Abstract
In wheat (Triticum spp.), modifying inflorescence (spike) morphology can increase grain number and size and thus improve yield. Here, we demonstrated the potential for manipulating and predicting spike morphology, based on 44 traits. In 12 wheat cultivars, we observed that detillering (removal of branches), which alters photosynthate distribution, changed spike morphology. Our genome-wide association study detected close associations between carbon partitioning (e.g. tiller number, main shoot dry weight) and spike morphology (e.g. spike length, spikelet density) traits in 210 cultivars. Most carbon-partitioning traits (e.g. tiller dry weight, harvest index) demonstrated high prediction abilities (>0.5). For spike morphology, some traits (e.g. total and fertile spikelet number, spike length) displayed high prediction abilities (0.3–0.5), but others (e.g. spikelet fertility, spikelet density) exhibited low prediction abilities (<0.2). Grain size traits were closely correlated in field and greenhouse experiments. Stepwise regression analysis suggests that significantly associated traits in the greenhouse explain 35.35% of the variation in grain yield and 67.63% of the variation in thousand-kernel weight in the field. Therefore, the traits identified in this study affect spike morphology; these traits can be used to predict and improve plant architecture and thus increase yield.
Introduction
The morphology of the inflorescence (termed the spike) of small-grain cereals is crucial in determining grain yield. The components of the spike (e.g. spikelets, which are the basic units of the inflorescence and contain florets, glumes, and lemma), also influence each other. The number and arrangement of these spike components affect spike length, spike weight, spike chaff (i.e. the non-grain biomass in the spike), grain number per spike, grain weight per spike, and spikelet number per spike, which all contribute to final grain yield per spike1–3. To produce varieties with the most efficient grain production in different environments, we need to know how to predict and manipulate spike morphology in wheat.
The formation of branches, termed tillers, affects grain number and grain weight, and is therefore closely related to yield4–7. Donald (1968) proposed that uniculm wheat, which does not form tillers, has the potential for greater yield compared to wheat with tillers8. Detillering can be used to study the relationship between tillering and the spike morphology factors discussed above. It has been reported that the increase in grain yield after tiller removal was mainly due to the increase of grain number per spikelet in wheat9.
In addition to tiller removal, other studies have removed spike components such as spikelets, florets, glumes and lemmas to assess their interactions with each other. For example, Pinthus and Millet (1978) found a small increase in grain number and a marked increase in grain weight in the remaining spikelets after spikelet removal in wheat10. They also showed that grain weight was determined by factors that affect grain volume11. Similarly, Wang et al. (1998) found that spikelet removal considerably increased grain number and grain weight per spikelet12. Removal of glumes and lemmas from wheat spikelets during the grain filling period resulted in less growth than in intact florets13.
In addition to measuring spike morphology traits, we examined some important indicators that are related to known differences in spike morphology and assimilate distribution. For example, the spike fertility index (determined as grain number per gram of spike chaff), spikelet density (spikelet number per centimeter of spike length), spikelet fertility (the ratio of fertile to total spikelet number) and floret fertility (the ratio between the maximum number of floret primordia and final grain number within individual spikelet) reveal relationships between the spike components discussed above and also demonstrate the overall effects on spike growth and development. We hypothesized that the wheat spike adapts to stress and environmental conditions by balancing the relationships between different components of the spike. Therefore, we can try to maximize the grain yield by manipulating and predicting spike morphology traits in wheat.
In this report, we first demonstrated the potential for manipulating spike morphology through a detillering experiment. Moreover, we conducted a genome-wide association study (GWAS) and genome-wide selection (GS), demonstrating the potential for genetically manipulating and predicting spike morphology in wheat. Furthermore, correlation and stepwise analyses revealed the relationship between greenhouse and field data, as well as critical spike morphometric traits for the determination of grain yield.
Materials and Methods
Plant material and growth conditions
Experiments were carried out at the Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany (51°49′23″N, 11°17′13″E, altitude 112 m). The 12 hexaploid spring wheat cultivars were selected according to released years (Table S1) for detillering experiment. Control and tiller removal experiments based on the 12 wheat cultivars were conducted in the field and greenhouse simultaneously. Tillers were removed two to three times per week. Eighty plants per cultivar (forty plants for control and the other forty plants for tiller removal) were planted under field and greenhouse conditions. The 80 individuals from each cultivar were used to make sure there are enough plants for phenotypic measurement, because there are variations between individual plants within one genotype and not all the plants can be used for the phenotypic measurements. The GABI-WHEAT population contains 358 European winter and 14 spring wheat varieties. Of these, we selected 210 winter cultivars based on the different alleles at the photoperiod (Ppd) and reduced height (Rht) loci14 (Supplementary dataset). The genotyping work for Ppd-D1 (chromosome 2D) and Rht-D1 (chromosome 4D) was carried out according to the markers in previous study15,16. Detailed information about the genotyping process can be found in previous work17.
Phenotyping for the 210 winter wheat accessions was conducted in the greenhouse. The growth conditions for all the cultivars, including the 210 winter wheat accessions for GWAS, and the 12 spring wheat accessions for the detillering experiment, can be found in a previous work3. In brief, forty plants were planted for each cultivar. The grains were sown in 96-well trays on the same date and germinated under greenhouse conditions (photoperiod and temperature, 16:8 h, 20:16 °C, light: dark) for 14 d. Seedlings at the two- to three-leaf stage were transferred to 4 °C to vernalize for 63 d. The vernalized seedlings were transferred to a hardening stage (photoperiod and temperature, 12:12 h, 15:15 °C, light: dark) for 7 d to gradually acclimatize. Finally, all the plants were trans- planted into 0.5 l pots (one plant per pot) under controlled greenhouse conditions (photoperiod and temperature, 16:8 h, 20:6 °C, light: dark). Supplemental light (c. 250 lmol m−2 s−1 photosynthetically active radiation) was supplied with low- intensity incandescent light and plants were irrigated when required.
Phenotypic measurements
In this study, 44 traits were measured in the 210 wheat accessions (Table 1). Spike length (cm), spike chaff per spike (g), spike dry weight (g), main stem dry weight (g), grain weight per spike (g), grain number per spike, and total and fertile spikelet numbers were measured at physiological maturity. Six plants for each cultivar were randomly selected for trait measurements. Spike length was measured without awn. The main stem was measured by the weight of the main shoot without spike but included leaves. In addition, final grain number per spikelet was measured in spikelets at three positions of the spike: apical (the third spikelet from the top of the spike), central (the spikelet in center of the spike), and basal positions (the third spikelet from the bottom of the spike). Spike fertility index, spikelet density, and fertility are determined by spikelet number per centimeter of spike length, grain number (per spike) per gram of spike chaff (per spike) at harvest18, and ratio of fertile and total spikelet numbers. Here, it should be noted that basal spikelets of the spike are from low-middle positions of the spike and do not include the extreme basal spikelets at the bottom of the spike. Infertile spikelets are defined as spikelets that did not set grain at all (i.e. were completely empty); whereas fertile spikelets produced at least one grain.
| 1 |
| 2 |
| 3 |
Table 1.
The 44 assimilate distribution and spike morphology traits in this study.
| Running number | Trait | Affected plant parts |
|---|---|---|
| 1 | Grain A | main shoot |
| 2 | Grain C | main shoot |
| 3 | Grain B | main shoot |
| 4 | grain number for tillers | tiller |
| 5 | grain weight for tillers (g) | tiller |
| 6 | TKW (g) | tiller |
| 7 | grain area (mm2) | tiller |
| 8 | grain width (mm) | tiller |
| 9 | grain length (mm) | tiller |
| 10 | tiller number | tiller |
| 11 | tiller DW (g) | tiller |
| 12 | stem length (cm) | main shoot |
| 13 | leaf number | main shoot |
| 14 | leaf DW (g) | main shoot |
| 15 | main DW (g) | main shoot |
| 16 | spike length (cm) | main shoot |
| 17 | total spikelet number | main shoot |
| 18 | fertile spikelet number | main shoot |
| 19 | spike DW (g) | main shoot |
| 20 | main stem DW (g) | main shoot |
| 21 | grain number/spike | main shoot |
| 22 | grain weight/spike | main shoot |
| 23 | spike chaff (g) | main shoot |
| 24 | TKW (g) | main shoot |
| 25 | grain area (mm2) | main shoot |
| 26 | grain width (mm) | main shoot |
| 27 | grain length (mm) | main shoot |
| 28 | spikelet density | main shoot |
| 29 | spike fertility index | main shoot |
| 30 | grain weight/spike chaff | main shoot |
| 31 | spikelet fertility | main shoot |
| 32 | spike DW/main stem DW | main shoot |
| 33 | plant DW (g) | whole plant |
| 34 | grain number | whole plant |
| 35 | grain weight (g) | whole plant |
| 36 | TKW(g) | whole plant |
| 37 | grain area (mm2) | whole plant |
| 38 | grain width (mm) | whole plant |
| 39 | grain length (mm) | whole plant |
| 40 | harvest index | whole plant |
| 41 | tiller DW/main DW | tiller/main |
| 42 | Max.Floret A | main shoot |
| 43 | Max.Floret C | main shoot |
| 44 | Max.Floret B | main shoot |
Note: A and B indicate the third spikelet from the top (apical) and bottom (base) of the spike while C (central) indicates spikelets from the middle of the spike; DW, dry weight; TKW, thousand kernel weight. Grain number A, C, B is the grain number per spikelet at different spikelet positions. Max. Floret A, C, B is the maximum number of floret primordia per spikelet at different spikelet positions. Spike chaff is the DW of spike without grains. Main DW, main stem DW, total spikelet number mean the main shoot DW, main shoot DW without spike, spikelet number per spike on the main shoot, respectively. Tiller DW/main DW, spike DW/main stem DW, grain weight/spike chaff imply the ratios between tiller DW and main shoot DW, between spike DW and main stem DW, between grain weight and spike chaff. Spike fertility index is the ratio between grain number and spike chaff of the main shoot. Spikelet fertility is the ratio between the number of fertile spikelet and total spikelet. Harvest index is obtained as the ratio between grain weight and total aboveground dry weight. The attribution of traits suggests the traits from main shoot or tillers. Grain size traits (grain length, width, area, TKW) are measured for main shoot, tillers and the whole plant (including main shoot and tillers), respectively.
The grain yield (tons/hectare) for all the 210 winter wheat accessions were determined at eight environments in the field. In this study, we reanalyzed some data to support the results with different SNP markers and approaches for different purposes.
Plant materials and analyses of phenotypic data
The phenotypic data within each trial were analyzed using the following mixed model:
| 4 |
where P were the phenotypic values in each trial, G referred to the genetic values, and e denotes the residuals. G and e were assumed to be random effects in order to estimate the repeatability. The repeatability for each experiment was calculated as: where VG was the genetic variance, Ve was the variance of residuals, and R referred to the number of replicates. Phenotypic values of 3 traits in different trials with repeatability estimates below 0.5 were removed from further analysis.
We used the following linear mixed model to estimate the best linear unbiased estimations (BLUEs) of the genotypes across the experiments:
| 5 |
where P represents the phenotypic values in each trial, G referred to the genetic values, T was the effect of each trial, and e denotes the residuals. G was treated as fixed effect to estimate BLUEs and as random effect to estimate the variance component. E and e were always set as random effects. Moreover, we assumed heterogeneous error variance in each trial. The heritability was calculated as , where O was the number of average overlapped trials.
Genome-wide association analyses
To investigate the influence of population admixture, we first used the block relaxation algorithm in the ADMIXTURE program19 to estimate individual ancestry proportions given an optimal number (k = 10) of hypothetical ancestral populations. Then, we performed a principal component analysis (PCA) on the wheat population of 210 cultivars. The first 10 principal components were used for subsequent analysis analyses according to the ADMIXTURE results. The PCA results did not reveal any apparent population structure among the varieties (Fig. S1). Therefore, we carried out a genome-wide association study (GWAS) with a mixed linear model using EMMA20. Since many associated single nucleotide polymorphism (SNP) markers were detected, we chose an overall cutoff significance level of −log10 (P-value) ≥ 3.0, which means that one false positive is expected in one-thousand events. It is above or close to the threshold of false discovery rate for most traits.
Marker-based estimation of heri- tability (or narrow-sense heritability, h2) was implemented based on a mixed model to compute the restricted maximum likelihood (REML)-estimates of the genetic and residual variance. Repeata- bility and heritability were calculated by the HERITABILITY R pack- age21.
Genome-wide prediction
We used two genome-wide prediction models in combination with five-fold cross validation in order to evaluate the accuracy to predict genetic values for untested genotypes. The first model exploits additive effects:
| 6 |
The second model use additive and additive time additive effects:
| 7 |
where y was the vector of phenotypic records, μ referred to the overall mean, ga were the additive genetic effect and gaa were the additive × additive genetic effects. We assumed that μ is a fixed effect, , , and , where the matrices Ga, and Gaa are the relationship matrices corresponding to additive and additive × additive epistatic genetic effects. Details of the implementation of the models have been described in Zhao et al. (2015).
Results
Modifying spike morphology through manipulation of assimilate partitioning
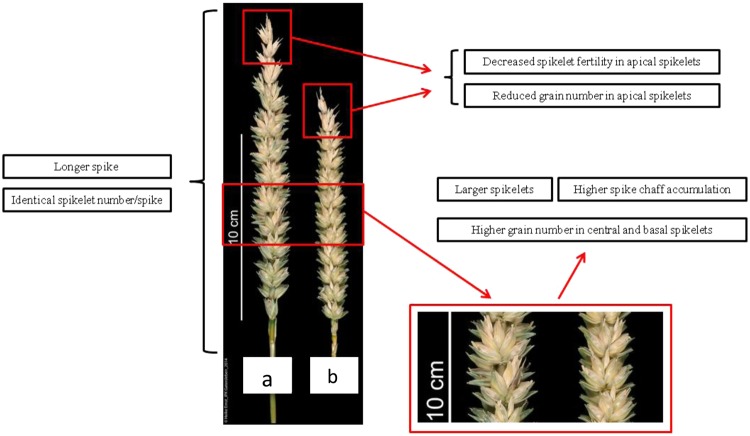
We conducted a detillering experiment to examine the potential of spike morphology manipulation by regulating allocation distribution (Fig. 1, Tables 2 and S2–8). Under field and greenhouse growth conditions, the average of spikelet density (spikelet number per centimeter of spike length) was significantly decreased by tiller removal in all the 12 cultivars (Fig. 1, Tables S2 and S3). Detillering lead to a substantial decrease of spikelet density, because tiller removal had no significant effect on total spikelet number per spike, spike length was significantly increased by tiller removal in all the 12 genotypes compared with control under both greenhouse growth conditions (Fig. 1, Tables S2 and S3).
Figure 1.
The spikes (three weeks after anthesis) in detillering (a) and control (b) treatments in the genotype ‘Breustedt’s Lera’. The traits under detillering treatment are described compared to control.
Table 2.
Grain number per spike, spike chaff per spike (g), and spike fertility index in 12 cultivars under control and tiller removal treatments in the greenhouse at harvest.
| Cultivar | Grain number per spike | Spike chaff per spike | Spike fertility index | |||
|---|---|---|---|---|---|---|
| Control | Detillering | Control | Detillering | Control | Detillering | |
| 1- Adlung’s Alemannen | 57.83 ± 6.52a | 50.67 ± 10.93a | 1.02 ± 0.13b | 1.30 ± 0.10a | 56.81 ± 2.11a | 39.13 ± 7.82b |
| 2- NOS Nordgau | 52.00 ± 6.23a | 52.40 ± 13.16a | 0.61 ± 0.06b | 0.98 ± 0.14a | 85.14 ± 6.19a | 53.37 ± 9.33b |
| 3- Peragis Garant | 44.00 ± 5.29a | 52.60 ± 16.62a | 0.64 ± 0.09b | 0.83 ± 0.21a | 69.89 ± 7.98a | 62.54 ± 6.96a |
| 4- Heine’s Peko | 39.33 ± 3.08a | 34.50 ± 10.63a | 0.83 ± 0.10b | 1.19 ± 0.17a | 47.86 ± 3.69a | 28.57 ± 6.24b |
| 5- Hohenheimer Franken II | 31.00 ± 3.95a | 28.40 ± 6.39a | 0.67 ± 0.12b | 1.01 ± 0.11a | 47.80 ± 11.77a | 28.02 ± 4.15b |
| 6- Probat | 52.83 ± 9.15a | 41.50 ± 6.66a | 0.80 ± 0.12b | 1.15 ± 0.15a | 69.33 ± 13.09a | 36.70 ± 8.95b |
| 7- Breustedt’s Lera | 41.00 ± 7.94a | 47.33 ± 16.07a | 0.63 ± 0.10b | 1.21 ± 0.13a | 64.69 ± 6.10a | 40.99 ± 10.88b |
| 8- Arin | 43.67 ± 5.28b | 64.67 ± 10.60a | 0.61 ± 0.09b | 0.84 ± 0.11a | 72.10 ± 6.18a | 76.72 ± 7.13a |
| 9- Kolibri | 44.00 ± 5.44b | 67.67 ± 4.76a | 0.72 ± 0.05b | 1.19 ± 0.08a | 64.04 ± 4.48a | 56.84 ± 3.38b |
| 10- Ralle | 45.20 ± 3.35b | 57.33 ± 10.52a | 0.70 ± 0.07b | 1.03 ± 0.10a | 65.13 ± 3.64a | 55.67 ± 6.48b |
| 11- Nandu | 46.67 ± 6.35b | 66.50 ± 5.68a | 0.63 ± 0.11b | 1.16 ± 0.15a | 74.12 ± 3.55a | 58.02 ± 5.63b |
| 12- Fasan | 45.77 ± 12.47b | 65.17 ± 11.97a | 0.57 ± 0.14b | 0.98 ± 0.15a | 80.05 ± 5.58a | 66.89 ± 8.17b |
| Average | 45.33 ± 9.16b | 53.34 ± 15.84a | 0.70 ± 0.16b | 1.07 ± 0.19a | 66.45 ± 12.99a | 51.26 ± 16.44b |
Data are presented as the mean ± SD, n = 6; different letters per trait indicate significant differences between control and treated plants (p < 0.05).
Detillering also considerably decrease the average of spike fertility index (grain number per gram of spike chaff) for all the 12 cultivars. The combined influence on grain number per spike and spike chaff per spike resulted in a marked decreases in spike fertility index (with one exception, cv. Arin) in the field and greenhouse (Tables 2 and S4). The average of spikelet fertility rate (the ratio of fertile and total spikelet number) for all the 12 cultivars in control plants was considerably higher than in detillered plants (Tables S5 and S6).
Here, we found that final grain number per spikelet was increased at central or basal spikelets by tiller removal in greenhouse and field conditions (Tables S7 and S8), which may have resulted in less final grain number per spikelet at apical spikelet positions, possibly due to the competition between spikelets at different positions. At apical spikelets, especially in the greenhouse, there were large variations in the grain number per spikelet (Table S7, S8), mainly because of completely infertile spikelets. Infertile spikelets set no grains, producing a grain number per spikelet of zero. The nearly infertile spikelets only set one or two grains per spikelet.
Shared QTLs between assimilate distribution and spike morphology
Based the 210 winter wheat accessions, narrow sense heritability showed a broad range from 0.16 to 0.93. Of all 44 traits, 88.63% (39 traits) displayed narrow-sense heritability (h2) above 0.4, and 63.63% (28 traits) traits showed a h2 over 0.7 (Table S9), providing the genetic basis for the GWAS.
We conducted GWAS of all the 44 traits to determine the associated SNP markers that can be used to modify the spike morphology (Supplementary Dataset). Based on the GWAS results, we further assessed the shared QTLs between assimilate partitioning (e.g. tiller DW, main shoot DW, main stem DW, Table 1) and spike morphology (e.g. spike length, spikelet density, Table 1), since the main goal of this study was to investigate how the assimilate flow regulates spike morphology.
As shown in Table 3, shared QTLs on 2A (64.3 cM), 5A (5.69 cM), 5B (166.5 cM), 6B (49.0 cM) and 7B (135.7 cM) indicated the involvement of main shoot DW, main stem DW, ratio between tiller DW and main shoot DW, ratio between spike DW and main stem DW, and spike chaff in determination of spike length. It suggested that the assimilate partitioning between spike and stem as well as between tiller and main shoot, affected spikelet size/spike chaff which further lead to the modification of spike length. The association of grain number per spike on the main shoot with main stem DW, ratio between spike DW and main stem DW and spike chaff was revealed by the QTLs on 1A (26.1 cM), 1B (46.0 cM) and 7B (59.1 cM). It implied roles of assimilate allocation between spike DW and main stem DW, and spike chaff and grains in determining grain number per spike. As expected, the spike chaff per spike is associated with the ratio of tiller DW and main shoot DW, and the ratio between spike DW and main stem DW, which is indicated by shared QTLs on 1B (46.0 cM), 2B (87.5 cM) and 4A (156.3 cM). Spike fertility index can be influenced by the ratio between tiller DW and main DW, and the ratio between grain weight and spike chaff, which is revealed by 7A (1.6 cM, 89.8 cM). The shared QTLs on 3A (59.8 cM, 61.4 cM), 5B (45.8) and 7A (1.6 cM) indicated the importance of assimilate distribution between tiller and main shoot, grain and spike chaff in the improvement of spikelet fertility. Furthermore, grain number per spikelet at apical (A, the third spikelet from the top) and central (C, in the center of the spike) spikelet can be determined by the assimilate partitioning between tiller DW and main DW, spike DW and main stem DW, grains and spike chaff, which is implied by QTLs on 1B (41.3 cM), 2A (57.1 cM, 64.3 cM), 3A (59.0 cM, 59.8 cM) and 7A (13.4 cM).
Table 3.
SNP markers associated with assimilate allocation and spike morphology traits.
| Traits | Main DW | Tiller DW/main DW | Main stem DW | Spike DW/main stem DW | Grain weight per spike | Spike chaff | Grain weight/spike chaff |
|---|---|---|---|---|---|---|---|
| Total spikelet number | 2A, 64.3 | ||||||
| Spike length | 5B, 166.5 | 5A, 11.0 | 5B, 166.5 | 2A, 64.3 | 6B, 49; 7B, 135.7 | ||
| Grain number per spike | 7B, 59.1 | 1A, 26.1 | 1B, 46 | ||||
| Spike chaff | 2B, 87.5; 4A, 156.3 | 1B, 46 | |||||
| Spike fertility index | 7A, 1.6 | 7A, 89.8 | |||||
| Spikelet fertility | 3A, 59.8; 7A, 1.6 | 5B, 45.8 | 3A, 61.4 | ||||
| Grain number (A) | 2A, 57.1; 3A, 59.0 | 2A, 64.3 | 3A, 59.8 | ||||
| Grain number (C) | 7A, 13.4 | 1B, 41.3 | 1B, 41.3 | 1B, 41.3 |
Note: A and C indicate the third spikelet from apical position and the spikelet in central position of the spike, respectively; DW, dry weight; TKW, thousand kernel weight. Grain number is the grain number per spikelet. Spike chaff is the DW of spike without grains on the main shoot. Main DW, main stem DW, total spikelet number mean the main shoot DW, main shoot DW without spike, spikelet number per spike on the main shoot, respectively. Tiller DW/main DW, spike DW/main stem DW, grain weight/spike chaff imply the ratios between tiller DW and main shoot DW, between spike DW and main stem DW, between grain weight and spike chaff. Spike fertility index is the ratio between grain number and spike chaff of the main shoot. Spikelet fertility is the ratio between the number of fertile spikelet and total spikelet. All data are from the main shoot, except data that have been named by tiller. SNP, single nucleotide polymorphism.
We genotyped the Rht-D1 (Chromosome 4D) in the 210 wheat accessions and detected that allelic variants at Rht-D1 influence spike morphology traits. The dwarf allele of Rht-D1 significantly increased grain number per spikelet at apical positions of spike (grain A, P < 0.001); but it did not show effects on central and basal positions of spike (grain C, B). The dwarf allele significantly decreased spike DW of main shoot (P < 0.05), which may attributable to its effects on grain weight. Since the dwarf allele markedly decreased grain size (grain area, width, length and TKW, all P < 0.001); but has no obvious effects on spike chaff, it significantly increased the spike fertility index (ratio between grain number and spike chaff).
Correlation between traits in field (grain yield) and greenhouse
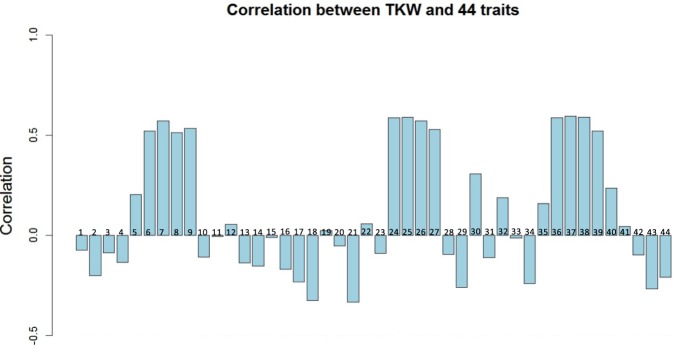
Using the 210 winter wheat accessions, we also assessed the correlations between the 44 traits in the greenhouse with TKW and grain yield (ton per hectare) in the field to see the relationships between yield-related traits between the field and greenhouse (Figs 2 and S2, Tables S10 and S11). We observed that individual grain area, grain width, length, TKW in the greenhouse displayed the strongest correlations (>0.5 Pearson correlation coefficient) with TKW in the filed (Fig. 3, Table S10). It suggests a the high stability of grain size between field and greenhouse. The associations of the 44 traits with grain yield in the field are not as strong as TKW (Fig. S2, Table S11). The ratio between tiller DW and main shoot DW displays the closest positive connection with grain yield in field, indicating that similar DW between tillers and main shoot (low differences between tiller DW and main shoot DW) is helpful to the improvement of grain yield (Fig. S2, Table S11). In addition, grain number per spike, grain weight per spike, tiller number, tiller DW and ratio between spike DW and stem DW also exhibited relatively strong connections with grain yield in the field (Fig. S2, Table S11), implying that not only the tiller growth but also assimilate distribution between spike and stem is beneficial to the improvement of grain yield. This is supported by the strong negative correlation between grain yield and stem DW, which suggests that reducing assimilate distribution to the stem is helpful for the improvement of grain yield. Moreover, leaf number and DW show close correlation with grain yield, suggesting that the superabundant leaf number and DW may also compete assimilates with the spike.
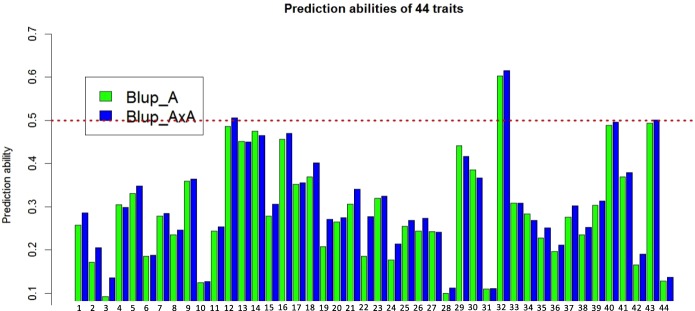
Figure 2.
Cross-validated prediction abilities of genome-wide prediction exploiting additive (Blup_A) or additive and additive × additive effects (Blup_AxA) for all 44 traits. The traits corresponding to each number can be found in Table 1.
Figure 3.
Correlations between TKW in field and the 44 traits (spike morphology and assimilate partitioning traits) in greenhouse, the corresponding number of the running number is shown in Table 1.
Stepwise Regression for yield and TKW in field
Using the data from the 210 winter wheat accessions, the 44 traits in this study were subjected to a stepwise analysis to determine the significant variables contributing to the variation of TKW and grain yield (Tables 4 and 5). Results suggested that the most important variables contributing to TKW (field)) were TKW, main stem DW, ratio between grain weight and spike chaff, grain number per spike, leaf number in greenhouse, which can explain 27.20%, 12.42%, 12.29%, 11.63% and 4.19% of the seen variation for TKW in the field, respectively (Table 4). The effects of all the five traits on grain yield were significant (TKW P = 4.09 × 10−9 < 0.001, main stem DW P = 1.65 × 10−5 < 0.001, ratio between grain weight and spike chaff P = 1.79 × 10−5 < 0.001, grain number per spike P = 2.80 × 10−5 < 0.001, leaf number P = 0.008 < 0.01), the effects of TKW and the ratio between grain weight and spike chaff were positive, but the effects of grain number per spike, leaf number, main stem DW were negative (Table 5). The positive and negative effects can be explained by the tradeoff between grain size and number as well as the competition for assimilates between spike and main stem.
Table 4.
Stepwise regression for TKW in the field based on the 44 traits in the greenhouse.
| Yield component | Percentage of square | Effect value | Fisher test value |
|---|---|---|---|
| TKW (tillers) | 27.20 | 0.05336 | 4.09E-09*** |
| Stem DW (main shoot) | 12.42 | −4.34451 | 1.65E-05*** |
| Grain weight/spike chaff (main shoot) | 12.29 | 2.24634 | 1.79E-05*** |
| Grain number for tillers (tillers) | 11.63 | −1.4021 | 2.80E-05*** |
| Leaf number (main shoot) | 4.19 | −0.15695 | 0.008125** |
| Total | 67.73 |
Note: the traits from tillers or main shoot are indicated in parentheses. Grain weight/spike chaff is the ratio between spike weight and spike chaff on the main shoot. TKW, thousand kernel weight. *, **, *** suggest the significance of Fisher test value on 0.05, 0.01, 0.001 level, respectively.
Table 5.
Stepwise regression for grain yield in the field based on the 44 traits in the greenhouse.
| Yield components | Percentage of square | Effect value | Fisher test value |
|---|---|---|---|
| Grain weight/spike chaff (main shoot) | 13.84 | 0.25361 | 0.000571*** |
| Leaf number (main shoot) | 11.31 | −0.14824 | 0.001676** |
| Grain length (main shoot) | 5.22 | 0.14363 | 0.029014* |
| Grain width (tillers) | 4.98 | −0.22353 | 0.032889* |
| Total | 35.35 |
Note: individual grain width means the grains from tillers; the other three traits are from main shoot. Grain weight/spike chaff is the ratio between spike weight and spike chaff on the main shoot. *, **, *** suggest the significance of Fisher test value on 0.05, 0.01, 0.001 level, respectively.
The most important variables contributing to grain yield in the field were the ratio between grain weight and spike chaff, leaf number on the main shoot, grain length and grain width, which can explain 13.84%, 11.31%, 5.22% and 4.98% of the variation for TKW in the field, respectively (Table 5). The effects of grain width and leaf number were negative, while the effects of grain length and the ratio between grain weight and spike chaff are positive (Table 5). It appears that long and thin grains are beneficial for grain setting based on the negative effects of grain width and positive effects of grain length. The positive effects of the ratio between grain weight and spike chaff indicates increased assimilates allocation towards grains improves grain yield in wheat.
In summary, in this study two traits (leaf number on the main shoot and ratio between grain weight and spike chaff) appeared to closely influence to both grain yield and TKW; while 35.35% of grain yield variation and 67.73% of the TKW variation in the field can be explained by the traits with significant effects in the greenhouse (Tables 4 and 5).
Prediction ability of assimilate distribution and spike morphology traits
The correlation between the 44 traits (assimilate distribution and spike morphology traits) and the stepwise regression analysis suggest that grain yield and TKW are closed associated with assimilate distribution and spike morphology traits. Therefore, it is necessary to know if it is reliable to predict these traits using the SNP markers and show the prediction ability. In the 210 winter wheat accessions, the prediction ability for each trait was estimated as the Pearson correlation coefficient between the predicted values and the BLUEs. We observed moderate to high prediction ability ranging from 0.10 to 0.62 across the 44 traits. The model with additive × additive interactions was on average 2% more accurate than the standard additive model (Fig. 3).
Most of assimilate partitioning traits (e.g. tiller DW, harvest index, spike fertility index) demonstrated high prediction abilities (>0.5). For the determination of spike morphology, some traits (e.g. total and fertile spikelet number, spike length) displayed relatively high prediction abilities (0.3–0.5), while others (e.g. spikelet fertility, spikelet density) exhibited relatively low prediction abilities (<0.2). Interestingly, it was detected that prediction ability for maximum number of floret primordia per spikelet (max. floret) is quite high in central spikelet (~0.5), but it is relatively low in apical and basal spikelets (<0.2), suggesting the independence of genetic regulation for max. floret at the three spikelet positions in this study.
Discussion
Interaction among spike morphology traits in wheat
The evident effects of tiller removal on spikes were mainly on spike length and chaff, grain number per spike, grain number per spikelet (mainly central and basal), and fertile spikelet number.
Final grain number per spikelet at central and basal spikelets of the spike was increased, while it was decreased at apical positions (S7, S8). Previous studies showed that spikelet removal resulted in a higher grain number per spikelet10,12. We also found that an increase in grain number per spikelet (central and basal spikelets) after tiller removal decreased fertile spikelet number per spike. Final grain number per spikelet at apical spikelets showed large variation and the apical spikelet have a lower chance to set grain compared with central and basal spikelets of the same spike, which is consistent with previous work22,23.
Fertile spikelet number was decreased, but total spikelet (infertile + fertile spikelet) number was unchanged (Tables S7 and S8), indicating that the decrease in fertile spikelet number per spike is mainly caused by the increase of infertile spikelets at the apical part of the spike in detillered plants.
Grain number per spike, spike length, and spike chaff were consistently increased in detillered plants (Tables 2 and S4). Consequently, spike fertility index, spikelet density and fertility were all markedly decreased by tiller removal. Grain number per spikelet were increased at the central and basal spikelets, but decreased at apical spikelets (Tables S7 and S8); hence fertile spikelet number was decreased (Tables S5 and S6). The combined influence of these four traits on grain number per spike is as follows: the increased grain number per spikelet at central and basal spikelets did not only compensate the loss caused by decreased grain number per spikelet at apical spikelets, but also finally improved the overall grain number per spike (Tables 2 and S4). The increase of chaff per spike was attributed to the increase of spikelet and floret organ size (i.e. for example glumes, lemma, palea) and spike length (predominantly rachis internode length; Fig. 1). The increase of grain number per spikelet resulted in the increase of spikelet fertility and size at central and basal spikelets (Fig. 1). We found increased spike length and unchanged total spikelet (fertile + infertile spikelets) numbers in this experiment, consistent with previous work in the wheat tin (tiller inhibition) mutant5,24.
Assimilates distribution on spike morphology traits
Tillering is a core component of plant architecture25. Varying tiller number may produce significant differences in spike morphology26–28. A major tiller inhibition (tin) gene (a wheat cellulose synthase-like gene) can restrict tiller production4,25,29,30. Higher kernel weights in tin lines were realized even with the greater kernel number per spike as there was little difference in grain size4,31.
The effects of the tin gene on spike morphology traits were supported by the results in this report. Here, we observed that the ratio between tiller DW and main shoot DW shares SNP markers with spike chaff per spike, spike fertility index, spikelet fertility and grain number per spikelet. These shared markers suggest the close association of spike morphology traits with assimilate distribution between tiller and main shoot.
The utilization of dwarfing genes Rht-B1b and Rht-D1b in wheat significantly increased grain yield, because of their effects on grain number and size. In this study, we found that the ratio between spike DW and main stem DW shares SNP markers with spikelet number per spike, spike length, grain number per spike, grain number per spikelet, and spike chaff per spike. This implies the connection of assimilate partitioning between spike and stem with spike morphology traits, which is consistent with the effects of allelic variants of Rht loci on spike morphology.
Important traits for the determination of grain yield and size
As expected, the ratio between grain weight and spike chaff can positively and significantly influence both grain yield and TKW. The ratio reveals the assimilates allocation between grain and the spike bracts (glume, lemma, and palea) which are the main photosynthesis parts of wheat spike32,33. Photosynthesis by spikes may make a larger contribution to final grain yield than flag leaves when drought develops during grain filling32,34,35. The length and width of glume and lemma can significantly affect grain size in wheat36.
Although photosynthesis by wheat spikes (glume, lemma, palea) makes a large contribution to final grain yield37–39, the excessive growth of glume, lemma and palea will increase their competition strength for assimilates, which may further reduce the assimilates allocation to grain growth and finally decrease final grain yield. This may explain the positive contribution of the ratio between grain weight and spike chaff to grain yield.
Leaf development is critical for the determination of grain yield, since the leaves are the main photosynthetic part40–43. Also, maintaining green leaves during grain filling possibly leads to increased grain yield, since the duration of leaf senescence during grain filling has been shown to affect both carbon and nitrogen partitioning and remobilization44–47. However, the redundant leaves may result in improved competition strength for assimilates and they similarly decrease of assimilate allocation to spike growth. This can explain the negative contribution of leaf number to grain yield.
Electronic supplementary material
Acknowledgements
We thank Karin Lipfert and Heike Ernst for the help on figure design, and Mechthild Pürschel for excellent technical assistance. The authors received financial support from a scholarship of the Chinese Scholarship Council to Z.G., the HEISENBERG Program of the German Research Foundation (DFG), grant no. SCHN 768/8-1, to T.S., from the EU-FP7 KBBE-2011-5 ‘ADAPTAWHEAT’ project number 289842 (to T.S. and M.W.G.) and from the German Bundesministerium für Bildung und Forschung (0315947 A to M.W.G. and 0315947B to M.S.R.).
Author Contributions
T.S. conceived the project. T.S. and Z.G. designed the experiments. Z.G. conducted phenotyping measurement in the greenhouse trials. Y.Z., D.J. and Z.G. managed the main data analysis, including GWAS and genomic selection analysis, figure design and generation, and J.C.R. supervised the data analysis in R. M.S.R. and M.W.G. developed and mapped the SNP markers. All the authors were involved in the preparation of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31977-3.
References
- 1.Millet E. Relationships Between Grain Weight and the Size of Floret Cavity in the Wheat Spike. Ann. Bot. 1986;58:417–423. doi: 10.1093/oxfordjournals.aob.a087220. [DOI] [Google Scholar]
- 2.Fischer RA, Hille RisLambers D. Effect of environment and cultivar on source limitation to grain weight in wheat. Aust. J. Agric. Res. 1978;29:443–458. doi: 10.1071/AR9780443. [DOI] [Google Scholar]
- 3.Guo Z, et al. Genome-wide association analyses of 54 traits identified multiple loci for the determination of floret fertility in wheat. New Phytol. 2017;214:257–270. doi: 10.1111/nph.14342. [DOI] [PubMed] [Google Scholar]
- 4.Duggan BL, Richards RA, Van Herwaarden AF, Fettell NA. Agronomic evaluation of a tiller inhibition gene (tin) in wheat. I. Effect on yield, yield components, and grain protein. Aust. J. Agric. Res. 2005;56:169–178. doi: 10.1071/AR04152. [DOI] [Google Scholar]
- 5.Kebrom TH, Richards RA. Physiological perspectives of reduced tillering and stunting in the tiller inhibition (tin) mutant of wheat. Functional Plant Biology. 2013;40:977–985. doi: 10.1071/FP13034. [DOI] [PubMed] [Google Scholar]
- 6.Whipple CJ, et al. Grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc. Natl. Acad. Sci. 2011;108:E506–E512. doi: 10.1073/pnas.1102819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang D, et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. 2014;111:11199–11204. doi: 10.1073/pnas.1411859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald CM. The breeding of crop ideotypes. Euphytica. 1968;17:385–403. doi: 10.1007/BF00056241. [DOI] [Google Scholar]
- 9.Mohamed GES, Marshall C. Physiological Aspects Of Tiller Removal In Spring Wheat. J. Agric. Sci. 1979;93:457–463. doi: 10.1017/S0021859600038156. [DOI] [Google Scholar]
- 10.Pinthus MJ, Millet E. Interactions Among Number of Spikelets, Number of Grains and Grain Weight in the Spikes of Wheat (Triticum aestivum L)*. Ann. Bot. 1978;42:839–848. doi: 10.1093/oxfordjournals.aob.a085523. [DOI] [Google Scholar]
- 11.Millet E, Pinthus MJ. The association between grain volume and grain weight in wheat. J. Cereal Sci. 1984;2:31–35. doi: 10.1016/S0733-5210(84)80005-3. [DOI] [Google Scholar]
- 12.Wang Z, Yin Y, He M, Cao H. Source-sink manipulation effects on postanthesis photosynthesis and grain setting on spike in winter wheat. Photosynthetica. 1998;35:453–459. doi: 10.1023/A:1006817714739. [DOI] [Google Scholar]
- 13.Radley M. The effect on wheat grain growth of the removal or ABA treatment of glumes and lemmas. J. Exp. Bot. 1981;32:129–140. doi: 10.1093/jxb/32.1.129. [DOI] [Google Scholar]
- 14.Kollers, S. et al. Whole Genome Association Mapping of Fusarium Head Blight Resistance in European Winter Wheat (Triticum aestivum L.). PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 15.Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.) Theor. Appl. Genet. 2007;115:721–733. doi: 10.1007/s00122-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 16.Ellis MH, Spielmeyer W, Gale KR, Rebetzke GJ, Richards RA. ‘Perfect’ markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor. Appl. Genet. 2002;105:1038–1042. doi: 10.1007/s00122-002-1048-4. [DOI] [PubMed] [Google Scholar]
- 17.Zanke, C. D. et al. Whole genome association mapping of plant height in winter wheat (Triticum aestivum L). PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 18.González FG, Terrile II, Falcón MO. Spike fertility and duration of stem elongation as promising traits to improve potential grain number (and yield): Variation in modern Argentinean wheats. Crop Sci. 2011;51:1693–1702. doi: 10.2135/cropsci2010.08.0447. [DOI] [Google Scholar]
- 19.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 21.Kruijer W, et al. Marker-based estimation of heritability in immortal populations. Genetics. 2014;199:379–398. doi: 10.1534/genetics.114.167916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrante A, Savin R, Slafer GA. Floret development and grain setting differences between modern durum wheats under contrasting nitrogen availability. J. Exp. Bot. 2013;64:169–184. doi: 10.1093/jxb/ers320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subedi KD, Gregory PJ, Summerfield RJ, Gooding MJ. Pattern of grain set in boron-deficient and cold-stressed wheat (Triticum aestivum L.) J. Agric. Sci. 2000;134:25–31. doi: 10.1017/S0021859699007303. [DOI] [Google Scholar]
- 24.Kebrom TH, et al. Inhibition of Tiller Bud Outgrowth in the tin Mutant of Wheat Is Associated with Precocious Internode Development. PLANT Physiol. 2012;160:308–318. doi: 10.1104/pp.112.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller, C., Evers, J. B. & Rebetzke, G. Canopy architectural and physiological characterization of near-isogenic wheat lines differing in the tiller inhibition gene tin. Front. Plant Sci. 5 (2014). [DOI] [PMC free article] [PubMed]
- 26.Kebrom TH, Spielmeyer W, Finnegan EJ. Grasses provide new insights into regulation of shoot branching. Trends in Plant Science. 2013;18:41–48. doi: 10.1016/j.tplants.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Kuraparthy V, Sood S, Dhaliwal HS, Chhuneja P, Gill BS. Identification and mapping of a tiller inhibition gene (tin3) in wheat. Theor. Appl. Genet. 2007;114:285–294. doi: 10.1007/s00122-006-0431-y. [DOI] [PubMed] [Google Scholar]
- 28.Evers JB, van der Krol AR, Vos J, Struik PC. Understanding shoot branching by modelling form and function. Trends in Plant Science. 2011;16:464–467. doi: 10.1016/j.tplants.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Hyles J, et al. Repeat-length variation in a wheat cellulose synthase-like gene is associated with altered tiller number and stem cell wall composition. J. Exp. Bot. 2017;68:1519–1529. doi: 10.1093/jxb/erx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards RA. A tiller inhibitor gene in wheat and its effect on plant growth. Aust. J. Agric. Res. 1988;39:749–757. doi: 10.1071/AR9880749. [DOI] [Google Scholar]
- 31.Moeller C, Rebetzke G. Performance of spring wheat lines near-isogenic for the reduced-tillering ‘tin’ trait across a wide range of water-stress environment-types. F. Crop. Res. 2017;200:98–113. doi: 10.1016/j.fcr.2016.10.010. [DOI] [Google Scholar]
- 32.Guo Z, Schnurbusch T. Costs and benefits of awns. J. Exp. Bot. 2016;67:2533–2535. doi: 10.1093/jxb/erw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum A. Photosynthesis and transpiration in leaves and ears of wheat and barley varieties. J. Exp. Bot. 1985;36:432–440. doi: 10.1093/jxb/36.3.432. [DOI] [Google Scholar]
- 34.Johnson R, Moss D. Effect of water stress on 14CO2 fixation and translocation in wheat during grain filling. Crop Sci. 1976;16:1023–1028. [Google Scholar]
- 35.Rebetzke GJ, Bonnett DG, Reynolds MP. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J. Exp. Bot. 2016;67:2573–2586. doi: 10.1093/jxb/erw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamoto Y, Kajimura T, Ikeda TM, Takumi S. Evidence from principal component analysis for improvement of grain shape- and spikelet morphology-related traits after hexaploid wheat speciation. Genes Genet. Syst. 2012;87:299–310. doi: 10.1266/ggs.87.299. [DOI] [PubMed] [Google Scholar]
- 37.Rangan P, Furtado A, Henry RJ. New evidence for grain specific C4 photosynthesis in wheat. Sci. Rep. 2016;6:31721. doi: 10.1038/srep31721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun D, et al. OsMADS16 genetically interacts with OsMADS3 and OsMADS58 in specifying. Mol. Plant. 2013;6:743–756. doi: 10.1093/mp/sst003. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt R, et al. SALT-RESPONSIVE ERF1 is a negative regulator of grain filling and gibberellin-mediated seedling establishment in rice. Mol. Plant. 2014;7:404–421. doi: 10.1093/mp/sst131. [DOI] [PubMed] [Google Scholar]
- 40.Huang, C.-F. et al. Elevated auxin biosynthesis and transport underlie high vein density in C4 leaves. Proc. Natl. Acad. Sci. 201709171, 10.1073/pnas.1709171114 (2017). [DOI] [PMC free article] [PubMed]
- 41.Lake JA, Quick WP, Beerling DJ, Woodward FI. Signals from mature to new leaves. Nature. 2001;411:154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- 42.Cañas RA, et al. Exploiting the Genetic Diversity of Maize Using a Combined Metabolomic, Enzyme Activity Profiling, and Metabolic Modeling Approach to Link Leaf Physiology to Kernel Yield. Plant Cell. 2017;29:919–943. doi: 10.1105/tpc.16.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckmann D, Schlüter U, Weber APM. Machine Learning Techniques for Predicting Crop Photosynthetic Capacity from Leaf Reflectance Spectra. Mol. Plant. 2017;10:878–890. doi: 10.1016/j.molp.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Bogard M, et al. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs. J. Exp. Bot. 2011;62:3621–3636. doi: 10.1093/jxb/err061. [DOI] [PubMed] [Google Scholar]
- 45.Distelfeld A, Avni R, Fischer AM. Senescence, nutrient remobilization, and yield in wheat and barley. Journal of experimental botany. 2014;65:3783–3798. doi: 10.1093/jxb/ert477. [DOI] [PubMed] [Google Scholar]
- 46.Li F, et al. Autophagic Recycling Plays a Central Role in Maize Nitrogen Remobilization. Plant Cell. 2015;27:1389–1408. doi: 10.1105/tpc.15.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Woo HRR, Nam HGG. Toward Systems Understanding of Leaf Senescence: An Integrated Multi-Omics Perspective on Leaf SenescenceResearch. Molecular Plant. 2016;9:813–825. doi: 10.1016/j.molp.2016.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.