Abstract
Fire ants are widely studied, invasive and venomous arthropod pests. There is significant biomedical interest in immunotherapy against fire ant stings. However, mainly due to practical reasons, the physiological effects of envenomation has remained poorly characterized. The present study takes advantage of a recently-described venom protein extract to delineate the immunological pathways underlying the allergic reaction to fire ant venom toxins. Mice were injected with controlled doses of venom protein extract. Following sensitization and a second exposure, a marked footpad swelling was observed. Based on eosinophil recruitment and production of Th2 cytokines, we hereby establish that fire ant proteins per se can lead to an allergic response, which casts a new light into the mechanism of action of these toxins.
Introduction
The Red Imported Fire Ant (RIFA) Solenopsis invicta Buren (Insecta: Formicidae) is one of the most dangerous invasive pests on a global scale1,2. These aggressive ants have been inadvertently shipped from South America to many territories around the world over the last century, and are now causing severe problems in regions as far apart as Vietnam, China, Australia, the United States, and the Galapagos archipelago1,3. When disturbed, these ants will viciously defend their nests with a venomous sting which can be particularly dangerous to the children4 and the elderly5.
Anaphylactic reactions to fire ant stings are recurrent for a fraction of previously sensitized victims, where systemic hypersensitive reactions can pose life-threatening complications6,7. Since the prevalence of sensitized subjects in invaded areas is reportedly relatively high8, there is a growing demand for immunotherapy methods in regions recently invaded by RIFA9,10. Immediate effects of the stings are mainly caused by a major (>95%) fraction of toxic alkaloids, but the later allergic responses11,12 are solely ascribed to venom proteins.
Nonetheless, in spite of over 40 years of research13, the allergic reaction caused by fire ant venom has remained poorly characterized. This is primarily because each ant carries venom proteins that amounts to only 0.1% of total body weight14,15, rendering bioassays unfeasible. Fortunately, a simple method enabling the isolation of venom proteins from fire ants in large quantities has been recently devised14, enabling a range of biological tests which were previously impossible, such as injecting animal models with venom protein fractions.
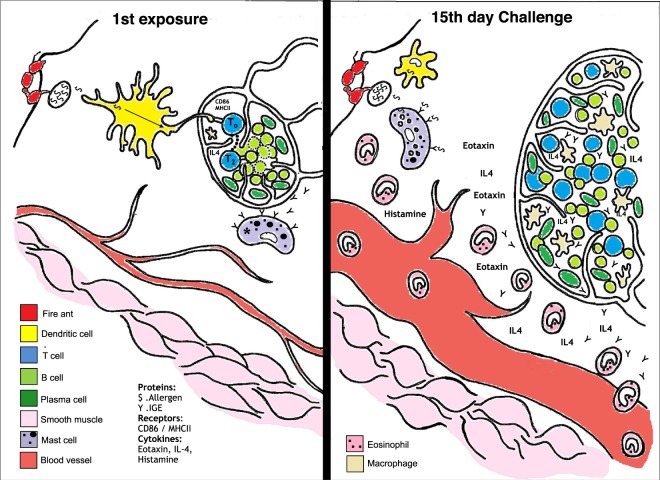
The network of immune cells and expressed factors involved in an allergic response is complex and context-dependent. We provide a simplified general diagram illustrating the proposed allergic reaction induced by injected venom in Fig. 1. Allergens upon entering the system are endocytosed and processed by phagocytes (e.g. macrophages, dendritic cells) in order to generate peptides. The physico-chemical properties and enzymatic activity of allergens play a central role in the activation and maturation of dendritic cells, which are fundamental steps for proper antigen presentation and immune response triggering. The dendritic cells subsequently migrate to the nearest draining lymph node to present the generated peptides (antigens) coupled to a major histocompatibility complex class II (MHCII) molecule to naive T lymphocytes16. Three signals are required during the antigen presentation in order to induce proper T cell activation: the first is provided by the recognition of the complex MHCII/peptide by the T cell receptor (TCR) present in a naive T lymphocyte. The binding between the phagocyte costimulatory molecules (e.g. CD86) and the CD28 receptor present at the T cell surface provides the second signal. The third signal is given by soluble cytokines released from the antigen presenting cell (APC) to act on the T cell. Together, these signals can activate a naive T cell that will differentiate into distinct T helper subsets17,18.
Figure 1.
Hypothesized allergic reaction following a fire ant sting. Upon a first exposure, (left panel) peripheral resident cells recruit blood leukocytes that will differentiate into macrophages and dendritic cells. These phagocytes will endocytose and process the venom’s antigens. Dendritic cells exposed to venom components will be activated and mature, thus promoting their ability to trigger the adaptive immune response. In this scheme, a dendritic cell migrates into a lymph node to present fire ant venom-derived peptides via MHCII to a naive T cell in the presence of the costimulatory molecule CD86. Upon activation, T cells become Th2 cells and proliferate under secretion of IL-4, and which will eventually activate an antigen-compatible B lymphocyte. Activated lymphocytes undergo clonal expansion inside the lymph node. A key event at the immunization stage takes place within ca. 7–10 days: activated B cells mature into IgE-secretory plasma cells, and the secreted allergen-specific IgE bind to peripheral mast-cells’ FcεRI (this immunization event is marked with an *). Upon a second exposure (right panel), antigens will trigger a specific, amplified reaction: (i) mast cells carrying antigen-specific IgE are activated to secrete inflammatory factors (not tested in the present study); (ii) specific lymphocytes secrete IgE and IL-4 promoting further cell activation; (iii) the amplified reaction increases edema and promote intense recruiting of eosinophils.
In the context of allergy, the participation of type-2 T helper cells (Th2) is central. Once activated, Th2 lymphocytes will undergo clonal expansion leading to lymph node hyperplasia and production of IL-4, the major Th2 cytokine19,20. In the context of venoms, many toxins can directly trigger Th2 responses21–24. This T cell subset produces cytokines that can promote leukocyte recruitment and the synthesis of further Th2-related cytokines17. A second exposure to venom antigens triggers a stereotypical allergic response marked by acute vascular permeability and later eosinophil recruitment at the site of the sting. This condition can be life threatening when the allergic reaction is triggered systemically progressing to anaphylactic shock22.
In this context, the present manuscript utilizes an extract of RIFA venom proteins to describe the general features of the immunological response from injections into male mice. These venom proteins are demonstrated to be independent from alkaloids and adjuvants in triggering an immunological response, which brings a new biological meaning to the role of these toxins.
Results and Discussion
In short, all challenged mice demonstrated clear signs of allergic response, demonstrating the ant venom proteins will immunize even at the lowest tested doses, and in the absence of adjuvants upon first exposure. Details are given as per the pertinent sections below.
Ant venom promotes eosinophil recruitment to peritoneal cavity
To test the premise that venom proteins are the causative factors of allergic reactions to fire ant stings, we sensitized mice via venom proteins into the hind footpad. Fourteen days later, the same mice were challenged by a second injection into the peritoneal cavity. The observed increase in peritoneal cellularity in venom-sensitized mice was mainly due to significant eosinophil recruitment. Injection of venom proteins into non-sensitized (naive) mice promotes merely mild, non-significant eosinophil recruitment (Fig. 2). Interestingly, inoculating ovalbumin (OVA) into mice previously sensitized with combined venom proteins + OVA promotes recruitment of eosinophils comparable to positive controls of combined OVA + aluminum hydroxide (as an adjuvant). This result suggests a potential role of venom proteins as adjuvants in allergic sensitization.
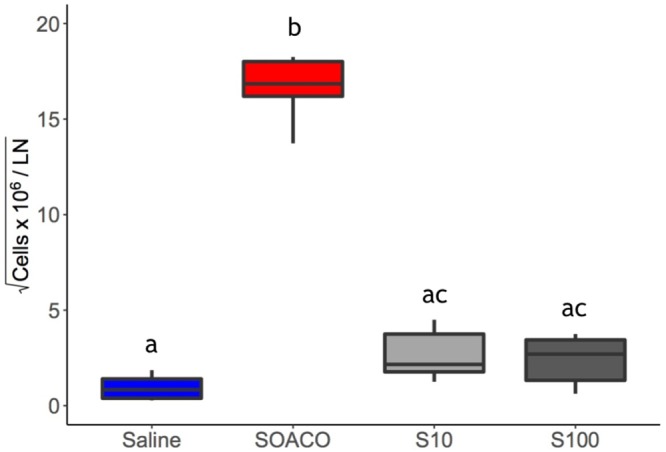
Figure 2.
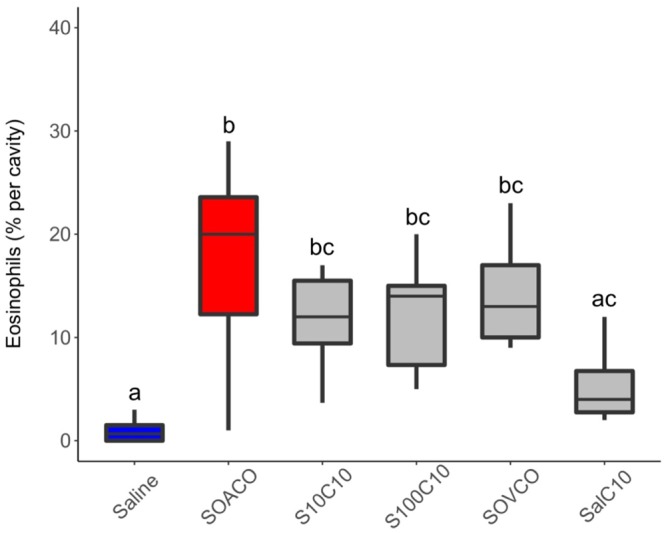
Ant venom promotes eosinophil recruitment to peritoneal cavity. Mice were previously injected (sensitized: S) with saline (Sal), OVA + AlOH3 (SOACO), ant venom (10 μg = S10; 100 μg = S100), or OVA + 10 μg ant venom (SOVCO). After 2 weeks, mice were submitted to an intraperitoneal challenge (Challenge: C) with saline, OVA (CO), or 10 μg ant venom extract (C10). Twenty-four hours after the challenge, the peritoneal cells were harvested and the eosinophils were counted. Measurements are expressed as the percentage of eosinophils among the peritoneal cell population. Boxplot of pooled internal replicates from three independent experiments, where the vertical lines are upper and lower limits, and the internal line are median values. Different letters indicate statistical significance (p < 0.05) between groups as compared by Kruskal-Wallis followed by Dunn’s Multiple Comparisons Test.
Eotaxin production triggered by ant venom
Peritoneal eotaxin accumulation in mice challenged with venom protein was higher in saline-injected mice (Fig. 3). The increased eotaxin levels of venom-inoculated mice were comparable to concomitant positive controls (see red bar ‘SOACO’ on Fig. 3). Taken together, Figs. 2 and 3 strongly suggest that venom proteins per se can actively promote sensitization-dependent eosinophil recruitment to the peritoneal cavity after a second exposure through the production of eosinophilotactic signals such as eotaxin.
Figure 3.
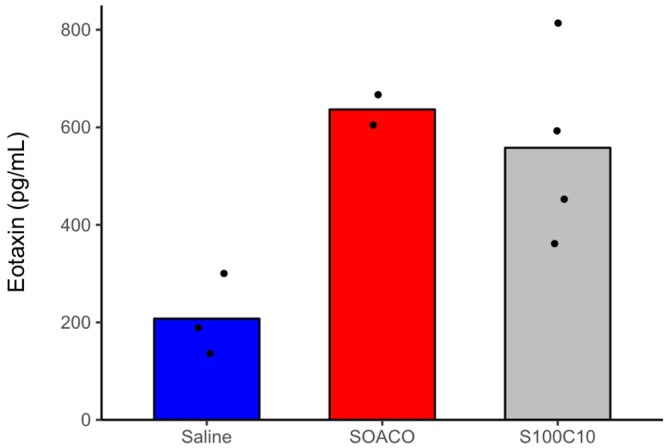
Fire ant venom induces eotaxin production in the peritoneal cavity. Mice were previously immunized with saline, ovalbumin + Al(OH)3 (SOACO), or 100 μg ant venom extract (S100C10). After 2 weeks, mice were submitted to an intraperitoneal challenge with saline, ovalbumin, or 10 μg ant venom extract, respectively. Within 24 h after the challenge, mice peritoneal exudates were harvested and eotaxin quantification was performed by ELISA. Vertical axis numbers are calculated concentrations of eotaxin in pg/mL. Bars represent means from pooled replicates deriving from two independent experiments; dots are raw values. Treatment S100C10 was statistically different from Saline control by nonparametric Wilcoxon-Mann-Whitney test at alpha = 0.05.
Dendritic cells activation by ant venom
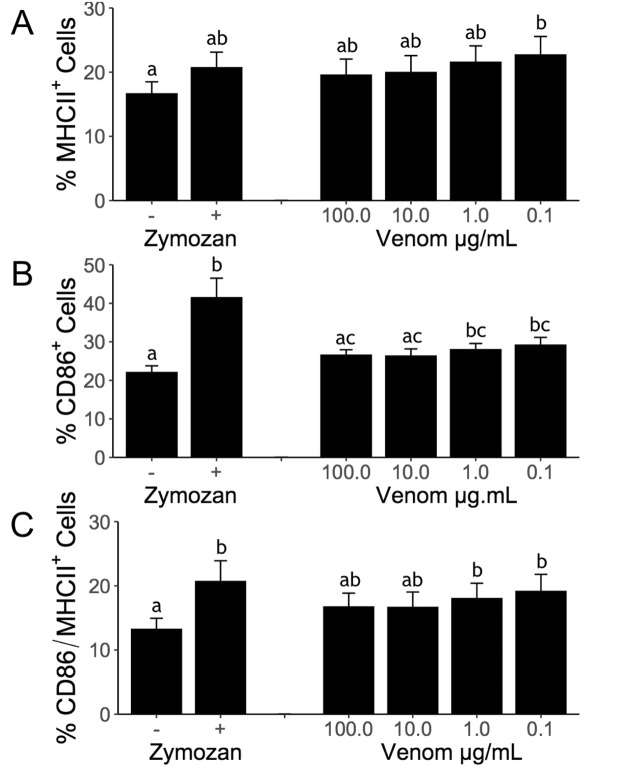
To investigate a potential role of RIFA venom proteins as adjuvants in immunization, we evaluated murine bone marrow-derived dendritic cells (BMDC) after exposure to venom proteins or alkaloids from the venom (Fig. 4A–C). Dendritic cells are responsible for the induction of adaptive immune responses, and seen as ‘sentinels’ for the immune system25. The venom alkaloids proved extremely cytotoxic even at 10 µg/mL (data not shown) indicating these venom components may not be relevant direct immunogenic stimuli for dendritic cells. Meanwhile, the venom proteins elicited an activation response, as shown by the upregulation of MHCII and CD86 (Fig. 4). This demonstrates that the venom proteins can directly activate dendritic cells to promote antigen presentation, of which the increased expression of MHCII is a first marker signal of immunological activation. Following expression of MHCII, secondary signals (costimulatory signals) are needed to elicit a response from lymphocytes, including CD86. The fact that the highest doses of venom generated a weaker response may indicate the presence of intrinsically dynamic components, such as proteinase-regulated zymogens and enzymatic inhibitors. Toxin activation by dilution could be a simple mechanism to achieve high levels of damage towards injected victims whilst ensuring a natural resistance by the ants. In fact at least one phospholipase inhibitor is described from the venom of RIFA26, indicating secreted proteases might be involved in the activation of zymogens.
Figure 4.
Ant venom activates dendritic cells in vitro. BMDCs were stimulated with zymosan (1:5) or with different concentrations of fire ant venom extract. After a 24 h incubation period, the expression of MHCII (A), CD86 (B), and MHCII + CD86 (C) were evaluated. Bars represent means and errors of three independent experiments. Different letters indicate statistical significance (p-value < 0.05) between groups as compared by Kruskal-Wallis followed by Dunn’s Multiple Comparisons Test.
In vivo hypersensitivity
The literature reports that the hypersensitive reactions following RIFA stings may include swelling of distal body parts which might lead to a life-threatening throat obstruction in humans6,7, initially signaled by wheezing, difficulty in breathing, and a coarse voice. To test for allergen-triggered swelling, mice were sensitized with an intradermal inoculum of venom proteins into their right hind footpad (sensitization), and 14 days later the opposite hind footpad (left) was challenged with another inoculum. Both footpads were measured every 30 min from the moment of inoculation until up to 2 h after injections.
Naturally, all assayed mice displayed marked swelling in the injected footpad as a result of the inoculum, but the effects become visible as the injected volume is drained. Full drainage of the initial swelling is completed within 2 hours following the injections. After 2 weeks following the first exposure, a second inoculation (i.e. challenge) resulted in persistent footpad swelling of venom-exposed animals comparable to positive controls (OVA + Al2(OH)3) (Fig. 5). To address a possible role of venom proteins as adjuvants, mice were sensitized with an association between OVA and venom protein, or just OVA. When further challenged with OVA, only the mice that received OVA + venom proteins presented footpad swelling (Fig. 6). These results establish a sensitization-dependent swelling upon later challenge as an immunogenic response to fire ant venom proteins.
Figure 5.
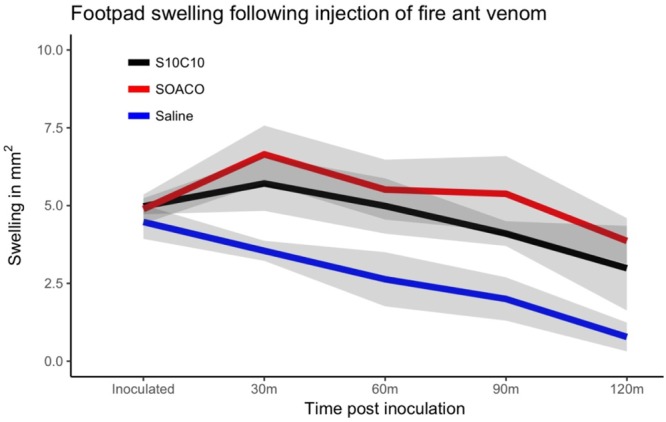
Footpad swelling after ant venom protein-fraction challenge. Mice were previously sensitized with saline, ovalbumin (OVA) + AlOH3 (SOACO), or fire ant venom extract (S10C10). After 2 weeks mice were respectively challenged with another exposure to saline, OVA or ant venom extract, and their footpad swelling was measured every 30 mins for 120 minutes. Lines represent means of the obtained swelling measures and the shaded area are standard errors from three independent experiments (N = 5 mice per group from 3 independent experiments).
Figure 6.
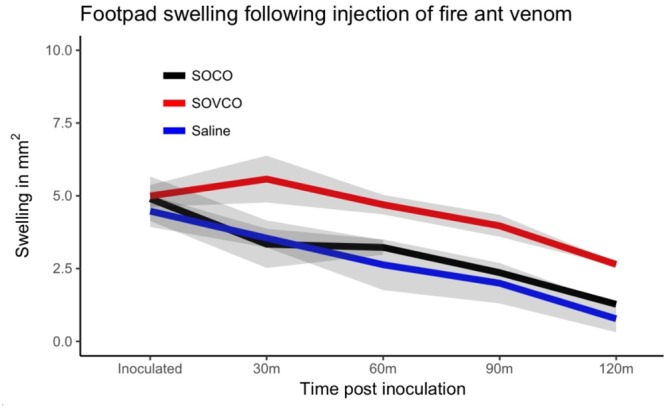
Adjuvant function of fire ant venom proteins. Mice were previously immunized with saline, ovalbumin + ant venom extract (SOVCO), or ovalbumin (SOCO). After 2 weeks the mice were challenged with new injections of saline or ovalbumin; footpad swelling was measured within every 30 mins for 120 minutes. Lines represent means; shaded area are standard errors measured at each analysis time point (N = 5 mice per group from 3 independent experiments).
Draining lymph node response
We assessed the hyperplasia of the draining lymph node (popliteal) 14 days after sensitization (Fig. 7). The lymph nodes of positive control mice (OVA + Al(OH)3) became greatly swollen two weeks after the sensitization. Venom-sensitized mice showed marked but less intense lymph node hyperplasia when sensitized with venom proteins. These lymph nodes were dissected out of the euthanized mice to evaluate the number of cells and the production of IL-4, the major Th2 cytokine, in order to test whether venom proteins induced priming of T cells in vivo. As indicated by the size of the respective lymph nodes, venom protein-sensitized mice presented an increase in the total cell number per lymph node when compared to the negative controls. Interestingly, lymph node hyperplasia from mice sensitized with the highest dose of venom proteins (100 μg) was not statistically different from the negative control, although a clear trend is apparent (Fig. 7). The increased cell number is indicative of lymphocyte proliferation triggered by venom proteins exposure, although this proliferative activity was less intense than in the positive control (OVA + Al(OH)3). However, when stimulated in vitro for cytokine production, cells from the draining lymph nodes of venom-sensitized mice produced significant amounts of IL-4 (Fig. 8), comparable to OVA + Al(OH)3 sensitized positive controls, with an apparent tendency to greater production after 48 h (see whiskers on right hand side of axis on Fig. 8).
Figure 7.
Fire ant venom induces lymph node response. Mice were previously immunized with either OVA + AlOH3 (SOA) or with 10 µg (S10) or 100 µg (S100) of of ant venom proteins extract. Two weeks after first exposure, the draining (popliteal) lymph nodes (LN) were isolated and cell totals were determined. The box plot represents upper and lower limits and quartiles from six independent experimental results; the internal line is the median. Different letters indicate statistical significance (p < 0.05) between groups as compared by Kruskal-Wallis followed by Dunn’s Multiple Comparisons Test.
Figure 8.
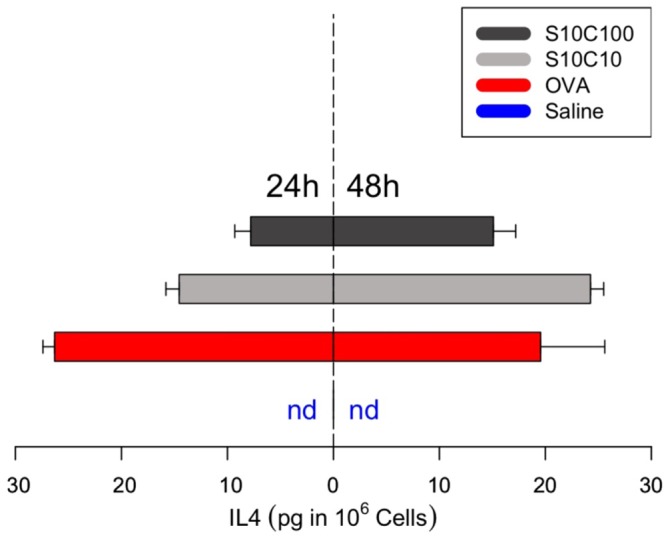
Ant venom induces cytokine response in lymph node cells. Mice were previously immunized with saline, ovalbumin + AlOH3 (SOA) or with 10 µg (S10) or 100 µg (S100) of ant venom proteins. After 2 weeks, popliteal lymph nodes were dissected and macerated for cell extraction, followed by stimulation with α-CD3. The cytokine IL-4 was quantified from lymph nodes supernatants after 24 h (left side) and 48 h (righthand side) of incubation. Bars are mirrored for size comparison representing the means (n = 2 independent experiments) while the whiskers are the interval towards the maximum value; nd = not detectable.
Venom proteins as adjuvant
At least four different allergens are described from RIFA venom proteins13. However, the venom components driving the adaptive immune response have not been empirically identified. Crude fire ant venom is mainly composed of alkaloids, which are cytotoxic and insoluble13,26 and cause local tissue damage. Tissue damage is accompanied by a steep inflammatory response, typically followed by later pustule formation. Given the local inflammatory reaction and edema that follows fire ants stings, it is tempting to hypothesize that alkaloids would play a necessary biological role as adjuvants for ensuring the adaptive response (as in Fig. 1) to the few accompanying venom antigens, roughly akin to the role of aluminum adjuvants in injected vaccines27,28. However, venom alkaloids herein proved to be highly toxic to dendritic cells, while the proteins were sufficient to promote antigen presentation (increased MHC-II) and co-stimulatory (increased CD86) capacity (Fig. 4). To test this hypothesis, we injected naive mice with a mixture of venom proteins and ovalbumin in the absence of an aluminum adjuvant. Ovalbumin is innocuous when injected alone, but becomes a potent allergen when administered in combination with an adjuvant like aluminum hydroxide (Fig. 5), thus widely employed as a control in immunology experiments.
Mice previously sensitized with 100 μg of ovalbumin in the presence of 10 µg of fire ant venom protein extract (SOVCO) produced a significant increase in footpad volume when later challenged with ovalbumin, as compared to challenged mice previously sensitized with ovalbumin alone (SOCO) (Fig. 6). The amount of swelling was similar to OVA + Al(OH)3 sensitized mice (SOACO) (Fig. 5). A similar adjuvant effect was observed when the OVA challenge injection was performed into the peritoneal cavity, where eosinophil influx was determined 24 h later (SOCO: 5.24 ± 1.53%; SOVCO: 12.15 ± 4.55%, p < 0.05). Since the extraction of venom proteins is not specifically demonstrated to eliminate potential microbial contaminants (e.g. LPS) such compounds could be involved in the observed effects.
To assess this possibility, we designed additional assays with heat-treated venom. Injection of venom induced significant eosinophil recruitment only when animals were previously exposed to the venom, indicating that neither contaminants nor the venom extract itself will trigger eosinophilic response without a previous sensitization (Fig. 2). Still, a possible role of contaminants in sensitization remained. To address this point, mice were sensitized with 100 µg of OVA combined with 10 µg of previously heat inactivated (100 °C/60 min) venom extract proteins (SOVhiCO).When SOVhiCO mice were challenged with OVA they elicited comparable amounts of eosinophils in the peritoneal cavity as mice sensitized with OVA alone (SOCO) (Fig. S2). Thus, heat exposure completely eliminated the adjuvant property of the venom extract, suggesting that it is fundamentally enzymatic, given that bacterial contaminants such as LPS are thermo-resistant29.
Fire ant venom proteins are relatively few in comparison to the venom composition of other social insects26. Still, some of the identified proteins may contribute to the observed adjuvant effects. Toxins identified from the venom extract include proteins involved in tissue damage (phospholipases A1 and A2, disintegrin/metalloproteinase, and myotoxins), and neurotoxicity (toxin PsTX-60, U5-ctenotoxins Pk1a, alpha-toxins Tc48a, and Scolopendra toxin)26. Phospholipase A2 from honeybee venom is known as a potent inducer of activation and maturation of dendritic cells30, suggesting that this enzyme may be playing a similar role in RIFA venom. In fact, previous immunological tests with honeybee venom indicated that venom-purified phospholipase A2 alone could lead to an adaptive response via Th2 phenotype31,32. Activity of PLA2 activity in RIFA venom was herein demonstrated for the first time with a fluorogenic substrate assay33. Remarkably the assay performed with 10 µg of the venom protein yielded the same activity intensity as 13 µg of Bothrops jararaca venom (Fig. S1). These results also demonstrate that enzymatic activity of the venom is maintained through extraction, lyophilization, and storage.
Conclusions
The present observations delineate the general pathways by which RIFA venom proteins can drive the primary physiological effects of an allergic response, i.e. the basic mechanisms of immunosensitization induced by fire ant venom proteins depicted in Fig. 1. Immunosensitization was manifested in all venom-exposed individuals, visually realized by footpad swelling. Peritoneal cell counts indicated a prototypical Th2 immune reaction based on augmented recruitment of eosinophils probably due to the production of eotaxin. The production of IL-4 by draining lymph node cells illustrates the cellular immune aspects of the allergic reaction. Co-injection of fire ant venom proteins with ovalbumin induced sensitization to ovalbumin, demonstrating an adjuvant activity for the venom itself decoupled of a pro-inflammatory effect of venom alkaloids.
From our standpoint such first insights into the immunoreaction to fire ant venom proteins, as tested in mice, pave the way for further tests. For example, it would be interesting to investigate individual- and species-specific variability, and the response to purified venom fractions. Identifying the most immunologically active peptides is paramount for developing more cost-effective, safer immunotherapy strategies. The venom extraction technology13,14 could include further purification steps (e.g. gel exclusion chromatography) to test finer fractions in animal models towards validation for immunotherapy. As a next step, trying to revert the acquired sensitivity of venom-injected mice could provide a simple model for powerful studies of immunotherapeutic protocols. Moreover, other aspects involved in immune/allergic responses for RIFA (e.g. venom fractions) and others related species are under investigation.
Methods
Venom sample
Nests of RIFA were excavated from the public gardens at Ilha do Fundão, Rio de Janeiro, Brazil into a plastic bucket rimmed with Teflon paint, and the ants were separated from the soil by slow flooding34. Species identification was based on a distinctive medial clypeal tooth and clearly-defined frons mark present on major workers, apart from typical color pattern of workers and the distinctively black males35. Based on recurrent field observations of excavated nests in this region by EGPF, local RIFA populations seem to be consistently monogyne3 (i.e. low density of mounds, presence of single reproductive queen, and relatively large major workers). The ants were allowed to dry and cleanse for 2 hours, and then submitted to a rapid method of venom extraction13,14, which relies on immersing live ants in a biphasic solvent mix (distilled water: hexane at 1: 5). The aqueous phase was subsequently lyophilized (henceforth ‘RIFA venom proteins’) and maintained at −20 °C until reconstitution for use. RIFA venom proteins extract obtained through method14 is equivalent to commercially available preparations as demonstrated previously by 2D electrophoresis14 and mass spectrometry26. Upon each day of experimentation, a dedicated sample of RIFA venom proteins was reconstituted in saline (sterile 0.9% NaCl) for fresh use.
Venom injections and footpad swelling
Male Mus musculus BALB/c mice aged 8–12 weeks were reared at 25 °C and 70% humidity in metal cages, and given water and special chow ad libitum. All methods used in this study were approved by the Ethics Committee on the Use of Animals of Health Sciences Center of Federal University of Rio de Janeiro, Brazil (CEUA/CCS/UFRJ). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences, USA, and National Council for Controlling Animal Experimentation, Ministry of Science, Technology and Innovation (CONCEA/MCTI), Brazil. Males were always siblings (to avoid fights) being held at the number of four or five per cage. Hypodermic plastic syringes were used; all intradermal injections were performed on mice anesthetized with ketamine 112.5 mg/kg and xylazine 10.0 mg/kg unless stated otherwise. The first venom presentation (i.e. the sensitizing inoculum) was injected into the right hind footpad using either 10.0 or 100.0 µg of total venom protein per animal. A third sensitization test was made using 10.0 μg of venom protein previously incubated at 100 °C in a water bath for 60 min, to test for enzymatic adjuvant role (SOVhiCO). Fourteen days after sensitization, a second exposure to venom (i.e. challenge) was made into the left hind footpad. This interval was established to allow for immunization of tested animals against the fire ant venom allergens, as detailed in Fig. 1 (see the asterisk on left-hand panel). Immediately before and after each venom injection, the width and height of the challenged and opposing paws were measured with a caliper, and measurements were taken every 30 min for 2 h, during which animals were monitored. Negative control injections used saline solution (sensitization - S; and challenge - C), and positive controls (SOACO) used ovalbumin (OVA – 100.0 μg/animal) with adjuvant aluminum hydroxide (5.0 mg/animal) upon sensitization, or only OVA upon challenge. All footpad inoculations were carried out in a 30.0 μL injection. Experimental groups were sensitized and challenged with venom protein unless stated otherwise. Each experiment employed groups of 4–5 mice and were repeated at least 3 times independently, unless specified otherwise.
Leukocyte recruitment to the peritoneal cavity
Sensitized mice were intraperitoneally challenged with 0.9% sterile saline, OVA, or venom proteins with the quantities of OVA and venom previously described for a total injection volume of 500.0 μL per individual. Twenty-four hours after the challenge, peritoneal cavities were washed with PBS containing 0.1% heparin and the resulting suspensions were used for total and differential cell counting, using a Neubauer chamber and cytospun slides, respectively. The slides were stained with panoptic stain. The peritoneal exudates were harvested for chemokine quantification (Eotaxin - BDBiosciences) by ELISA according to the manufacturer’s protocol.
Lymph node cytokine production
Mice were individually euthanized in CO2 chambers and the draining lymph node (popliteal) of the right hind footpad was dissected out and homogenized. Lymph node cells were cultivated for 48 h in the presence of immobilized α-CD3 (1.0 μg/mL) for cytokine production. After the incubation period, supernatants were harvested and used to quantify IL-4 (BD Biosciences) by ELISA according to the manufacturer’s protocols.
Phospholipase A2 (PLA2) activity in venom
PLA2 activity was measured using the fluorogenic substrate 1-acyl-2-{12-[(7-nitro-2-1,3-benzoxadiazol-4-yl) amino] dodecanoyl}-sn-glycero-3-phosphocholine (aka ‘12NBD PC’, Avanti Polar Lipids Inc), an analogous of phosphatidilcholine. Assays were done in 96 well plates in a medium composed of Tris-HCl buffer 20 mM, pH 7.5, CaCl2 2.0 mM and 10.0 μg of venom protein of S. invicta. The reaction was triggered by the addition of 20.0 µM of the substrate 12NBD PC; after which the release of fatty acids was measured for 1 h in a plate-reading fluorimeter (Victor™ X5 Multilabel-Perkin Elmer) set for excitation and emission wavelengths of 460 nm and 534 nm, respectively. The negative control was reaction medium without added venom, and the positive control sample contained 1.0 μl (∼13.0 µg/µl) of Bothrops jararaca venom36.
Statistical Analyses
All statistical analyses and plots were generated with RStudio v.1.0.136, using the packages ‘plyr’, ‘reshape2’, ‘ggplot2’, ‘dunn.test’. All numeric raw data are provided as Supplementary Materials along with the scripts37, to ensure output reproducibility and verification of specific details. Results from different internal and independent replicates were either compared for statistical differences using non-parametric Kruskal-Wallis followed by multiple comparison Dunn’s test (in comparing multiple treatments) or by Wilcoxon-Mann-Whitney test (in comparing two treatments). Our conclusions were also compatible with analytical patterns obtained by parametric methods (i.e. assuming Gaussian distribution; not shown).
Electronic supplementary material
Acknowledgements
We wish to thank Leticia Coli and Debora Fogel for providing access to liquid nitrogen and lyophilizers; to the responsible technicians who provided the animals used in this manuscript. Eric Lucas has kindly revised a final version of this manuscript for readability and provided useful suggestions. Venom extract from B. jararaca was provided by André Lopes Fuly.
Author Contributions
D.Z.M., E.G.P.F., E.A.M. and B.L.D. designed research; B.L.D. supervised the study; D.Z.M., E.G.P.F., A.P.M., A.F.A., D.G. and L.E.P. performed experiments; D.Z.M., E.G.P.F., A.P.M., D.G., L.E.P., E.A.M. and B.L.D. analyzed data; E.G.P.F. wrote the R scripts behind statistics and plots; E.A.M. and B.L.D. contributed with new reagents/analytic tools; D.Z.M. and E.G.P.F. wrote the paper; E.A.M. and B.L.D. provided lab space, instrumentation and funding; all authors edited and gave critical input on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel Zamith-Miranda and Eduardo G. P. Fox contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32327-z.
References
- 1.Ascunce MS, et al. Global invasion history of the fire ant Solenopsis invicta. Science. 2011;331:1066–1068. doi: 10.1126/science.1198734. [DOI] [PubMed] [Google Scholar]
- 2.Morrison LW, Porter SD, Daniels E, Korzukhin MD. Potential global range expansion of the invasive fire ant. Solenopsis invicta. Biological Invasions. 2004;6:183–191. doi: 10.1023/b:binv.0000022135.96042.90. [DOI] [Google Scholar]
- 3.Tschinkel, W. R. The fire ants. (Harvard University Press, 2006).
- 4.More DR, Kohlmeier RE, Hoffman DR. Fatal anaphylaxis to indoor native fire ant stings in an infant. The American journal of forensic medicine and pathology. 2008;29:62–63. doi: 10.1097/PAF.0b013e3181651b53. [DOI] [PubMed] [Google Scholar]
- 5.de Shazo RD, Williams DF, Moak ES. Fire ant attacks on residents in health care facilities: A report of two cases. Annals of Internal Medicine. 1999;131:424–429. doi: 10.7326/0003-4819-131-6-199909210-00005. [DOI] [PubMed] [Google Scholar]
- 6.Stafford CT. Hypersensitivity to Fire Ant Venom. Annals of Allergy, Asthma & Immunology. 1996;77:87–99. doi: 10.1016/S1081-1206(10)63493-X. [DOI] [PubMed] [Google Scholar]
- 7.Prahlow JA, Barnard JJ. Fatal Anaphylaxis Due to Fire Ant Stings. The American journal of forensic medicine and pathology. 1998;19:137–142. doi: 10.1097/00000433-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Partridge ME, et al. Prevalence of allergic sensitization to imported fire ants in children living in an endemic region of the southeastern United States. Annals of Allergy, Asthma & Immunology. 2008;100:54–58. doi: 10.1016/S1081-1206(10)60405-X. [DOI] [PubMed] [Google Scholar]
- 9.Potiwat, R. et al. Solenopsis geminata (tropical fire ant) anaphylaxis among Thai patients: its allergens and specific IgE-reactivity. Asian Pac J Allergy Immunol. Earlier, 10.12932/AP-100217-0012. [DOI] [PubMed]
- 10.Haymore BR, McCoy RL, Nelson MR. Imported fire ant immunotherapy prescribing patterns in a large health care system during a 17-year period. Annals of Allergy, Asthma & Immunology. 2009;102:422–425. doi: 10.1016/S1081-1206(10)60515-7. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KT, Flood AA. Hymenoptera stings. Clinical techniques in small animal practice. 2006;21:194–204. doi: 10.1053/j.ctsap.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Parrino J, Kandawalla NM, Lockey RF. Treatment of local skin response to imported fire ant sting. Southern medical journal. 1981;74:1361–1364. doi: 10.1097/00007611-198111000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Fox, E. G. P. In Venom Genomics and Proteomics (eds P. Gopalakrishnakone & Juan J. Calvete) 149–167 (Springer Netherlands, 2016).
- 14.Fox EGP, et al. Simple, rapid method for the extraction of whole fire ant venom (Insecta: Formicidae: Solenopsis) Toxicon. 2013;65:5–8. doi: 10.1016/j.toxicon.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Baer H, et al. Protein components of fire ant venom (Solenopsis invicta) Toxicon. 1979;17:397–405. doi: 10.1016/0041-0101(79)90267-8. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. The Journal of experimental medicine. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nature immunology. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/S1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 21.Habermann E. Bee and Wasp Venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 22.Bircher AJ. Systemic immediate allergic reactions to arthropod stings and bites. Dermatology. 2005;210:119–127. doi: 10.1159/000082567. [DOI] [PubMed] [Google Scholar]
- 23.Madero MF, et al. Characterization of allergens in four South American snake species. International archives of allergy and immunology. 2009;150:307–310. doi: 10.1159/000222684. [DOI] [PubMed] [Google Scholar]
- 24.Muller UR. Insect venoms. Chemical immunology and allergy. 2010;95:141–156. doi: 10.1159/000315948. [DOI] [PubMed] [Google Scholar]
- 25.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature reviews. Immunology. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 26.Pinto JRAS, et al. Proteomic view of the venom from the fire ant Solenopsis invicta Buren. Journal of proteome research. 2012;11:4643–4653. doi: 10.1021/pr300451g. [DOI] [PubMed] [Google Scholar]
- 27.Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Frontiers in immunology. 2012;3:406. doi: 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghimire TR. The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm. SpringerPlus. 2015;4:181. doi: 10.1186/s40064-015-0972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorbet MB, Sefton MV. Endotoxin: The uninvited guest. Biomaterials. 2005;26:6811–6817. doi: 10.1016/j.biomaterials.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 30.Perrin-Cocon L, et al. Secretory phospholipase A2 induces dendritic cell maturation. Eur J Immunol. 2004;34:293–2302. doi: 10.1002/eji.200324797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano M, et al. Involvement of carbohydrate on phospholipase A2, a bee‐venom allergen, in in vivo antigen‐specific IgE synthesis in mice. Allergy. 1999;54:811–818. doi: 10.1034/j.1398-9995.1999.00096.x. [DOI] [PubMed] [Google Scholar]
- 32.Palm NW, et al. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitsiouli EI, Nakos G, Lekka ME. Differential determination of phospholipase A2 and PAF-acetylhydrolase in biological fluids using fluorescent substrates. J Lipid Res. 1999;40:2346–2356. [PubMed] [Google Scholar]
- 34.Banks, W. A. Techniques for collecting, rearing, and handling imported fire ants (1981).
- 35.Pitts JP, Hugh MCJ, Ross KG. Cladistic analysis of the fire ants of the Solenopsis saevissima species-group (Hymenoptera: Formicidae) Zoologica Scripta. 2005;34:493–505. doi: 10.1111/j.1463-6409.2005.00203.x. [DOI] [Google Scholar]
- 36.Sobrinho JC, et al. Anti-platelet aggregation activity of two novel acidic Asp49-phospholipases A2 from Bothrops brazili snake venom. Int J Biol Macrom. 2018;107:1014–1022. doi: 10.1016/j.ijbiomac.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 37.Fox, E. G. P., Zamith, D., Monteiro, A. P. & Gama, D. Raw files behind the published paper ‘The allergic response mediated by fire ant venom proteins’ by Zamith & Fox et al., Mendeley Data, v.1, 10.17632/dk68nhdvz4.1 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.