Abstract
The intrinsic oscillatory activity of central pattern generators underlies motor rhythm. We review and discuss recent findings that address the origin of Caenorhabditis elegans motor rhythm. These studies propose that the A- and mid-body B-class excitatory motor neurons at the ventral cord function as non-bursting intrinsic oscillators to underlie body undulation during reversal and forward movements, respectively. Proprioception entrains their intrinsic activities, allows phase-coupling between members of the same class motor neurons, and thereby facilitates directional propagation of undulations. Distinct pools of premotor interneurons project along the ventral nerve cord to innervate all members of the A- and B-class motor neurons, modulating their oscillations, as well as promoting their bi-directional coupling. The two motor sub-circuits, which consist of oscillators and descending inputs with distinct properties, form the structural base of dynamic rhythmicity and flexible partition of the forward and backward motor states. These results contribute to a continuous effort to establish a mechanistic and dynamic model of the C. elegans sensorimotor system. C. elegans exhibits rich sensorimotor functions despite a small neuron number. These findings implicate a circuit-level functional compression. By integrating the role of rhythm generation and proprioception into motor neurons, and the role of descending regulation of oscillators into premotor interneurons, this numerically simple nervous system can achieve a circuit infrastructure analogous to that of anatomically complex systems. C. elegans has manifested itself as a compact model to search for general principles of sensorimotor behaviours.
This article is part of a discussion meeting issue ‘Connectome to behaviour: modelling C. elegans at cellular resolution’.
Keywords: central pattern generators, motor neuron, projection-premotor interneuron, proprioception, functional compression, neuromechanical model
1. Origin and regulation of motor rhythms
Neurons and neural circuits with intrinsic oscillatory activities underlie rhythmicity of motor behaviours, from respiration, heartbeat, gastric motility to locomotion [1–5]. In the absence of rhythmic inputs from the descending neural networks or sensory organs, isolated vertebrate spinal cords, and their invertebrate counterpart, the ventral nerve cords, retain a capacity to generate rhythmic and patterned motor neuron activity or fictive locomotion [6–10]. Hence oscillators for locomotory activities reside within the spinal or ventral nerve cords, where premotor interneurons form local circuits with excitatory motor neurons to orchestrate motor outputs [1,2,11].
Selective recruitment, modulation and coordination of locomotory oscillators constitute the form and transit between different motor patterns. This process is regulated at multiple levels. Within the spinal cords of rodents and fish, distinct pools of spinal premotor interneurons play dedicated roles in rhythm generation and pattern coordination. Different modes of movements correlate with selective recruitment of excitatory and inhibitory premotor interneuron groups [1,12]. Mechanosensory interneurons provide local proprioceptive and sensory feedbacks to the spinal premotor interneurons [13–17]. While excitatory premotor interneurons are generally considered to originate the rhythm, motor neurons are integral components of the oscillatory output. In the crayfish and leech swimmerets, the Caenorhabditis elegans and Drosophila larva motor circuit, as well as the zebrafish spinal cord, motor neurons that receive mixed electric and chemical synaptic inputs can retrogradely affect the activity of premotor interneurons [18–22]. Lastly, the oscillatory activities of local central pattern generators (CPGs) are regulated by descending inputs from the brain, via synaptic and neuromodulatory pathways, for initiation, reconfiguration and coordination. For example, in the lamprey and rodents, pools of interneurons of the retinospinal region or the brainstem project into the spinal cord, and their activities can either turn on or shut down different motor patterns [23–26].
2. CPGs for body bending reside in the C. elegans ventral nerve cord
Despite a deep understanding of anatomy [27–31] and theoretical studies [32–34], direct experimental evidence that addresses the potential existence and identity of C. elegans CPGs has been lacking. Obtaining a mechanistic understanding of the C. elegans motor rhythm has been difficult. Our progress has been hindered by the technical difficulty of in situ C. elegans electrophysiology experiments [35], and mystified by the membrane physiology of C. elegans neurons, which lack voltage-gated sodium currents, and with an exception [36], do not appear to fire action potentials [37]. Its anatomical organization of the nervous system also seems to be unconventional: unlike most spinal and ventral nerve cords, where premotor interneurons and motor neurons co-reside, only the soma of motor neurons, of both the excitatory and inhibitory classes, occupy the C. elegans ventral cord. Among them, the A- and some B-class excitatory motor neurons are main executors of body undulation during backward and forward movement, respectively. Recent studies reveal that these motor neurons function as the respective CPGs for backward and forward movements [38–40].
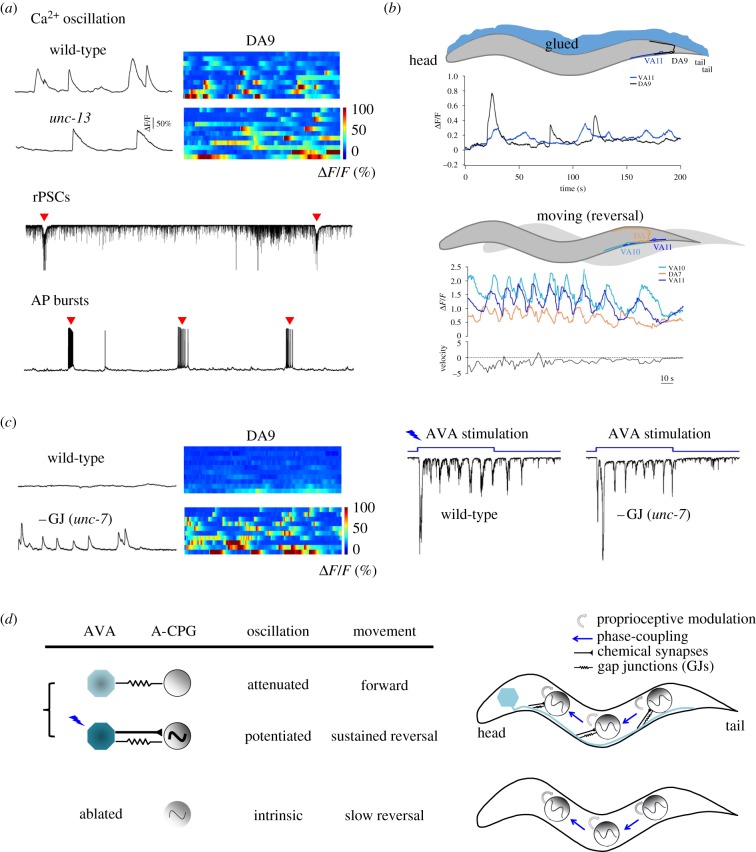
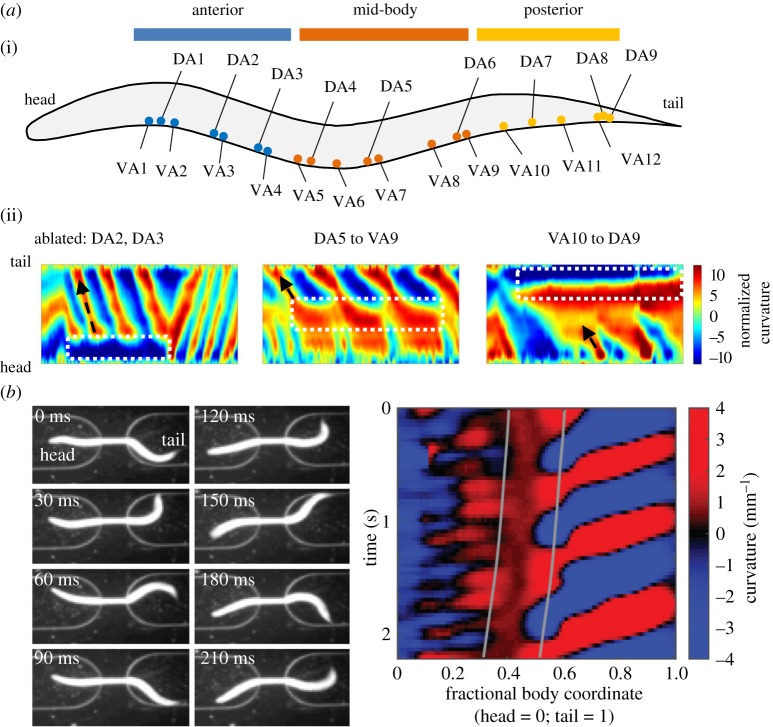
The A-class motor neurons exhibited intrinsic oscillatory activities (figure 1a) that were sufficient to execute slow backward movements without premotor interneurons (figure 1b). After the removal of all premotor interneuron inputs, the A-class excitatory motor neurons continued to generate oscillatory and phase-coupled activities (figure 1b) that triggered rhythmic action potential bursts in body wall muscles (figure 1a). Robust calcium oscillation maintained in posterior A-class motor neurons even when chemical synaptic transmission was further eliminated from the entire nervous system in this preparation (figure 1a). Selective ablation of a subgroup of the A-class motor neurons led to disruption of local bending, but did not inhibit rhythmic bending in neighbouring body segments [39] (figure 2a). These results suggest that the A-class motor neurons can function as non-bursting oscillators to drive body bending and to generate backward movements (figure 1b).
Figure 1.
An integrative model for backward locomotion: local reversal oscillators are phase-coupled via proprioception, and dually regulated by descending inputs. (a) The A-class motor neurons exhibit intrinsic, oscillatory activities that are sufficient to drive backward movement. (Top panel) Calcium oscillation in the posterior A motor neuron was observed in animals where chemical synaptic transmission and all premotor interneurons were removed from the nervous system. Left, sample traces; right, raster plot of recording from multiple animals. (Bottom panel) A dissected ventral cord muscle preparation from an animal where all premotor interneurons were removed exhibited anterior A-class motor-dependent rhythmic postsynaptic currents (rPSCs) and action potential (AP) bursts, both denoted by red arrowheads. (b) The A-class motor neurons may use intrinsic proprioceptive properties to self-organize phase-coupling during backward movement. (Top panels) A comparison of calcium activities exhibited by posterior A-class motor neurons in an immobilized (left) and a freely moving (reversal) animal (right), where all premotor interneurons were removed from the nervous system. Movements strengthened both calcium oscillation of and phase-coupling among A-class motor neurons. (c) The AVA premotor interneurons provide descending inputs that dually regulate the A-class motor neuron's oscillation through a mixed gap junction and chemical synapse configuration. Gap-junction-mediated coupling between AVA and A-class motor neurons shunts their intrinsic oscillation, whereas chemical synapses allow optogenetically activated AVA to potentiate their oscillation. (d) A model: backward movement is driven by oscillation from a chain of distributed CPGs (the A-class motor neurons), phase-coupled by proprioceptive feedback and regulated by descending inputs. Figure panels adapted from [39,53].
Figure 2.
Multiple A-class motor neurons function as oscillators during backward movements. (a) Ablation of A-class motor neurons that reside in a restricted body segments did not prevent bending wave propagation at adjacent regions. (i) Schematics of the soma position of the A-class motor neurons, arbitrarily separated into the anterior, mid- and posterior segments. (ii) Representative kymographs of bending curvature along the body of animals missing A-class motor neurons in the anterior (left), mid- (centre) and posterior (right) body segments. (b) An animal with its mid-body trapped inside a microfluidic device continued to generate anteriorly propagating bending waves in the unrestrained anterior and posterior segment with different frequencies, as shown in a series of video frames (left), and by kymograph of time-varying curvature along the body (right) where vertical lines mark the anterior and posterior limits of the straight channel. Panel (a) adapted from [39]. See Materials and Methods.
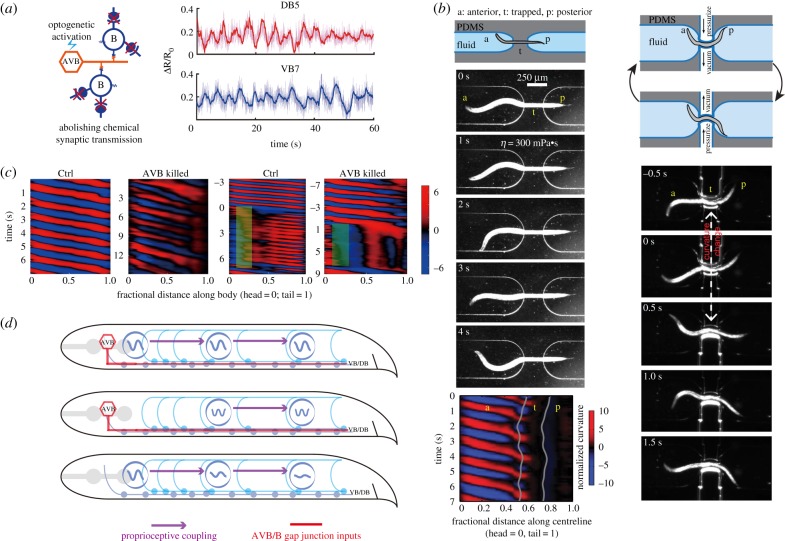
The B-class motor neurons also exhibit oscillatory activities, but with more restricted capacities. The mid-body B-class motor neurons exhibited oscillation when activated by the electric synaptic inputs from premotor interneurons projecting along the ventral nerve cord [40]. The oscillation of mid-body B-class motor neurons was observed when chemical synaptic transmission of the entire nervous system was eliminated (figure 3a).
Figure 3.
A biophysical model for forward locomotion that integrates local oscillators, proprioceptive couplings and descending inputs from premotor interneurons. (a) The B-class motor neurons in the mid-body exhibited rhythmic calcium activities upon optogenetic activation of premotor interneuron AVBs. Note that imaging experiments were carried out in mutants unc-13 (e51) where chemical synaptic transmission in the whole nervous system was largely abolished. (b) Local and directional (anterior to posterior) proprioceptive couplings propagate body undulations. When a mid-body region of a worm was constrained in a straight microfluidic channel, the posterior body region emerged from the channel would remain still and straight. Curvature kymograph showed that the bending waves could only propagate to the anterior limit of the channel. When dynamic curvature change in the worm mid-body was imposed by a pneumatic microfluidic device, rapid curvature changes and bending waves followed in the posterior body. (c) Descending inputs from AVB interneurons are required for mid-body oscillations. Curvature kymographs show that, in an AVB-ablated worm, the bending amplitude decayed monotonically towards the tail during forward locomotion. When an anterior body region of a wild-type worm was immobilized via optogenetic inhibition of B-class motor neurons or muscle cells, higher frequency and low-amplitude bending waves emerged from the mid-body. Ablating AVB premotor interneurons would abolish the mid-body bending waves. (d) Local body oscillators, proprioceptive coupling between B-class motor neurons and AVB-B gap junction coupling work synergistically to drive and propagate a coordinated undulatory wave from the head to the tail. When a strong and time-varying proprioceptive signal from an anterior body region is absent, AVB-B gap junction coupling induces mid-body high-frequency undulation. In the absence of AVB-B gap junction inputs, proprioceptive couplings are less effective in propagating bending waves, leading to rapidly decaying bending amplitude towards the tail. Figure panels adapted from [40,41].
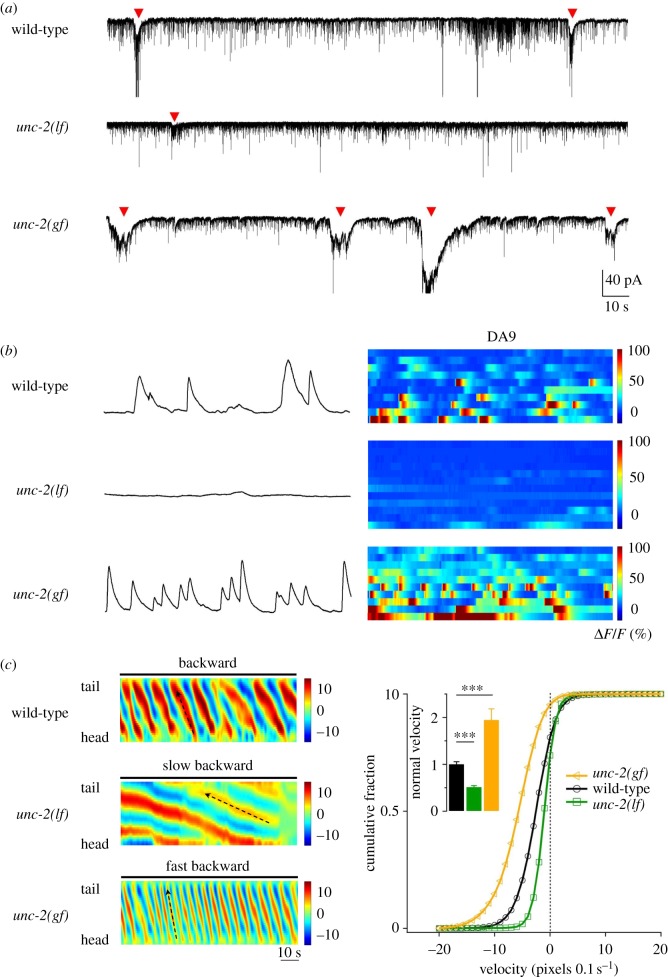
A plethora of sodium, calcium and potassium channels underlie a CPG's intrinsic membrane potential oscillations [42,43]. UNC-2, the C. elegans orthologue of the P/Q/N-type high-voltage-activated calcium channel, is one of the components [39]. The reduction and gain of UNC-2 conductance in the A-class motor neurons led to a decrease and increase, respectively, of the amplitude and frequency of muscle rPSC bursting (figure 4a) and their calcium oscillation (figure 4b). These effects were observed in the absence of premotor interneurons (figure 4a,b).
Figure 4.
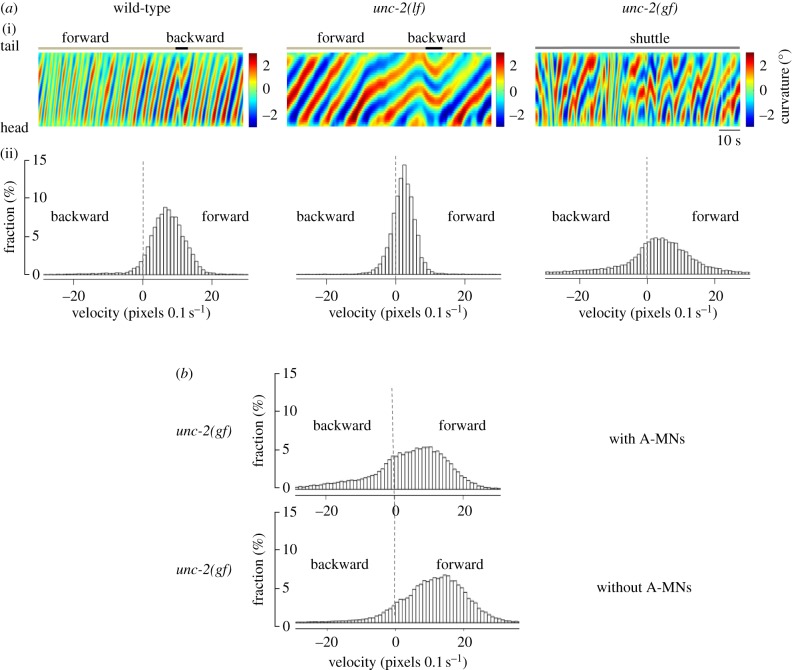
A high-voltage-gated calcium current UNC-2 underlies intrinsic membrane oscillation for oscillators for backward movement. (a,b) The decrease (lf) and increase (gf) of UNC-2 currents in A-class motor neurons led to decreased and increased amplitude and frequency of anterior A-motor neuron-dependent rhythmic rPSCs in dissected ventral cord muscle preparations, denoted by arrowheads (a), and reduced and increased frequency of calcium oscillation in a posterior A-class motor neuron DA9 (b). (c) These animals exhibited decreased and increased velocity during backward movement, as shown by representative bending curvature kymographs and the distribution of the instantaneous velocity of wild-type and unc-2 mutant animals. Note that all premotor interneurons and B-class motor neurons were removed from the nervous system in these animals, reiterating the sufficiency of an intrinsic activity of A-class motor neurons to drive cohesive anterior bending propagation and organized backward movement. Figure panels adapted from [39].
Whether UNC-2-mediated calcium conductance also underlies the B-class motor neuron oscillation has not been addressed, but indirect evidence supports this notion. Animals with reduced UNC-2 activity exhibited reduced velocity during both forward and backward movements (figure 5a). In the absence of A-class motor neurons, animals with increased UNC-2 activity continued to exhibit increased forward velocity (figure 5b). High-voltage-activated calcium conductance probably represents a conserved, endogenous constituent of the membrane intrinsic oscillation. In isolated lamprey spinal neuron soma, the N-type calcium currents prominently potentiate bursting, and are coupled with the burst-terminating calcium-activated potassium currents [44,45]; the intrinsic, high-frequency gamma band oscillation in the rat pedunculopontine nucleus requires the N- and/or P/Q-type calcium currents [46–48].
Figure 5.
UNC-2 may also underlie the activity of oscillators for forward movements. (a) The decrease (lf) and increase (gf) of UNC-2 activity led to velocity decrease and increase in both forward and backward movements. (i) Representative curvature kymographs in respective genetic background. Wild-type animal exhibits active movements consisting of anterior to posterior bending wave propagation, with occasional and short reverse movements. (ii) Histograms of instantaneous velocity distribution by animals of respective genotypes. Positive and negative values refer to forward and backward movements, respectively. (b) Forward velocity continued to exhibit an increase after the A-class motor neurons were ablated in unc-2(gf) animals, shown as the histogram of velocity distribution. Both lines of evidence support the idea that UNC-2 activity directly affects the forward circuit.
The complete channel composition of C. elegans oscillators awaits further dissection. Future work should also address mechanisms that underlie the difference between the high and low intrinsic activities of the A- and B-class motor neurons. The involvement of other ventral cord motor neurons in rhythm generation requires further investigation. The D-class GABAergic motor neurons modulate, but are not necessary for, body bending [39,40,49], arguing against them being CPGs. The perturbation of AS, another class cholinergic and excitatory motor neurons, affects both forward and backward movement; whether they harbour intrinsic oscillatory activity is unknown [50].
3. Proprioception entrains and coordinates CPG-driven body undulation
Proprioception is a prominent modulator of CPG's oscillatory properties [14,51]. Mechanisms must be in place to coordinate CPGs' activities distributed along the C. elegans body to form a cohesive propagating bending wave [34].
During forward movements in microfluidic devices, local and directional proprioceptive coupling between the adjacent body regions plays a critical role in the propagation of undulation [40,41]. When the mid-body region was trapped in a microfluidic channel with a defined curvature, the unrestrained posterior body region exhibited the same curvature in the same direction (figure 3b). When dynamic curvature change was imposed with a pneumatic microfluidic device, rapid curvature changes and bending waves followed in the posterior body [41] (figure 3b).
Whereas no dedicated local sensory neurons exist in the ventral nerve cord, the B-class motor neurons are found to transduce the bend-sensitive signals during forward movements. When a body segment was compelled to bend towards the dorsal side, the dorsal muscle-innervating B-class motor neuron sustained a higher level of intracellular calcium activity than the ventral muscle-innervating B-class, and vice versa during ventral bending [41]. The bend-sensitivity of B-class motor neurons allows the curvature change in an anterior body to define the curvature of the posterior neighbour, facilitating the propagation of bending waves from head to tail.
Optogenetic experiments reveal that proprioception entrains the B-class motor neuron's oscillation. When proprioceptive signal from the most anterior body region was eliminated by either optogenetically inhibiting the head muscles or anterior B-type motor neurons, the mid-body generated rhythmic bending activity, with a higher frequency than normal undulation [38,40] (figure 3c). Hence, the B-class motor neurons situated in the anterior and posterior body region may operate at different intrinsic frequencies, but directional proprioceptive coupling entrains their activities to generate coherent body undulation (figure 3c,d).
Whether and how proprioception regulates backward movements requires further examination. Several lines of indirect evidence support the notion of proprioceptive entrainment and coupling of reversal CPGs. When all premotor interneurons and B-class motor neurons were removed, animals continued to exhibit slow but organized, anteriorly propagating body undulation, as well as phase-coupled calcium oscillation of multiple A-class motor neurons [39] (figure 1b). This contrasted the case when these animals were glued down, in which the A-class motor neurons exhibited less robust or coordinated calcium oscillation [39] (figure 1b). Sparse ablation of a small number of the A-class motor neurons only blocked local body bending ([39] (figure 2a). When the mid-body was constrained by a straight microfluidic channel, the unconstrained anterior and posterior body regions continued to exhibit undulation at different frequencies (figure 2b). These observations imply that the A-class motor neurons are also proprioceptive, and use proprioception for self-organized phase-coupling (figure 1d).
Proprioceptive coupling probably plays a critical role for phase-locked coherent bending during both directional movements, but the forward and reversal circuit may incorporate such an ingredient differently. Most B-class motor neurons, with low intrinsic oscillatory activity, may rely strongly on proprioception for sequential activation during propagation. Multiple A-class motor neurons, with higher intrinsically oscillatory activities, may mainly use their proprioceptive property to self-organize a cohesive wave during propagation.
4. Descending pathways by the projection-premotor interneurons control movements
C. elegans ventral cord excitatory motor neurons integrate roles of rhythm generation and proprioception to organize and execute body undulations. Consistent with the A- and B-class motor neurons exhibiting differences in their intrinsic activities, the projection-premotor interneurons use different strategies to modulate their activities.
The B-class motor neurons exhibit a low level of intrinsic oscillatory activities compared with the A-class motor neurons [39]. The AVB premotor interneurons project along the ventral cord to form gap junctions with all B-class motor neurons. These electrical couplings allow depolarized AVB to trigger the bifurcation of mid-body B-class motor neurons' oscillatory activities to promote forward movement [40] (figure 3c). The A-class motor neurons, by contrast, have intrinsically high level of activities. The premotor interneurons AVA project along the ventral nerve cord to form both gap junctions and chemical synapses with all A-class motor neurons [28,52]. Their electrical couplings shunt the A-class motor neurons’ intrinsic oscillation, reducing the propensity of spontaneous reversals [39,53] (figure 1c,d). However, when AVA were stimulated to overcome the shunting effect, depolarized AVAs would potentiate the A-class motor neuron oscillation through chemical and electrical synapses to sustain long reversals [39,53,54] (figure 1c,d).
The interplay between the two descending pathways partly explains how C. elegans executes the two distinct motor programmes (figures 1d and 3d) with an inherent bias for forward movement [40,53] (figure 6). By shunting the reversal CPGs through electric coupling with the AVA premotor interneurons, animals favour forward movement by default. Upon depolarization, the AVA premotor interneurons potentiate A-class motor neurons to sustain backward movements. Upon hyperpolarization, they use electrical synapses to facilitate efficient inhibition of all A-class motor neuron activities [39,53]. Similarly, the depolarization and hyperpolarization of the AVB premotor interneurons could potentiate and halt forward locomotion, respectively, through their electrical coupling with the B-class motor neurons [40].
Figure 6.
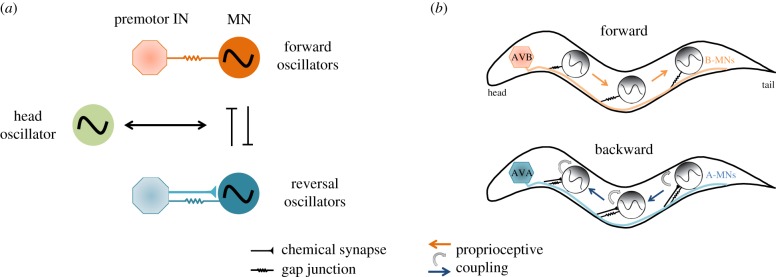
Schematics of a model of C. elegans locomotion as dynamic coupling of multiple motor states. (a) Head oscillation and body undulation are separately controlled. Descending inputs and directional phase-couplings allow distributed local oscillators to drive body undulation during forward and backward locomotion, respectively. A mutually inhibitory motif is introduced to flexibly control the two motor programme sub-circuits. Head–body undulation can be bi-directionally coupled with the forward or backward body undulation to generate different motor programmes. (b) The spatial layout of descending projection-premotor interneurons, local motor neuron CPGs and proprioceptive couplings between motor neurons for body undulation that drive forward and backward movements.
Bi-directional couplings between the descending pathways to and between excitatory motor neurons can have additional physiological implications. The ablation of the projection-premotor interneurons and, specifically, genetic ablation of their gap junction input to excitatory motor neurons lead to kink, a motor state in which the forward and backward circuit fails to establish the usual imbalance in their activity output [39,53]. The weakly rectifying electrical couplings between the AVA premotor interneurons and A-class motor neurons [55] may allow activated motor neurons to antidromically amplify the excitatory chemical synaptic inputs from AVA, prolonging evoked reversals [55]. Gap junction coupling between the AVB interneurons and B-class motor neurons [28,52] may similarly enable retrograde regulation of the dynamics of AVB, subsequently modulating the coupling of multiple B-motor neurons [40].
In fish and rodents, dedicated reticulospinal neurons project from the brain stem to the spinal cord, where they innervate either excitatory or inhibitory spinal premotor interneurons. Activating those that innervate excitatory spinal premotor interneurons can initiate locomotion [23,25,56,57], whereas activating those that innervate inhibitory spinal premotor interneurons can terminate locomotion [24]. In the C. elegans motor circuit, premotor neurons project along the ventral nerve cord to innervate excitatory ventral cord motor neurons, and can both facilitate and halt movements. Hence, functionally, C. elegans premotor interneurons more closely resemble the projection neurons in more motor circuits.
5. Towards a full mechanistic and computational model of C. elegans locomotion
There have been continuous efforts to model C. elegans locomotion [33,34,58–61]. Cohen and colleagues [32,62,63] developed proprioceptive coupling models for bending wave undulation, providing insights into gait adaptation [64–66]. Recently, Olivares and co-workers [32] constructed a CPG model based on local network motifs along the ventral nerve cord; however, proposed biophysical mechanisms only partly agree with available experimental data; caveats remain that the direction of proposed CPG coupling is opposite to what has been discussed above.
With a mechanistic dissection of the oscillators and their regulation for forward and backward locomotion, we can aim for a phenomenological model with minimum assumptions and parameters that recapitulates in vivo neuromuscular activities and locomotory behaviours in a changing environment. Models may be developed at different levels of abstraction, guided by the principle of Occam's razor. New modelling ingredients are only added when they are required to explain experimental observations and to generate testable predictions.
Here, we propose to integrate local oscillators, proprioceptive feedback, descending inputs and neuromuscular dynamics to define the computational algorithms for generation of undulations, and their posterior- and anterior-directed propagation during backward and forward movements, respectively (figures 1d, 3d and 6b). Roles of other motor neurons, such as the GABAergic D-class motor neurons, which facilitate contra-lateral inhibition, and the AS-class motor neurons, should be incorporated in the model. Some progress has been made to characterize the forward movements [40,41], but much work remains, and no efforts have been made for the backward movements. While multiple CPG models can serve as the base to model A- and B-class motor neuron oscillations, we must first obtain further experimental dissection of their intrinsic membrane conductance, through a combination of calcium- and voltage-imaging, electrophysiological recordings and molecular genetic analyses of candidate channel mutants.
Models for other motor primitives, including head oscillation, head casting and body turning [58,67–70] may be similarly constructed. With an understanding of individual modules, we are better positioned to use the connectome [28] as a road map to model how they are coordinated, such as the interaction between head oscillation and body undulation during forward and backward movements (figure 6a).
During C. elegans sensorimotor transformation, a rapid segregation from sensory to motor representation may occur in or after the first-layer interneurons [68,71–76]. Recurrent connections between local interneurons and projection (premotor) interneurons are prominent in the C. elegans connectome [28]. These connections underlie the rich and organized dynamics that dictate selection and transition between different motor states.
Deciphering the computation that interneurons collectively perform remains a major task. Tackling this problem requires identifying function motifs between interneurons, defining the input–output function of each neuron and probing the dynamics of synaptic plasticity of their connections through experimental and computational approaches. Dynamical systems theory may provide insights into constructing experimentally testable models. The premise is that such models can be developed with rigour and completeness, ultimately connecting the motor control algorithms and their implementation through ion channels, synapses and neurons.
6. Towards a comparative approach for general principles for circuit architecture
Previous studies have revealed cellular and molecular features that seem to place C. elegans at odds with other animal models. In most locomotory networks, premotor interneurons and motor neurons exhibit rhythmic action potential bursts, but most C. elegans neurons, premotor interneurons and motor neurons included, do not fire classic action potentials [37,55,71,77,78].
While the C. elegans motor neurons exhibit the property of non-bursting oscillators, the C. elegans body wall muscles generate L-type voltage-gated calcium-current-dependent action potentials [79–82]. Optogenetic activation of either premotor interneurons or A-class motor neurons triggered rhythmic action potential bursts in body wall muscles [39,54]. These results indicate that the C. elegans locomotory network can achieve motor rhythmicity through a combined oscillatory and bursting property of motor neurons and muscles. In the absence of voltage-activated sodium channels, high-voltage-activated calcium channels take on the deterministic roles in the rhythmic output of neural circuits.
These results unveil a simplified, but functionally homologous motor circuit infrastructure for C. elegans: the body wall muscles convert graded synaptic transmission to digital signalling through their bursting property; the ventral cord motor neurons integrate the role of rhythm generation and proprioception; the premotor interneurons play the role of projection interneurons to activate, halt or reconfigure CPGs.
Taken together, functional compression occurs at the numerically constrained C. elegans motor circuit: a single neuron or neuron class assumes the role of multiple layers of the microcircuit of the spinal and ventral nerve cords. Such a property allows a small nervous system to serve as compact models to dissect organizational logic of neural circuits, exemplified by the example of intricate roles of the conserved, mix synapse configuration at the invertebrate nerve and vertebrate spinal cords.
7. Materials and Methods
Figure 2b. A young wildtype (N2) adult C. elegans was loaded into a microfluidic device filled with the NGM media. The mid-body of the worm was trapped by a straight channel (around ∼ 200 µm). This animal attempted spontaneous forward and backward locomotion that was distinguished by the bending waves exhibited by its head and tail. Body curvature of the worm was analysed and visualized with custom-written MATLAB script as described in [41].
Figure 5(a). We recorded 1-day old wildtype (N2), unc-2(lf) (CB55 unc-2(eff)) and unc-2(gf) (ZM9040 unc-2(hp647)) adults during spontaneous and free crawling on thinly seeded OP50 plates, and quantified their bending curvature and velocities. Recordings and analysis were carried out as described in [39,53]. (b) Locomotor behaviours of unc-2(gf) animals with and without the A-class motor neurons were recorded using ZM7819 (unc-2(hp647); hpIs366) 1-day old adults. To ablate the A-class motor neurons, ZM7819 L1 stage larva cultured on standard NGM plates were exposed to the blue LED light for 40 minutes with lids open. Animals were allowed to recover in darkness and grew to 24 h post-L4 stages before recording. After recording, each adult were mounted to a wide-field compound fluorescent microscope to examine whether all A-class motor neurons had been ablated. Only data obtained from animals where all A-class motor neurons were ablated were used for quantification. Non-LED light exposed ZM7819 L1 larva were cultured and imaged in parallel. They were considered the ‘wildtype’ control. hpIs366 is an integrated transgenic array that expresses mitochondria-targetted miniSOG in the A-class (and a few other) neurons. Detailed description of hpIs366 construction, neuron ablation, behaviral recording, and velocity analyses were described in [39].
Data accessibility
This article has no additional data.
Competing interests
Authors have no competing interests.
Funding
This work was funded by the CAS Hundred Talents Plan and the National Science Foundation of China (NSFC-31471051 and NSFC-91632102 to Q.W.), the Strategic Priority Research Program of the Chinese Academy of Sciences (Pilot study, grant XDPB10 to Q.W.), the National Science Foundation of China (NSFC-31671052 to S.G.), the Natural Science foundation of Hubei (2018CFA039 to S.G.), Wuhan Morning Light Plan of Youth Science and Technology (2017050304010295 to S.G.), the Junior Thousand Talents Program of China (to S.G.) and the Canadian Institute of Health Research (CIHR-MOP93619, MOP123250 to M.Z.).
Acknowledgements
We are grateful for discussions with our colleagues, most prominently Christopher Fang-Yen and Alexander Gottschalk.
References
- 1.Kiehn O. 2016. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238. ( 10.1038/nrn.2016.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder E. 2001. Moving rhythms. Nature 410, 755 ( 10.1038/35071196) [DOI] [PubMed] [Google Scholar]
- 3.Selverston AI, Moulins M. 1985. Oscillatory neural networks. Annu. Rev. Physiol. 47, 29–48. ( 10.1146/annurev.ph.47.030185.000333) [DOI] [PubMed] [Google Scholar]
- 4.Grillner S. 2006. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 52, 751–766. ( 10.1016/j.neuron.2006.11.008) [DOI] [PubMed] [Google Scholar]
- 5.Grillner S. 1985. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 228, 143–149. ( 10.1126/science.3975635) [DOI] [PubMed] [Google Scholar]
- 6.Wallen P, Williams TL. 1984. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J. Physiol. 347, 225–239. ( 10.1113/jphysiol.1984.sp015063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiehn O, Iizuka M, Kudo N. 1992. Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci. Lett. 148, 43–46. ( 10.1016/0304-3940(92)90800-M) [DOI] [PubMed] [Google Scholar]
- 8.Juvin L, Simmers J, Morin D. 2007. Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators. J. Physiol. 583, 115–128. ( 10.1113/jphysiol.2007.133413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guertin P, Angel MJ, Perreault MC, McCrea DA. 1995. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J. Physiol. 487, 197–209. ( 10.1113/jphysiol.1995.sp020871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggman KL, Abarbanel HD, Kristan WB Jr. 2006. From crawling to cognition: analyzing the dynamical interactions among populations of neurons. Curr. Opin Neurobiol. 16, 135–144. ( 10.1016/j.conb.2006.03.014) [DOI] [PubMed] [Google Scholar]
- 11.Kiehn O. 2006. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306. ( 10.1146/annurev.neuro.29.051605.112910) [DOI] [PubMed] [Google Scholar]
- 12.Bjornfors ER, El Manira A. 2016. Functional diversity of excitatory commissural interneurons in adult zebrafish. eLife 5, e18579 ( 10.7554/eLife.18579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClellan AD, Jang W. 1993. Mechanosensory inputs to the central pattern generators for locomotion in the lamprey spinal cord: resetting, entrainment, and computer modeling. J. Neurophysiol. 70, 2442–2454. ( 10.1152/jn.1993.70.6.2442) [DOI] [PubMed] [Google Scholar]
- 14.Pearson KG. 1995. Proprioceptive regulation of locomotion. Curr. Opin Neurobiol. 5, 786–791. ( 10.1016/0959-4388(95)80107-3) [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Friesen WO. 2004. Entrainment of leech swimming activity by the ventral stretch receptor. J. Comp. Physiol. A 190, 939–949. ( 10.1007/s00359-004-0549-9) [DOI] [PubMed] [Google Scholar]
- 16.Akay T, Tourtellotte WG, Arber S, Jessell TM. 2014. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc. Natl Acad. Sci. USA 111, 16 877–16 882. ( 10.1073/pnas.1419045111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess D, Buschges A. 1999. Role of proprioceptive signals from an insect femur-tibia joint in patterning motoneuronal activity of an adjacent leg joint. J. Neurophysiol. 81, 1856–1865. ( 10.1152/jn.1999.81.4.1856) [DOI] [PubMed] [Google Scholar]
- 18.Heitler WJ. 1978. Coupled motoneurones are part of the crayfish swimmeret central oscillator. Nature 275, 231–234. ( 10.1038/275231a0) [DOI] [PubMed] [Google Scholar]
- 19.Szczupak L. 2014. Recurrent inhibition in motor systems, a comparative analysis. J. Physiol. Paris. 108, 148–154. ( 10.1016/j.jphysparis.2014.05.004) [DOI] [PubMed] [Google Scholar]
- 20.Rela L, Szczupak L. 2003. Coactivation of motoneurons regulated by a network combining electrical and chemical synapses. J. Neurosci. 23, 682–692. ( 10.1523/JNEUROSCI.23-02-00682.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Ampatzis K, Bjornfors ER, El Manira A. 2016. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 529, 399–402. ( 10.1038/nature16497) [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga T, Kohsaka H, Nose A. 2017. Gap junction-mediated signaling from motor neurons regulates motor generation in the central circuits of larval Drosophila. J. Neurosci. 37, 2045–2060. ( 10.1523/JNEUROSCI.1453-16.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagglund M, Borgius L, Dougherty KJ, Kiehn O. 2010. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 13, 246–252. ( 10.1038/nn.2482) [DOI] [PubMed] [Google Scholar]
- 24.Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O. 2015. Descending command neurons in the brainstem that halt locomotion. Cell. 163, 1191–1203. ( 10.1016/j.cell.2015.10.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan JT, Brodin L, Dale N, Grillner S. 1987. Reticulospinal neurons activate excitatory amino-acid receptors. Brain Res. 408, 321–325. ( 10.1016/0006-8993(87)90397-0) [DOI] [PubMed] [Google Scholar]
- 26.Capelli P, Pivetta C, Soledad Esposito M, Arber S. 2017. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377. ( 10.1038/nature24064) [DOI] [PubMed] [Google Scholar]
- 27.McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. 1993. The GABAergic nervous system of Caenorhabditis elegans. Nature 364, 337–341. ( 10.1038/364337a0) [DOI] [PubMed] [Google Scholar]
- 28.White JG, Southgate E, Thomson JN, Brenner S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340. ( 10.1098/rstb.1986.0056) [DOI] [PubMed] [Google Scholar]
- 29.Pereira L, et al. 2015. A cellular and regulatory map of the cholinergic nervous system of C. elegans. eLife 4, e12432 ( 10.7554/eLife.12432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duerr JS, Han HP, Fields SD, Rand JB. 2008. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 506, 398–408. ( 10.1002/cne.21551) [DOI] [PubMed] [Google Scholar]
- 31.Gendrel M, Atlas EG, Hobert O. 2016. A cellular and regulatory map of the GABAergic nervous system of C. elegans. eLife 5, e17686 ( 10.7554/eLife.17686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivares E, Izquierdo EJ, Beer RD. 2017. Potential role of a ventral nerve cord central pattern generator in forward and backward locomotion in Caenorhabditis elegans. Network Neurosci. ( 10.1162/netn_a_00036) [DOI] [PMC free article] [PubMed]
- 33.Karbowski J, Schindelman G, Cronin CJ, Seah A, Sternberg PW. 2008. Systems level circuit model of C. elegans undulatory locomotion: mathematical modeling and molecular genetics. J. Comput. Neurosci. 24, 253–276. ( 10.1007/s10827-007-0054-6) [DOI] [PubMed] [Google Scholar]
- 34.Gjorgjieva J, Biron D, Haspel G. 2014. Neurobiology of Caenorhabditis elegans locomotion: where do we stand? Bioscience 64, 476–486. ( 10.1093/biosci/biu058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman MB, Lindsay TH, Lockery SR, Richmond JE. 2012. Electrophysiological methods for Caenorhabditis elegans neurobiology. Methods Cell Biol. 107, 409–436. ( 10.1016/B978-0-12-394620-1.00014-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Q, Kidd PB, Dobosiewicz M, Bargmann C. 2018. The C. elegans AWA olfactory neuron fires calcium-mediated all-or-none action potentials. BioRxiv 359935 ( 10.1101/359935) [DOI] [PubMed] [Google Scholar]
- 37.Goodman MB, Hall DH, Avery L, Lockery SR. 1998. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron 20, 763–772. ( 10.1016/S0896-6273(00)81014-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouad AD, et al. 2018. Distributed rhythm generators underlie Caenorhabditis elegans forward locomotion. eLife 7, e29913 ( 10.7554/eLife.29913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao S, et al. 2018. Excitatory motor neurons are local oscillators for backward locomotion. eLife 7, e22915 ( 10.7554/eLife.29915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu T, Huo J, Shao S, Po M, Kawano T, Lu Y, Wu M, Zhen M, Wen Q. 2018. Descending pathway facilitates undulatory wave propagation in Caenorhabditis elegans through gap junctions. Proc. Natl Acad. Sci. USA 115, E4493–E4502. ( 10.1073/pnas.1717022115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen Q, et al. 2012. Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron 76, 750–761. ( 10.1016/j.neuron.2012.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izhikevich EM. 2007. Dynamical systems in neuroscience: the geometry of excitability and bursting, xvi, 441 p Cambridge, MA: MIT Press. [Google Scholar]
- 43.Harris-Warrick RM. 2002. Voltage-sensitive ion channels in rhythmic motor systems. Curr. Opin Neurobiol. 12, 646–651. ( 10.1016/S0959-4388(02)00377-X) [DOI] [PubMed] [Google Scholar]
- 44.Wikstrom MA, El Manira A. 1998. Calcium influx through N- and P/Q-type channels activate apamin-sensitive calcium-dependent potassium channels generating the late afterhyperpolarization in lamprey spinal neurons. Eur. J. Neurosci. 10, 1528–1532. ( 10.1046/j.1460-9568.1998.00194.x) [DOI] [PubMed] [Google Scholar]
- 45.el Manira A, Tegner J, Grillner S. 1994. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J. Neurophysiol. 72, 1852–1861. ( 10.1152/jn.1994.72.4.1852) [DOI] [PubMed] [Google Scholar]
- 46.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. 2011. Mechanism behind gamma band activity in the pedunculopontine nucleus. Eur. J. Neurosci. 34, 404–415. ( 10.1111/j.1460-9568.2011.07766.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. 2013. Spatiotemporal properties of high-speed calcium oscillations in the pedunculopontine nucleus. J. Appl. Physiol. 115, 1402–1414. ( 10.1152/japplphysiol.00762.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luster B, D'Onofrio S, Urbano F, Garcia-Rill E. 2015. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol. Rep.3, e12431. ( 10.14814/phy2.12431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donnelly JL, et al. 2013. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 11, e1001529 ( 10.1371/journal.pbio.1001529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolstenkov O, et al. 2018. Functionally asymmetric motor neurons coordinate locomotion of Caenorhabditis elegans. BioRxiv 244434.
- 51.Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. 2000. How animals move: an integrative view. Science 288, 100–106. ( 10.1126/science.288.5463.100) [DOI] [PubMed] [Google Scholar]
- 52.Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. 2009. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 4, 16 ( 10.1186/1749-8104-4-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawano T, Po MD, Gao S, Leung G, Ryu WS, Zhen M. 2011. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron 72, 572–586. ( 10.1016/j.neuron.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 54.Gao S, et al. 2015. The NCA sodium leak channel is required for persistent motor circuit activity that sustains locomotion. Nat. Commun. 6, 6323 ( 10.1038/ncomms7323) [DOI] [PubMed] [Google Scholar]
- 55.Liu P, Chen B, Mailler R, Wang ZW. 2017. Antidromic-rectifying gap junctions amplify chemical transmission at functionally mixed electrical-chemical synapses. Nat. Commun. 8, 14818 ( 10.1038/ncomms14818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiele TR, Donovan JC, Baier H. 2014. Descending control of swim posture by a midbrain nucleus in zebrafish. Neuron 83, 679–691. ( 10.1016/j.neuron.2014.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Severi KE, Portugues R, Marques JC, O'Malley DM, Orger MB, Engert F. 2014. Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83, 692–707. ( 10.1016/j.neuron.2014.06.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izquierdo EJ, Lockery SR. 2010. Evolution and analysis of minimal neural circuits for klinotaxis in Caenorhabditis elegans. J. Neurosci. 30, 12 908–12 917. ( 10.1523/JNEUROSCI.2606-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts WM, et al. 2016. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. eLife 5, e12572 ( 10.7554/eLife.12572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen N, Sanders T. 2014. Nematode locomotion: dissecting the neuronal–environmental loop. Curr. Opin Neurobiol. 25, 99–106. ( 10.1016/j.conb.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 61.Kunert JM, Proctor JL, Brunton SL, Kutz JN. 2017. Spatiotemporal feedback and network structure drive and encode Caenorhabditis elegans locomotion. PLoS Comput. Biol. 13, e1005303 ( 10.1371/journal.pcbi.1005303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyle JH, Berri S, Cohen N. 2012. Gait modulation in C. elegans: an integrated neuromechanical model. Front. Comput. Neurosci. 6, 10 ( 10.3389/fncom.2012.00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denham JE, Ranner T, Cohen N. 2018. Intrinsic and extrinsic modulation of C. elegans locomotion. bioRxiv ( 10.1101/312132) [DOI] [PMC free article] [PubMed]
- 64.Fang-Yen C, Wyart M, Xie J, Kawai R, Kodger T, Chen S, Wen Q, Samuel ADT. 2010. Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 107, 20 323–20 328. ( 10.1073/pnas.1003016107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berri S, Boyle JH, Tassieri M, Hope IA, Cohen N. 2009. Forward locomotion of the nematode C. elegans is achieved through modulation of a single gait. HFSP J. 3, 186–193. ( 10.2976/1.3082260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. 2008. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc. Natl Acad. Sci. USA 105, 20 982–20 987. ( 10.1073/pnas.0810359105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hendricks M, Ha H, Maffey N, Zhang Y. 2012. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature 487, 99–103. ( 10.1038/nature11081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray JM, Hill JJ, Bargmann CI. 2005. A circuit for navigation in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 102, 3184–3191. ( 10.1073/pnas.0409009101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Y, et al. 2016. An extrasynaptic GABAergic signal modulates a pattern of forward movement in Caenorhabditis elegans. eLife 5, e14197 ( 10.7554/eLife.14197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pierce-Shimomura JT, Dores M, Lockery SR. 2005. Analysis of the effects of turning bias on chemotaxis in C. elegans. J. Exp. Biol. 208, 4727–4733. ( 10.1242/jeb.01933) [DOI] [PubMed] [Google Scholar]
- 71.Kato S, Kaplan HS, Schrodel T, Skora S, Lindsay TH, Yemini E, Lockery S, Zimmer M. 2015. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669. ( 10.1016/j.cell.2015.09.034) [DOI] [PubMed] [Google Scholar]
- 72.Luo L, et al. 2014. Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 82, 1115–1128. ( 10.1016/j.neuron.2014.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venkatachalam V, et al. 2016. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E1082–E1088. ( 10.1073/pnas.1507109113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen JP, Shipley FB, Linder AN, Plummer GS, Liu M, Setru SU, Shaevitz JW, Leifer AM. 2016. Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E1074–E1081. ( 10.1073/pnas.1507110112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z, Liu J, Zheng M, Xu XZ. 2014. Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell 159, 751–765. ( 10.1016/j.cell.2014.09.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu H, Yang W, Wu T, Duan F, Soucy E, Jin X, Zhang Y. 2018. Cholinergic sensorimotor integration regulates olfactory steering. Neuron 97, 390–405. ( 10.1016/j.neuron.2017.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.CeS C. 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012–2018. ( 10.1126/science.282.5396.2012) [DOI] [PubMed] [Google Scholar]
- 78.Xie L, Gao S, Alcaire SM, Aoyagi K, Wang Y, Griffin JK, Stagljar I, Nagamatsu S, Zhen M. 2013. NLF-1 delivers a sodium leak channel to regulate neuronal excitability and modulate rhythmic locomotion. Neuron 77, 1069–1082. ( 10.1016/j.neuron.2013.01.018) [DOI] [PubMed] [Google Scholar]
- 79.Raizen DM, Avery L. 1994. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12, 483–495. ( 10.1016/0896-6273(94)90207-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jospin M, Mariol MC, Segalat L, Allard B. 2002. Characterization of K+ currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans. J. Physiol. 544, 373–384. ( 10.1113/jphysiol.2002.022293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao S, Zhen M. 2011. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 2557–2562. ( 10.1073/pnas.1012346108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu P, Ge Q, Chen B, Salkoff L, Kotlikoff MI, Wang ZW. 2011. Genetic dissection of ion currents underlying all-or-none action potentials in C. elegans body-wall muscle cells. J. Physiol. 589, 101–117. ( 10.1113/jphysiol.2010.200683) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.