. The authors concluded that cTn-I remained the best single test, with the incremental diagnostic sensitivity of serum H-FABP, but not copeptin. Furthermore, a recent dobutamine stress echocardiography (DSE) study reported significantly increased levels of serum H-FABP at 1 hour in the presence of DSE-induced ischemia, in contrast to DSE negative group, whose serum H-FABP remained unchanged before and 1 hour after the test24. However, in a study that was expected H-FABP to increase during exercise stress testing (EST), serum H-FABP tended to decline statistically significant from the basal level to 3 hours after the EST25. A recent systemic review of H-FABP in ACS found marked heterogeneity in the prognostic impact of H-FABP between studies, reflecting differences in sampling times and the population at risk. Hence, it may not be possible to routinely use H-FABP as a prognostic marker in patients with suspected ACS26.
Wunderlich et al. were the first to report that an early elevation of serum H-FABP and brain type fatty acid binding protein (B-FABP) concentration were significantly associated with the severity of neurological deficits and functional outcomes in patients after an acute ischemic stroke12. The peak levels of H-FABP and B-FABP occur 2 to 3 hours after an event and remain elevated for up to 120 hours. In addition, a high level of H-FABP is associated with large infarctions on brain computed tomography. Another investigation of 41 patients with acute stroke (31 with ischemic stroke, 10 with intracerebral hemorrhage) demonstrated that serum H-FABP and ischemic-modified albumin (IMA) levels increased within 4.5 hours27. Nonetheless, An et al. reported that H-FABP was not an independent marker in patients with ischemic stroke, and thus that its clinical usefulness is limited28. In the current study, we demonstrated the prognostic value of H-FABP in CV events in patients with SCHD after successful treatment, but that it had limited value in the prediction of nonfatal MI and ischemic stroke. In this study, although statistically non-significant, there was also a trend of higher rate of nonfatal MI and nonfatal stroke in the high H-FABP group.
The relationship between H-FABP and heart failure was first reported in the early 2000s, when the concentration of H-FABP was positively correlated with the concentration of BNP in patients with acute deterioration of heart failure29. Later, Setsuka et al.30 reported that H-FABP was present in the activation of tumor necrosis factor (TNF) and the Fas ligand system. This suggested a pathophysiological role of cardiomyocyte necrosis and/or apoptosis in patients with worsening heart failure. Moreover, Hoffmann et al.14 investigated H-FABP in acute heart failure, and found that additional H-FABP measurements improved the diagnostic specificity and positive predictive value of NT-proBNP tests. In addition, their patients in the highest H-FABP quartile had significantly higher rates of all-cause mortality (HR 2.1–2.5; p = 0.04) and risk of re-hospitalization for acute heart failure at 5 years (HR 2.8–8.3, p = 0.001). Our study also demonstrated that the SCHD patients with high H-FABP level had a higher risk for acute heart failure-related hospitalizations at 24 months.
There are several limitations of this study. First, even though the criteria for patient enrollment and the protocol for clinical follow-up were clearly defined, selection bias arising from clinical profiles, investigator participation and treatment adherence by the patients could not be completely excluded31. Second, this is a hospital based rather than a community-based study, and this design was potentially limited by geographic variations such as environmental exposure to risk factors of CV disease32. Third, all the patients were stable during enrollment and followed up regularly for clinical events in the out-patient clinics of the medical centers. Their medications may have been adjusted by the specific cardiologists during follow-up. Thus, the potential effects of different cardiovascular drugs on clinical outcomes could not be well addressed33. Fourth, the very few cases of the each secondary event category, insufficient statistical power of predictive value of H-FABP could be derived from the multivariate analyses.
In conclusion, H-FABP was an independent predictor for total CV events in the patients with SCHD at 24 months, mainly for CV and cerebrovascular deaths and acute heart failure-related hospitalization.
Methods
Study population
This NTBR was a prospective cohort study of patients with SCHD (aged ≧ 20 years) from nine medical centers in Taiwan31. At enrollment, all of the participants had undergone a percutaneous coronary intervention at least once and had been stable on medical treatment for at least 1 month. The exclusion criteria included hospitalization for any CV event within 3 months, and those unable or unwilling to be followed up during the following 1 year period. Specific clinical outcomes including all-cause, cardiovascular, cerebrovascular mortalities, and CV-related hospitalizations were confirmed using the Health and Welfare Data Science Center (HWDC) of Taiwan.
This study complied with the Declaration of Helsinki and was approved by the appropriate Health Authorities, independent Ethics Committees, and Institutional Review Boards (IRB) in each hospital as well as the Joint IRB Ethics Committee Review Board in Taiwan. All of the patients agreed to participate and signed the informed consent form.
Baseline clinical and biomarker data collection
After enrollment, data were prospectively collected by physicians and nurses whenever feasible. Baseline characteristics included sex, age, HTN, T2DM, hyperlipidemia, smoking, family history of premature CAD, BMI, number of stenotic coronary arteries, and biochemical data including renal function, lipid profile at enrollment in each hospital were recorded. Hs-CRP was performed automatically with chemiluminescent immunoassay methods, on a Beckman Coulter DXC 800 immunoassay platform (Beckman Coulter, Inc. CA, USA). NT-pro BNP and H-FABP were measured manually on EMD Millipore’s MILLIPLEX MAP Human CVD 1 Magnetic Bead kit (Millipore, Inc. MO, USA).
Clinical follow-up
Questionnaire and blood samples were obtained from the patients every 3 months in the first year and every 6 months thereafter for a total of 24 months. The primary CV outcome was composite CV events, defined as cardiovascular or cerebrovascular death, MI, stroke, angina-related hospitalization, PAOD-related hospitalization and heart failure. Heart failure was a composite of acute heart failure-related hospitalization, syncope, cardiopulmonary resuscitation, bradyarrhythmia, supraventricular tachyarrhythmia, ventricular arrhythmia, permanent pacemaker implantation and aortic dissection. The secondary outcomes included CV or cerebrovascular death, nonfatal MI, nonfatal stroke, and acute heart failure-related hospitalization.
Statistical analysis
The cut-off value of H-FABP was determined using ROC curve analysis between the patients with and without CV events from the blood sample obtained at enrollment. Baseline characteristics and CV outcomes were compared between the patients with high and low levels of H-FABP.
Results are expressed as median (interquartile ranges [IQRs]) for continuous variables, and qualitative variables are expressed in absolute frequencies (number of patients) and relative frequencies (percentage). Comparisons of continuous variables between groups were performed using ANOVA or Mann-Whitney U tests. The primary and secondary outcomes were described as overall percentages and expressed as means of proportions with a 95% confidence interval (CI). The Kaplan-Meier method was used to calculate events and survival rates. Hazard ratios (HRs) for the regression of Cox proportional hazards were used, along with the corresponding standard error, 95% CI, and p value. Independent baseline variables with a p value < 0.05 in the univariate analysis were included in the multivariate analysis. In all the tests, the two-tailed alpha significance level was 0.05. In addition, p values were reported up to three decimals, while those below 0.001 were reported as p < 0.001.
Electronic supplementary material
Acknowledgements
Biosignature project is supported by Academia Sinica (project number: BM10501010039) and Taiwan Clinical Trial Consortium of Cardiovascular Diseases (TCTC-CVD) (project number: MOST 106-2321-B-002-029-).
Author Contributions
S.K.H., Y.-W.W. and C.-C.W. conceived and designed the research. Y.-W.W., W.-K.T., H.-B.L., W.-H.Y., T.-H.L., H.-I.Y., K.-C.C., J.-H.W., J.-W.C. and C.-C.W. managed data collection. S.K.H. drafted the manuscript and designed the figures and tables. Y.-W.W. and C.-C.W. made critical revision of the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
A full list of consortium members appears in the Supplementary Information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yen-Wen Wu and Chau-Chung Wu contributed equally.
Change history
3/13/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Contributor Information
Yen-Wen Wu, Email: wuyw0502@gmail.com.
Chau-Chung Wu, Email: chauchungwu@ntu.edu.tw.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32210-x.
References
- 1.Boeckel JN, et al. Analyzing the release of copeptin from the heart in acute myocardial infarction using a transcoronary gradient model. Sci Rep. 2016;6:20812. doi: 10.1038/srep20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vengen IT, Enger TB, Videm V, Garred P. Pentraxin 3, ficolin-2 and lectin pathway associated serine protease MASP-3 as early predictors of myocardial infarction – the HUNT2 study. Sci Rep. 2017;7:43045. doi: 10.1038/srep43045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenenberger AW, et al. Incremental value of heart-type fatty acid-binding protein in suspected acute myocardial infarction early after symptom onset. Eur Heart J Acute Cardiovasc Care. 2016;5:185–192. doi: 10.1177/2048872615571256. [DOI] [PubMed] [Google Scholar]
- 4.Phelan CM, et al. The human mammary-derived growth inhibitor (MDGI) gene: genomic structure and mutation analysis in human breast tumors. Genomics. 1996;34:63–68. doi: 10.1006/geno.1996.0241. [DOI] [PubMed] [Google Scholar]
- 5.Glatz JF, et al. Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988;961:148–152. doi: 10.1016/0005-2760(88)90141-5. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy AM, et al. Performances of the heart fatty acid protein assay for the rapid diagnosis of acute myocardial infarction in ED patients. Am J Emerg Med. 2015;33:326–330. doi: 10.1016/j.ajem.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Liebetrau C, et al. Release kinetics of early ischaemic biomarkers in a clinical model of acute myocardial infarction. Heart. 2014;100:652–657. doi: 10.1136/heartjnl-2013-305253. [DOI] [PubMed] [Google Scholar]
- 8.Uitterdijk A, et al. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: how fast and accurate can myocardial infarct size and no-reflow be predicted? Am J Physiol Heart Circ Physiol. 2013;305:1104–1110. doi: 10.1152/ajpheart.00447.2013. [DOI] [PubMed] [Google Scholar]
- 9.Shirakabe A, et al. The serum heart-type fatty acid-binding protein (HFABP) levels can be used to detect the presence of acute kidney injury on admission in patients admitted to the non-surgical intensive care unit. BMC Cardiovasc Disorders. 2016;16:174. doi: 10.1186/s12872-016-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol. 2017;28:3699–3707. doi: 10.1681/ASN.2017010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer M, et al. Heart-type fatty acid-binding protein and myocardial creatine kinase enable rapid risk stratification in normotensive patients with pulmonary embolism. J Crit Care. 2016;35:174–179. doi: 10.1016/j.jcrc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Wunderlich MT, et al. Release of brain-type and heart-type fatty acid-binding proteins in serum after acute ischaemic stroke. J Neurol. 2005;252:718–724. doi: 10.1007/s00415-005-0725-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZC, Dai HW, Yu YH, Yang JD, Hu CB. Usefulness of heart-type fatty acid-binding protein in patients with severe sepsis. J Crit Care. 2012;27:415.e13–415.e18. doi: 10.1016/j.jcrc.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann U, et al. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure - diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc Disord. 2015;15:50. doi: 10.1186/s12872-015-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunes F, et al. Serum H-FABP levels in patients with hypothyroidism. Wien Klin Wochenschr. 2014;126:727–733. doi: 10.1007/s00508-014-0612-7. [DOI] [PubMed] [Google Scholar]
- 16.Ozbek M, et al. Serum heart type fatty acid binding protein levels are not changed in hyperthyroidism. Minerva Endocrinol. 2016;41:298–301. [PubMed] [Google Scholar]
- 17.Marković DZ, et al. Addition of biomarker panel improves prediction performance of American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) calculator for cardiac risk assessment of elderly patients preparing for major non-cardiac surgery: a pilot study. Aging Clin Exp Res. 2018;30:419–431. doi: 10.1007/s40520-017-0805-9. [DOI] [PubMed] [Google Scholar]
- 18.Sari M, Kilic H, Ariturk OK, Yazihan N, Akdemir R. Diabetic patients have increased perioperative cardiac risk in heart-type fatty acid-binding protein-based assessment. Med Princ Pract. 2015;24:53–57. doi: 10.1159/000368756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otaki Y, et al. Association of heart-type fatty acid-binding protein with cardiovascular risk factors and all-cause mortality in the general population: the Takahata study. PLoS One. 2014;9:e94834. doi: 10.1371/journal.pone.0094834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aartsen WM, et al. Heart fatty acid binding protein and cardiac troponin T plasma concentrations as markers for myocardial infarction after coronary artery ligation in mice. Pflugers Arch. 2000;439:416–422. doi: 10.1007/s004249900180. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto F, et al. Human heart-type cytoplasmic fatty acid-binding protein (H-FABP) for the diagnosis of acute myocardial infarction. Clinical evaluation of H-FABP in comparison with myoglobin and creatine kinase isoenzyme MB. Clin Chem Lab Med. 2000;38:231–238. doi: 10.1515/CCLM.2000.034. [DOI] [PubMed] [Google Scholar]
- 22.O’Donoghue M, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 23.Collinson P, Gaze D, Goodacre S. Comparison of contemporary troponin assays with the novel biomarkers, heart fatty acid binding protein and copeptin, for the early confirmation or exclusion of myocardial infarction in patients presenting to the emergency department with chest pain. Heart. 2014;100:140–145. doi: 10.1136/heartjnl-2013-304716. [DOI] [PubMed] [Google Scholar]
- 24.Akinci S, et al. Effect of dobutamine stress echocardiography on serum heart fatty acid binding protein levels. Acta Cardiol. 2017;72:161–166. doi: 10.1080/00015385.2017.1291181. [DOI] [PubMed] [Google Scholar]
- 25.Arı H, et al. Relationship between heart-type fatty acid-binding protein levels and coronary artery disease in exercise stress testing: an observational study. Anadolu Kardiyol Derg. 2011;11:685–691. doi: 10.5152/akd.2011.189. [DOI] [PubMed] [Google Scholar]
- 26.Jones JD, et al. The Prognostic value of heart type fatty acid binding protein in patients with suspected acute coronary syndrome: a systematic review. Curr Cardiol Rev. 2017;13:189–198. doi: 10.2174/1573403X13666170116121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herisson F, Delaroche O, Auffray-Calvier E, Duport BD, Guillon B. Ischemia-modified albumin and heart fatty acid-binding protein: could early ischemic cardiac biomarkers be used in acute stroke management? J Stroke Cerebrovasc Dis. 2010;19:279–282. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 28.An SA, et al. Limited clinical value of multiple blood markers in the diagnosis of ischemic stroke. ClinBiochem. 2013;46:710–715. doi: 10.1016/j.clinbiochem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Goto T, et al. Circulating concentrations of cardiac proteins indicate the severity of congestive heart failure. Heart. 2003;89:1303–1307. doi: 10.1136/heart.89.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setsuta K, et al. Ongoing myocardial damage in chronic heart failure is related to activated tumor necrosis factor and Fas/Fas ligand system. Circ J. 2004;68:747–750. doi: 10.1253/circj.68.747. [DOI] [PubMed] [Google Scholar]
- 31.Leu HB, et al. Identification of new biosignatures for clinical outcomes in stable coronary artery disease - The study protocol and initial observations of a prospective follow-up study in Taiwan. BMC Cardiovasc Disord. 2017;17:42. doi: 10.1186/s12872-017-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien SC, et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. 2017;241:1–5. doi: 10.1016/j.ijcard.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Huang CC, et al. Optimal achieved blood pressure for patients with stable coronary artery disease. Sci Rep. 2017;7:10137. doi: 10.1038/s41598-017-10628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.