Abstract
Cortical folding, or gyrification, coincides with several important developmental processes. The folded shape of the human brain allows the cerebral cortex, the thin outer layer of neurons and their associated projections, to attain a large surface area relative to brain volume. Abnormal cortical folding has been associated with severe neurological, cognitive and behavioural disorders, such as epilepsy, autism and schizophrenia. However, despite decades of study, the mechanical forces that lead to cortical folding remain incompletely understood. Leading hypotheses have focused on the roles of (i) tangential growth of the outer cortex, (ii) spatio-temporal patterns in the birth and migration of neurons, and (iii) internal tension in axons. Recent experimental studies have illuminated not only the fundamental cellular and molecular processes underlying cortical development, but also the stress state, mechanical properties and spatio-temporal patterns of growth in the developing brain. The combination of mathematical modelling and physical measurements has allowed researchers to evaluate hypothesized mechanisms of folding, to determine whether each is consistent with physical laws. This review summarizes what physical scientists have learned from models and recent experimental observations, in the context of recent neurobiological discoveries regarding cortical development. Here, we highlight evidence of a combined mechanism, in which spatio-temporal patterns bias the locations of primary folds (i), but tangential growth of the cortical plate induces mechanical instability (ii) to propagate primary and higher-order folds.
This article is part of the Theo Murphy meeting issue ‘Mechanics of development’.
Keywords: cortical folding, gyrification, growth, modelling, instability, stress
1. Introduction
(a). General features and terminology of cortical folding
The brains of most large mammals—including humans, some other primates, dolphins, whales, cows, sheep, pigs, cats, dogs and ferrets—possess wrinkled, or folded, outer (cortical) surfaces [1]. In contrast, the brains of many other species, such as mice, rats and new-world monkeys, are smooth. Folding allows for a relatively large cortical surface area in relation to brain volume; the more complex the folding pattern, the greater the area-to-volume ratio. As shown in figure 1a (bottom), the human brain exhibits an intricate pattern of convex folds (gyri) and valleys (sulci). The first, or primary, folds emerge in consistent locations across individuals and between species. Folds that emerge later, called secondary and tertiary folds, appear more random in their locations and orientations [1,2]. The brains of other large animals, such as the dolphin, whale, cow or pig, also exhibit these higher-order gyri and sulci and greater inter-individual variability at maturity. Species with smaller brains, such as the ferret, cat and macaque monkey, are characterized by simpler, more stereotyped folding patterns (figure 1a, top) consisting of mainly primary sulci and gyri [1,3,4].
Figure 1.
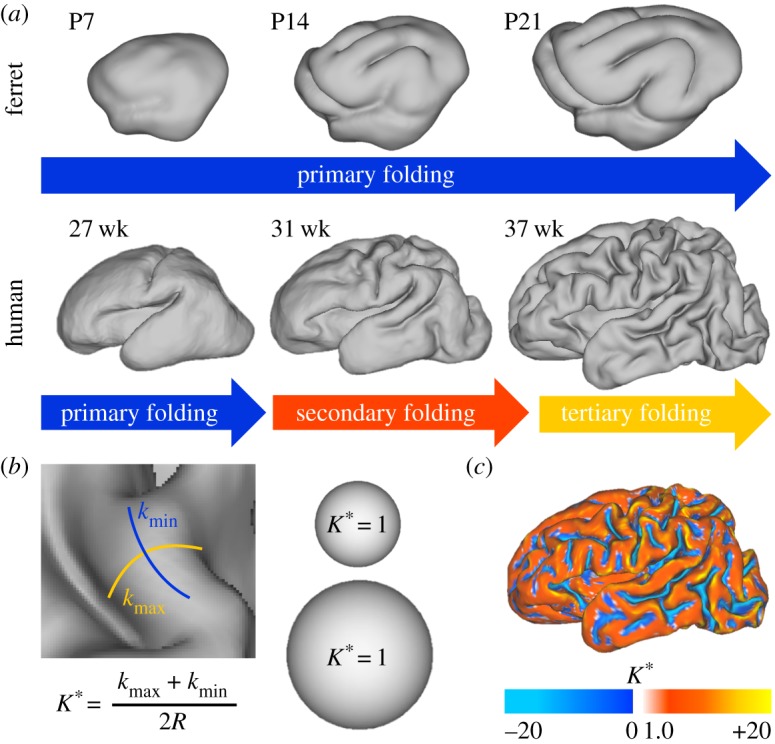
Measuring cortical folding. (a) The progression of cortical folding is shown after birth in ferret (top) and preterm human (bottom). (b) Dimensionless mean curvature (K*), the average of maximum and minimum principal curvatures (kmax and kmin) multiplied by the surface's characteristic radius (R), offers a useful metric of local folding. As shown for two spheres of different radius, K* is unaffected by size (K* = 1 everywhere on a sphere, regardless of scale). (c) Map of dimensionless mean curvature displays the complexity of the 37 week human surface in (a). P = postnatal day, wk = weeks postmenstrual age. (Online version in colour.)
In humans, the folding process takes place during gestational weeks 16–40 [5–7], spanning the second and third trimesters of pregnancy. In infants born prematurely (figure 1a, bottom), much of this critical process occurs postnatally in a neonatal intensive care unit (NICU), so it is possible that folding may be impacted by clinical management. In ferrets (figure 1a, top), cortical folding occurs between two to four weeks after birth [4,8–10]. In all cases, cortical folding occurs during a period of relatively rapid surface expansion, and the degree of folding increases with surface expansion [5–7].
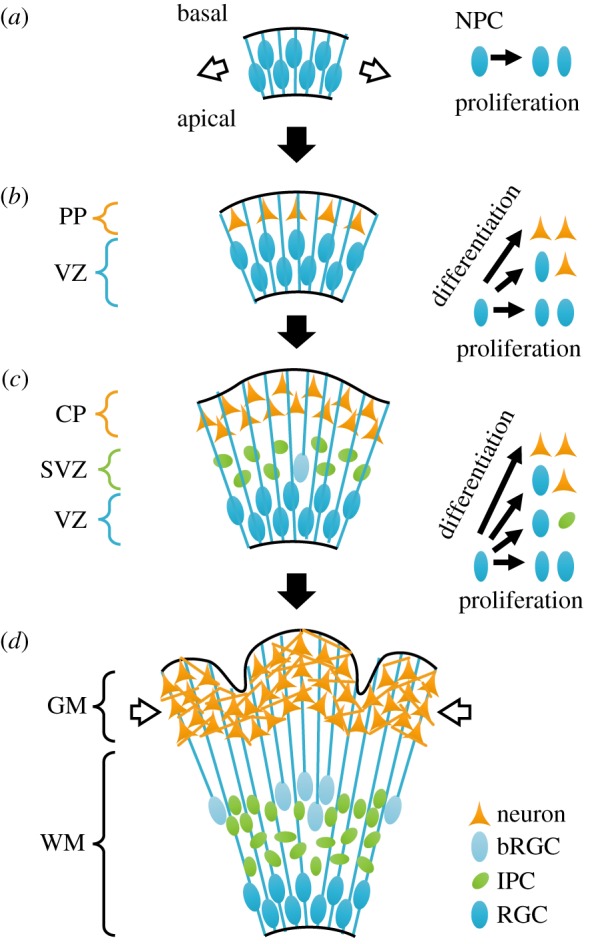
Metrics of cortical folding are important to objectively describe complexity of the folded surface. For instance, the gyrification index (GI) offers a useful global measure: cortical surface area divided by the area of its smooth ‘convex hull’ (the minimum surface area needed to fully enclose the brain). As a dimensionless ratio, GI is invariant under simple scaling. Local metrics of folding such as surface curvature (K) and sulcal depth (D) are also useful to describe spatial variations in folding. However, both curvature and sulcal depth are dimensional, so that any proportionately enlarged surface will have smaller curvature and larger sulcal depth than the original. To use these surface properties in comparisons between brains of differing sizes, dimensionless versions of these parameters, normalized by a ‘characteristic radius’ (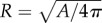 ), offer a useful alternative: K* = KR and D* = D/R (figure 1b,c).
), offer a useful alternative: K* = KR and D* = D/R (figure 1b,c).
(b). Overview of developmental neurobiology
Long before the onset of cortical folding, the embryonic brain emerges from the anterior portion of the neural tube. During early stages, the walls of the neural tube contain pseudostratified, radially aligned neuroepithelial progenitor cells (NPCs), and embryonic cerebrospinal fluid (CSF) fills the lumen. Embryonic CSF exerts a mechanical pressure that inflates the anterior neural tube [11,12], stretching the walls tangentially (figure 2a). Embryonic eCSF also drives proliferation [13,14], a process which may be mediated by mechanical feedback [15,16].
Figure 2.
Development in the gyrencephalic brain. (a) During early embryonic stages, the brain wall consists of pseudostratified neuroepithelial progenitor cells (NPCs, blue ellipses). Each cell maintains contact with both apical (inner) and basal (outer) surfaces. During embryonic stages, these cells experience mechanical tension (white arrows) [11,12]. (b) As development continues, some NPCs divide asymmetrically to produce differentiated neurons (orange triangles), which do not maintain contact with the apical wall. Instead, neurons migrate toward the basal, outer surface along the radial scaffold (blue lines) provided by NPCs, the precursors to radial glial cells (RGCs). (c) In later stages, some NPCs divide to produce intermediate progenitor cells (IPCs, green ellipses), and some lose contact with the apical surface to form basal radial glial cells (bRGCs, light blue ellipses). These proliferative cell types populate the subventricular zone (SVZ), between the ventricular zone (VZ, filled with RGCs) and cortical plate (CP, filled with neurons). (d) As neurons morphologically mature in the cortical plate, cortical grey matter (GM) expands faster than subcortical white matter (WM). At late stages, constrained growth of the grey matter may result in mechanical compression (white arrows). PP = preplate. (Online version in colour.)
By mid-gestation in humans (end of gestation in the ferret), the brain contains a relatively smooth pair of cerebral hemispheres. Similar to earlier stages, the CSF-filled lumen, or ventricle, of each hemisphere is lined by a neuroepithelial layer called the ventricular zone (VZ) (figure 2b). While some NPCs divide symmetrically to produce two new NPCs (proliferation), others divide asymmetrically to produce one NPC and one neuronal precursor [17]. In the latter case, the differentiated neural cell migrates toward the pial surface of the brain to populate the preplate (PP, figure 2b). The cortical plate (CP) later splits the PP into a pial marginal zone (MZ) and a deeper subplate zone (SP). Remaining NPCs start to resemble radial glial cells [17].
In some species, a distinct zone of cell proliferation, the subventricular zone (SVZ), emerges between the VZ and the CP (figure 2c). This layer contains intermediate progenitor cells (IPCs, also produced by asymmetric division of NPCs), which can divide to produce two new IPCs or two neurons. Neurons born in the SVZ migrate along the scaffold of radial glial cells into the CP. Furthermore, as layers develop and thicken, some radial glial cells (RGCs) lose contact with the apical brain surface. These basal radial glial cells (bRGC) can proliferate or differentiate into IPCs and neurons [18,19]. Smart et al. [20] defined further stratification in primates: the inner subventricular zone with randomly oriented cells, an inner fibrous layer, the outer subventricular zone with radially aligned cells, and an outer fibrous layer [20] or intermediate zone [21,22]. Most bRGCs are found in the outer subventricular zone and are more prevalent in animals with folded brains than animals without folding [18,19].
The onset of cerebral cortical folding takes place after the majority of cerebral cortical neurons have completed migration from their sites of origin (cell layers near the surface of the lateral ventricle) to outer layers which will become cerebral cortex (figure 2d) [8]. By the end of the second trimester (week 26) in humans, the cortical plate is poised for expansion. In subsequent weeks, cortical neurons mature and form connections. This phase is marked by dendritic arborization—the emergence of branching dendritic fibres from the neuronal soma (cell body). These changes in cellular morphology coincide with changes in cortical cytoarchitecture from a radially oriented anisotropic tissue, due to the dominance of apical dendrites of neurons and radial glial fibres, to a laminar organization, due to the addition of tangentially oriented structures [23]. This microstructural change can be detected by diffusion tensor imaging: In early cortical tissue, water diffuses more readily parallel to the radial glial fibres and apical dendrites, but the extent of diffusion anisotropy decreases with cytoarchitectural development of the cortex [24,25].
Several neurobiological factors stand out with respect to the mechanics of folding. (i) Basal radial glial cells in the subventricular zone, which is more pronounced in developing brains of gyrencephalic animals, amplify neurogenesis [18,26]. Intercalation of neurons into the developing cortex contributes to its expansion in surface area [27]. (ii) Once neurons arrive at the cortex, their maturation involves the elaboration of dendritic arbours, which induces further cortical expansion. (iii) If growing tissue is constrained by neighbouring tissue, expanding regions may be forced to bulge outward, compress, or buckle. (iv) Neural connections between distinct cortical areas, or between cortex and subcortical structures, are mediated by axons that ultimately are myelinated in mature white matter tracts. Developing axonal processes may exert mechanical influence on the cortex via tension or growth. To understand the potential mechanical effects of these neurobiological phenomena, we first review some key mechanical concepts.
(c). Key concepts in mechanics
Deformations of cells and tissues are governed by physical principles (such as the principle of force equilibrium), constitutive relationships (material properties) and the biological and chemical processes that underlie growth and remodelling.
Force equilibrium: The net force on a cell or tissue element is equal to the product of its mass and acceleration. Since acceleration is negligible for slow processes like cortical folding, the vector sum of external forces on such an element is almost zero. Such processes are described as ‘quasi-static’. In quasi-static models, perfect force equilibrium (zero net force) is assumed.
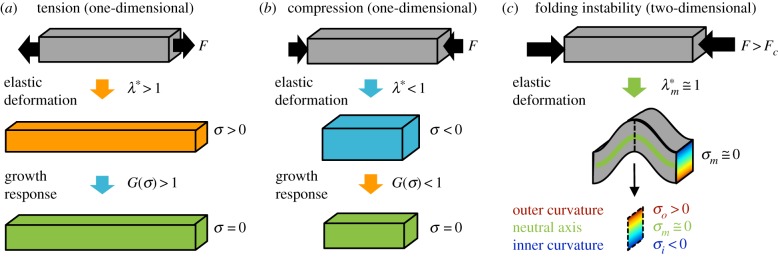
Stress: Stress is a measure of force, normalized by the area over which the force is applied. Pressure is a particular example of stress in which the force is normal to a given surface and the magnitude is independent of the surface orientation. Shear stresses describe forces tangent to a surface. Tensile stresses act to elongate a material element (figure 3a), while compressive stresses shorten a material element (figure 3b). The units of stress are N m−2, or Pa.
Figure 3.
Elastic, viscoelastic and unstable behaviour in response to mechanical force. (a–c) Application of axial force (F) is shown for a hypothetical bar-shaped element of tissue. In all cases, the sum of forces on the bar is equal, denoted by black arrows in opposing directions. (a) Under pulling forces, the bar will stretch elastically (λ* > 1), resulting in tensile stress (σ > 0). In the case of either viscoelastic tissue or growing tissue, sustained tension may lead to permanent growth and relaxation of the original stress. (b) Conversely, under pushing forces, the bar will shorten elastically (λ* < 1), resulting in compressive stress (σ < 0) and—potentially—tissue shrinking to relieve stress. (c) Under sufficiently high compressive force, the same bar may buckle to a lower energy configuration. In the resulting fold, stress is tensile along the outer curvature region (red) and compressive along the inner curvature region (blue) due to bending. The neutral axis (green) represents the intermediate location where stresses due to bending are zero. (Online version in colour.)
Strain: Strain describes the deformation of a body or element of material normalized by size. For example, a rod in tension will typically get longer and thinner. Axial deformation is the new length divided by the old length (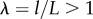 ), and axial strain is the (positive) change in length divided by the original length. Shear strain describes the change in angle between the sides of a deformed cubic element. Strain and deformation are dimensionless.
), and axial strain is the (positive) change in length divided by the original length. Shear strain describes the change in angle between the sides of a deformed cubic element. Strain and deformation are dimensionless.
Material properties: Constitutive (material) properties describe the relationship between forces and deformations. Intrinsic material properties, which are independent of the size and shape of an object, relate stress to strain. For example, in a linear elastic material, stress is simply proportional to strain. Young's modulus, E (units Pa), is the ratio of axial stress in a stretched elastic rod to axial strain. Shear modulus, G (Pa), similarly relates the shear stress in an elastic material to shear strain. Highly deformable soft materials are termed ‘hyperelastic’ and are characterized by a strain energy density function (the work per unit volume required to deform the material). Several models of brain folding assume brain tissue is elastic [28] or hyperelastic [29,30]. However, brain tissue is widely understood to be viscoelastic [31], such that sustained tension can lead to stress relaxation as the tissue lengthens passively. Stress-induced tissue growth can mimic viscoelastic relaxation. In figure 3a, elastic stretch (tension) leads to positive growth and a new lengthened configuration with no stress. Conversely, compression typically leads to negative growth and a shortened configuration with no stress. Some models of brain folding, such as the model in figure 5, incorporate viscoelastic behaviour explicitly to account for growth [32–34].
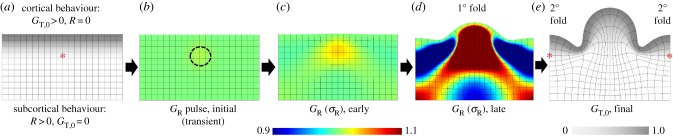
Figure 5.
Evolution of folding based on initial radial patterning and growth-induced instability. (a) As described in Bayly et al. [33], cortex (grey) was defined to grow tangentially at a pre-determined rate (GT,0), while growth of subcortical layers evolves in response to stress GR(σR) and GT(σT), with rate constant R. (b) A small, transient perturbation in radial growth was applied (region circled by dotted line) to influence the location of the first fold. (c) After the perturbation, mechanical feedback, combined with tangential growth of the cortex, causes increased radial stress and growth in this region. (d) A primary (1°) gyrus later develops above the initial perturbation, and regions of higher radial growth develop to the right and left. (e) As the model continues, secondary (2°) gyri emerge from these new regions of higher radial growth. Red asterisks denote local growth concentrations beneath prospective gyri. (Online version in colour.)
Mechanical feedback: Cells both generate and respond to mechanical force [35]. For example contractile forces arise from interactions of myosin motor proteins and actin filaments. Actin polymerization itself can exert forces as filaments lengthen [36,37]. Proliferation, maturation of cells and accumulation of extracellular matrix material all tend to increase tissue dimensions (tissue growth), which will generate forces against constraining boundaries. On the other hand, applied forces can influence motor stepping rates, actin polymerization and gene expression, which in turn affect the processes of proliferation and growth.
Stability: Equilibria can be stable or unstable. For example, if a pure axial load is applied to a rod, it will shorten slightly but stay straight (figure 3b). If the load is large enough (above a critical level), the straight, compressed solution still exists, but lower-energy buckled states may also exist. Any slight perturbation will lead to a large deformation (an instability) and the rod will take a new, buckled shape (figure 3c). Importantly, the buckled shape is not different for every perturbation, but reflects a characteristic ‘mode’ of instability. However, the specific state that emerges, i.e. whether the rod buckles to the left or to the right, may depend on the perturbation. In addition, any imperfection in the rod (deviation from perfect straightness and symmetry) will affect the final configuration. In the context of the developing cerebral cortical sheet, deformations resulting from compressive stress in the cortical plate due to surface area expansion would be anticipated to adopt the more stable, low-energy configurations.
2. Models of cortical folding
(a). Overview of hypothetical folding mechanisms
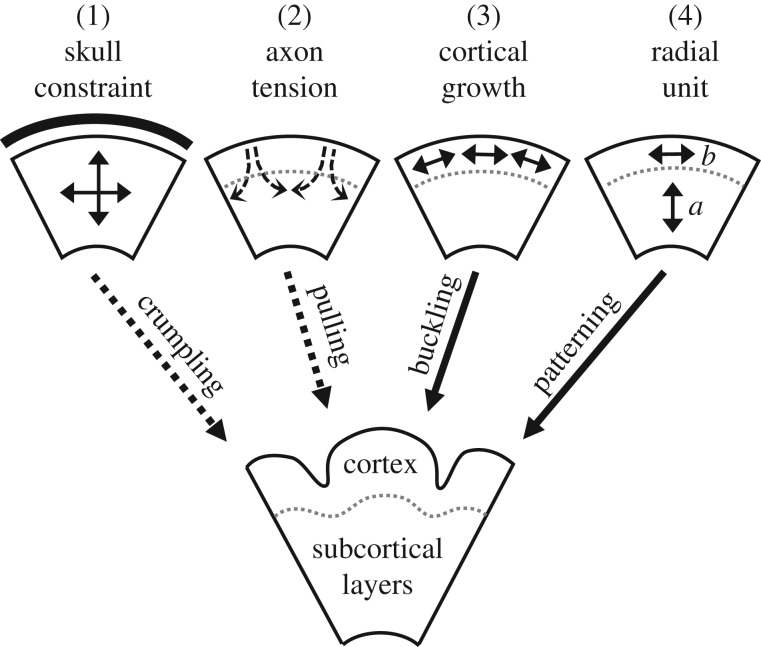
Four hypotheses have dominated research and speculation into the mechanisms of cortical folding: (1) the skull constrains growth of the brain and causes compressive stresses and buckling; (2) tension in axons connecting adjacent regions of the cortex draws those regions together to form gyri; (3) tangential expansion of outer cortical layers, greater than in inner layers, causes folding by a mechanical instability; (4) programmed patterns of growth cause more neurons to emerge, proliferate, reach the cortex and expand in some regions (gyri) more than others (sulci). As shown in figure 4, these models are not necessarily mutually exclusive.
Figure 4.
Competing (or complementary) hypotheses for cortical folding. Dotted arrows (1 and 2) denote mechanisms not consistent with prior experiments. Additional experiments are needed to clarify the true mechanism(s) of cortical folding, which could include a combination of these hypotheses.
(b). Skull constraint
A plate or shell-like structure, expanding tangentially inside a rigid container, would have to fold. This concept has been proposed by several authors to explain cortical folding (as reviewed by Welker [1]). Raghavan and co-authors [38] developed an explicit mechanical model of cortical folding based on this hypothesis. However, experimental studies by Barron, in which underlying brain tissue was removed to create space between the brain and skull [39], showed that interactions with the skull are not needed to produce folding. In addition, the skull increases in size to accommodate brain growth [1]. Thus the role of skull in cortical folding has largely been discounted.
(c). Axonal tension
Axons in the living brain exist in a state of tension [40–42], and axons in vitro will grow or shrink to maintain this tension [43,44]. Based on these observations, Van Essen [45] hypothesized that axons draw together opposite sides of gyral folds, generating axonal tension while helping minimize total ‘wiring’ length of neuron-to-neuron connections in the brain [45].
Experiments have clearly confirmed tensile stress in white matter tracts [40,41] of both the smooth mouse brain and the folded ferret brain. In these studies, tension was directly assessed by making small cuts into regions of cortical grey matter and subcortical white matter. In grey matter the cuts remained closed, but in white matter (axonal) tracts, cuts perpendicular to the axon fibres opened up.
However, the observed directions of tension are not consistent with the original axonal tension hypothesis. In the folded brain, cuts along the radial axis of each gyrus remained closed, indicating no tension pulling the walls of the gyrus together. On the other hand, circumferential cuts, perpendicular to the axis of each gyrus, opened up. These observations are consistent with two others: (i) Axonal tracts are primarily oriented radially in gyri (i.e. from the interior of the brain to the gyral crest), not across each gyrus [41], and (ii) tension follows the axonal fibre direction. Thus, axonal tension does not play the role originally postulated by Van Essen [45], though the stress state of axons in white matter may be important in other ways.
(d). Differential tangential growth
(i). Elastic buckling models
During folding, the cortical plate must expand faster than subcortical brain layers to attain its large surface area relative to brain volume. This ‘differential growth’, meaning different growth rates in different layers, was proposed to explain cortical folding [28] based on two mechanical principles: (i) tangential expansion of an elastic layer, connected to an elastic foundation which does not expand, induces tangential compression in the expanding layer; (ii) a thin elastic layer under sufficiently large compression will become unstable and buckle (figure 3c). If the thin layer is supported by an elastic foundation, the buckled shape will be sinusoidal, with the wavelength determined by the relative stiffnesses (determined by the elastic moduli) of the layer and foundation.
Richman et al. [28] proposed the first quantitative model of folding based on differential growth. They analysed a model with two connected, growing layers of similar elastic material (equal elastic modulus in each layer, E1 = E2) connected to a soft elastic core (Ecore = E1/10). Growth was largest in the outer layer, so that compressive stress was induced in that layer. With these parameters, the authors obtained a theoretical prediction of wavelength that was consistent with the average wavelength (gyral peak-to-peak distance) of folding in the human brain.
In reality, the material properties in the outer and inner layers of the brain appear to be very similar. Xu et al. [41] measured the regional mechanical properties of the ferret brain by indentation but found no significant differences between cortical and subcortical regions during the period of folding. Data from other studies of sheep [46] and pig brains [47] show similar mechanical properties in grey and white matter.
More recent studies have shown buckling-type instabilities in growing outer layers with elastic modulus similar to that of the inner layer. For example, Dervaux & Ben Amar [48] built a physical model of differential growth using circular slices of gel, in which the outermost layer expanded by swelling. This outer layer exhibited folding with wavelength determined by the thickness and modulus of the outer layer. Theoretical predictions agreed closely with the observed shapes. Tallinen et al. [29,30] extended experimental studies of differential growth into 3D, first using hemispheres of the elastomer polydimethylsiloxane (PDMS) in which the outer layer was expanded by swelling induced by immersion in hexane. By controlling the modulus and thickness of the swelling surface relative to the radius of the hemisphere, they obtained wrinkled surfaces that qualitatively resembled folded brains. Additional experiments, in which the starting condition approximated the smooth fetal human brain before folding, resulted in even more lifelike 3D gyrification. These authors [29,30] also performed numerical simulations of growing hyperelastic (soft, highly deformable) materials and obtained shapes that closely matched their experimental observations.
(ii). Stress-induced growth and viscoelastic instability
Models based strictly on uniform surface growth on an elastic foundation fail to replicate two key features of mammalian cortical folding. First, primary folds that are highly conserved between individuals have not been consistently and accurately reproduced. Second, models fail to recapitulate observed growth of the subcortical white matter. Developmental neuroanatomists emphasize that more superficial subcortical layers assume the folded shape of the cortex but deeper layers remain smooth [4,49]. White matter architecture is critical to normal brain development, so useful models should capture the structural development of the subcortical layers.
In vitro studies have shown that axons, the primary component of white matter, grow in response to tension and reach an equilibrium length that maintains a small, but finite, level of tension [43,44,50,51]. Thus, the white matter layer will probably respond to tensile stress by growing in the direction of tension and maintaining tension along the direction of axonal fibres (figure 3a). This prediction is consistent with the oriented tension observed in white matter [40,41]. Since tension-induced axonal growth takes time, stress induced by relatively fast loading will relax with a characteristic time constant. This behaviour mimics the response of a viscoelastic material, in which the relaxation time depends on the ratio of ‘viscous’ resistance to elastic stiffness.
If the inner subcortical tissue is modelled as a viscoelastic material, a distinct type of viscoelastic instability (buckling on a viscoelastic foundation) occurs in response to growth of the cortical plate [33]. Because a viscoelastic foundation appears stiffer when loading is applied faster, the occurrence of viscoelastic instability, and the wavelength of the resulting surface, depends on the rate of cortical growth relative to the rate of relaxation in the subcortical region.
Toro & Burnod [32] incorporated stress-induced growth into a computer model of folding by including discrete, radial, viscoelastic fibres (representing the subcortical tissue zones) connected to an expanding elastic ring (representing the cortical plate). Simulations reproduced many qualitative features of folding, specifically the evolution of a wavelike folding pattern in the outer ring. They modulated the emergence and wavelength of folds by varying the growth rate, cortical thickness and stiffness of the outer layer. Folds were produced in specific locations by geometrical perturbations (small changes in initial shape) or spatial variations in model parameters (thickness or stiffness, for example). This elegant model demonstrated clearly that simple physics can produce rich folding behaviour, and it illustrated the qualitative effects of physical parameters.
Moving beyond one-dimensional, discrete elements, continuum mechanical models can more accurately represent the behaviour of tissue in 2D or 3D, as they capture stresses and deformations in multiple directions. By representing the subcortical region as a continuous viscoelastic foundation, Bayly et al. [33] obtained a theoretical prediction (a mathematical formula) for the effects of relative growth rate on the wavelength of cortical folds. The tangential dimension of the outer (cortical) layer was defined to grow at a rate of GT,0 (week−1). For example, if the tangential dimension doubles in one week then tangential growth GT = 2 (dimensionless) and GT,0 = 1 week−1. In the inner (subcortical) layer, growth was assumed to occur in both radial and tangential directions (GR and GT), but only in response to radial and tangential stresses (σR and σT). This is a form of stress relaxation, described by the rate constant, R. In this viscoelastic material framework, any initial stress (σ0) will decrease to the equilibrium stress (σe) according to the form 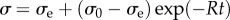 [33]. Thus, the dimensionless ratio ΓG = GT,0/R describes how fast the cortex grows (GT,0) relative to the subcortical, stress-dependent growth response (R). Since growth occurs slowly (on the time scale of days or weeks), inertial effects are negligible, and cortical folding is treated as a quasi-static process.
[33]. Thus, the dimensionless ratio ΓG = GT,0/R describes how fast the cortex grows (GT,0) relative to the subcortical, stress-dependent growth response (R). Since growth occurs slowly (on the time scale of days or weeks), inertial effects are negligible, and cortical folding is treated as a quasi-static process.
The formula for folding wavelength (λ), as a function of relative growth rate (ΓG) cortical thickness (h), and ratio of elastic moduli (β = μ/μf) derived by Bayly et al. [33] is:
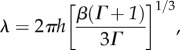 |
2.1 |
where Γ is a root of the characteristic polynomial: 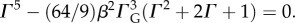 This formula predicts that wavelength increases with cortical thickness and modulus (μ) of cortex relative to modulus (μf) of subcortical regions. Furthermore, this formula predicts that the larger the ratio of cortical to subcortical growth rates (ΓG), the shorter the folding wavelength. The effects of cortical thickness and elastic moduli in the two layers are consistent with experiments and simulations from other groups (i.e. Dervaux & Ben Amar [48]; Tallinen et al. [29,30]; Toro & Burnod [32]).
This formula predicts that wavelength increases with cortical thickness and modulus (μ) of cortex relative to modulus (μf) of subcortical regions. Furthermore, this formula predicts that the larger the ratio of cortical to subcortical growth rates (ΓG), the shorter the folding wavelength. The effects of cortical thickness and elastic moduli in the two layers are consistent with experiments and simulations from other groups (i.e. Dervaux & Ben Amar [48]; Tallinen et al. [29,30]; Toro & Burnod [32]).
The predicted effect of relative growth rate on folding wavelength was also observed in computer simulations [33], which provides complementary (albeit also theoretical) evidence in support of the prediction. Figure 5 demonstrates the simulated evolution of subcortical growth (stress relaxation) and brain folding based on a numerical (finite element) model of the behaviour described above. Importantly, initial conditions had a strong effect on the locations of major folds, and could under some conditions overwhelm the effects of relative growth rate, thickness and material properties. In many cases, initial perturbations (figure 5b) were amplified by differential growth (figure 5c), producing predictable ‘primary’ folds (figure 5d), while the viscoelastic instability produced superimposed ‘secondary’ folds of variable location (figure 5e).
Subsequent numerical simulations by Budday and co-authors [52,53] extended the modelling of stress-dependent growth into 3D and confirmed that the ratio between growth rates in outer and inner layers affects folding wavelength. Budday et al. [52] specifically hypothesize that the two factors, relative growth rate and cortical thickness, may explain the pathologies of folding comprising lissencephaly, pachygyria and polymicrogyria. Holland et al. [54] further extended this model to include subcortical anisotropy, reasoning that more axon elongation will occur in the direction of axon orientation than in other directions. Their simulations predicted that subcortical axon orientation can alter the locations of gyri and sulci, suggesting that tissue anisotropy is another important parameter.
(iii). Cellular mechanisms of differential tangential growth
Mathematical modelling of differential growth may have proceeded beyond the current understanding of the cell biology of cortical and subcortical growth. For example, the precise mechanism by which the cortical plate expands tangentially (in area), but not much radially (in thickness), is not completely clear. Since the period of folding is later than the periods of most neuronal birth, proliferation and migration, these events probably set the stage for cortical expansion, rather than drive it directly. Richman and co-authors [28] originally suggested that neuronal maturation, particularly dendritic arborization, may provide a dominant mechanism of tangential growth, due to the coincidence in timing of cortical folding and morphological differentiation of cells in the cerebral cortex [28]. More recent observations with diffusion-weighted MRI further support this possibility [24,55,56]. Before folds appear in the cerebral cortex, water diffusion in the cortical plate is strongly anisotropic. Radial diffusion is less restricted than diffusion tangent to the surface, apparently due to the effects of morphologically undifferentiated, and tangentially oriented, glial and neuronal cell processes restricting diffusion perpendicular to their axes. As neurons mature and extend branching dendrites, these cell projections provide new pathways for unrestricted diffusion in tangential directions, rendering diffusion more isotropic. In ferrets, the timing of the loss of water diffusion anisotropy [36] coincides with morphological differentiation of pyramidal neurons, as assessed by Golgi staining methods [57,58]. Within-subject comparisons of water diffusion anisotropy and dendrite orientation complexity [55] have further confirmed that the loss of anisotropy correlates with increasing arborization. Lastly, longitudinal in utero diffusion tensor imaging measurements have demonstrated correlations between the loss of cortical diffusion anisotropy and folding in the fetal rhesus macaque brain [56].
An alternative, or additional, mechanism for cortical expansion is late intercalation of previously born neurons from subcortical layers [27]. Several authors [17–19,26] have noted a histologically distinct layer found in gyrencephalic animals, the outer subventricular zone, where basal radial glial cells (bRGCs) proliferate or produce intermediate progenitors; by contrast, bRGCs in the lissencephalic mouse divide only once to directly generate two neurons [19,26]. The resulting increase in bRGCs and intermediate progenitors, both of which can produce neurons, boosts the final number of neuronal cells in the cortex of folded brains. The bRGCs themselves appear to fan out [18,59] perhaps providing a wider path for migrating neurons (figure 2d) and enabling more to reach the cortex.
It is now important to determine the biomechanical implications of this biological difference between gyrencephalic and lissencephalic species. Interestingly, dissection experiments in mouse revealed tangential compressive stress in the cortex [40,41], suggesting some increase in cortical expansion relative to subcortical layers, though this difference appears insufficient to induce folding. Future studies are needed to understand how enhanced mechanisms of cortical neurogenesis contribute to differential growth. An additional consideration is that increased cortical thickness relative to (initial) brain size can increase the growth threshold for inducing folding [33,52,60].
(e). Patterned growth
(i). Growth beneath gyri
In addition to growth differences between layers, growth of the brain may be heterogeneous within each layer. While growth that differs between layers (i.e. the ‘differential growth’ in models described in the previous section) is sufficient to cause folding, spatial patterns along a single layer have also been reported [17,61,62]. Such spatial patterns, if consistent among individuals of a given species, might underlie the consistently located primary folds that dominate the geometry of the ferret brain and dictate many features of human neuroanatomy.
Smart & McSherry [3,4] noted that the subcortical tissue expands heterogeneously in the radial direction, with its largest radial expansion under gyri. They interpret this to mean that gyrification is driven by non-uniform radial expansion (mushrooming) of the subcortical layers, and that effects of this heterogeneous radial growth are amplified by increased radial expansion in the gyral crowns relative to sulcal valleys.
In contrast, in the differential (tangential) growth model of Bayly et al. [33], heterogeneous radial expansion of the subcortical tissue results from folding of the outer layer (secondary folds in figure 5d,e). How can one distinguish whether the subcortical layers ‘push’ the cortex outward to form a gyrus, or whether the folding cortex ‘pulls’ the inner layers outward via stress-induced growth? The existence of tension, rather than compression, along the radial axis of each gyrus provides direct physical evidence for the folding cortex ‘pulling’ on underlying gyral tissue [41].
Nevertheless, birth and proliferation of neurons and glial cells may be different under gyri and sulci, particularly those of primary folds. Kriegstein et al. [17] showed that proliferative layers were thicker under incipient gyri than incipient sulci. Later, the Kriegstein and Borrell groups [18,19,26] showed that proliferation in the oSVZ enriches neuronal density in the cortical plate. As noted above, Borrell and co-authors [18,19] have shown that basal radial glial cells (bRGCs) fan out tangentially under gyri in the developing ferret. These tangentially spreading bRGCs are not found below sulci in the developing ferret brain, nor anywhere in the developing mouse brain. The accumulation of evidence suggests that heterogeneous patterns of bRGC birth and proliferation do exist and play a significant role in determining the location of primary folds.
(ii). Reconciling patterned and tangential growth hypotheses
Two key points should be kept in mind with respect to the role of patterned birth and proliferation. First, these patterns probably modulate tangential expansion of the cortex as much as, or more than, radial expansion. Thus, observations of heterogeneous neurogenesis are consistent with previously described differential growth models (§2d), albeit with tangential growth that is non-uniform within the cortical layer. Second, studies in animals that exhibit mainly primary folds, such as the ferret, necessarily emphasize processes that drive primary fold formation.
In the differential growth model by Bayly et al. [33], the location of the first (primary) gyrus was specified by a local increase in radial growth, but subsequent (secondary) folds evolved from mechanical instability. As depicted in figure 5, such models suggest that the patterned (radial) growth hypothesis and differential (tangential) growth hypotheses are not mutually exclusive.
An additional, intriguing, possibility is that mechanical stress itself may initiate patterns of proliferation, differentiation, migration and maturation. Studies in chicken embryos suggest that neuroepithelial progenitor cells, precursors to basal radial glial cells in the oSVZ, proliferate in response to mechanical feedback [13,15,16]. Substrate mechanical properties [63] and mechanical tension [64] have been shown to affect the differentiation of stem cells into neurons. Franze and colleagues [65,66] have shown that axons are mechanosensitive, and several authors have observed that the emergence of neurites [43,51], as well as synapses [67], are affected by tension.
For instabilities induced by uniform cortical growth, Bayly et al. [33] showed that mechanical stress becomes heterogeneous in the subcortical layers before any noticeable change in shape occurs. Local radial stress (e.g. in the proliferative oSVZ) may be sufficient to induce radial growth (e.g. patterning of the oSVZ) below a prospective gyrus. In this case, a ‘primary’ fold could emerge due to mechanical instability, but in a precise location based subtle factors that induce nonuniform stress in subcortical layers. As the geometry changes, so do the patterns of subcortical stress. As in figure 5, local stress in subcortical layers may induce radial growth and additional ‘secondary’ folds. In a similar way, stress patterns—and therefore folding patterns—may be influenced by physical factors including initial geometry, anisotropy of cell process orientations [54], regional variations in mechanical properties [32] or regional variations in cortical growth.
On a larger scale, spatial and temporal variations in cortical areal expansion have been observed in magnetic resonance imaging (MRI) studies of ferret, macaque and human brain (figure 6) during folding. Spatial variations appear to span multiple gyri and sulci, and patterns change over multiple days (ferret) [9] or weeks (human) [7], consistent with patterns in cortical maturation and functional development. Although a region of interest based approach was adopted in macaque [56], regional patterns identified were similar to those found in ferrets and humans. By incorporating these regional patterns of tangential expansion, future 3D models may better recapitulate primary folding in human [29,30], or subtle folding differences observed in neurological disorders like epilepsy, autism, schizophrenia and bipolar disorder.
Figure 6.
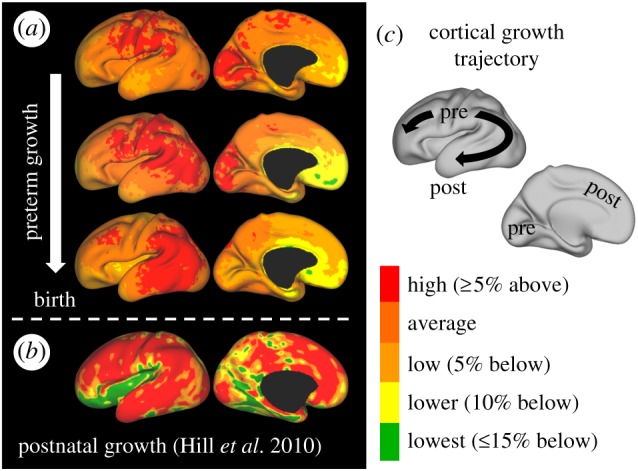
Dynamic patterns of tangential growth in human brain development. (a) In preterm infants, patterns of cortical expansion (red) change from 28–30 weeks (top) to 30–34 weeks (middle) to 34–38 weeks (bottom). (b) In healthy infants, areas of highest cortical expansion (red) are consistent with the trajectory from preterm development. (c) Schematic illustrating the trajectory of the maximum growth region from primary motor, sensory and visual cortices (labelled ‘pre’) to frontal, parietal and temporal lobes (labelled ‘post’). Pre, prenatal/preterm; post, postnatal. Reproduced from [7] with permission. (Online version in colour.)
3. Conclusion and outlook
Recent work has clarified both the mechanics and cell biology of cortical folding in the brain, while leaving significant questions unanswered. Key points made in studies reviewed above include:
-
(1)
Axonal tension exists in folded brains but does not drive folding. In particular, axonal tension is not selectively applied between walls of gyri to draw them together and create convex folds. However, tension-induced growth of white matter, and the amplitude and direction of residual axonal tension, probably modulate folding patterns.
-
(2)
Heterogeneities in cortical growth exist within and across layers, and probably determine the locations of primary sulci. Tangential and radial growth are both heterogeneous, with effects that may be induced or amplified by mechanical feedback.
-
(3)Differential tangential growth (expansion of the cortex at a greater rate than subcortical layers) explains many features of cortical folding.
-
(a)Uniform expansion of the outer cortical layer, constrained by an elastic inner core of similar modulus, produces folding similar to that seen in brains of gyrencephalic species, with wavelength determined by cortical thickness, relative stiffness differences (if any) and initial surface imperfections (if any).
-
(b)Uniform cortical expansion, constrained by stress-dependent (or viscoelastic) growth of the inner core, additionally explains the observed growth and stress state of subcortical white matter. Folding wavelength is determined by cortical thickness, relative stiffness difference (if any), initial surface imperfections (if any) and growth rate of cortex relative to subcortical layers.
-
(a)
Open questions remain. How are cells generating force during folding? Furthermore, how do these cells respond to mechanical force during folding? Definitively and quantitatively, researchers must determine the contributions of specific cellular activities to the rapid expansion of the cortex. Although much progress has been made in recent years, the causes and effects of patterned neurogenesis remain a topic of active research. Quantitative studies of the mechanobiology of neural tissue, and the continued development of models to rigorously assess biological hypotheses, will accelerate progress toward answering these questions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
NIH Grants R01 NS055951 (P.V.B.), NIH T32 EB018266 (K.E.G.), R01 AA021981 (C.D.K.). C.D.K. also acknowledges salary support from NIH grant OD011092. Data were provided by the Washington University Neonatal Development and Research (WUNDER) group (T. Inder and D. Van Essen; NIH RO1HD057098).
References
- 1.Welker W. 1990. Why does the cortex fissure and fold: a review of determinants of gyri and sulci. In Cerebral cortex: comparative structure and evolution of cerebral cortex (eds Jones EG, Peters A), pp. 3–136. New York, NY: Plenum Press. [Google Scholar]
- 2.Le Gros Clark WE. 1945. Deformation patterns in the cerebral cortex. In Essays on growth and form (eds Le Gros Clark WE, Medawar PB), pp. 1–22. London, UK: Oxford University Press. [Google Scholar]
- 3.Smart IH, McSherry GM. 1986. Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J. Anat. 147, 27–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Smart IH, McSherry GM. 1986. Gyrus formation in the cerebral cortex in the ferret. I. Description of the external changes. J. Anat. 146, 141–152. [PMC free article] [PubMed] [Google Scholar]
- 5.Sun T, Hevner RF. 2014. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat. Rev. Neurosci. 15, 217–232. ( 10.1038/nrn3707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Encinas JL, et al. 2011. Maldevelopment of the cerebral cortex in the surgically induced model of myelomeningocele: implications for fetal neurosurgery. J. Pediatr. Surg. 46, 713–722. ( 10.1016/j.jpedsurg.2010.11.028) [DOI] [PubMed] [Google Scholar]
- 7.Garcia KE, et al. 2018. Dynamic patterns of cortical expansion during folding of the preterm human brain. Proc. Natl Acad. Sci. USA 115, 3156–3161. ( 10.1073/pnas.1715451115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal J, Takahashi M, Silva M, Tiao G, Walsh CA, Sheen VL. 2007. Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J. Anat. 210, 66–77. ( 10.1111/j.1469-7580.2006.00674.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutsen AK, Kroenke CD, Chang YV, Taber LA, Bayly PV. 2012. Spatial and temporal variations of cortical growth during gyrogenesis in the developing ferret brain. Cereb. Cortex 23, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnette AR, Neil JJ, Kroenke CD, Griffith JL, Epstein AA, Bayly PV, Knutsen AK, Inder TE. 2009. Characterization of brain development in the ferret via MRI. Pediatr. Res. 66, 80–84. ( 10.1203/PDR.0b013e3181a291d9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jelinek R, Pexieder T. 1968. The pressure of encephalic fluid in chick embryos between the 2nd and 6th day of incubation. Physiol. Bohemoslov. 17, 297–305. [PubMed] [Google Scholar]
- 12.Pexieder T, Jelinek R. 1970. Pressure of the CSF and the morphogenesis of the CNS. II. Pressure necessary for normal development of brain vesicles. Folia Morphol. 18, 181–192. [PubMed] [Google Scholar]
- 13.Desmond ME, Levitan ML, Haas AR. 2005. Internal luminal pressure during early chick embryonic brain growth: descriptive and empirical observations. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 285, 737–747. ( 10.1002/ar.a.20211) [DOI] [PubMed] [Google Scholar]
- 14.Lowery LA, Sive H. 2009. Totally tubular: the mystery behind function and origin of the brain ventricular system. Bioessays 31, 446–458. ( 10.1002/bies.200800207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmond ME, Knepper JE, DiBenedetto AJ, Malaugh E, Callejo S, Carretero R, Alonso MI, Gato A. 2014. Focal adhesion kinase as a mechanotransducer during rapid brain growth of the chick embryo. Int. J. Dev. Biol. 58, 35–43. ( 10.1387/ijdb.130305md) [DOI] [PubMed] [Google Scholar]
- 16.Garcia KE, Okamoto RJ, Bayly PV, Taber LA. 2017. Contraction and stress-dependent growth shape the forebrain of the early chicken embryo. J. Mech. Behav. Biomed. Mater. 65, 383–397. ( 10.1016/j.jmbbm.2016.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriegstein A, Noctor S, Martinez-Cerdeno V. 2006. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 7, 883–890. ( 10.1038/nrn2008) [DOI] [PubMed] [Google Scholar]
- 18.Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. 2011. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694. ( 10.1093/cercor/bhq238) [DOI] [PubMed] [Google Scholar]
- 19.De Juan Romero C, Borrell V. 2015. Coevolution of radial glial cells and the cerebral cortex. Glia 63, 1303–1319. ( 10.1002/glia.22827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. 2002. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53. ( 10.1093/cercor/12.1.37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 9, 110–122. ( 10.1038/nrn2252) [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Pettersson DR, Studholme C, Kroenke CD. 2015. Characterization of laminar zones in the mid-gestation primate brain with magnetic resonance imaging and histological methods. Front. Neuroanat. 9, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coogan TA, Van Essen DC. 1996. Development of connections within and between areas V1 and V2 of macaque monkeys. J. Comp. Neurol. 372, 327–342. ( 10.1002/(SICI)1096-9861(19960826)372:3%3C327::AID-CNE1%3E3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 24.Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. 2009. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb. Cortex 19, 2916–2929. ( 10.1093/cercor/bhp061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinstry RC, et al. 2002. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb. Cortex 12, 1237–1243. ( 10.1093/cercor/12.12.1237) [DOI] [PubMed] [Google Scholar]
- 26.Hansen DV, Lui JH, Parker PR, Kriegstein AR. 2010. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. ( 10.1038/nature08845) [DOI] [PubMed] [Google Scholar]
- 27.Striedter GF, Srinivasan S, Monuki ES. 2015. Cortical folding: when, where, how, and why? Annu. Rev. Neurosci. 38, 291–307. ( 10.1146/annurev-neuro-071714-034128) [DOI] [PubMed] [Google Scholar]
- 28.Richman DP, Stewart RM, Hutchinson JW, Caviness VS. 1975. Mechanical model of brain convolutional development. Science 189, 18–21. ( 10.1126/science.1135626) [DOI] [PubMed] [Google Scholar]
- 29.Tallinen T, Chung JY, Biggins JS, Mahadevan L. 2014. Gyrification from constrained cortical expansion. Proc. Natl Acad. Sci. USA 111, 12 667–12 672. ( 10.1073/pnas.1406015111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallinen T, Chung JY, Rousseau F, Girard N, Lefevre J, Mahadevan L. 2016. On the growth and form of cortical convolutions. Nat. Phys. 12, 588–593. ( 10.1038/nphys3632) [DOI] [Google Scholar]
- 31.Chatelin S, Constantinesco A, Willinger R. 2010. Fifty years of brain tissue mechanical testing: from in vitro to in vivo investigations. Biorheology 47, 255–276. [DOI] [PubMed] [Google Scholar]
- 32.Toro R, Burnod Y. 2005. A morphogenetic model for the development of cortical convolutions. Cereb. Cortex 15, 1900–1913. ( 10.1093/cercor/bhi068) [DOI] [PubMed] [Google Scholar]
- 33.Bayly PV, Okamoto RJ, Xu G, Shi Y, Taber LA. 2013. A cortical folding model incorporating stress-dependent growth explains gyral wavelengths and stress patterns in the developing brain. Phys. Biol. 10, 016005 ( 10.1088/1478-3975/10/1/016005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budday S, Steinmann P, Kuhl E. 2015. Secondary instabilities modulate cortical complexity in the mammalian brain. Philos. Mag. 95, 3244–3256. ( 10.1080/14786435.2015.1024184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammoto T, Ingber DE. 2010. Mechanical control of tissue and organ development. Development 137, 1407–1420. ( 10.1242/dev.024166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollard TD, Borisy GG. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. ( 10.1016/S0092-8674(03)00120-X) [DOI] [PubMed] [Google Scholar]
- 37.Carlsson AE, Bayly PV. 2014. Force generation by endocytic actin patches in budding yeast. Biophys. J. 106, 1596–1606. ( 10.1016/j.bpj.2014.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghavan R, Lawton W, Ranjan SR, Viswanathan RR. 1997. A continuum mechanics-based model for cortical growth. J. Theor. Biol. 187, 285–296. ( 10.1006/jtbi.1997.0450) [DOI] [Google Scholar]
- 39.Barron DH. 1950. An experimental analysis of some factors involved in the development of the fissure pattern of the cerebral cortex. J. Exp. Zool. 113, 553–581. ( 10.1002/jez.1401130304) [DOI] [Google Scholar]
- 40.Xu G, Bayly PV, Taber LA. 2009. Residual stress in the adult mouse brain. Biomech. Model. Mechanobiol. 8, 253–262. ( 10.1007/s10237-008-0131-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu G, Knutsen AK, Dikranian K, Kroenke CD, Bayly PV, Taber LA. 2010. Axons pull on the brain, but tension does not drive cortical folding. J. Biomech. Eng. 132, 071013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G, Kemp PS, Hwu JA, Beagley AM, Bayly PV, Taber LA. 2010. Opening angles and material properties of the early embryonic chick brain. J. Biomech. Eng. 132, 011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennerll TJ, Lamoureux P, Buxbaum RE, Heidemann SR. 1989. The cytomechanics of axonal elongation and retraction. J. Cell Biol. 109, 3073–3083. ( 10.1083/jcb.109.6.3073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidemann SR, Buxbaum RE. 1990. Tension as a regulator and integrator of axonal growth. Cell Motil. Cytoskeleton 17, 6–10. ( 10.1002/cm.970170103) [DOI] [PubMed] [Google Scholar]
- 45.Van Essen DC. 1997. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. ( 10.1038/385313a0) [DOI] [PubMed] [Google Scholar]
- 46.Feng Y, Okamoto RJ, Namani R, Genin GM, Bayly PV. 2013. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. J. Mech. Behav. Biomed. Mater. 23, 117–132. ( 10.1016/j.jmbbm.2013.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prange MT, Margulies SS. 2002. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J. Biomech. Eng. 124, 244–252. ( 10.1115/1.1449907) [DOI] [PubMed] [Google Scholar]
- 48.Dervaux J, Ben Amar M. 2008. Morphogenesis of growing soft tissues. Phys. Rev. Lett. 101, 068101 ( 10.1103/PhysRevLett.101.068101) [DOI] [PubMed] [Google Scholar]
- 49.Borrell V. 2018. How cells fold the cerebral cortex. J. Neurosci. 38, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bray D. 1984. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 102, 379–389. ( 10.1016/0012-1606(84)90202-1) [DOI] [PubMed] [Google Scholar]
- 51.Chada S, Lamoureux P, Buxbaum RE, Heidemann SR. 1997. Cytomechanics of neurite outgrowth from chick brain neurons. J. Cell Sci. 110, 1179–1186. [DOI] [PubMed] [Google Scholar]
- 52.Budday S, Raybaud C, Kuhl E. 2014. A mechanical model predicts morphological abnormalities in the developing human brain. Sci. Rep. 4, 5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budday S, Steinmann P, Kuhl E. 2015. Physical biology of human brain development. Front. Cell. Neurosci. 9, 257 ( 10.3389/fncel.2015.00257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holland MA, Miller KE, Kuhl E. 2015. Emerging brain morphologies from axonal elongation. Ann. Biomed. Eng. 43, 1640–1653. ( 10.1007/s10439-015-1312-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jespersen SN, Leigland LA, Cornea A, Kroenke CD. 2012. Determination of axonal and dendritic orientation distributions within the developing cerebral cortex by diffusion tensor imaging. IEEE Trans. Med. Imaging 31, 16–32. ( 10.1109/TMI.2011.2162099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Studholme C, Grigsby PL, Frias AE, Cuzon Carlson VC, Kroenke CD. 2017. Folding, but not surface area expansion, is associated with cellular morphological maturation in the fetal cerebral cortex. J. Neurosci. 37, 1971–1983. ( 10.1523/JNEUROSCI.3157-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayly PV, Taber LA, Kroenke CD. 2014. Mechanical forces in cerebral cortical folding: a review of measurements and models. J. Mech. Behav. Biomed. Mater. 29, 568–581. ( 10.1016/j.jmbbm.2013.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zervas M, Walkley SU. 1999. Ferret pyramidal cell dendritogenesis: changes in morphology and ganglioside expression during cortical development. J. Comp. Neurol. 413, 429–448. ( 10.1002/(SICI)1096-9861(19991025)413:3%3C429::AID-CNE6%3E3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 59.Fernandez V, Llinares-Benadero C, Borrell V. 2016. Cerebral cortex expansion and folding: what have we learned? EMBO J. 35, 1021–1044. ( 10.15252/embj.201593701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Razavi MJ, Zhang T, Li X, Liu T, Wang X. 2015. Role of mechanical factors in cortical folding development. Phys. Rev. E 92, 032701. [DOI] [PubMed] [Google Scholar]
- 61.de Juan Romero C, Bruder C, Tomasello U, Sanz-Anquela JM, Borrell V. 2015. Discrete domains of gene expression in germinal layers distinguish the development of gyrencephaly. EMBO J. 34, 1859–1874. ( 10.15252/embj.201591176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Juan Romero C, Borrell V. 2017. Genetic maps and patterns of cerebral cortex folding. Curr. Opin Cell Biol. 49, 31–37. ( 10.1016/j.ceb.2017.11.009) [DOI] [PubMed] [Google Scholar]
- 63.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. ( 10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 64.Chang YJ, Tsai CJ, Tseng FG, Chen TJ, Wang TW. 2013. Micropatterned stretching system for the investigation of mechanical tension on neural stem cells behavior. Nanomedicine 9, 345–355. ( 10.1016/j.nano.2012.07.008) [DOI] [PubMed] [Google Scholar]
- 65.Franze K. 2013. The mechanical control of nervous system development. Development 140, 3069–3077. ( 10.1242/dev.079145) [DOI] [PubMed] [Google Scholar]
- 66.Koser DE, et al. 2016. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 19, 1592–1598. ( 10.1038/nn.4394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siechen S, Yang S, Chiba A, Saif T. 2009. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc. Natl Acad. Sci. USA 106, 12 611–12 616. ( 10.1073/pnas.0901867106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D. 2010. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci. 30, 2268–2276. ( 10.1523/JNEUROSCI.4682-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.