Abstract
Background: Dehydration is common among ischemic stroke patients and is associated with early neurological deterioration and poor outcome. This study aimed to test the hypothesis that dehydration status is associated with decreased cerebral perfusion and aggravation of ischemic brain injury.
Methods: Diffusion-weighted imaging and arterial spin labeling perfusion MR imaging were performed on rats with middle cerebral artery occlusion (MCAO) by using a 9.4T MR imaging scanner to measure the volume of infarction and relative cerebral blood flow (rCBF) after infarction. Twenty-five rats were assigned to either a dehydration group or normal hydration group, and dehydration status was achieved by water deprivation for 48 h prior to MCAO.
Results: The volume of the infarction was significantly larger for the dehydration group at the 4th h after MCAO (p = 0.040). The progression in the infarct volume between the 1st and 4th h was also larger in the dehydration group (p = 0.021). The average rCBF values of the contralateral normal hemispheres at the 1st and the 4th h were significantly lower in the dehydration group (p = 0.027 and 0.040, respectively).
Conclusions: Our findings suggested that dehydration status is associated with the progression of infarct volume and decreases in cerebral blood flow during the acute stage of ischemic stroke. This preliminary study provided an imaging clue that more intensive hydration therapies and reperfusion strategies are necessary for the management of acute ischemic stroke patients with dehydration status.
Keywords: dehydration, cerebral blood flow, acute stroke, MRI, middle cerebral artery occlusion
Introduction
Clinical guidelines suggest the assessment of volume status and emphasize the importance of adequate hydration after stroke (1). Recent studies have demonstrated that dehydration is common among stroke subjects (2, 3), and hydration status is associated with stroke-in-evolution (4, 5), discharge outcome and admission costs in acute ischemic stroke (6–8). The reasons for these observations are based on the concept that dehydration status may lead to cerebral hypoperfusion and decreased collateral blood flow, which may exacerbate the ischemic brain injury (6, 9, 10). In an animal study, Paczynski et al. reported that both extremes of hydration and dehydration spectrum would lead to more severe brain edema and midline brain structure shifting (11). However, the evidence for the effect of dehydration status on cerebral perfusion and the evolution of the ischemic core after occlusion of major arteries have, until now, never been discussed in the literature.
Magnetic resonance (MRI) diffusion-weighted imaging (DWI) identifies tissue wherein diffusion is restricted as a result of cytotoxic edema due to ischemia, which leads to a reduction in the apparent diffusion coefficient (ADC). Arterial spin-labeling (ASL) is an MRI technique that can be used to identify and quantify cerebral perfusion. It provides noninvasive, quantitative measurements of cerebral blood flow (CBF) with relative insensitivity to permeability (12).
The goal of our current study was to investigate the role of hydration status in the cerebral perfusion and infarct core using an acute middle cerebral artery occlusion (MCAO) rat model. Dehydration was achieved by water deprivation for 48 h prior to MCAO. DWI and ASL imaging were used to measure the size of infarct cores and relative cerebral blood flow (rCBF), respectively. We hypothesized that dehydration status would be associated with decreased cerebral perfusion and aggravation of ischemic brain injury.
Methods
Stroke animal model
Adult male Sprague-Dawley rats, weighing 250–300 g, were maintained at a constant temperature (21 ± 2°C) and humidity in a facility recognized by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) under a 12/12-h light/dark cycle with free access to food and water. All experimental procedures were approved and supervised by the Animal Committee of Chang Gung Memorial Hospital and were in compliance with the guidelines for animal care and use set forth by that Committee (Approval No.20160301803). Focal cerebral ischemia was induced via extracranial intraluminal MCAO, following the previously described method of Longa et al. (13). Briefly, the left common carotid artery, the external carotid artery (ECA) and the internal carotid artery (ICA) were sequentially exposed following anesthetization of the animal by intramuscular injection of a mixture of zoletil50 25 mg/kg and xylazine 10 mg/kg. A silicon-coated 30-mm length of 4–0 polyamide monofilament non-absorbable surgical suture was inserted via the ECA into the ICA until the bifurcation of the left middle cerebral artery (MCA) and the anterior cerebral artery in order to block the circulation in the left MCA territory. After 1 h of vessel occlusion, blood flow was restored (reperfusion) by the withdrawal of the inserted suture. The rats were then sent for the 1st MRI scan and sacrificed with carbon dioxide gas after the 2nd MRI scan that was started at the 4th h after MCAO.
Dehydration protocols
A total of 25 Sprague-Dawley rats were randomly assigned to either the euhydration/MCAO or dehydration/MCAO group. Each rat in the dehydration group was individually housed in a metabolic cage for the 48 h prior to the MCAO surgery. Water deprivation for 48 h was accomplished by removing the water bottle from the cage (14, 15). Between the 1st and the 2nd MRI scans, rats randomized into euhydration group had free access to water while those in dehydration group were restricted from drinking water.
MRI acquisition and analysis
MRI was performed using a 9.4-T horizontal-bore animal MR scanning system (Biospec 94/20, Bruker, Ettlingen, Germany). The MRI were started at 1 and 4 h post MCAO for each rat to generate DWI ADC and ASL CBF maps with scan time around 40 min. Before the MR imaging, animals were anesthetized with 3.0% isoflurane in room air and were placed inside the magnet. During the MRI measurements, the anesthesia delivery to the rats was maintained using a gas mixture of ~1.5-2.0% isoflurane and oxygen. Animals were physiologically monitored throughout the MR imaging experiments.
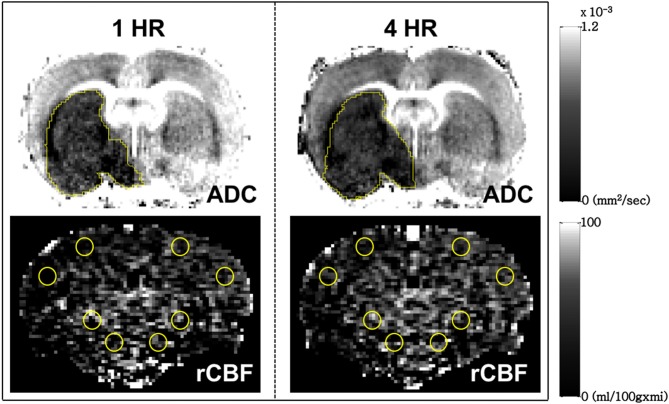
DWI scans were obtained to produce ADC maps using spin-echo EPI with the following parameters: matrix = 128 × 128; field of view = 2 × 2 cm2; TR = 3000 ms; echo time (TE) = 27 ms; number of slices = 9, which were 1-mm thick with a 0.5-mm gap; three directions = x, y, z; and b values = 0, 100, 300, 500, 800, and 1,000 s/mm2. The ASL CBF images were acquired using flow alternating inversion recovery (FAIR)-echo planar imaging (EPI) in a single coronal slice (the selection of the slice was based on our experience of the slice with a maximal area of infarction) with the following parameters: matrix = 96 × 96; field of view = 2 × 2 cm2; spectral width = 250 kHz; repetition time (TR) = 13000 ms; echo time (TE) = 16 ms; 3-mm selected slab thickness; 1-mm slices with 4-mm labeling coverage and 25 TI values. Twenty-five pairs of images (total time 11 min and 53 s) were acquired for signal averaging. The relative CBF (rCBF) map was calculated from the ASL images using the Algebra Tool on the Bruker console. The volume of the infarction and the ADC value were manually measured on the ADC map using polygon selection on Paravision 5.1 (Bruker, Ettlingen, Germany). The rCBF was measured by manual drawing circular regions of interest (ROI) on the ASL images. Four ROIs were selected in each cerebral hemisphere, with 2 of them located in cortical regions and the others in subcortical regions. The rCBF for each hemisphere was defined as the average values measured across the 4 ROIs (Figure 1).
Figure 1.
Measurement of infarct volume (Upper row) and relative cerebral blood flow (Lower row) by manual drawing regions of interest on the coronal view of the brain of a typical male Sprague Dawley rat used in this study. The relative cerebral blood flow for each hemisphere was defined as the average value measured from the 4 circular ROIs in cortical and subcortical brain regions.
Statistical analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS) statistical software (version 18, Chicago, IL, USA). Variables were expressed as the mean ± standard deviation and were compared by performing Mann–Whitney U tests. The correlation between progression of infarct volume and rCBF was tested by the Person correlation. The level of statistical significance was set at P < 0.05.
Results
Fifteen rats were included in dehydration group and 10 in control group. Five rats in the dehydration group died before the 1st MRI scan and other 2 died during the 1st MRI scan, thus, excluded. Three rats in control group completed the 1st MRI scan but died before the 2nd MRI scan; thus, only seven rats from the control group had data from the 2nd MRI scan. The results are shown in the Table 1. (The data of individual measurement are presented in the Supplementary Table 1 and Supplementary Figure 1). The mean ADC values of the selected areas were 456.1 and 441.6 10−6mm2/sec at the 1st h for euhydration and dehydration groups respectively, which confirmed the definition of tissue infarction. Although there was no significant difference between the volumes of the infarctions during the 1st h within each of the groups, the volume of the infarction was significantly larger in the dehydration group at the 4th h after MCAO (p = 0.040) than that of the control group. The absolute volume of the infarct progression between the 1st and 4th h was also larger in the dehydration group (p = 0.021). There were no significant differences between the measured absolute ADC values of each group at the 2 time points. The rCBF values of the infarct side were lower in dehydration group than in the control group at the 1st and 4th h after MCAO but did not reach statistical significance. On the contrary, the rCBF values of the contralateral normal side were significantly lower in the dehydration group than in the control group at the 1st and 4th h after MCAO (p = 0.027 and 0.040, respectively). However, the rCBF value at the 4th h and the rCBF ratio (infarct/normal), as well as the recovery of rCBF within 4 hours, were not significantly different between the two groups. There was a negative correlation between the rCBF of normal hemisphere at the 4th h and the progression of infarct size for all rats (Person correlation, r = −0.628, p = 0.012). In subgroup analysis, there was a moderate correlation between rCBF of normal hemisphere at the 4th h and the progression of infarct size for the dehydrated rats but did not reach statistical significance (r = −0.628, p = 0.095).
Table 1.
Comparison of infarct volume and CBF based on ADC and ASL in two groups.
| Control (n = 10 for 1 h and 7 for 4 h) | Dehydration (n = 8) | p-value | |
|---|---|---|---|
| Volume of infarction (mm3) | |||
| 1 h after MCAO | 39.4 (15.3) | 59.9 (46.2) | 0.573 |
| 4 h after MCAO | 75.9 (22.2) | 105.5 (46.3) | 0.040* |
| Ratio of progression | 1.46 | 2.34 | 0.121 |
| Absolute volume of progression | 24.1(9.4) | 45.6 (36.8) | 0.021* |
| ADC value (× 10−6 mm2/sec) | |||
| Infarct side | |||
| 1 h after MCAO | 456.1 (53.6) | 441.6 (54.9) | 0.573 |
| 4 h after MCAO | 525.5 (75.0) | 449.5 (46.3) | 0.867 |
| Normal side | |||
| 1 h after MCAO | 829.2 (45.6) | 789.4 (40.8) | 0.122 |
| 4 h after MCAO | 851.5 (41.3) | 816.0 (44.4) | 0.694 |
| Ratio (Infarct/Normal) | |||
| 1 h after MCAO | 0.55 | 0.56 | 0.573 |
| 4 h after MCAO | 0.61 | 0.55 | 0.955 |
| Relative CBF (ml/100 g−1min−1) | |||
| Infarct side | |||
| 1 h after MCAO | 10.6 (5.2) | 5.7 (2.6) | 0.101 |
| 4 h after MCAO | 10.9 (5.6) | 7.7 (3.1) | 0.397 |
| Normal side | |||
| 1 h after MCAO | 25.6 (5.5) | 19.8 (4.8) | 0.027* |
| 4 h after MCAO | 24.1 (2.8) | 20.7 (2.9) | 0.040* |
| Ratio (Infarct/Normal) | |||
| 1 h after MCAO | 0.42 | 0.30 | 0.408 |
| 4 h after MCAO | 0.44 | 0.37 | 0.613 |
| Recovery of perfusion in 4 h | |||
| Absolute value of infarct side | 1.1 (3.4) | 2.0 (2.6) | 0.189 |
| Absolute value of normal side | −0.1 (4.8) | 0.9 (4.4) | 0.121 |
| Ratio (4 h/1 h of infarct side) | 1.1 (0.3) | 1.6 (1.1) | 0.054 |
| Ratio (Infarct/Normal) | 1.1 (0.5) | 1.6 (1.3) | 0.336 |
p < 0.05.
Discussion
This study demonstrated that hydration status may be associated with the progression of infarct volume and the cerebral blood flow after MCAO. Although there was no significant difference between the initial infarct volumes, the volumes of the infarctions among the dehydrated rats were larger at 4th h. Interestingly, dehydration status had a negative impact on cerebral perfusion, which was more prominent in the contralateral normal hemisphere. This finding extended previously reported work that summarized the high prevalence of dehydration among stroke patients (2, 3), the significant impact of dehydration on early neurological deterioration and performance on disability scales (4, 6–8), by adding a physiological basis and imaging evidence. It also supports recent evidence indicating that hydration therapy should be a central feature of acute stroke management (1, 10, 16).
Dehydration and its associated changes in blood viscosity can result in decreased cerebral blood flow (17). Dehydration has been shown to impair cerebral autoregulation following exercise and during a cold pressure test, such that dehydration led to greater changes in mean middle cerebral artery velocity compared with normal hydration status (18, 19). The results of the current study are in line with these studies and provide the first line of evidence suggesting that dehydration status is associated with lower cerebral blood flow, as we observed in the non-infarct healthy hemisphere.
Only few imaging studies have provided imaging evidence regarding the effect of hydration status on the volume of the infarction and cerebral perfusion. In a human study using MRI, we demonstrated that the development of leptomeningeal collaterals after acute MCAO was associated with the patient's hydration status (9). In the current study, we evaluated the volume of infarction and cerebral perfusion with an MCAO rat model using high field MRI to better control for other factors. The reason for an insignificant difference in infarct volume between two groups at the 1st h after MCAO might be due to the pseudo-normalization phenomenon of ADC at early phase of reperfusion (20). Further histological studies are necessary to clarify this.
Our study, nevertheless, had several limitations. First, although we followed the dehydration protocol of previous studies with water deprivation for 48 h, we did not measure the body weight, blood, and urine parameters, such as osmolality and urine-specific gravity, to better quantify the severity of dehydration. Second, the mortality rate of the MCAO rats was very high, especially in the dehydration group. Approximately 50% of the rats died before the first MRI scan had been completed and were excluded from further analysis. This might be due to poor physiological status of the dehydrated rats before and after MCAO. This produces a potential study bias since rats with larger infarctions or more severe decreases in cerebral perfusion might be excluded. Third, this study did not completely fulfill the standard of good laboratory in allocation concealment. Fourth, the rCBF values were relatively low compare to the literatures. This might be due to the partial volume effect, insufficient inversion of the selective pulse, hypocapnia effect as well as noises from the MR system. Furthermore, rats randomized into dehydration group were isolated housed while 2 rats in euhydration group were housed together. This might result in different effect of social isolation.
In conclusion, this preliminary study demonstrated that dehydration status may be associated with the progression of infarct volume and the decrease in cerebral blood flow during the acute stage of ischemic stroke. This preliminary study provided an imaging clue that more intensive hydration therapies and reperfusion strategies are necessary for the management of acute ischemic stroke patients with dehydration status.
Author contributions
Y-HT: literature review, manuscript writing, tables making; L-CL: study design; I-NL and J-LY: animal study; C-HS and M-YY: imaging collection; Y-CH and H-HW: imaging analysis; J-TY: final manuscript review, and editing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by the Chang Gung Medical Research Fund, Chang Gung Memorial Hospital, Chiayi, Taiwan (CORPG6D0123, CORPG6D0153, and CMRPG6G0411).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00786/full#supplementary-material
References
- 1.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2013) 44:870–947. 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez GJ, Cordina SM, Vazquez G, Suri MFK, Kirmani JF, Ezzeddine MA, et al. The hydration influence on the risk of stroke (THIRST) study. Neurocrit Care (2009) 10:187–94. 10.1007/s12028-008-9169-5 [DOI] [PubMed] [Google Scholar]
- 3.Rowat A, Graham C, Dennis M. Dehydration in hospital-admitted stroke patients: Detection, frequency, and association. Stroke (2012) 43:857–9. 10.1161/STROKEAHA.111.640821 [DOI] [PubMed] [Google Scholar]
- 4.Lin LC, Hsiao K-Y, Tsai YH, Lai SL, Lei CC, Hsiao CT. Hydration status and stroke-in-evolution after ischemic stroke: a preliminary study. Int J Stroke (2013) 8:E52. 10.1111/ijs.12114 [DOI] [PubMed] [Google Scholar]
- 5.Lin LC, Yang JT, Weng HH, Hsiao CT, Lai SL, Fann WC. Predictors of early clinical deterioration after acute ischemic stroke. Am J Emerg Med. (2011) 29:577–81. 10.1016/j.ajem.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 6.Bhalla A, Sankaralingam S, Dundas R, Swaminathan R, Wolfe CD, Rudd AG. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke (2000) 31:2043–8. 10.1161/01.STR.31.9.2043 [DOI] [PubMed] [Google Scholar]
- 7.Liu CH, Lin SC, Lin JR, Yang JT, Chang YJ, Chang CH, et al. Dehydration is an independent predictor of discharge outcome and admission cost in acute ischaemic stroke. Eur J Neurol. (2014) 21:1184–91. 10.1111/ene.12452 [DOI] [PubMed] [Google Scholar]
- 8.Schrock JW, Glasenapp M, Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg. (2012) 114:881–4. 10.1016/j.clineuro.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 9.Chang S-W, Huang Y-C, Lin L-C, Yang J-T, Weng H-H, Tsai Y-H, et al. Effect of dehydration on the development of collaterals in acute middle cerebral artery occlusion. Eur J Neurol. (2016) 23:494–500 10.1111/ene.12841 [DOI] [PubMed] [Google Scholar]
- 10.Suwanwela NC, Chutinet A, Mayotarn S, Thanapiyachaikul R, Chaisinanunkul N, Asawavichienjinda T, et al. A randomized controlled study of intravenous fluid in acute ischemic stroke. Clin Neurol Neurosurg. (2017) 161:98–103. 10.1016/j.clineuro.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 11.Paczynski RP, Venkatesan R, Diringer MN, He YY, Hsu CY, Lin W. Effects of fluid management on edema volume and midline shift in a rat model of ischemic stroke. Stroke (2000) 31:1702–8. 10.1161/01.STR.31.7.1702 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y-C, Liu H-L, Lee J-D, Yang J-T, Weng H-H, Lee M, et al. Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MRI in patients with acute stroke. PLoS ONE (2013) 8:e69085. 10.1371/journal.pone.0069085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke (1989) 20:84–91. 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 14.Faraco G, Wijasa TS, Park L, Moore J, Anrather J, Iadecola C. Water deprivation induces neurovascular and cognitive dysfunction through vasopressin-induced oxidative stress. J Cereb Blood Flow Metab. (2014) 34:1–9. 10.1038/jcbfm.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. Dehydration Parameters and Standards for Laboratory Mice. J Am Assoc Lab Anim Sci. (2013) 52:233–9. 10.1371/journal.pone.0020691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CJ, Tsai YY, Hsiao KY, Tsai YH, Lee MH, Huang YC, et al. Urine-specific gravity-based hydration prevents stroke in evolution in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:1885–91. 10.1016/j.jstrokecerebrovasdis.2017.06.044 [DOI] [PubMed] [Google Scholar]
- 17.Muizelaar JP, Wei EP, Kontos HA, Becker DP. Cerebral blood flow is regulated by changes in blood pressure and in blood viscosity alike. Stroke (1986) 17:44–8. 10.1161/01.STR.17.1.44 [DOI] [PubMed] [Google Scholar]
- 18.Moralez G, Romero SA, Rickards CA, Ryan KL, Convertino VA, Cooke WH. Effects of dehydration on cerebrovascular control during standing after heavy resistance exercise. J Appl Physiol. (2012) 112:1875–83. 10.1152/japplphysiol.01217.2011 [DOI] [PubMed] [Google Scholar]
- 19.Perry BG, Bear TLK, Lucas SJE, Mündel T. Mild dehydration modifies the cerebrovascular response to the cold pressor test. Exp Physiol. (2016) 101:135–42. 10.1113/EP085449 [DOI] [PubMed] [Google Scholar]
- 20.Rojas S, Martín A, Justicia C, Falcón C, Bargalló N, Chamorro Á, et al. Modest MRI signal intensity changes precede delayed cortical necrosis after transient focal ischemia in the rat. Stroke (2006) 37:1525–32. 10.1161/01.STR.0000221713.06148.16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.